Abstract
Progestins may have effects to reduce depressive behavior, in part through actions of its metabolite, 5α-pregnan-3α-ol-20-one (3α,5α-THP) at GABAA receptors, rather than through intracellular progestin receptors. In this study, we examined the effects of progesterone (10 mg/kg, subcutaneous injection) versus vehicle control (propylene glycol) on the depressive behavior of young and aged mice in the tail suspension test. In Experiment 1, we first characterized progesterone's anti-depressant effects by utilizing young (4–6-month-old) intact or ovariectomized female, and intact or gonadectomized male, C57BL/6 mice. Young female mice showed more depressive behavior than the young male mice. Compared with vehicle administration, progesterone reduced depressive behavior of ovariectomized female, but not male or intact female mice. In Experiment 2, mice were aged (20–24-month-old) intact wild type or progestin receptor knockout mice. Progestin receptor knockout mice showed less depressive behavior than wild type mice. Administration of progesterone to wild type and progestin receptor knockout mice reduced depressive behavior. Together, these data suggest that progesterone can decrease depressive behavior of young adult ovariectomized female, aged wild type and progestin receptor knockout mice. Thus, progesterone's effect to reduce depressive behavior of aged mice may not require actions at the intracellular progestin receptors.
Keywords: aging, allopregnanolone, depression, neurosteroids, non-genomic, senescent
Introduction
Depression is a serious and widespread mental disorder that may be influenced by many factors including steroid hormones. Findings have suggested that progestins may have effects upon depressive behaviors in both people and animals. Women, compared with men, who do not experience profound changes in progestins, are more susceptible to depression and/or anxiety disorders. Throughout the life-span, women experience varied, and occasionally dramatic, changes in their hormonal and reproductive cycles. Among some women, hormonal and/or reproductive events may influence the onset or expression of depression and/or anxiety disorders, such as premenstrual syndrome, premenstrual dysphoric disorder (PMDD), and post-partum depression, syndromes which occur when endogenous progestin levels are changing (Angst et al., 2001; Backstrom et al., 2003; Endicott et al., 1999; Glick and Bennett, 1981; Markou et al., 2005; Pearlstein et al., 2005; Rapkin et al., 2002). Indeed, among some individuals, progesterone may exacerbate rather than mitigate, symptoms of PMDD, depression, mood or bipolar disorder (Freeman et al., 2002, 2009; Hardoy et al., 2006). However, among others, natural, age-related decline in steroid hormones may be associated with increased vulnerability to depression and/or suicidality (Lebowitz et al., 2006). Thus, progestins have been implicated in the pathophysiology of some depressive disorders.
Findings from animal models also support a role for progesterone in depressive behaviors. For example, when rodents are in the proestrous phase of the estrous cycle and have high levels of progestins, they show less depressive behavior compared with rodents that are in low progestin phases of their cycle (Barros and Ferigolo, 1998; Becker and Cha, 1989; Bitran et al., 1991; Frye et al., 2000; Gulinello et al., 2003; Marvan et al., 1997; Walf et al., 2006b). Pregnant rats, which have sustained, higher levels of progestins, show less depressive behavior in the forced swim test than do post-parturient rats, with lower levels of progestins (Frye and Walf, 2004). Ovariectomy (OVX), removal of the primary source of endogenous ovarian hormones, increases depressive behaviors of female rats in the forced swim test (Bekku and Yoshimura, 2005; Bekku et al., 2006; Frye and Walf, 2002; Frye and Wawrzycki, 2003; Walf et al., 2006b) and administration of progesterone can reverse these effects (Espallergues et al., 2009; Frye and Walf, 2004, 2009; Frye et al., 2004; Walf et al., 2006b). Thus, progesterone may reduce depressive behaviors of mice (albeit these effects of progesterone are associated with changes in estradiol as well).
Progesterone's actions may occur, in part, through its classic membrane receptors, progestin receptors (PRs). Mifepristone (RU486), a duel PR and glucocorticoid receptor (GR) agonist, is being investigated clinically for its effects at reducing psychotic depressive symptoms (Blasey et al., 2009; Van Look and von Hertzen, 1995). Its antidepressant effects are reported within a week of its use in psychotic depression and it demonstrates efficacy in other mood and cognitive disorders through these mechanisms (DeBattista and Belanoff, 2006). Animal models confirm clinical studies, where RU486 has rapid antidepressant effects through synaptic alternation in the hippocampus (Murphy, 1997; Wu et al., 2007). However, whether these effects are via PRs or GRs cannot be determined. In fact, its antidepressant effects are often described as being mediated through GRs (Gallagher et al., 2008; Murphy, 1997; Nihalani and Schwartz, 2007), which implies there may be a PR-independent mechanism for some of progesterone's antidepressant effects.
Interestingly, recent clinical findings indicate that progesterone's antidepressant effects may involve actions of its metabolite, 5α-pregnan-3α-ol-20-one (3α,5α-THP). Among some women at menopause, reduced levels of 3α,5α-THP and other neurosteroids have been associated with depression and other mood disorders (Freeman et al., 2002; Girdler et al., 2001; Pearlstein, 1995). Some patients with depressive disorders have reduced plasma concentrations and/or cerebrospinal fluid (CSF) levels of 3α,5α-THP (Romeo et al., 1998; Stahl, 1997; Uzunova et al., 1998). Administration of antidepressants, such as fluoxetine or fluvoxamine, normalize decreased 3α,5α-THP concentrations concomitant with reducing depressive symptomology (Uzunova et al., 2004, 2006). However, recent findings also suggest that the increased CSF levels of 3α,5α-THP may represent non-specific pharmacologic effects of antidepressants, rather than markers of therapeutic response (Schule et al., 2007). Thus, further investigation of 3α,5α-THP's role in affective processes is needed.
