Abstract
Rationale
Greater incidence of anxiety and depressive disorders of women compared to men may be due in part to progesterone (P) and its neuroactive metabolite, 5α-pregnan-3α-ol-20-one (3α,5α-THP), acting in limbic regions, such as the amygdala.
Objective
If P’s metabolism via 5α-reduction to 3α,5α-THP in the amygdala is critical for antianxiety and antidepressive behavior, then blocking 5α-reductase in the amygdala of female rats is likely to attenuate the antianxiety and anti-depressive effects of high progestin levels from both endogenous and exogenous sources.
Methods
Naturally receptive female rats with high endogenous estrogen (E2) and P and ovariectomized (ovx) rats administered E2 (10 μg) and P (500 μg) subcutaneously were administered finasteride (10 μg/μl), a Type II 5α-reductase inhibitor, or vehicle to the amygdala. Anxiety behavior (open field, elevated plus maze, defensive freezing) and depressive behavior (Porsolt forced swim test) were assessed.
Results
There were similar effects of finasteride administration to the amygdala to attenuate antianxiety behavior in naturally receptive and ovx, hormone-primed rats. Finasteride administration significantly decreased central entries in the open field, decreased open arm time in the elevated plus maze, increased defensive freezing in response to footshock, and increased time spent immobile compared to vehicle.
Conclusions
Thus, formation and subsequent actions of 3α,5α-THP in the amygdala may be important for antianxiety and antidepressive effects.
Keywords: Progestin, Allopregnanolone, 5α-pregnan-3α-ol-20-one, Neurosteroid, Sex differences, Mood
Introduction
Fluctuations in progestins may underlie behavioral changes observed in animal models of anxiety and depression behavior. In naturally receptive rats, concentrations of estradiol (E2), progesterone (P), and P’s 5α-reduced metabolite 5α-pregnan-3α-ol-20-one (3α,5α-THP) are increased relative to that of rats in other phases of the estrous cycle and male rats (Frye and Bayon 1999). Pregnancy is also associated with significant increases in 3α,5α-THP and is followed by a precipitous decline in 3α,5α-THP with parturition (Frye and Bayon 1999). Coincident with these changes in progestins, the behavior of female rats in tasks assessing anxiety and depressive behavior also varies. Naturally receptive rats demonstrate greater antianxiety behavior than do nonreceptive rats, such as increased central entries in the open field, open arm activity in the elevated plus maze, time in social interactions with a conspecific, and decreased responses to footshock in the defensive freezing task (Fernandez-Guasti and Picazo 1992; Mora et al. 1996; Frye et al. 2000). Similarly, naturally receptive rats in proestrus or pregnant rats have decreased depressive behavior in the Porsolt forced swim test compared to rats in other stages of the estrous cycle, postpartum rats, or male rats (Frye and Walf 2002, 2004a). Together, these data demonstrate that endogenous variations in progestins may modify anxiety and depressive behavior of female rodents.
Extirpation and replacement studies also support a role of progestins, such as 3α,5α-THP, for affective behavior. Ovariectomy-induced increases in burying or freezing behavior in response to footshock are attenuated with administration of P or 3α,5α-THP (Picazo and Fernandez-Guasti 1995; Martinez-Mota et al. 2000; Frye and Walf 2004b). Similar effects of P or 3α,5α-THP administration to ovariectomized (ovx) rats to alter anxiety and depression behavior have been observed in the open field, elevated plus maze, forced swim, and corticotropin-releasing-factor-enhanced startle task (Bitran et al. 1991; Frye and Walf 2004b; Martinez-Mota et al. 1999; Toufexis et al. 2004). However, not all studies demonstrate an antianxiety effect of progestins. For example, administration of P via three systemic injections over 48 or 72 h or administration of 3α,5α-THP via three injections over 48 h similarly decreased open arm time (i.e., increased anxiety behavior) of intact female rats compared to those not administered these regimen of progestins (Gulinello et al. 2001; Gulinello and Smith 2003). It may be that baseline levels of progestins modulate anxiety behavior of rodents. For instance, anxiolytic effects of 3α,5α-THP were only observed with increased endogenous or exogenous P levels (Laconi et al. 2001). Together, these data demonstrate that altering circulating progestins through ovariectomy and progestin administered subcutaneously (SC) can mediate anxiety and depressive behavior of female rodents.
Although the brain targets for progestins’ effects on anxiety and depressive behavior are not entirely clear, the amygdala may be one region of importance. The amygdala is well known as a brain region important for modulating fear and anxiety behavior (LeDoux 2000). Rats exposed to tasks typically used to assess anxiety behavior, such as the open field and elevated plus maze, show activation of the amygdala as indicated by greater c-fos mRNA induction and/or labeling of FOS-positive neurons in this region compared to rats not exposed to these tasks (Silveira et al. 1993; Emmert and Herman 1999). Another way the amygdala has been investigated as a brain region of importance for affective behavior is through a kindling model. For instance, open arm activity in the elevated plus maze is decreased in male rats with medial amygdala kindling compared to nonkindled control rats (Adamec and Shallow 2000). These data suggest that further investigation of progestins’ effects in the amygdala on affective behavior is warranted.
The amygdala may be a putative site of progestins’ effects to modulate anxiety and depressive behavior of rodents. In support, P administered systemically or directly to the amygdala produces similar effects to increase central entries in an open field, increase open arm exploration, and decrease freezing in response to footshock (Frye and Walf 2004b). Furthermore, these effects to reduce anxiety and depressive behavior may be due to actions of 3α,5α-THP. First, the enzymes required to metabolize P to 3α,5α-THP, 5α-reductase and 3α-hydroxysteroid dehydrogenase, have been localized to the amygdala (Khanna et al. 1995; Li et al. 1997). Second, systemic hormone regimen that produced antianxiety behavior also produces physiological levels of 3α,5α-THP in the amygdala (Frye and Bayon 1999; Frye and Vongher 1999; Frye and Walf 2004a). Third, administration of 3α,5α-THP directly to the amygdala increases open arm duration in the elevated plus maze and punished responding in a modified Geller–Seifter conflict test (Akwa et al. 1999). Together, these data suggest that the amygdala may be one central target of 3α,5α-THP for its effects to reduce anxiety and depressive behavior.
