Abstract
Plasmodium falciparum malaria remains a major public health threat for which there is no licensed vaccine. Abs play a key role in malaria immunity, but Ab-mediated protection is only acquired after years of repeated infections, leaving children in endemic areas vulnerable to severe malaria and death. Many P. falciparum Ags are extraordinarily diverse and clonally variant which likely contribute to the inefficient acquisition of protective Abs. However, mounting evidence suggests that there is more to the story and that infection-induced dysregulation of B cell function also plays a role. Here we review progress toward understanding the B cell biology of P. falciparum infection, focusing on what has been learned from population-based studies in malaria-endemic areas. We suggest ways in which advances in immunology and genomics-based technology can further improve our understanding of the B cell response in malaria and perhaps illuminate new pathways to the development of effective vaccines.
Introduction
Malaria is caused by mosquito-borne parasites of the genus Plasmodium. Of the five Plasmodium species that infect humans, P. falciparum is the deadliest, each year causing approximately 225 million cases of malaria and nearly one million deaths, the majority among African children and pregnant women (http://www.who.int/malaria/world_malaria_report_2011/en/) (1). Optimism that a first-generation malaria vaccine, RTS,S, may soon be licensed has been tempered by the interim results of an ongoing phase 3 clinical trial in Africa which indicated that the vaccine confers short-lived protection from malaria in only ~30% of infants (2). Clearly, the ongoing effort to develop a highly effective vaccine would benefit from a more detailed understanding of malaria immunity.
P. falciparum has a complex life cycle (Fig 1) in which only the blood stage of infection is associated with disease, typically an undifferentiated febrile illness which in a minority of cases progresses to severe disease and death (3). Epidemiologic studies in areas where individuals are repeatedly infected with P. falciparum show that immunity to severe, life-threatening malaria is usually acquired early in childhood, whereas immunity to uncomplicated febrile malaria is not reliably acquired until early adulthood (4), and once acquired, appears to wane (5), at least partially (6), in the absence of ongoing P. falciparum exposure. Despite decades of exposure, sterile immunity to infection develops rarely, if at all (4), as adults often carry blood stage parasites without symptoms. In this review we focus our attention on naturally-acquired immunity to the blood stage of P. falciparum infection where B cells are known to play a critical role in protection.
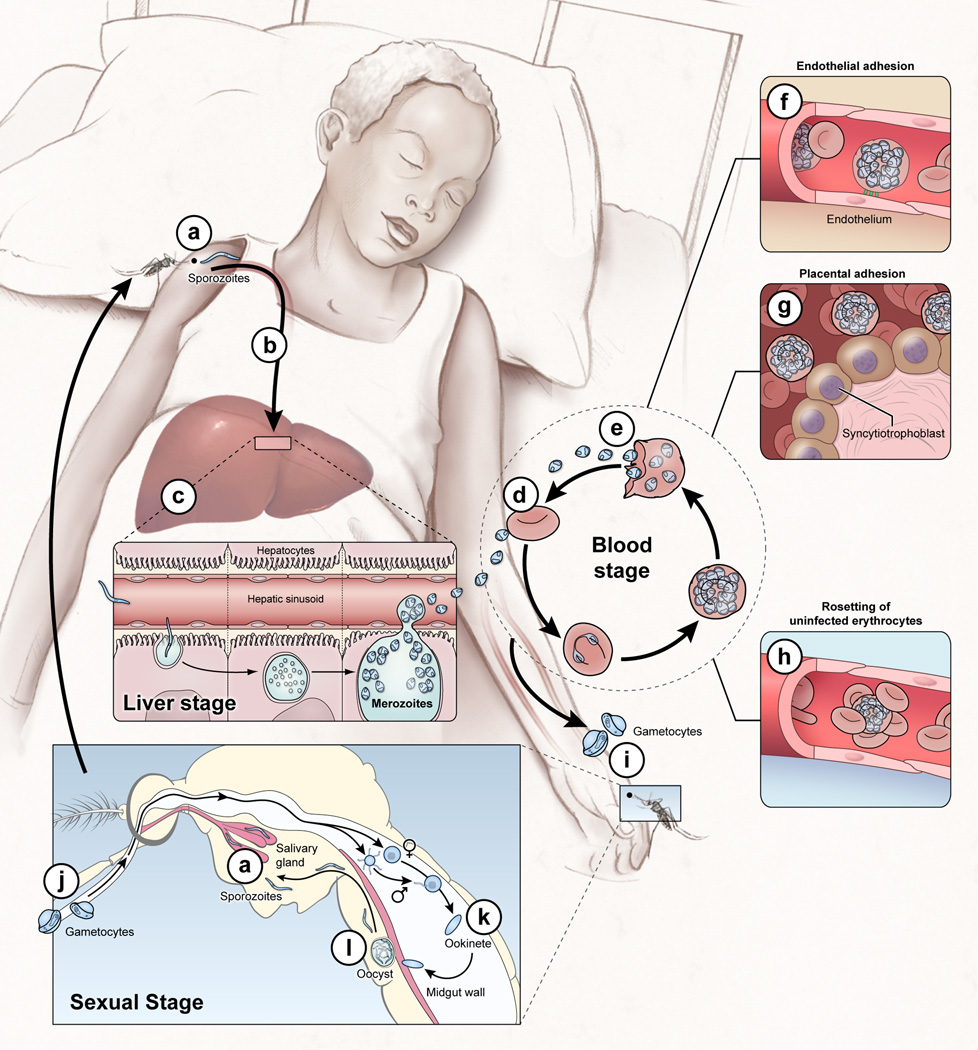
Figure 1. The P. falciparum life cycle: no shortage of Ab targets.