Progesterone's antidepressant effects are related to actions of 3α,5α-THP. First, progesterone and/or 3α,5α-THP similarly decrease depressive behavior of rodents (Frye and Walf, 2002; Hirani et al., 2002; Walf and Frye, 2007). Second, the antidepressant effects of progesterone are reduced when drugs that block conversion of progesterone to 3α,5α-THP are co-administered (Hirani et al., 2002; Walf et al., 2006b). Third, the antidepressive effect of progesterone in the forced swim test are attenuated among 5α-reductase knockout mice that cannot convert progesterone to 3α,5α-THP (Frye et al., 2004). In other animal models of depression, socially isolating mice decreases 3α,5α-THP levels, but can be reversed by progesterone or 3α,5α-THP administration (Dong et al., 2001; Guidotti et al., 2001). 3α,5α-THP has actions through gamma-aminobutyric acid (GABAA)/benzodiazepine receptors complexes (GBRs) rather than via traditional actions at intracellular PRs (Bethea et al., 2002; Gulinello, et al., 2003; Lan and Gee, 1994; Lancel et al., 1996; Nicol et al., 1999). Thus, progesterone's actions on mood and/or anxiety-related behaviors may be dependent on conversion to 3α,5α-THP and some of these actions may be mediated by GBRs rather than by classical actions at PRs.
It is important to understand the nature of progesterone's effects on depressive-like behaviors and possible underlying mechanism by which progesterone has antidepressive effects. In this experiment, we utilized young C57BL/6 and aged PR knockout (PRKO) and wild type mice to investigate whether progesterone's effects on depressive behavior in the tail suspension test require the PRs. We hypothesized that if progesterone has effects that reduce depressive behavior, then administration of progesterone should reduce mice immobility in the tail suspension task. Further, if progesterone's antidepressant effects occur independently of actions at the PRs, then administration of progesterone to wild type and PRKO mice should similarly reduce immobility.
Methods
Animals and housing
The paradigm was pre-approved by the Institutional Animal Care and Use Committee at the University at Albany – SUNY.
Mice were group-housed in a room with a reversed 12/12 hour light/dark cycle (lights on 08:00). Animals had free access to Purina rat chow (RHM #3000) and water in their home cages.
In Experiment 1, young C57BL/6 mice (N = 56), between 4 and 6 months old, were bred in the Life Science Laboratory Animal Care Facility at the University at Albany – SUNY. Mice were randomly assigned to gonadal status (intact or castrated). Female mice were intact (n = 14) or OVX (n = 14) mice. Male mice were intact (n = 14) or gonadectomized (GDX) (n = 14). Seven mice in each condition were randomly assigned to be administered progesterone and the remaining seven in each condition were administered vehicle.
In Experiment 2, wild type or PRKO mice (N = 14), between 20 and 24 months old, were bred in the Social Sciences Building Animal Care Facility at the University at Albany – SUNY. Wild type (female n = 4 and male n = 3) or PRKO mice (female n = 3 and male n 4) remained intact due to their age, and the possibility of attrition associated with surgery. These mice were breeders that had been retired and were subsequently used in these experiments when our colonies had to be taken down because of a laboratory move. Regrettably, no younger counterparts were available for the PRKOs.
Genotype
Wild type and PRKO mice were genotyped by genomic DNA isolated from tails and subsequent analyses by polymerase chain reaction, according to methods previously described (Frye et al., 2006b). The following PR-specific primers were used: P1(5′-TAGACAGTGTCTTAGACTCGTTGTTG-3′),P2(5′GATGGGCACATGGATGAAATC-3′), and a neo gene-specific primer, N2 (5′-GCATGCTCCAGACTGCCTTGGGAAA-3′). Bands of approximately 565 and 500 base pairs were amplified for wild type and PRKO mice.
Surgery
All young mice (Experiment 1) were operated on around 3–4 months of age. Briefly, mice were administered sodium pentobarbital anesthesia (70 mg/kg). Females were either OVX (n = 14) or sham-operated (n = 14), and males were either GDX (n = 14) or sham-operated (n = 14). As with previous methods, female mice require 1–2 weeks after OVX for physiological levels of estrogen (E2) and progestins to be reduced (Frye and Walf, 2008; Walf et al., 2008a, b), while male mice require 4–6 weeks to clear endogenous androgens following GDX (Frye and Walf, 2009). After these time periods, mice were randomly assigned to progesterone or vehicle condition and were behaviorally tested (described below).
In Experiment 2, aged wild type or PRKO mice (between 20 and 24 months) remained intact for the following reasons. First, aged mice have significantly lower levels of sex hormones than younger mice (Nelson et al., 1992; Perrot-Sinal et al., 1998). Second, surgery may produce greater rates of attrition in aged mice compared with their younger counterparts. Thus, aged mice remained intact.
Hormone administration
All mice were randomly assigned to either progesterone (10 mg/kg) or vehicle (propylene glycol) condition. Progesterone was obtained from Sigma Chemical Co. (St Louis, MO, USA) and dissolved in propylene glycol to a concentration of 10 mg/ml. Mice received subcutaneous progesterone or vehicle injection 1 h prior to behavioral testing. This progesterone regimen produces levels of 3α,5α-THP around 35 pmol/g; it increases GABA-stimulated chloride influx in cortical synaptoneurosomes, and muscimol binding in the hippocampus among aged (20–24 months of age) wild type or PRKO mice (Frye et al., 2006a, b). We, and others, found that PRKO mice have decreased PR binding compared with their wild type counterparts (Frye et al., 2006a, b; Mani et al., 1997). Moreover, this progesterone regimen does not produce gross alterations in locomotion and/or coordination (Frye et al., 2006a). After mice were injected with progesterone (10 mg/kg) or vehicle, they were returned to their home cages for 1 h until behavioral testing.