Whether P’s metabolism to 3α,5α-THP via actions of 5α-reductase in the amygdala is required for progestins’ antianxiety and antidepressive effects was investigated. Female rats with an intact hypothalamic–pituitary–gonadal axis (naturally receptive) and those that were ovx and primed with E2 and P regimen, which is typically utilized to induce sexual receptivity, were examined to be able to parse out differences, if any, of endogenous and exogenous progestins. Experiments tested the hypothesis that if 5α reduction of P to 3α,5α-THP in the amygdala is critical for the antianxiety and antidepressive effects of progestins, then administration of a 5α-reductase inhibitor to the amygdala would be expected to attenuate the antianxiety and antidepressive behavior of naturally receptive and ovx, hormone-primed rats.
Materials and methods
These methods were preapproved by the Institutional Animal Care and Use Committee at S.U.N.Y.-Albany.
Subjects
Adult, female Long–Evans rats (N=34), approximately 55 days old, were obtained from the breeding colony at S.U.N. Y.-Albany (original stock from Taconic Farms, German-town, NY). Rats were group-housed (four per cage) in polycarbonate cages (45×24×21 cm) in a temperature-controlled room (21±1°C) in the Laboratory Animal Care Facility. The rats were maintained on a 12 h/12 h reversed light cycle (lights off at 0800 hours) with ad libitum access to Purina Rat Chow and tap water.
Surgery
All rats were stereotaxically implanted with bilateral guide cannulae aimed at the medial amygdala (from bregma AP=−2.4, ML=±3.75, DV=−7.3; Paxinos and Watson 1986) under Rompum (12 mg/kg; Bayer Corp., Shawnee Mission, KS) and Ketaset (80 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) anesthesia 1 week before testing. Cannulae consisted of 23-gauge, thin-wall, stainless steel guide tubing. Some rats (n=16) were ovx using the same anesthesia protocol 1 week prior to implantation of guide cannulae.
Determination of behavioral estrus
Intact female rats were paired daily with a male to determine if they were in behavioral estrus (i.e., naturally receptive) or not. As per previously published methods, behavioral estrus was verified by the female rats assuming a lordosis posture in response to mounting by a sexually experienced male rat (Frye and Walf 2002). After screening (~0800–1000 hours), rats were behaviorally tested.
Hormone-priming
Ovx rats received subcutaneous injections of 17 β-estradiol (E2; Sigma, 5 μg) at hours 0 and 24 and P (Sigma; 500 μg) at hour 44. All rats were tested at hour 48.
Finasteride administration
Rats were administered bilateral infusions of the Type II 5α-reductase inhibitor finasteride (10 μg/μl) or vehicle (β-cyclodextran) to the amygdala 2 h before testing (Rhodes and Frye 2001; Frye and Walf 2002).
Behavioral testing
Testing occurred in the brightly lit testing room adjacent to the animal housing facility between 0900 and 1300 hours. Ovx females were tested on two occasions. First, rats were tested in the open field, elevated plus maze, and defensive freezing task consecutively in one testing session without a rest period between tasks. Second, rats were tested in the Porsolt forced swim test in another testing session approximately 1 week later (i.e., the next time intact rats were naturally receptive, or 1 week following the initial hormone-priming in ovx rats). NB: Two naturally receptive rats in the finasteride group were not tested in the Porsolt forced swim test due to problems with the patency of their cannulae.
Open field task
The open field task was used in accordance with previously published methods (McCarthy et al. 1995; Frye et al. 2000). The open field is a white, melamine box (76×57×35 cm) with a 48-square grid floor, with each square measuring 9.5 cm per side. After placement in one corner of the box, the number of peripheral and central entries made by a rat during a 5-min period is recorded. Central entries were defined by rats’ entrance into all but the 24 peripheral squares. Central entries are considered indicative of antianxiety behavior.
Elevated plus maze
The elevated plus maze is situated 50 cm above the ground and consists of four arms each measuring 49 cm long and 10 cm wide. Two arms of this maze are concealed by a 30-cm-high wall (closed arms), and the other two are not concealed (open arms). At the start of this 5-min task, a rat is placed at the intersection of the open and closed arms, and the time spent in the open and closed arms, as well as entries into these arms, are recorded (Pellow and File 1986; Frye et al. 2000). Open arm activity is considered to be antianxiety behavior.
Defensive freezing task
The defensive freezing task was used, as per previously published methods (Treit et al. 1981; Frye and Walf 2004a). During this task, a rat was placed in the testing chamber, which was made of clear Plexiglas (26.0×21.2×24.7 cm) and filled with wood-chip bedding to a height of 5.0 cm. Rats received a footshock (6.66 mA of unscrambled shock) when both forepaws touched a pedestal (2.5 cm in diameter and 10.0 cm in height) in the chamber that was connected to a shock source (Lafayette Model A615B, Lafayette, IN). The time that rats spend freezing for 15 min after footshock was recorded. Freezing behavior is considered an index of anxiety/fear behavior in this task.
Porsolt forced swim task
Rats were tested in a single trial of the Porsolt forced swim test, as per previously published methods (Frye and Walf 2002). The duration a rat spent struggling, swimming, and immobile in a cylindrical container filled with 30 cm of 30°C water for 10 min was recorded. Struggling is defined by quick movements of the forelimbs, which break the surface of the water, and attempts to climb the walls of the chamber. Swimming does not resemble struggling and is defined by movements of the forelimbs and/or hindlimbs under the surface of the water. Immobility is defined as the absence of any movement in the water (i.e., floating) and is considered indicative of depressive behavior.