The P. falciparum life cycle in humans includes the asymptomatic liver stage; the blood stage which causes disease; and the sexual gametocyte blood stage which infects mosquitoes that transmit the parasite. Infection begins when Anopheles mosquitos inject sporozoites into the skin and blood (a) which migrate to the liver and invade a small number of hepatocytes (b). Each sporozoite gives rise to thousands of asexual parasites called merozoites (c). ~1 week after hepatocyte invasion merozoites exit the liver into the bloodstream and begin a 48 hr cycle (d) of RBC invasion, replication, RBC rupture, and merozoite release (e). Once inside RBCs the parasite exports variant surface antigens (VSAs) such as PfEMP1 to the RBC surface. VSAs mediate binding of iRBCs to the microvascular endothelium of various organs (f) and placental tissue (g) allowing parasites to avoid splenic clearance and promoting the inflammation and circulatory obstruction associated with clinical syndromes such as cerebral malaria (coma) and pregnancy-associated malaria. VSA-mediated rosetting (binding of iRBCs to RBCs) may also contribute to disease (h). A small number of blood-stage parasites differentiate into sexual gametocytes (i) which are taken up by mosquitos (j) where they differentiate into gametes that fuse to form a motile zygote, the ookinete (k), where meiosis occurs. The ookinete crosses the midgut wall and forms an oocyst (l) that develops into sporozoites that enter the mosquito salivary gland to complete the life cycle (a).
Abs that sterilely protect by neutralizing sporozoites (a) and/or blocking hepatocyte invasion (b) are rarely if ever acquired through natural infection (4). Abs induced by the RTS,S vaccine target the circumsporozoite (CS) protein on the sporozoite surface (98) and correlate with sterile protection in malaria-naïve adults (99); but in African children RTS,S generally protects against disease not infection and the correlation between Abs and protection is less clear (100, 101). Abs are a key component of naturally-acquired blood stage immunity (7) but the Ag targets and mechanisms of protection are incompletely understood and likely multifaceted. Abs may contribute to protection by clearing merozoites (102) and iRBCs (e) through opsonization (103) or complement-mediated lysis (104); inhibiting merozoite invasion of RBCs (d) (105); and/or blocking adhesion of iRBCs to vascular endothelium (f) (106). Non-neutralizing Abs may contribute to protection through Ab-dependent monocyte-(107) or NK cell-mediated cytotoxicity. Blood stage vaccines have focused primarily on generating Abs to merozoite proteins but with limited success (54, 108), probably due to Ag polymorphism (14, 108) and redundant RBC invasion pathways (109). Of note, the conserved merozoite protein PfRh5 appears to be essential for RBC invasion (80) and may be susceptible to vaccine-inducible cross-strain neutralizing Ab (110). Protection from pregnancy-associated malaria correlates with IgG specific for VAR2CSA, a conserved protein which mediates adherence to placental tissue (g) (111). Vaccine-induced Abs targeting Ags on gametocytes and gametes that are ingested with the blood meal (i–l) could prevent parasite development in the mosquito and block or reduce transmission (112).