Tail suspension
The tail suspension test is an animal model of depressive behavior, which was introduced as a screening test for antidepressant activity by Steru et al. (1985). There are several advantages of the tail suspension test for evaluating depressive behavior of rodents. First, the procedure is a very simple and objective test. Second, the results from this approach are in agreement with those from other validated animal models of depression, such as the Porsolt forced swim test (Steru et al., 1985). Moreover, a critical analysis by Porsolt (Porsolt et al., 1977), indicates that tail suspension and behavioral despair are the best animal model of depression, based on non-dependence on a mechanism, pharmacological validity, existence of genetic determinants, availability of a mouse version, procedural simplicity, and reproducibility. In this experiment, we utilized the procedures of Steru et al. (1985). We used a slightly modified version that was introduced by Steru. Briefly, mice were individually suspended 45 cm above an apparatus (50 cm high × 19 cm wide), (Stoelting Co., IL, USA) (Bilkei-Gorzo et al., 2002) by a gauze-covered binder clip affixed 1 cm from the tip of the tail (Kulkarni and Dhir, 2008; Zhang et al., 2008). For 6 min, the total duration of immobility (freezing time) was recorded each minute as an indicator of depressive behavior. Immobility is a typical motionless position defined as the absence of active behaviors (i.e. searching behavior with intense movements, such as struggling). Following the test, mice were removed from the tail suspension apparatus and returned to their home cages.
In Experiment 2, limited numbers of aged wild type and PRKO mice were available. Given this, a two-week, repeated-measure design was utilized. Therefore, once mice were tested in either the progesterone or vehicle condition, they were re-tested in the opposite condition the following week. Whether progesterone or vehicle was received initially was counterbalanced across subjects (Frye et al., 2006b). There was no evidence for test decay between performances in the first and second week of testing.
Data analyses
For Experiment 1, a three-way analysis of variance (ANOVA) was utilized to evaluate the effects of sex (female, male), gonadal status (intact, OVX/GDX), and hormone condition (progesterone, vehicle). For Experiment 2, a two-between (sex; female, male; genotype; PRKO, wild type), one-within (condition; progesterone or vehicle), factor ANOVAs were used. The alpha level for statistical significance was p < 0.05. Where appropriate, ANOVAs were followed by Fisher's post-hoc tests to determine group differences.
Results
Experiment 1: young mice
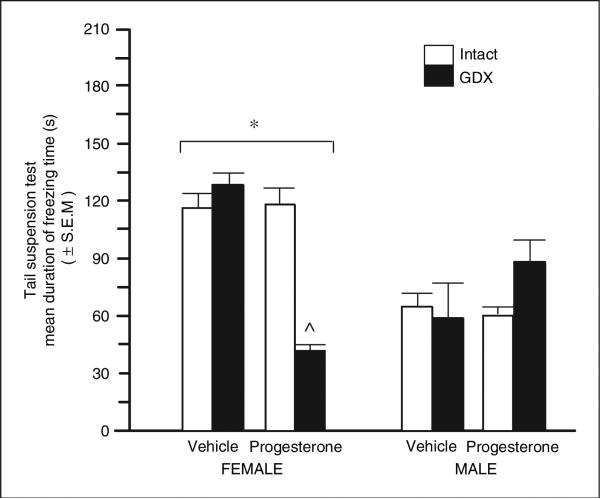
There was a significant effect of sex (F(1,48) = 17.26, p = 0.001). Female mice showed more depressive behavior (mean immobility = 104 ± 7 s) than did male mice (69 ± 7 s). There was a significant interaction between sex, gonadal status, and progesterone (F(1,48) = 10.59, p = 0.002) that was due to progesterone significantly decreasing depressive behavior only among OVX female mice (Figure 1).
Figure 1.
Mean duration of freezing (s) in the tail suspension test of young adult (4–6 months of age) intact (white bars) or ovariectomized (black bars) female (left; n = 7 in each condition) and male young adult intact or gonadectomized (GDX) (right; n = 7 in each condition) C57/BL6 mice administered vehicle control (inset left) or progesterone (inset right). *Denotes a significant main effect for females and males to differ (p < 0.05), ^Indicates significant interaction wherein progesterone decreased depressive behaviors of female GDX mice but not other groups.
Experiment 2: aged PRKO mice
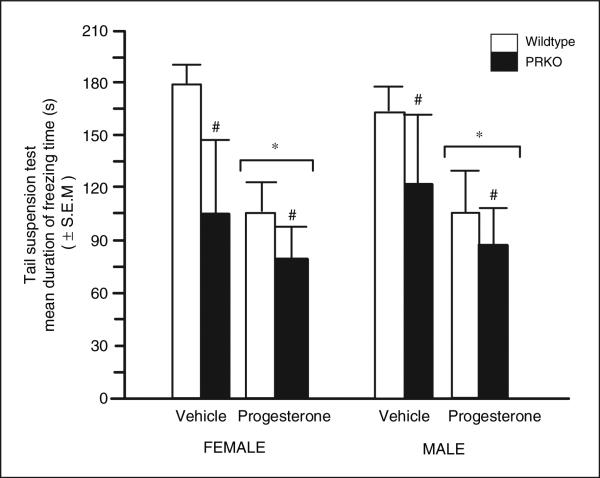
Progesterone (F(1,10) = 6.43, p = 0.02) and genotype (F(1,10) = 5.30, p = 0.04), but not sex, significantly influenced immobility time of aged wild type and PRKO mice. Progesterone (mean immobility = 93.6 ± 21.1 s), compared with vehicle (141.7 ± 26.9 s), decreased immobility in the tail suspension task. PRKOs showed less immobility (96.1 ± 30.8 s) than did wild type mice (138.8 ± 17.3 s). Aged female or male wild type and PRKO mice demonstrated less depressive behavior in response to progesterone, compared with vehicle, administration (Figure 2).
Figure 2.
Mean duration of freezing (s) in the tail suspension of aged adult (20–24 months of age) intact female (left panel; wild type n = 4, middle left; progestin receptor knockout (PRKO) n = 3) and intact male (right panel; wild type n = 3, far right panel; PRKO n = 4) mice administered vehicle control (inset left) or progesterone (inset right). #Denotes a significant main effect for wild type and PRKO mice to differ (p < 0.05). *Denotes a significant main effect for vehicle and progesterone-administered mice to differ (p < 0.05).
Discussion
The present results are consistent with the hypothesis that progesterone can decrease depressive behavior and that actions through the PRs may not be required for acute effects of progesterone in aged mice. In support, young female mice showed more depressive behavior than young male mice. Compared with vehicle administration, progesterone to young OVX mice, but neither intact female, nor male mice, significantly reduced freezing time. Administration of progesterone to aged intact female and male wild type and PRKO mice reduced freezing time. Together, these data suggest that progesterone may have effects that reduce depressive behavior in OVX females and that some of these effects of progesterone may be independent of actions at the PRs.