Perfusion and histological analyses
To determine cannulae location, rats were exsanguinated with 0.9% saline and 10% formalin. Brains were then frozen and sliced at 40-μm thickness on a cryostat. Slices were then stained with cresyl violet, and infusion locations were determined by light microscopy. The majority of rats (n=30) received bilateral infusions to the medial amygdala. The area that encompasses all of these effective infusions is illustrated in Fig. 1. Four rats had infusions beyond the medial amygdala. Their infusion sites are also represented in Fig. 1. As their behavioral responses differed from those with infusions to the medial amygdala, the data from these rats were excluded from statistical analyses. Their data are presented in Table 1.
Fig. 1.
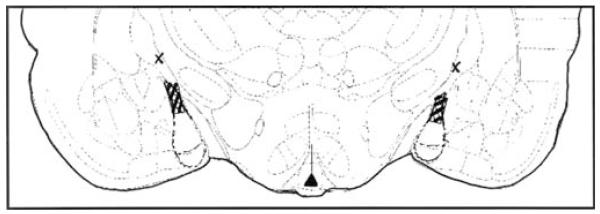
Hatched region depicts area of effective infusions of finasteride to the medial amygdala. Xs denote area of ineffective finasteride infusion outside the medial amygdala
Table 1.
Data from animals with missed cannulae placement
| Hormone and finasteride condition |
Behavioral data | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Central open field entries |
Total open field entries |
Open arm time (s) |
Total arm entries |
Freezing time (s) |
Duration of immobility (s) |
|
| Naturally receptive and vehicle (n=1) |
44 | 191 | 30 | 9 | 260 | 170 |
| Naturally receptive and finasteride (n=1) |
23 | 154 | 0 | 2 | 738 | 344 |
| Hormone-primed and finasteride (n=2) |
45±1 | 188±38 | 90±21 | 15±2 | 180±13 | 263±22 |
Naturally receptive rats had one cannula to the amygdala and one to the basal nucleus of Meynert. Both hormone-primed rats had cannula placement to the basal nucleus of Meynert
Statistical analyses
Multiple one-way analyses of variance (ANOVAs) were used to examine the main effects of finasteride on behavioral indices in the tasks used. Where appropriate, Fisher’s least significant difference post hoc tests were used to determine differences among groups. α level for the determination of statistical significance was p≤0.05.
Results
Open field task
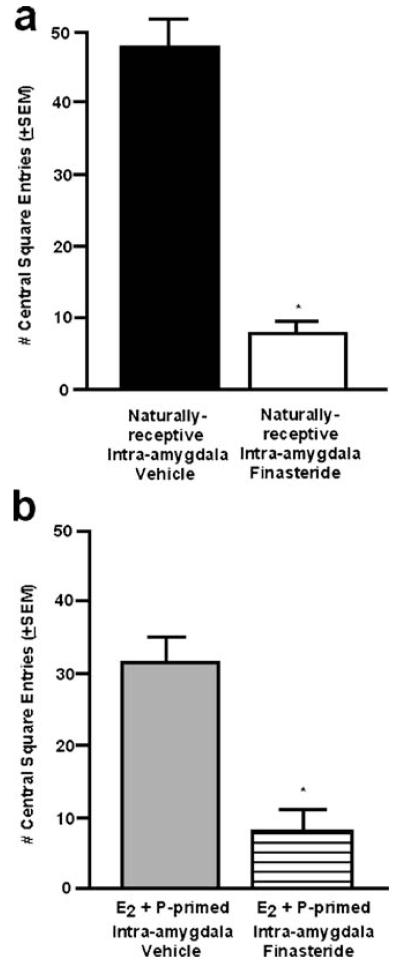
There was a significant main effect of intra-amygdala finasteride administration on the number of total [F(1,14)=7.64; P≤0.02] and central entries [F(1,14)=6.64; P≤0.02] made in the open field. Finasteride administration to the amygdala (80±11) significantly decreased the number of total entries made by naturally receptive rats compared with those administered vehicle (202±42). Rats administered finasteride to the amygdala made significantly fewer central entries in the open field compared to rats administered vehicle (Fig. 2a).
Fig. 2.
a The mean (+SEM) number of central entries made in the open field task of naturally receptive female rats administered vehicle (closed) or finasteride (open) to the amygdala. b The mean (+SEM) number of central entries made in the open field task of ovx, E2- and P-primed rats administered vehicle (gray) or finasteride (striped) to the amygdala. * denotes significant difference from vehicle group (p≤0.05)
Similar effects of finasteride on behavior in the open field were observed in ovx, E2- and P-primed rats. There was no main effect of finasteride (102±21) compared to vehicle administration (152±17) on total entries in the open field. However, there was a significant main effect of intraamygdala finasteride administration on central [F(1,12)=12.84; P≤0.01] entries in the open field. Ovx rats administered E2 and P SC and finasteride to the amygdala entered significantly fewer central squares in the open field compared to ovx rats administered E2+P SC and vehicle to the amygdala (Fig. 2b).
Elevated plus maze
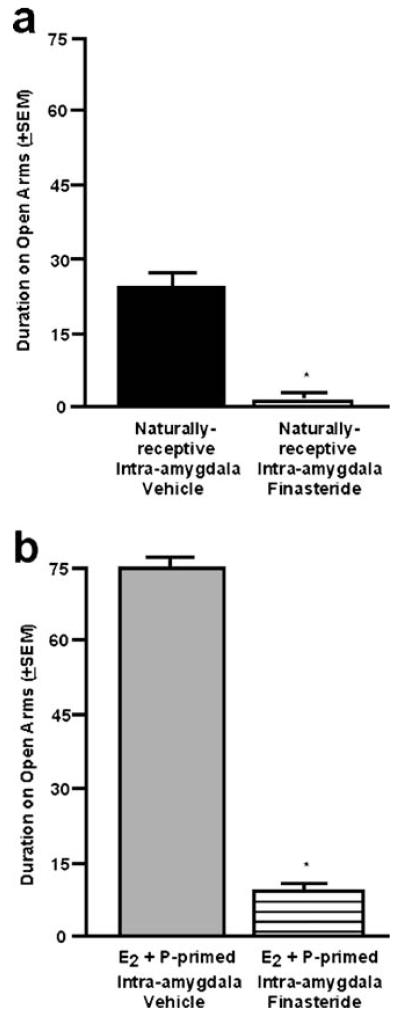
There was a significant main effect of finasteride administration to the amygdala on time spent on the open arms of the elevated plus maze [F(1,14)=35.63; P≤0.01]. Naturally receptive rats administered finasteride to the amygdala spent significantly less time on the open arms of the elevated plus maze compared to those administered vehicle to the amygdala (Fig. 3a). There were no significant differences among groups for total entries made in the elevated plus maze (finasteride, 6±1; vehicle, 11±2).