The central role of Abs in naturally-acquired malaria immunity was shown by early experiments in which the transfer of purified IgG from malaria-immune West African adults to children with malaria in West Africa (7), East Africa (8) or Thailand (9) led to rapid and profound reductions in parasite numbers in the blood and resolution of fever. Over 50 years after understanding that Abs mediate malaria immunity, it remains a major challenge to determine which of the ~ 5,400 predicted P. falciparum proteins (10) elicit protective Abs and the mechanisms by which these Abs protect (described in Fig 1) (11). Indeed, absent this information there are currently no unambiguous immune correlates of protection from malaria. It also remains incompletely understood why the acquisition of Ab-mediated immunity is so slow to develop (12, 13). The gradual acquisition of protective Abs has been proposed to be due in large part to the extensive genetic diversity of many P. falciparum Ags (14) as well as the parasite’s ability to clonally vary the Ags it expresses on the surface of infected RBCs (iRBCs) (15). The reasoning goes that it may take years living in an endemic area for an individual to be exposed to a sufficient number of parasite clones to generate a protective Abs. However, the data supporting this view are not ironclad. Here we review studies that point toward an alternative but non-mutually exclusive explanation for the gradual acquisition of Ab immunity to malaria—that P. falciparum subverts B cell responses making the acquisition of protective Abs inefficient, particularly as compared to responses to other pathogens in which long-lived Ab-mediated protection is acquired after a single or few exposures.
The Ab Response to Malaria
How clone-specific are Abs that protect against malaria?
The proposal that the slow acquisition of protective Abs in individuals living in malaria endemic areas is due to the time required to be exposed to a large number of P. falciparum clones predicts that Abs to P. falciparum variant antigens should be highly clone specific. A major target of protective Abs in malaria appears to be P. falciparum erythrocyte membrane protein 1 (PfEMP1) expressed on the surface of iRBCs (16). PfEMP1s are encoded by the highly polymorphic var multigene family (~60 genes/genome) and their expression is clonally variant (15). PfEMP1s mediate adhesion of iRBCs to microvascular endothelium, allowing the parasite to sequester in capillaries of various organs and avoid destruction in the spleen (Fig 1) (3). In addition, sequestration of iRBCs in organs such as the brain and placenta has been linked to the pathogenesis of clinical syndromes such as cerebral malaria and pregnancy-associated malaria (Fig 1) (17). Several studies support the hypothesis that repeated infections are required to elicit a protective repertoire of PfEMP1-specific Abs (16, 18–23). However, other studies suggest that there is a limited subset of common PfEMP1s Ags that are important targets of immunity (24–27) or that protective Abs target cross-reactive or conserved epitopes on PfEMP1s (28–33). The observation that purified IgG from immune West African adults was therapeutic in children not only in West Africa but in East Africa (8) and Thailand as well (9) suggests a significant degree of Ab cross-reactivity to genetically diverse P. falciparum parasites (34). Together these observations raise the possibility that the gradual acquisition of protective Abs may not simply be a reflection of the time required for an individual to be exposed to all the parasite clones circulating in a given endemic area.
How long-lived is the Ab response to P. falciparum?
It is well established that long-lived protective Ab responses, that are induced by many pathogens and vaccines after one or a few exposures (35), depend on the generation of high affinity memory B cells (MBCs) and long-lived plasma cells (LLPCs) (36–38). LLPCs reside in the bone marrow where they constitutively secrete Abs and provide a critical first line of defense against re-infection, whereas MBCs mediate recall Ab responses after re-exposure to their Ag by rapidly proliferating and differentiating into PCs.