The results of this study confirmed previous findings that female rodents show more depressive behavior than male rodents. Here, we observed more depressive behavior among young female mice than young male mice in the tail suspension test. These findings are analogous to those of others using different animal models. In the forced swim test, female rats, compared with males, have a longer duration of immobility (Frye and Walf, 2004; Paré and Redei, 1993; Walf et al., 2006b). Moreover, in the forced swim test, males show more recuperative behavior, such as head swinging, than do females (Drossopoulou et al., 2004). In a novel animal model of depression, young female Wistar rats showed more indications of vulnerability to depression induction than did males (Sun and Alkon, 2006). Thus, as seen in clinical studies and other animal models of depression, young female mice showed more depressive behavior than did young male mice.
Despite young female mice showing more depression behavior than young male mice, progesterone only had significant antidepressant effects among young OVX mice. One explanation for this may be that changes in the levels of ovarian hormones over the estrous and/or reproductive cycles may have influenced depressive behavior of intact female mice. For example, when rodents have high levels of ovarian hormones, such as occurs during proestrous or pregnancy, less depressive behavior is observed in the forced swim test compared with that of rats with low ovarian hormones (i.e. diestrous, post-parturient, or male rats) (Frye and Walf, 2004; Walf and Frye, 2007; Walf et al., 2006b). Rats that were three days post-partum displayed more depressive behavior than did cycling rats (Molina-Hernández et al., 2000). One drawback of the present experiment is that we neither controlled, nor evaluated, the phases of the estrous cycle in which young, intact female mice were tested. Despite this limitation, we did examine behavior of intact aged female mice that were between 20 and 24 months of age. Notably, about 80% of 17-month-old female C57BL/6NIA mice had irregular or absent cycles and 100% of their 25-month-old counterparts were acyclic (Frick et al., 2000). Based upon these findings, we expect that the majority of aged intact mice from Experiment 2 had very low levels of sex hormones, compared with young intact female mice. We observed that aged mice in Experiment 2 generally demonstrated more depressive behavior than did younger mice from Experiment 1. Although the aged female mice might be comparable to the OVX younger ones in terms of gonadal hormonal status, that does not explain the difference between the GDX young male mice and the aged intact males.
Just as endogenous decline in gonadal steroids with aging may precipitate vulnerability to increases in depressive behavior, extirpation of the gonads may also increase the likelihood of demonstrating depressive behavior. In this experiment, OVX increased depressive behavior over that of intact female mice. Progesterone administration to OVX, but not intact female and male mice, significantly reduced depressive behavior compared with vehicle administration. These findings are similar to those of previous reports that OVX increases depressive behaviors over that of intact and/or sham-operated rodents and administration of ovarian hormones can reverse these depressant effects (Bekku et al., 2006; Bernardi et al., 1989; Galea et al., 2001; Stoffel and Craft, 2004). Indeed, acute administration of progesterone to OVX rats increases antidepressive behavior in the forced swim test (Frye and Walf, 2002). Notably, the regimen of progesterone that has previously demonstrated antidepressant effects in the forced swim test when administered to young OVX mice, involves a higher concentration of progesterone (50, 1000, or 10,000 μg/kg) and chronic administration (subcutaneous injections four times daily) (Bernardi et al., 1989), compared with our dosing (10 mg/kg subcutaneous injection 1 h prior to testing. Previously, we observed that this same progesterone regimen increases anti-anxiety behavior of young and aged wild type/PRKO mice (Frye et al., 2006a). The pattern and magnitude of progesterone's effects are clearly consistent across our studies, which speaks to the veracity of the results, despite the small sample size and the large variability in the response to progesterone. In contrast, others have shown that acute administration of progesterone (10 mg/kg) to female intact albino mice (Laca strain) produced significant increases in immobility in the forced swim test (Kaur and Kulkarni, 2002). Given that there are well-known differences between mouse strains in response to steroid administration and their behavioral effects (Svare, 1988), further investigations of dose-dependent effects of progesterone will be required to elucidate a clearer understanding of the nature of progesterone's antidepressant effects.
Progesterone's metabolite, 3α,5α-THP, may underlie some of progesterone's antidepressant effects. Our work, and others, has elucidated progesterone, which binds to PRs, as having antidepressant effects (Espallergues et al., 2009; Frye and Walf, 2009). However, recent reports of progestins’ effects on increasing anti-anxiety and antidepressive behavior suggest that these effects are mediated in part through actions at GBRs rather than via progesterone's traditional actions through PRs (Bethea et al., 2002; Gulinello et al., 2003; Lan and Gee, 1994; Lancel et al., 1999; Nicol et al., 1999). 3α,5α-THP is one of the most potent positive allosteric modulators of GBRs (Compagnone and Mellon, 2000; Lambert et al., 1995; Paul and Purdy, 1992) and interacts with GBRs at low concentrations to increase the frequency and/or duration of openings of GABA-gated chloride channels (Lambert et al., 1995; Majewska et al., 1986; Paul and Purdy, 1992) or by changing specific GBR subunit gene expression (Guidotti et al., 2001). In Experiment 2, progesterone administration to aged wild type and PRKO mice reduced depressive behavior in the tail suspension test. In our previous investigation of anti-anxiety effects in aged wild type and PRKO mice, we (and previously others) found that PRKO mice are devoid of PRs (Frye et al., 2006b; Mani et al., 1997), but they readily convert progesterone to 3α,5α-THP and have enhanced GABA-stimulated chloride influx (Frye et al., 2006a). 3α,5α-THP's effects to reduce depressive behavior of rodents can be blocked with the co-administration of GBR antagonists in the forced swim test (Hirani et al., 2002; Rodríguez-Landa et al., 2007). Thus, progesterone's depression-reducing effects may not require actions at the PRs.
