Abstract
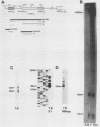
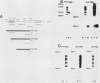
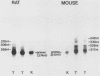
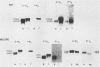
During spermatogenesis, several genes are expressed in a germ cell-specific manner. Previous studies have demonstrated that rat and mouse spermatogenic cells produce a 1,700-nucleotide proenkephalin RNA, while somatic cells that express the proenkephalin gene contain a 1,450-nucleotide transcript. Using cDNA cloning, RNA protection, and primer extension analyses, we showed that transcription of the rat and mouse spermatogenic-cell RNAs is initiated downstream from the proenkephalin somatic promoter in the first somatic intron (intron As). In both species, the germ cell cap site region consists of multiple start sites distributed over a length of approximately 30 base pairs. Within rat and mouse intron As, the region upstream of the germ cell cap sites is GC rich and lacks TATA sequences. A consensus binding site for the transcription factor SP1 was identified in intron As downstream of the proenkephalin germ cell cap site region. These features are characteristic of several previously described promoters that lack TATA sequences. Homologies were also identified between the proenkephalin and rat cytochrome c spermatogenic-cell promoters, including the absence of a TATA box, a multiple start site region, and several common sequences. This promoter motif thus may be shared with other genes expressed in male germ cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adra C. N., Boer P. H., McBurney M. W. Cloning and expression of the mouse pgk-1 gene and the nucleotide sequence of its promoter. Gene. 1987;60(1):65–74. doi: 10.1016/0378-1119(87)90214-9. [DOI] [PubMed] [Google Scholar]
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blum M., McEwen B. S., Roberts J. L. Transcriptional analysis of tyrosine hydroxylase gene expression in the tuberoinfundibular dopaminergic neurons of the rat arcuate nucleus after estrogen treatment. J Biol Chem. 1987 Jan 15;262(2):817–821. [PubMed] [Google Scholar]
- Boer P. H., Adra C. N., Lau Y. F., McBurney M. W. The testis-specific phosphoglycerate kinase gene pgk-2 is a recruited retroposon. Mol Cell Biol. 1987 Sep;7(9):3107–3112. doi: 10.1128/mcb.7.9.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Gascoigne N. R., Kavaler J., Lee N. E., Davis M. M. Somatic recombination in a murine T-cell receptor gene. Nature. 1984 May 24;309(5966):322–326. doi: 10.1038/309322a0. [DOI] [PubMed] [Google Scholar]
- Chretien S., Dubart A., Beaupain D., Raich N., Grandchamp B., Rosa J., Goossens M., Romeo P. H. Alternative transcription and splicing of the human porphobilinogen deaminase gene result either in tissue-specific or in housekeeping expression. Proc Natl Acad Sci U S A. 1988 Jan;85(1):6–10. doi: 10.1073/pnas.85.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrivia J. C., Uhler M. D., McKnight G. S. Characterization of genomic clones coding for the C alpha and C beta subunits of mouse cAMP-dependent protein kinase. J Biol Chem. 1988 Apr 25;263(12):5739–5744. [PubMed] [Google Scholar]
- Cole K. D., Kandala J. C., Kistler W. S. Isolation of the gene for the testis-specific H1 histone variant H1t. J Biol Chem. 1986 Jun 5;261(16):7178–7183. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobner P. R., Kislauskis E., Wentworth B. M., Villa-Komaroff L. Alternative 5' exons either provide or deny an initiator methionine codon to the same alpha-tubulin coding region. Nucleic Acids Res. 1987 Jan 12;15(1):199–218. doi: 10.1093/nar/15.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass J., Cox B., Quinn B., Civelli O., Herbert E. Expression of the prodynorphin gene in male and female mammalian reproductive tissues. Endocrinology. 1987 Feb;120(2):707–713. doi: 10.1210/endo-120-2-707. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Scarpulla R. C. Both upstream and intron sequence elements are required for elevated expression of the rat somatic cytochrome c gene in COS-1 cells. Mol Cell Biol. 1988 Jan;8(1):35–41. doi: 10.1128/mcb.8.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J. E., Collard M. W., Douglass J. O. Translational control of germ cell-expressed mRNA imposed by alternative splicing: opioid peptide gene expression in rat testis. Mol Cell Biol. 1989 Oct;9(10):4381–4389. doi: 10.1128/mcb.9.10.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes S., Weisz-Carrington P., Daum H., 3rd, Smith J., Green L., Wright K., Stein G., Stein J. A rat histone H4 gene closely associated with the testis-specific H1t gene. Exp Cell Res. 1987 Dec;173(2):534–545. doi: 10.1016/0014-4827(87)90293-x. [DOI] [PubMed] [Google Scholar]
- Haugen A., Mann D., Murray C., Weston A., Willey J. C. Interleukin-1 alpha gene intron containing variable repeat region coding for the SP1 transcription factor recognition sequence is polymorphic. Mol Carcinog. 1989;2(2):68–71. doi: 10.1002/mc.2940020204. [DOI] [PubMed] [Google Scholar]
- Heidaran M. A., Kistler W. S. Transcriptional and translational control of the message for transition protein 1, a major chromosomal protein of mammalian spermatids. J Biol Chem. 1987 Sep 25;262(27):13309–13315. [PubMed] [Google Scholar]
- Heidaran M. A., Kozak C. A., Kistler W. S. Nucleotide sequence of the Stp-1 gene coding for rat spermatid nuclear transition protein 1 (TP1): homology with protamine P1 and assignment of the mouse Stp-1 gene to chromosome 1. Gene. 1989 Jan 30;75(1):39–46. doi: 10.1016/0378-1119(89)90381-8. [DOI] [PubMed] [Google Scholar]
- Howells R. D., Kilpatrick D. L., Bhatt R., Monahan J. J., Poonian M., Udenfriend S. Molecular cloning and sequence determination of rat preproenkephalin cDNA: sensitive probe for studying transcriptional changes in rat tissues. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7651–7655. doi: 10.1073/pnas.81.23.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Johnson P. A., Peschon J. J., Yelick P. C., Palmiter R. D., Hecht N. B. Sequence homologies in the mouse protamine 1 and 2 genes. Biochim Biophys Acta. 1988 May 6;950(1):45–53. doi: 10.1016/0167-4781(88)90071-1. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Kew D., Jin D. F., Kim F., Laddis T., Kilpatrick D. L. Translational status of proenkephalin mRNA in the rat reproductive system. Mol Endocrinol. 1989 Aug;3(8):1191–1196. doi: 10.1210/mend-3-8-1191. [DOI] [PubMed] [Google Scholar]
- Kew D., Kilpatrick D. L. Expression and regulation of the proenkephalin gene in rat Sertoli cells. Mol Endocrinol. 1989 Jan;3(1):179–184. doi: 10.1210/mend-3-1-179. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Borland K., Jin D. F. Differential expression of opioid peptide genes by testicular germ cells and somatic cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5695–5699. doi: 10.1073/pnas.84.16.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Rosenthal J. L. The proenkephalin gene is widely expressed within the male and female reproductive systems of the rat and hamster. Endocrinology. 1986 Jul;119(1):370–374. doi: 10.1210/endo-119-1-370. [DOI] [PubMed] [Google Scholar]
- Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol Cell Biol. 1989 Nov;9(11):5134–5142. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Selection of initiation sites by eucaryotic ribosomes: effect of inserting AUG triplets upstream from the coding sequence for preproinsulin. Nucleic Acids Res. 1984 May 11;12(9):3873–3893. doi: 10.1093/nar/12.9.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey J. R. Nucleotide sequence of the promoter region of a tissue-specific human retroposon: comparison with its housekeeping progenitor. Gene. 1987;61(3):291–298. doi: 10.1016/0378-1119(87)90192-2. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan J. L., Driscoll C. E., LeVan K. M., Goldberg E. Epitopes of human testis-specific lactate dehydrogenase deduced from a cDNA sequence. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5311–5315. doi: 10.1073/pnas.84.15.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muffly K. E., Jin D. F., Okulicz W. C., Kilpatrick D. L. Gonadal steroids regulate proenkephalin gene expression in a tissue-specific manner within the female reproductive system. Mol Endocrinol. 1988 Oct;2(10):979–985. doi: 10.1210/mend-2-10-979. [DOI] [PubMed] [Google Scholar]