Fig. 3.
a The mean (+SEM) duration spent on the open arms of the elevated plus maze of naturally receptive female rats administered vehicle (closed) or finasteride (open) to the amygdala. b The mean (+SEM) duration spent on the open arms of the elevated plus maze of ovx, E2- and P-primed rats administered vehicle (gray) or finasteride (striped) to the amygdala. * denotes significant difference from vehicle group (p≤0.05)
Similar to effects observed in naturally receptive rats, there were differences among ovx, hormone-primed rats administered finasteride and those administered vehicle to the amygdala for open arm time in the plus maze [F(1,12)=11.21; P≤0.01]. Ovx, E2- and P-primed rats administered intra-amygdala finasteride spent significantly less time on the open arms than did vehicle-administered rats (Fig. 3b). There were no significant differences among groups for total entries made in the elevated plus maze (finasteride, 8±2; vehicle, 14±2).
Defensive freezing task
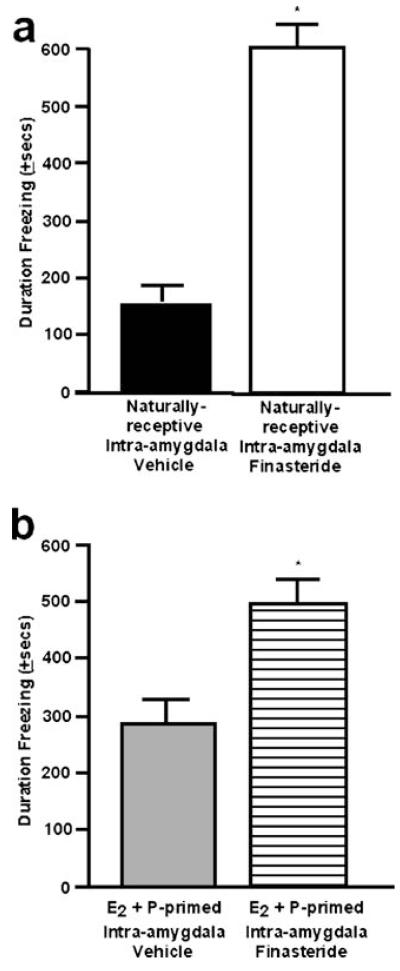
There was a significant main effect of intra-amygdala finasteride administration on the duration spent freezing in response to footshock in the defensive freezing task [F(1,14)=51.14; P≤0.01]. Finasteride administration to the amygdala significantly increased the time spent freezing compared to vehicle administration to naturally receptive rats (Fig. 4a).
Fig. 4.
a The mean (+SEM) duration spent freezing post-footshock in the defensive freezing task of naturally receptive female rats administered vehicle (closed) or finasteride (open) to the amygdala. b The mean (+SEM) duration spent freezing post-footshock in the defensive freezing task of ovx, E2- and P-primed rats administered vehicle (gray) or finasteride (striped) to the amygdala. * denotes significant difference from vehicle group (p≤0.05)
Similar effects of finasteride on behavior in the defensive freezing task were observed in ovx, E2- and P-primed rats [F(1,12)=6.89; P≤0.02]. Ovx, hormone-primed rats administered finasteride spent significantly more time freezing in response to footshock in the task compared with rats administered vehicle (Fig. 4b).
Porsolt forced swim test
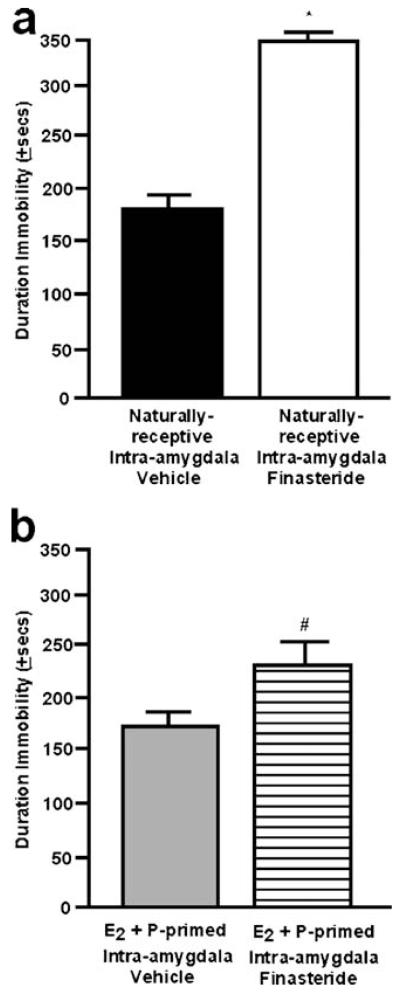
There was a significant main effect of finasteride administration to the amygdala on immobility behavior in the Porsolt forced swim task [F(1,12)=136.99; P≤0.01]. Naturally receptive rats administered intra-amygdala finasteride had significantly longer immobility duration than did rats administered intra-amygdala vehicle (Fig. 5a). Similarly, finasteride administered to the amygdala significantly reduced time spent swimming (finasteride, 88±13; vehicle, 272±61) [F(1,12)=6.59; P≤0.02]. There were no differences among groups for struggling behavior in this task (finasteride, 153±17; vehicle, 167±36).