Although there is significant heterogeneity in the magnitude, quality and longevity of Ab responses following infection or vaccination, in general, Ab responses are long-lived. For instance, estimates of half-lives of IgG responses range from 11 years for tetanus vaccines to >300 years for measles vaccines (35, 38). In sharp contrast, the half-lives of P. falciparum-specific Ab responses generally appear to be much shorter, particularly in young children. For example, in a 12-week study of Kenyan children following acute malaria, the half-lives of IgG responses specific for 5 merozoite surface Ags (39) were estimated to be 9.8 days for IgG1 and 6.1 days for IgG3. In additional longitudinal studies, P. falciparum-specific Ab responses have been reported to decline to undetectable or nearly undetectable levels within 3–9 months of documented malaria episodes in children (40–48). It is of interest that Ab responses to conserved (or semi-conserved) P. falciparum Ags (42) or conserved regions of polymorphic Ags (45), to which individuals presumably were repeatedly exposed, were similarly short lived. Together these studies in children indicate that P. falciparum infection does not reliably induce a stable pool of LLPCs. Short-lived P. falciparum-specific IgG responses have also been observed in older children and adults (42, 45, 49). On the other hand, there are studies that point toward more stable IgG responses with increasing age in areas of stable P. falciparum transmission (40, 44, 46, 50, 51), however, it is important to note that in such areas it is difficult to separate the effects of age and cumulative P. falciparum exposure on malaria immunity. Data from transmigrant studies in Indonesia suggest that adults may acquire clinical immunity to malaria more rapidly than children (52, 53), but this bears further investigation as the risk of clinical malaria was similar among previously unexposed children and adults during a malaria epidemic in Madagascar (6).
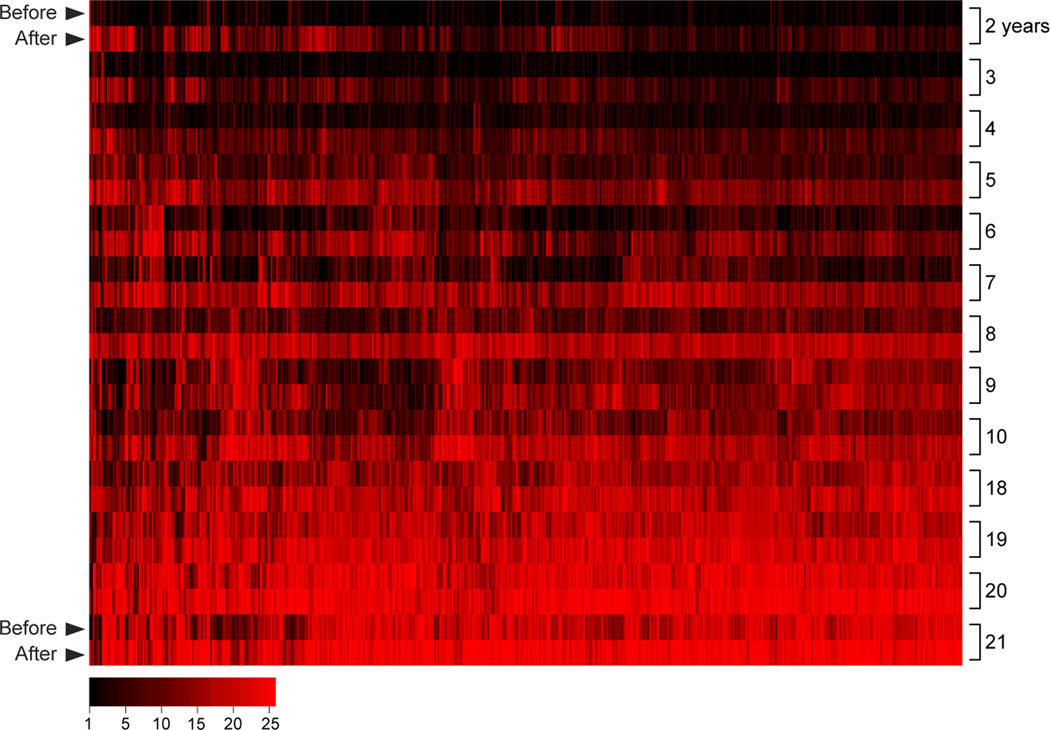
How might these differences in the estimates of the duration of Ab responses be reconciled? It is possible that studies that reported short-lived P. falciparum-specific Ab responses were biased by the small number of Ags examined. Indeed, the studies cited above measured IgG responses specific for one or a few Ags, often blood-stage merozoite surface Ags being developed as vaccine candidates (54) that together represent <0.5% of the predicted P. falciparum proteome (10). However, a recent study which employed protein microarray technology (55) to assess the IgG response to ~25% of the P. falciparum proteome, including proteins expressed at all stages of the parasite life cycle, showed that children exposed to intense seasonal P. falciparum transmission generate IgG responses to hundreds of P. falciparum Ags each transmission season. However, these declined rapidly during the 6 month dry season, a period of little to no P. falciparum transmission (Fig 2). These data suggest a preferential induction of short-lived plasma cells (SLPCs) during P. falciparum infection that is not restricted to a subset of intrinsically less immunogenic proteins.