Other animal models and clinical studies indicate that 3α,5α-THP has antidepressant effects. Administration of 3α,5α-THP to OVX rats reduces duration of immobility in the forced swim test (Frye and Walf, 2004). Moreover, these effects of 3α,5α-THP in the forced swim test are observed when it is administered intraperitoneally, intracerebroventricularly (Khisti and Chopde, 2000; Khisti et al., 2000), or directly to the nucleus accumbens (Molina-Hernandez et al., 2005). Further, high levels of 3α,5α-THP are observed in the hippocampus of proestrous female rats, which show reduced immobility time in the forced swim test (Frye and Walf, 2002; Frye et al., 2000). 3α,5α-THP has also been implicated in clinical depression. Some antidepressant medications enhance the production of 3α,5α-THP (Frye and Seliga, 2003; Griffinn and Mellon, 1999; Marx et al., 2003). Patients with severe depression have decreased CSF levels of 3α,5α-THP, which is normalized by treatment with a fluoxetine regimen that improves depressive symptomology concomitant with increasing 3α,5α-THP levels (Guidotti et al., 2001). Thus, progesterone's antidepressant effects may be due, in part, to 3α,5α-THP's actions through the GBRs.
It is particularly important to investigate the effect of progestins on depressive behavior, and examine the ability of aged animals to respond to hormonal manipulations in order to understand fully the costs and benefits of the hormone's effects on depression. Together with data from animal models, levels of progesterone and depression-like behavior among some women need to be considered. Women experience changes in their levels of progestins through their life-span and reproductive cycle. For instance, levels of 3α,5α-THP increase just before the onset of puberty (Fadalti et al., 1999). Across the menstrual cycle, the levels of 3α,5α-THP remain low in the follicular phase and are elevated in the mid-luteal phase (Rapkin et al., 1997; Schmidt et al., 1994). The onset of premenstrual syndrome, which includes psychological symptoms such as anxiety, depression and irritability, is variable but most of these negative symptoms occur during the late luteal phase, when the levels of progestins are low (Angst et al., 2001; Endicott et al., 1999; Hardoy et al., 2008; Pearlstein et al., 2005; Rapkin et al., 1997, 2002; Soares and Zitek, 2008). During pregnancy, levels of 3α,5α-THP reach their highest, and this is in part due to increased progesterone production from the corpus luteum during the first 3 months, which is the trimester most often associated with increased anxiety and/or depression (Paoletti et al., 2006). Further, there are reports of non-significant findings in changes in estrogen and progesterone levels in women experiencing post-partum depression, albeit the authors did not analyze 3α,5α-THP levels, which may have yielded different results (Klier et al., 2007). The levels of ovarian hormones start to change perimenopausally and circulating levels of ovarian hormones decline within a few years of menopause (Ballinger et al., 1987; Longcope, 1990; Markou et al., 2005). This menopausal transition is associated with increased incidence of affective and depressive symptoms (Markou et al., 2005). Some premenopausal and menopausal women experience negative physical and/or negative neuropsychological symptoms (Joffe et al., 2003; Pearlstein et al., 1997), which can be reversed by hormone therapy (Fitzpatrick et al., 2000; Sherwin, 1991; Sherwin and Gelfand, 1989). Among some women, anxiety or panic disorders emerge with increased emotional activities during the postmenopausal period (Claudia et al., 2004). Given that the population is aging and women are living longer in a state of ovarian hormone decline, it is essential to understand more about the effects and mechanisms by which hormones mediate depression.
In sum, our current experiment indicates that administration of progesterone to young mice and aged wild type and PRKO mice reduced depressive behavior in the tail suspension test. These findings imply that progesterone's effects may be in part independent of actions at the PRs and may involve 3α,5α-THP's action through the GBRs. These findings are relevant in elucidating the role of progestins in the etiology, therapeutic treatment, and/or prognosis of depressive disorders. Information on the role and actions of progestins in menopause is lacking. As such, further research is required to understand fully the effect of progestins in reducing depressive behavior and/or depression-like symptoms. Although the present findings indicate actions at the PRs may not be required for antidepressant effects in mice, in humans the PR and GR agonist RU486 seems to have therapeutic efficacy. Indeed, a multisite trial indicated that patients with plasma levels of RU486 >1800 ng/ml were more likely to show improvements in their psychotic depression than did placebo controls (Blasey et al., 2009). However, the effects of RU486 on PRs versus GRs cannot be parsed out, and evidence remains strong and consistent for antidepressive effects of 3α,5α-THP as the likely mediator behind progesterone's antidepressant effects.
Acknowledgement
This research was supported by grants from the National Science Foundation (IBN98-96263, IBN03-16083) and the National Institute of Mental Health (MH06769801). I am very grateful to Dr Bert W O'Malley and Dr John P Lydon for the mice that they provided for this research. Kanako Sumida and Mary Unger provided technical assistance.