Fig. 5.
a The mean (+SEM) duration spent immobile in the forced swim task of naturally receptive female rats administered vehicle (closed) or finasteride (open) to the amygdala. b The mean (+SEM) duration spent immobile in the forced swim task of ovx, E2- and P-primed rats administered vehicle (gray) or finasteride (striped) to the amygdala. * denotes significant difference from vehicle group (p≤0.05)
There were similar effects observed in the forced swim test behavior of ovx, hormone-primed rats administered finasteride or vehicle to the amygdala. Ovx, E2- and P-primed rats administered intra-amygdala finasteride spent more time immobile than did vehicle-administered rats; however, these data only approached statistical significance [F(1,12)=3.95; P≤0.07; Fig. 5b]. There were no differences among groups for swimming (finasteride, 206±35; vehicle, 246±21) or struggling (finasteride, 155±15; vehicle, 181±13) behavior in this task.
Discussion
Our hypothesis that metabolism of P to 3α,5α-THP in the amygdala is required for progestins’ antianxiety and anti-depressive effects in naturally receptive and ovx, E2- and P-primed rats was supported. Naturally receptive and ovx, hormone-primed rats administered the 5α-reductase inhibitor finasteride to the amygdala made significantly fewer central entries in the open field, spent less time on the open arms of the elevated plus maze, and spent more time freezing in response to footshock than did rats administered vehicle to the amygdala. Similarly, naturally receptive and ovx, E2- and P-primed rats administered intra-amygdala finasteride spent significantly more time immobile in the forced swim test than did those administered vehicle. Together, these data suggest that antianxiety and antidepressive effects of P may involve its 5α-reduction and subsequent actions of 3α,5α-THP in the amygdala.
The results from the present study confirm previous reports about the importance of P’s metabolism to 3α,5α-THP for progestins’ antianxiety and/or antidepressive effects in female rats and mice. For instance, P administration to ovx, wild-type mice, but not those deficient in the Type II 5α-reductase enzyme, increases whole-brain 3α,5α-THP levels, increases open arm activity in the plus maze, and decreases time spent immobile in the forced swim test compared to ovx, vehicle-administered mice (Frye et al. 2004). Similarly, antianxiety and antidepressive behavior of rats with high endogenous progestin levels (naturally receptive or pregnant) is attenuated by systemic administration of finasteride that could alter 3α,5α-THP levels throughout the brain (Rhodes and Frye 2001; Frye and Walf 2002, 2004b). Interestingly, hippocampal 3α,5α-THP levels are decreased in rats administered systemic finasteride coincident with these changes in anxiety and depressive behavior (Rhodes and Frye 2001; Frye and Walf 2002, 2004b). Furthermore, previous studies have shown similar effects of systemic and intra-hippocampal finasteride to reduce hippocampal 3α,5α-THP levels and to decrease antianxiety and antidepressive behavior of naturally receptive rats (Rhodes and Frye 2001; Frye and Walf 2002). Thus, the present data confirm these previous reports about the importance of P’s 5α-reduction in the hippocampus for antianxiety and antidepressive behavior and extend these results to suggest that the amygdala is also involved in the circuitry underlying these behaviors.
Separate reports of similar effects of systemic, intrahippocampal, and intra-amygdala finasteride to attenuate antianxiety and antidepressive behavior of naturally receptive rats, as discussed above, suggest a common circuitry involving these brain regions for these behavioral effects of 3α,5α-THP. It may be that the amygdala alters the tone of the hippocampus to produce different behavioral effects. For instance, with emotional memory, the amygdala may first process the incoming emotional stimuli, in particular negative emotional stimuli (LeDoux 1995; Akirav and Richter-Levin 1999; Wilensky et al. 2000). This input from the amygdala to higher cortical regions or the hippocampus ultimately alters long-term emotional memory processes (Leigland et al. 2004). Thus, our present results may reflect 3α,5α-THP’s modulation of this amygdala–hippocampal circuit.
In addition to the hippocampus and amygdala, affective behavior is likely modulated by other limbic regions controlled by the hypothalamic–pituitary–adrenal axis (HPA), such as the bed nucleus of the stria terminalis (BNST) (Lee and Davis, 1997; Walker et al. 2003; Herman et al. 2004). There are projections from the basolateral amygdala to the BNST (and central amygdala) that project to hypothalamic and/or brainstem regions to mediate anxiety and fear responses through actions via the HPA (Walker et al. 2003). It has been proposed that the BNST and central amygdala produce similar effects on affective behavior, but that the BNST is more important for long-duration responses (i.e., anxiety) and the central amygdala for acute responses (i.e., fear), to aversive and/or threatening stimuli (Walker and Davis 1997). There is evidence for progestins to alter behaviors that are influenced by activation of the central amygdala and BNST. P and 3α,5α-THP reduce corticotropin-releasing hormone (CRH)-induced startle in intact and ovx, E2- and P-primed rats (Walker et al. 2003; Toufexis et al. 2004). In an unconditioned fear model, ovx rats administered E2 and P systemically or to the medial amygdala had similar analgesic responses after exposure to trimethylthiazoline (Walf and Frye 2004). Thus, the present results expand this idea of BNST and central amygdala modulation of affective behavior to suggest that progestins in the medial amygdala may also be important for mitigating responses to anxiogenic and/or fear-inducing stimuli.
Another perspective may be that the effects observed are due to 3α,5α-THP mitigating HPA responsiveness. Both the amygdala and hippocampus are involved in modulation of the stress response (Herman et al. 2004). Notably, 3α,5α-THP dampens the HPA response (Patchev et al. 1996). Although the mechanism for 3α,5α-THP to reduce anxiety and depressive behavior was not directly tested in the present study, it likely involved γ-aminobutyric (GABAA) receptors. The mechanisms for the amygdala and hippocampus to modulate the HPA response include GABAergic inhibition (Herman et al. 2004). 3α,5α-THP is a potent allosteric modulator of GABAA receptors (Majewska et al. 1986). Furthermore, female, but not male, rats that demonstrated anxiogenic behavior in an acoustic startle model after P withdrawal had increased expression of the GABAA receptors α4 subunit in the amygdala (Gulinello et al. 2003). Together, these data suggest that decreasing 3α,5α-THP in the amygdala with finasteride administration may alter the tone of the HPA (via effects involving other limbic regions such as the hippocampus and/or BNST) and thus lead to decreased antianxiety and antidepressive behavior.