Figure 2. The P. falciparum-specific IgG response is broad but short-lived in children.
Shown is IgG reactivity (red) specific for 491 P. falciparum proteins (columns) in plasma collected from children (n=157) and adults (n=37) before and after an intense 6 month malaria season [(55) for detailed Methods and Results]. The average IgG level for each protein is stratified by 1-yr age groups from 2–21 yrs, and within each age group from before to after the malaria season (rows). A broad P. falciparum-specific IgG response is present after the 6-month malaria season in children as young as 2 yrs, however, the IgG response in young children is short-lived based on IgG levels at the end of the 6 month dry season, a period of little to no P. falciparum transmission. With increasing age, IgG levels at the end of the dry season gradually increase until plateauing in adulthood.
Even though Ab responses to P. falciparum Ags in children are short-lived, ultimately, by adulthood stable Ab levels are acquired. The study cited above showed that with each year of P. falciparum exposure, the level of IgG persisting through the dry season gradually increased until young adulthood when IgG levels were maintained at high levels (Fig 2). One simple explanation for the gradual buildup of P. falciparum-specific IgGs with age is the incremental accumulation of LLPCs with each year of exposure. How important for protection are long-lived Ab responses? An age-adjusted analysis in the same study showed that the overall level of P. falciparum-specific IgG present before but not after the malaria season predicted protection from malaria (55).
Taken together, these data suggest that the gradual acquisition of clinical immunity to malaria may reflect the need for repeated infections in order to ‘fill’ the P. falciparum-specific LLPC compartment to the point where steady-state Ab levels exceed a protective threshold. Conversely, MBC-mediated Ab boosting in response to acute infection, a process that generally peaks 6–10 days after Ag re-exposure, may not be rapid enough to prevent the onset of symptoms which can occur as early as 3 days after blood stage infection begins (56). Similar models have been postulated for other pathogens with short incubation periods such as Haemophilus influenzae type b (57), in which the infection progresses to a symptomatic threshold more rapidly than MBC-mediated Ab boosting, such that protection depends on already circulating Abs. In this light, it is likely that an effective blood-stage malaria vaccine will need to establish a critical level of circulating IgG produced by a stable pool of LLPCs, rather than depend on MBC-mediated Ab boosting. This may be especially important in areas of sporadic P. falciparum transmission where boosting of Ab responses does not occur through regular natural exposure. The same principle is likely applies to vaccines targeting sporozoites which are in the bloodstream and exposed to Abs only transiently before invading hepatocytes.
The continued use of high-throughput Ab profiling methods (58) in various epidemiological settings in conjunction with systems biology approaches (59) to describe the contribution of T cells and innate immunity will improve our understanding of the factors which influence the specificity, longevity and quality of Ab responses to malaria. A more detailed understanding of the mechanisms which ultimately confer protection against malaria and the reasons for their inefficient acquisition could help advance malaria vaccine development.