References
- Angst J, Sellaro R, Merikangas KR, Endicott J. The epidemiology of premenstrual psychological symptoms. Acta Psychiatr Scand. 2001;104:110–116. doi: 10.1034/j.1600-0447.2001.00412.x. [DOI] [PubMed] [Google Scholar]
- Backstrom T, Andreen L, Birzniece V, et al. The role of hormones and hormonal treatments in premenstrual syndrome. CNS Drugs. 2003;17:325–342. doi: 10.2165/00023210-200317050-00003. [DOI] [PubMed] [Google Scholar]
- Ballinger CB, Browning MC, Smith AH. Hormone profiles and psychological symptoms in peri-menopausal women. Maturitas. 1987;9:235–251. doi: 10.1016/0378-5122(87)90006-5. [DOI] [PubMed] [Google Scholar]
- Barros HM, Ferigolo M. Ethopharmacology of imipramine in the forced-swimming test: gender differences. Neurosci Biobehav Rev. 1998;23:279–286. doi: 10.1016/s0149-7634(98)00029-3. [DOI] [PubMed] [Google Scholar]
- Becker J, Cha J. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989;35:117–125. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- Bekku N, Yoshimura H. Animal model of menopausal depressive-like state in female mice: prolongation of immobility time in the forced swimming test following ovariectomy. Psychopharmacology (Berl) 2005;183:300–307. doi: 10.1007/s00213-005-0179-0. [DOI] [PubMed] [Google Scholar]
- Bekku N, Yoshimura H, Araki H. Factors producing a menopausal depressive-like state in mice following ovariectomy. Psychophamarmacology (Berl) 2006;187:170–180. doi: 10.1007/s00213-006-0395-2. [DOI] [PubMed] [Google Scholar]
- Bernardi M, Vergoni A, Sandrini M, Tagliavini S, Bertolini A. Influence of ovariectomy, estradiol and progesterone on the behavior of mice in an experimental model of depression. Physiol Behav. 1989;45:1067–1068. doi: 10.1016/0031-9384(89)90238-2. [DOI] [PubMed] [Google Scholar]
- Bethea C, Lu N, Gundlah C, Streicher J. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Michel K, Zimmer AJ. Diminished anxiety- and depression-related behaviors in mice with selective deletion of the Tac1 gene. Neurosci. 2002;22:10046–10052. doi: 10.1523/JNEUROSCI.22-22-10046.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Hilvers R, Kellogg C. Ovarian endocrine status modulates the anxiolytic potency of diazepam and the efficacy of gamma-aminobutyric acid-benzodiazepine receptor-mediated chloride ion transport. Behav Neurosci. 1991;105:653–662. doi: 10.1037//0735-7044.105.5.653. [DOI] [PubMed] [Google Scholar]
- Blasey CM, Debattista C, Roe R, Block T, Belanoff JK. A multi-site trial of mifepristone for the treatment of psychotic depression: a site-by-treatment interaction. Contemp Clin Trials. 2009;30:284–288. doi: 10.1016/j.cct.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Claudia P, Andrea C, Chiara C, et al. Panic disorder in menopause: a case control study. Maturitas. 2004;48:147–154. doi: 10.1016/j.maturitas.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- DeBattista C, Belanoff J. Mifepristone, versus placebo in the treatment of psychosis in patients with psychotic major depression. Biol Psychiatry. 2006;12:1343–1349. doi: 10.1016/j.biopsych.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, et al. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci U S A. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossopoulou G, Antoniou K, Kitraki E, et al. Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in rats. Neuroscience. 2004;126:849–857. doi: 10.1016/j.neuroscience.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Endicott J, Amsterdam J, Eriksson E, et al. Is premenstrual dysphoric disorder a distinct clinical entity? J Womens Health Gend Based Med. 1999;8:663–679. doi: 10.1089/jwh.1.1999.8.663. [DOI] [PubMed] [Google Scholar]
- Espallergues J, Givalois L, Temsamani J, Laruelle C, Maurice T. The 3B-hydroxysteriod dehydrogenase inhibitor trilostane shows antidepressant properties in mice. Psychoneuroendocrinology. 2009;34:644–659. doi: 10.1016/j.psyneuen.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Fadalti M, Petraglia F, Luisi S, et al. Changes of serum allopregnanolone levels in the first two years of life and during pubertal development. Pediatr Res. 1999;46:323–327. doi: 10.1203/00006450-199909000-00013. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick LA, Pace C, Wiita B. Comparison of regimens containing oral micronized progesterone or medroxyprogesterone acetate on quality of life in postmenopausal women: a cross-sectional survey. J Womens Health Gend Based Med. 2000;9:381–387. doi: 10.1089/15246090050020691. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Frye CA, Rickels K, Martin PA, Smith SS. Allopregnanolone levels and symptom improvement in severe premenstrual syndrome. J Clin Psychopharmacol. 2002;22:516–520. doi: 10.1097/00004714-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Frye CA, Sheng Li. Allopregnanolone levels before and after SSRI treatment for premenstrual symptoms. J Clin Psychopharmacol. 2009 doi: 10.1097/JCP.0b013e3181ad8825. in press. [DOI] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia S, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Olanzapine's effects to reduce fear and anxiety and enhance social interactions coincide with increased progestin concentrations of ovariectomized rats. Psychoneuroendocrinology. 2003;28:657–673. doi: 10.1016/s0306-4530(02)00049-5. [DOI] [PubMed] [Google Scholar]
- Frye CA, Sumida K, Dudek B, et al. Progesterone's effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology (Berl) 2006a;186:312–322. doi: 10.1007/s00213-006-0309-3. [DOI] [PubMed] [Google Scholar]
- Frye CA, Sumida K, Harney JP, Lydon J, O'Malley BW, Pfaff D. Mid-aged and aged wildtype and PRKO mice demonstrate rapid progesterone and 3α,5α-THP-facilitated lordosis. Psychopharmacology (Berl) 2006b;185:423–432. doi: 10.1007/s00213-005-0300-4. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–315. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Hippocampal 3α,5α-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav. 2004;78:531–540. doi: 10.1016/j.pbb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Progesterone to ovariectomized mice enhances cognitive performance in the spontaneous alteration, object recognition, but not placement, water maze and contextual and cued conditioned fear tasks. Neurobiol Learn Mem. 2008;90:171–177. doi: 10.1016/j.nlm.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Depression-like behavior of aged male and female mice is ameliorated with administration of testosterone or its metabolites. Physiol Behav. 2009;97:266–269. doi: 10.1016/j.physbeh.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5alpha-reductase. Brain Res. 2004;9:116–124. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Frye CA, Wawrzycki J. Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm Behav. 2003;44:319–326. doi: 10.1016/s0018-506x(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Galea L, Wide J, Barr A. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- Gallagher P, Watson S, Elizabeth Dye C, Young AH, Nicol Ferrier I. Persistent effects of mifepristone (RU-486) on cortisol levels in bipolar disorder and schizophrenia. J Psychiatr Res. 2008;42:1037–1041. doi: 10.1016/j.jpsychires.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Glick ID, Bennett SE. Psychiatric complications of progesterone and oral contraceptives. J Clin Psychopharmacol. 1981;1:350–367. doi: 10.1097/00004714-198111000-00003. [DOI] [PubMed] [Google Scholar]