- Oppi C., Shore S. K., Reddy E. P. Nucleotide sequence of testis-derived c-abl cDNAs: implications for testis-specific transcription and abl oncogene activation. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8200–8204. doi: 10.1073/pnas.84.23.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G. A., Basu S. K., Osborne T. F., Chin D. J., Gil G., Brown M. S., Goldstein J. L., Luskey K. L. HMG CoA reductase: a negatively regulated gene with unusual promoter and 5' untranslated regions. Cell. 1984 Aug;38(1):275–285. doi: 10.1016/0092-8674(84)90549-x. [DOI] [PubMed] [Google Scholar]
- Robinson M. O., McCarrey J. R., Simon M. I. Transcriptional regulatory regions of testis-specific PGK2 defined in transgenic mice. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8437–8441. doi: 10.1073/pnas.86.21.8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Douglass J., Herbert E. Isolation and characterization of the rat proenkephalin gene. J Biol Chem. 1984 Nov 25;259(22):14309–14313. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Sam J., Keith D. H., Tani K., Simmer R. L., Shively L., Lindsay S., Yoshida A., Riggs A. D. Sequence of the promoter region of the gene for human X-linked 3-phosphoglycerate kinase. Gene. 1984 Dec;32(3):409–417. doi: 10.1016/0378-1119(84)90016-7. [DOI] [PubMed] [Google Scholar]
- Sonnenberg J. L., Rauscher F. J., 3rd, Morgan J. I., Curran T. Regulation of proenkephalin by Fos and Jun. Science. 1989 Dec 22;246(4937):1622–1625. doi: 10.1126/science.2512642. [DOI] [PubMed] [Google Scholar]
- Staden R. The current status and portability of our sequence handling software. Nucleic Acids Res. 1986 Jan 10;14(1):217–231. doi: 10.1093/nar/14.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virbasius J. V., Scarpulla R. C. Structure and expression of rodent genes encoding the testis-specific cytochrome c. Differences in gene structure and evolution between somatic and testicular variants. J Biol Chem. 1988 May 15;263(14):6791–6796. [PubMed] [Google Scholar]
- Wolfes H., Kogawa K., Millette C. F., Cooper G. M. Specific expression of nuclear proto-oncogenes before entry into meiotic prophase of spermatogenesis. Science. 1989 Aug 18;245(4919):740–743. doi: 10.1126/science.2475907. [DOI] [PubMed] [Google Scholar]
- Yelick P. C., Balhorn R., Johnson P. A., Corzett M., Mazrimas J. A., Kleene K. C., Hecht N. B. Mouse protamine 2 is synthesized as a precursor whereas mouse protamine 1 is not. Mol Cell Biol. 1987 Jun;7(6):2173–2179. doi: 10.1128/mcb.7.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K., Maruyama K., Aizawa T., Yamamoto A. A new species of enkephalin precursor mRNA with a distinct 5'-untranslated region in haploid germ cells. FEBS Lett. 1989 Mar 27;246(1-2):193–196. doi: 10.1016/0014-5793(89)80281-9. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Sabol S. L. Glucocorticoids and cyclic AMP synergistically regulate the abundance of preproenkephalin messenger RNA in neuroblastoma-glioma hybrid cells. Biochem Biophys Res Commun. 1986 Aug 29;139(1):1–10. doi: 10.1016/s0006-291x(86)80071-7. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Williams C., Sabol S. L. Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J Biol Chem. 1984 Nov 25;259(22):14301–14308. [PubMed] [Google Scholar]
- Zurawski G., Benedik M., Kamb B. J., Abrams J. S., Zurawski S. M., Lee F. D. Activation of mouse T-helper cells induces abundant preproenkephalin mRNA synthesis. Science. 1986 May 9;232(4751):772–775. doi: 10.1126/science.2938259. [DOI] [PubMed] [Google Scholar]