The present data also confirm previous reports about the antianxiety and antidepressive effects of progestins and E2. It can be difficult to parse out the effects of progestins vs E2 in intact naturally receptive rats and ovx rats administered both E2 and P. First, both E2 and progestins have antianxiety and antidepressive effects in animal models (Nomikos and Spyraki 1988; Mora et al. 1996; Frye et al. 2000; Frye and Walf 2004a,b; Walf and Frye 2005a,b; Walf et al. 2004). The medial amygdala is a target of E2 (and progestins). Mating stimuli in ovx, hormone-primed rats is associated with coexpression of fos and estrogen receptor immunoreactivity in neurons of the medial amygdala (Greco et al. 2003). Furthermore, we have found similar effects of systemic and intra-amygdala E2 administration to increase antianxiety behavior of ovx rats (Frye and Walf 2004b). Second, blocking P’s metabolism to 3α,5α-THP completely attenuated the putative antianxiety and anti-depressive effects of E2 and progestins typically observed in naturally receptive rats in this study and previous ones (Rhodes and Frye 2001; Frye and Walf 2002). Notably, biosynthesis of these steroids may underlie these effects. Both E2 and 3α,5α-THP can be locally synthesized in the brain (Mellon and Griffin 2002; Hojo et al. 2004). The enzymes required for central biosynthesis, such as cytochrome P450 side chain cleavage, and 3β-hydroxysteroid dehydrogenase have been localized to limbic regions such as the hippocampus and amygdala (Mellon and Deschepper, 1993; Furukawa et al. 1998; Mellon and Griffin 2002; Sierra 2004). E2 increases synthesis of 3α, 5α-THP by increasing conversion of P to 3α,5α-THP via activity of 5α-reductase and, thereby, increases neuroactive progestins’ effects on behavior (Cheng and Karavolas 1973; Frye and Duncan 1996; Vongher and Frye 1999; Sinchak et al. 2003). Thus, the possibility of de novo progestin and/or E2 synthesis in the amygdala modulating the antianxiety and antidepressive effects observed in naturally receptive and hormone-primed female rodents is intriguing and requires further investigation.
Although the present data are compelling and suggest progestins may act on a common circuitry of the amygdala, hippocampus, and/or HPA axis to alter affective responses, there are some limitations to this study to consider. First, we were not able to measure central 3α,5α-THP levels of rats in the present study. We have previously demonstrated that comparable systemic and intrahippocampal administration of finasteride similarly reduces 3α,5α-THP levels in the hippocampus of naturally receptive rats (Rhodes and Frye 2001; Frye and Walf 2002, 2004b). Some differences can be noted between naturally receptive and ovx, hormone-primed rats in the elevated plus maze, such that ovx, E2- and P-primed rats had more salient antianxiety behavior than did naturally receptive rats. This may reflect a difference in central E2 and progestin levels produced by hormone-priming regimen. Notably, these differences were unique to the elevated plus maze and not noted in the three other behavioral tasks observed. This suggests that these group differences may be due to a task-specific effect. Furthermore, the present experiment focused on the effects of altering 5α-reductase activity in the amygdala in rats with physiological levels of E2 and P but did not address the importance of local de novo synthesis of progestins in ovx rats without hormone-priming. Second, the degree of site specificity is not entirely clear in the present studies. Although finasteride was administered to the medial amygdala of rats, we know that similar infusion regimen produce a spread of drug 1–2 mm from the infusion site (Frye and Edinger 2004). Finasteride administered to areas outside the amygdala region produced behavioral effects that were different from those observed with rats administered finasteride to the medial amygdala; however, any effective infusion likely affected the entire amygdala, not just the medial region. Other areas of the amygdala, such as the central and basolateral nuclei, are involved in modulating anxiety and fear behavior (LeDoux 1992, 1995, 2000; Davis 1992a,b; Davis and Whalen 2001). Furthermore, other regions of the brain besides the amygdala (or hippocampus) are likely targets of 3α,5α-THP’s effects to reduce anxiety and depressive behavior. For example, direct administration of 3α,5α-THP or drugs that increase 3α,5α-THP in the lateral septum enhances affective behavior (Bitran et al. 1999, 2000; Molina-Hernandez et al. 2002, 2003). Third, it is not clear from this experiment if different effects would be produced by longer finasteride treatment. For instance, not all animal studies have demonstrated that finasteride decreases antianxiety behavior. In one such study, finasteride administration for 3 days did not alter elevated plus maze behavior of lactating females (Kellogg and Barrett 1999). Interestingly, among men and women treated with finasteride for androgenetic alopecia for 9–19 weeks, increased Hamilton Depression scores and anxiety symptoms have been reported (Altomare and Capella 2002). These psychotropic side effects abated when treatment was discontinued and, in two patients that began taking finasteride again, depression symptoms developed within 2 weeks of treatment. Fourth, we do not know if the effects of finasteride to the amygdala could be abrogated by 3α,5α-THP replacement to the amygdala. This would be a strong experimental design to consider implementing in the future to further investigate possible site specificity of 3α,5α-THP’s effects in the amygdala. Thus, although the present data support an important role of P’s 5α-reduction in the amygdala for progestins’ anti-anxiety and antidepressive effects, it is not reasonable to conclude that other CNS sites of progestins’ actions beyond the medial amygdala are not targets of these effects.