- Griffin L, Mellon S. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96:13512–13517. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Dong E, Matsumoto K, Pinna G, Rasmusson AM, Costa E. The socially-isolated mouse: a model to study the putative role of allopregnanolone and 5alpha-dihydroprogesterone in psychiatric disorders. Brain Res Brain Res Rev. 2001;37:110–115. doi: 10.1016/s0165-0173(01)00129-1. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Smith SS. Progesterone withdrawal increases the anxiolytic actions of gaboxadol: role of alpha4beta-delta GABAA receptors. Neuroreport. 2003;14:43–46. doi: 10.1097/01.wnr.0000050303.92401.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardoy MC, Sardu C, Dell'osso L, Carta MG. The link between neurosteroids and syndromic/syndromal components of the mood spectrum disorders in women during the premenstrual phase. Clin Pract Epidemol Ment Health. 2008;4:3. doi: 10.1186/1745-0179-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardoy MC, Serra M, Carta MG, Contu P, Pisu MG, Biggio G. Increased neuroactive steroid concentrations in women with bipolar disorder and major depressive disorder. J Clin Psychopharmacol. 2006;26:379–384. doi: 10.1097/01.jcp.0000229483.52955.ec. [DOI] [PubMed] [Google Scholar]
- Hirani K, Khisti R, Chopde C. Behavioral action of ethanol in Porsolt's forced swim test: modulation by 3alpha-hydroxy-5 alpha-pregnan-20-one. Neuropharmacology. 2002;43:1339–1350. doi: 10.1016/s0028-3908(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Joffe H, Soares CN, Cohen LS. Assessment and treatment of hot flushes and menopausal mood disturbance. Psychiatr Clin North Am. 2003;26:563–580. doi: 10.1016/s0193-953x(03)00045-5. [DOI] [PubMed] [Google Scholar]
- Kaur G, Kulkarni S. Evidence for serotonergic modulation of progesterone-induced hyperphagia, depression and algesia in female mice. Brain Res. 2002;943:206–215. doi: 10.1016/s0006-8993(02)02624-0. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Chopde CT. Serotonergic agents modulate antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice. Brain Res. 2000;865:291–300. doi: 10.1016/s0006-8993(00)02373-8. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice forced swim test. Pharmacol Biochem Behav. 2000;67:137–143. doi: 10.1016/s0091-3057(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Klier CM, Muzik M, Dervic K, et al. The role of estrogen and progesterone in depression after birth. J Psychiatr Res. 2007;41:273–279. doi: 10.1016/j.jpsychires.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Dhir A. On the mechanism of antidepressant-like action of berbine chloride. Eur J Pharmacol. 2008;589:163–172. doi: 10.1016/j.ejphar.2008.05.043. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- Lan N, Gee K. Neuroactive steroid actions at the GABAA receptor. Horm Behav. 1994;28:537–544. doi: 10.1006/hbeh.1994.1052. [DOI] [PubMed] [Google Scholar]
- Lancel M, Faulhaber J, Holsboer F, Rupprecht R. Progesterone induces changes in sleep comparable to those of agonistic GABAA receptor modulators. Am J Physiol. 1996;271:763–772. doi: 10.1152/ajpendo.1996.271.4.E763. [DOI] [PubMed] [Google Scholar]
- Lancel M, Faulhaber J, Holsboer F, Rupprecht R. The GABA(A) receptor antagonist picrotoxin attenuates most sleep changes induced by progesterone. Psychopharmacology (Berl) 1999;141:213–219. doi: 10.1007/s002130050827. [DOI] [PubMed] [Google Scholar]
- Lebowitz BD, Pearson JL, Schneider LS, et al. Diagnosis and treatment of depression in late life: consensus statement update. J Am Med Assoc. 2006;278:1186–1190. [PubMed] [Google Scholar]
- Longcope C. Hormone dynamics at the menopause. Ann N Y Acad Sci. 1990;592:21–30. doi: 10.1111/j.1749-6632.1990.tb30313.x. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mani SK, Blaustein JD, O'Malley BW. Progesterone receptor function from a behavioral perspective. Horm Behav. 1997;31:244–255. doi: 10.1006/hbeh.1997.1393. [DOI] [PubMed] [Google Scholar]
- Markou A, Duka T, Prelevic GM. Estrogens and brain function. Hormones. 2005;4:9–17. doi: 10.14310/horm.2002.11138. [DOI] [PubMed] [Google Scholar]
- Marvan ML, Santana S, Chavez Chavez L, Bertran M. Inescapable shocks accentuate fluctuations of forced swimming immobility in different phases of the rat estrous cycle. Arch Med Res. 1997;28:369–372. [PubMed] [Google Scholar]
- Marx CE, VanDoren MJ, Duncan GE, Lieberman JA, Morrow AL. Olanzapine and clozapine increase the GABAergic neuroactive steroid allopregnanolone in rodents. Neuropsychopharmacology. 2003;28:1–13. doi: 10.1038/sj.npp.1300015. [DOI] [PubMed] [Google Scholar]
- Molina-Hernández M, Contreras C, Tellez-Alcantara P. Antidepressant-like effects of pregnancy and progesterone in Wistar rats as measured in the differential reinforcement of the low-rate 72 s task. Psychopharmacology (Berl) 2000;151:306–311. doi: 10.1007/s002130000496. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez M, Tellez-Alcantara NP, Garcia JP, Lopez JI, Jaramillo MT. Antidepressant-like actions of intra-accumbens infusions of allopregnanolone in ovariectomized Wistar rats. Pharmacol Biochem Behav. 2005;80:401–409. doi: 10.1016/j.pbb.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Murphy BE. Antiglucocorticoid therapies in major depression: a review. Psychoneuroendocrinology 1, Supplement. 1997;1:S25–S32. [PubMed] [Google Scholar]
- Nelson J, Felicio L, Osterburg H, Finch C. Differential contributions of ovarian and extraovarian factors to age-related reductions in plasma estradiol and progesterone during the estrous cycle of C57BL/6J mice. Endocrinology. 1992;130:805–810. doi: 10.1210/endo.130.2.1733727. [DOI] [PubMed] [Google Scholar]
- Nicol M, Hirst J, Walker D. Effects of pregnanolone on behavioural parameters and the responses to GABA(A) receptor antagonists in the late gestation fetal sheep. Neuropharmacology. 1999;38:49–63. doi: 10.1016/s0028-3908(98)00166-x. [DOI] [PubMed] [Google Scholar]
- Nihalani ND, Schwartz TL. Mifepristone, a glucocorticoid antagonist for the potential treatment of psychotic major depression. Curr Opin Investig Drugs. 2007;8:563–569. [PubMed] [Google Scholar]
- Paoletti AM, Romagnino S, Contu R, et al. Observational study on the stability of the psychological status during normal pregnancy and increased blood levels of neuroactive steroids with GABAA receptor agonist activity. Psychoneuroendocrinology. 2006;31:485–492. doi: 10.1016/j.psyneuen.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Paré WP, Redei E. Sex differences and stress response of WKY rats. Physiol Behav. 1993;54:1179–1185. doi: 10.1016/0031-9384(93)90345-g. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Pearlstein TB. Hormones and depression: what are the facts about premenstrual syndrome, menopause, and hormone replacement therapy? Am J Obstet Gynecol. 1995;173:646–653. doi: 10.1016/0002-9378(95)90297-x. [DOI] [PubMed] [Google Scholar]
- Pearlstein TB, Bachmann GA, Zacur HA, Yonkers KA. Treatment of premenstrual dysphoric disorder with a new drospirenone-containing oral contraceptive formulation. Contraception. 2005;72:414–421. doi: 10.1016/j.contraception.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Pearlstein TB, Rosen K, Stone AB. Mood disorders and menopause. Endocrinol Metab Clin North Am. 1997;26:279–294. doi: 10.1016/s0889-8529(05)70247-4. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Kavaliers M, Ossenkopp K. Spatial learning and hippocampal volume in male deer mice: relations to age, testosterone and adrenal gland weight. Neuroscience. 1998;86:1089–1099. doi: 10.1016/s0306-4522(98)00131-6. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90:709–714. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Mikacich JA, Moatakef-Imani B, Rasgon N. The clinical nature and formal diagnosis of premenstrual, postpartum, and perimenopausal affective disorders. Curr Psychiatry Rep. 2002;4:419–428. doi: 10.1007/s11920-002-0069-7. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Landa JF, Contreras CM, Bernal-Morales B, Gutiérrez-García AG, Saavedra M. Allopregnanolone reduces immobility in the forced swimming test and increases the firing rate of lateral septal neurons through actions on the GABAA receptor in the rat. J Psychopharmacol. 2007;21:76–84. doi: 10.1177/0269881106064203. [DOI] [PubMed] [Google Scholar]
- Romeo E, Stöhle A, Spalletta G, et al. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Purdy RH, Moore PH, Jr, Paul SM, Rubinow DR. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab. 1994;79:1256–1260. doi: 10.1210/jcem.79.5.7962316. [DOI] [PubMed] [Google Scholar]
- Schule C, Baghai TC, Di Michele F, et al. Effects of combination treatment with mood stabilizers and mirtazapine on plasma concentrations of neuroactive steroids in depressed patients. Psychoneuroendocrinology. 2007;32:669–680. doi: 10.1016/j.psyneuen.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. The impact of different doses of estrogen and progestin on mood and sexual behavior in postmenopausal women. J Clin Endocrinol Metab. 1991;72:336–343. doi: 10.1210/jcem-72-2-336. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Gelfand MM. A prospective one-year study of estrogen and progestin in postmenopausal women: effects on clinical symptoms and lipoprotein lipids. Obstet Gynecol. 1989;73:759–766. [PubMed] [Google Scholar]
- Soares CN, Zitek B. Reproductive hormone sensitivity and risk for depression across the female life cycle: a continuum of vulnerability? J Psychiatry Neurosci. 2008;33:331–343. [PMC free article] [PubMed] [Google Scholar]
- Stahl SM. Are two antidepressant mechanisms better than one? J Clin Psychiatry. 1997;58:339–340. doi: 10.4088/jcp.v58n0801. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Stoffel E, Craft R. Ovarian hormone withdrawal-induced “depression” in female rats. Physiol Behav. 2004;83:505–513. doi: 10.1016/j.physbeh.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Sun MK, Alkon DL. Differential gender-related vulnerability to depression induction and converging antidepressant responses in rats. J Pharmacol Exp Ther. 2006;316:926–932. doi: 10.1124/jpet.105.093948. [DOI] [PubMed] [Google Scholar]
- Svare B. Genotype modulates the aggression-promoting quality of progesterone in pregnant mice. Horm Behav. 1988;22:90–99. doi: 10.1016/0018-506x(88)90033-5. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sampson L, Uzunov DP. Relevance of endogenous 3alpha-reduced neurosteroids to depression and antidepressant action. Psychopharmacology (Berl) 2006;186:351–361. doi: 10.1007/s00213-005-0201-6. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Wrynn A, Kinnunen A, Ceci M, Kohler C, Uzunov D. Chronic antidepressants reverse cerebrocortical allopregnanolone decline in the olfactory-bulbectomized rat. Eur J Pharmacol. 2004;486:31–34. doi: 10.1016/j.ejphar.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Van Look PF, Von Hertzen H. Clinical use of antiprogestins. Hum Reprod. 1995;1:19–34. doi: 10.1093/humupd/1.1.19. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav. 2007;86:407–414. doi: 10.1016/j.pbb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout mice. Behav Neurosci. 2008a;122:974–981. doi: 10.1037/a0012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008b;89:513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Sumida K, Frye CA. Inhibiting 5alpha-reductase in the amygdala attenuates antianxiety and antidepressive behavior of naturally receptive and hormone-primed ovariectomized rats. Psychopharmacology (Berl) 2006b;186:302–311. doi: 10.1007/s00213-005-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LM, Han H, Wang QN, et al. Mifepristone repairs region-dependent alteration of synapsin I in hippocampus in rat model of depression. Neuropsychopharmacology. 2007;32:2500–2510. doi: 10.1038/sj.npp.1301386. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Masood A, et al. Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B). Neuropsychopharmacology. 2008;33:1611–1623. doi: 10.1038/sj.npp.1301537. [DOI] [PMC free article] [PubMed] [Google Scholar]