Our findings are important as they demonstrate that 5α-reduction of P in the amygdala may be important for the antianxiety and antidepressive behavior of naturally receptive and ovx, E2- and P-primed rats. Administration of the 5α-reductase inhibitor finasteride to the amygdala decreased the number of central entries in the open field, decreased open arm activity in the elevated plus maze, increased defensive freezing in response to footshock, and increased time spent immobile in the forced swim test. Thus, the amygdala may be an important target of progestins for their antianxiety and antidepressive effects.
Acknowledgements
This research was supported by grants from the National Science Foundation (IBN98-96263; IBN03-16083), National Institute of Mental Health (MH0676980), Whitehall Foundation (096-010), and Ronald McNair Research Program to support minority undergraduates. Technical assistance provided by Lesley Ann Cole, Kate Fagan-Solis, and Dr. Madeline Rhodes is greatly appreciated. All experiments comply with the current laws of the United States of America, where they were performed.
Contributor Information
Alicia A. Walf, Department of Psychology, The University at Albany-SUNY, 1400 Washington Avenue, Albany, NY 12222, USA
Kanako Sumida, Department of Psychology, The University at Albany-SUNY, 1400 Washington Avenue, Albany, NY 12222, USA.
Cheryl A. Frye, Department of Psychology, The University at Albany-SUNY, 1400 Washington Avenue, Albany, NY 12222, USA; Department of Biological Sciences, The University at Albany-SUNY, 1400 Washington Avenue, Albany, NY 12222, USA; Center for Neuroscience Research, The University at Albany-SUNY, 1400 Washington Avenue, Albany, NY 12222, USA
References
- Adamec R, Shallow T. Effects of baseline anxiety on response to kindling of the right medial amygdala. Physiol Behav. 2000;70:67–80. doi: 10.1016/s0031-9384(00)00247-x. [DOI] [PubMed] [Google Scholar]
- Akirav I, Richter-Levin G. Biphasic modulation of hippocampal plasticity by behavioral stress and basolateral amygdala stimulation in the rat. J Neurosci. 1999;19:10530–10535. doi: 10.1523/JNEUROSCI.19-23-10530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akwa Y, Purdy RH, Koob GF, Britton KT. The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–125. doi: 10.1016/s0166-4328(99)00101-1. [DOI] [PubMed] [Google Scholar]
- Altomare G, Capella GL. Depression circumstantially related to the administration of finasteride for androgenetic alopecia. J Dermatol. 2002;29:665–669. doi: 10.1111/j.1346-8138.2002.tb00200.x. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3α-hydroxy-5α[β]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3α-OH-5α-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999;850:217–224. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- Bitran D, Foley M, Audette D, Leslie N, Frye CA. Activation of peripheral mitochondrial benzodiazepine receptors in the hippocampus stimulates allopregnanolone synthesis and produces anxiolytic-like effects in the rat. Psychopharmacology. 2000;151:64–71. doi: 10.1007/s002130000471. [DOI] [PubMed] [Google Scholar]
- Cheng YJ, Karavolas HJ. Conversion of progesterone to 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnan-20-one by rat medical basal hypothalami and the effects of estradiol and stage of estrous cycle on the conversion. Endocrinology. 1973;93:1157–1162. doi: 10.1210/endo-93-5-1157. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992a;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol Sci. 1992b;13:35–41. doi: 10.1016/0165-6147(92)90014-w. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Emmert MH, Herman JP. Differential forebrain c-fos mRNA induction by ether inhalation and novelty; evidence for distinctive stress pathways. Brain Res. 1999;845:60–67. doi: 10.1016/s0006-8993(99)01931-9. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Picazo O. Changes in burying behavior during the estrous cycle; effect of estrogen and progesterone. Psychoneuroendocrinology. 1992;17:681–689. doi: 10.1016/0306-4530(92)90027-5. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3α,5α-THP and 3α-Diol. J Neuroendocrinol. 1999;11:839–847. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duncan JE. Estradiol benzoate potentiates neuroactive steroids’ effects on pain sensitivity. Pharmacol Biochem Behav. 1996;53:27–32. doi: 10.1016/0091-3057(95)00194-8. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger KL. Testosterone’s metabolism in the hippocampus may mediate its anti-anxiety effects in male rats. Pharmacol Biochem Behav. 2004;78:473–481. doi: 10.1016/j.pbb.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Frye CA, Vongher JM. 3α,5α-THP in the midbrain ventral tegmental area of rats and hamsters is increased in exogenous hormonal states associated with estrous cyclicity and sexual receptivity. J Endocrinol Invest. 1999;22:455–464. doi: 10.1007/BF03343590. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–315. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behav Neurosci. 2004a;118:306–313. doi: 10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Hippocampal 3α,5α-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav. 2004b;78:531–540. doi: 10.1016/j.pbb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5α-reductase. Brain Res. 2004;1004:116–124. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Furukawa A, Miyatake A, Ohnishi T, Ichikawa Y. Steroidogenic acute regulatory protein (StAR) transcripts constitutively expressed in the adult rat central nervous system: colocalization of StAR, cytochrome P-450SCC (CYP XIA1), and 3β-hydroxysteroid dehydrogenase in the rat brain. J Neurochem. 1998;71:2231–2238. doi: 10.1046/j.1471-4159.1998.71062231.x. [DOI] [PubMed] [Google Scholar]
- Greco B, Blasberg ME, Kosinski EC, Blaustein JD. Response of ERα-IR and ERβ-IR cells in the forebrain of female rats to mating stimuli. Horm Behav. 2003;43:444–453. doi: 10.1016/s0018-506x(03)00028-x. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J Pharmacol Exp Ther. 2003;305:541–548. doi: 10.1124/jpet.102.045120. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases α4 GABA(A) receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Orman R, Smith SS. Sex differences in anxiety, sensorimotor gating and expression of the α4 subunit of the GABAA receptor in the amygdala after progesterone withdrawal. Eur J Neurosci. 2003;17:641–648. doi: 10.1046/j.1460-9568.2003.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Mueller NK, Figueiredo H. Role of GABA and glutamate circuitry in hypothalamo–pituitary–adrenocortical stress integration. Ann N Y Acad Sci. 2004;1018:35–45. doi: 10.1196/annals.1296.004. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg CK, Barrett KA. Reduced progesterone metabolites are not critical for plus-maze performance of lactating female rats. Pharmacol Biochem Behav. 1999;63:441–448. doi: 10.1016/s0091-3057(99)00041-6. [DOI] [PubMed] [Google Scholar]
- Khanna M, Qin KN, Cheng KC. Distribution of 3α-hydroxysteroid dehydrogenase in rat brain and molecular cloning of multiple cDNAs encoding structurally related proteins in humans. J Steroid Biochem Mol Biol. 1995;53:41–46. doi: 10.1016/0960-0760(95)00019-v. [DOI] [PubMed] [Google Scholar]
- Laconi MR, Casteller G, Gargiulo PA, Bregonzio C, Cabrera RJ. The anxiolytic effect of allopregnanolone is associated with gonadal hormonal status in female rats. Eur J Pharmacol. 2001;417:111–116. doi: 10.1016/s0014-2999(01)00865-2. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Brain mechanisms of emotion and emotional learning. Curr Opin Neurobiol. 1992;2:191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion: clues from the brain. Annu Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigland LA, Schulz LE, Janowsky JS. Age related changes in emotional memory. Neurobiol Aging. 2004;25:1117–1124. doi: 10.1016/j.neurobiolaging.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Li X, Berties PJ, Karavolas HJ. Regional distribution of cytostolic and particulate 5α-dihydroprogesterone 3α-hydroxysteroid oxidoreductase in female rat brain. J Steroid Biochem Mol Biol. 1997;60:311–318. doi: 10.1016/s0960-0760(96)00195-1. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Martinez-Mota L, Contreras CM, Saavedra M. Progesterone reduces immobility in rats forced to swim. Arch Med Res. 1999;30:286–289. doi: 10.1016/s0188-0128(99)00024-x. [DOI] [PubMed] [Google Scholar]
- Martinez-Mota L, Estrada-Camarena E, Lopez-Rubalcava C, Contreras CM, Fernandez-Guasti A. Interaction of desipramine with steroid hormones on experimental anxiety. Psychoneuroendocrinology. 2000;25:109–120. doi: 10.1016/s0306-4530(99)00042-6. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Felzenberg E, Robbins A, Pfaff DW, Schwartz-Giblin S. Infusions of diazepam and allopregnanolone into the midbrain central gray facilitate open-field behavior and sexual receptivity in female rats. Horm Behav. 1995;29:279–295. doi: 10.1006/hbeh.1995.1020. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;629:283–292. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez M, Perez JG, Olivera Lopez JI. Female Wistar rats tested during late proestrus or during pregnancy and ovariectomized rats tested after receiving progesterone or allopregnanolone displayed reduced conflict behavior. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:839–844. doi: 10.1016/s0278-5846(01)00328-1. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez M, Tellez-Alcantara NP, Perez Garcia J, Olivera Lopez JI, Teresa Jaramillo M. Anti-conflict-like actions of intralateral septal infusions of allopregnanolone in Wistar rats. Pharmacol Biochem Behav. 2003;75:397–404. doi: 10.1016/s0091-3057(03)00133-3. [DOI] [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Spyraki C. Influence of oestrogen on spontaneous and diazepam-induced exploration of rats in an elevated plus maze. Neuropharmacology. 1988;27:691–696. doi: 10.1016/0028-3908(88)90077-9. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Hassan AH, Holsboer DF, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–540. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York, NY: 1986. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze as measure of anxiety in the rat. J Neurosci Methods. 1986;14:149–167. [Google Scholar]
- Picazo O, Fernandez-Guasti A. Anti-anxiety effects of progesterone and some of its reduced metabolites: an evaluation using the burying behavior test. Brain Res. 1995;680:135–141. doi: 10.1016/0006-8993(95)00254-n. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Inhibiting progesterone metabolism in the hippocampus of proestrous rats decreases anxiolytic, and enhances, exploration and analgesia. Cogn Affect Behav Neurosci. 2001;1:287–296. doi: 10.3758/cabn.1.3.287. [DOI] [PubMed] [Google Scholar]
- Sierra A. Neurosteroids: the StAR protein in the brain. J Neuroendocrinol. 2004;16:787–793. doi: 10.1111/j.1365-2826.2004.01226.x. [DOI] [PubMed] [Google Scholar]
- Silveira MC, Sandner G, Graeff FG. Induction of Fos immunoreactivity in the brain by exposure to elevated plus maze. Behav Brain Res. 1993;56:115–118. doi: 10.1016/0166-4328(93)90028-o. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK, Micevych P. Estrogen induces de novo progesterone synthesis in astrocytes. Dev Neurosci. 2003;25:343–348. doi: 10.1159/000073511. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Davis C, Hammond A, Davis M. Progesterone attenuates corticotropin-releasing factor-enhanced but not fear-potentiated startle via the activity of its neuroactive metabolite, allopregnanolone. J Neurosci. 2004;24:10280–10287. doi: 10.1523/JNEUROSCI.1386-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Pinel JP, Fibiger HC. Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacol Biochem Behav. 1981;15:619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- Vongher JM, Frye CA. Progesterone in conjunction with estradiol has neuroprotective effects in an animal model of neurodegeneration. Pharmacol Biochem Behav. 1999;64:777–785. doi: 10.1016/s0091-3057(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Anti-nociception following exposure to trimethylthiazoline, peripheral or intra-amygdala estrogen and/ or progesterone. Behav Brain Res. 2004;144:77–85. doi: 10.1016/s0166-4328(03)00067-6. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology. 2005a;30:1288–1301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERβ-selective estrogen receptor modulators produce anti-anxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005b doi: 10.1038/sj.npp.1300713. in press. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Anti-depressant effects of ERβ selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78:523–529. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J Neurosci. 2000;20:7059–7066. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]