The MBC Response to Malaria
It was initially speculated that P. falciparum infection might not generate MBCs based on anecdotal evidence of waning malaria immunity in the absence of P. falciparum exposure, and inconsistent Ab boosting upon re-infection (39), even to conserved Ags (42). However, several recent studies have now shown that P. falciparum-specific MBCs can indeed be generated in response to natural infection, albeit inefficiently. For example, a longitudinal study in Mali, where there is an annual 6 month malaria season, found that the frequency of MBCs specific for two blood stage Ags, AMA1 and MSP1, increased incrementally with each malaria season, from childhood to adulthood (43). Moreover, MBCs once acquired appear to be long lived. For example, a recent study in Kenya reported that P. falciparum-specific MBCs were found to persist longer than Abs in the absence of ongoing P. falciparum exposure (60). However, the Mali study showed that despite exposure to ~60 infective mosquito bites/month at the peak of the malaria season, only ~50% of adults had detectable P. falciparum-specific MBCs (43). A low prevalence (~30–50%) of P. falciparum-specific MBCs has also been reported in adults in studies conducted in The Gambia (61), Kenya (62) and Thailand (51), even in individuals with documented Plasmodium infection within 6 years (51). The findings for P. falciparum-induced MBCs are in sharp contrast to studies that show, for example, that smallpox vaccine-specific MBCs are generated and persist in nearly all vaccinees for 50+ years in the absence of Ag re-exposure (63). Thus, it appears that P. falciparum infection can generate long-lived MBCs but less efficiently, at least compared to vaccines for other pathogens. Although it might be predicted that the prevalence and frequencies of P. falciparum-specific MBCs would be higher in areas of intense versus sporadic P. falciparum transmission, studies to date suggest that this is not the case (43, 51, 61, 64), raising the possibility that chronic or persistent P. falciparum exposure may be detrimental to MBC generation.
What might underlie the inefficient acquisition of P. falciparum-specific LLPCs and MBCs?
Several mechanisms have been proposed to explain the relatively inefficient acquisition of P. falciparum-specific LLPCs and MBCs (Table 1). Because of limited space we comment on only a few possibilities for which there is recent data.
Table 1.
Potential factors contributing to the inefficient acquisition of protective Abs.
| Factor | References |
|---|---|
| Clonal Ag variation | (15) |
| Ag genetic diversity | (14) |
| GC disruption | (92) |
| Lack of Ab avidity maturation | (93) |
| Marginal zone B cell induction | (94) |
| B cell ‘exhaustion’ | (71) |
| Increased immature transitional B cells | (95) |
| CD4+ T cell exhaustion | (76) |
| Polyclonal B cell activation | (77, 96) |
| BAFF/BAFF-R dysregulation | (81) |
| TLR tolerance | (90) |
| Self-reactive antibodies/anergy | (97) |
| Clonal imprinting/OAS | (46) |
The relative inefficiency in acquiring P. falciparum-specific LLPC and MBCs may be related to dysregulated B cell Ag-driven differentiation, particularly in the setting of chronic P. falciparum exposure. Alterations in B cell function are well described in the context of chronic infections such as visceral leishmaniasis (65), HIV (66) and HCV (67). For example, HIV (68) and HCV (69) viremia is associated with an expansion of a subset of B cells identified by the cell surface markers CD19+CD20+CD21−CD27−CD10− and Fc-receptor-like-4 (FCRL4) (70). In HIV-infected individuals Moir et al (68) showed that these cells were hyporesponsive and suggested that these hyporesponsive, or ‘exhausted’ MBCs contribute to the humoral deficiencies observed in HIV-infected individuals.
Remarkably, an increase in a phenotypically similar subset of B cells has been observed in P. falciparum-exposed children and adults (71). In the context of malaria, this B cell subset has been referred to as ‘atypical’ rather than ‘exhausted’ because the function of these B cells and whether they are beneficial or detrimental in malaria remains unclear. An expansion of atypical MBCs has also been observed in malaria endemic Peru (72), The Gambia (61), Mali (73), Kenya (74) and Gabon (75), suggesting that P. falciparum-associated atypical MBC expansion is generalizable to genetically and geographically disparate populations; and that the degree of atypical MBC expansion correlates with P. falciparum transmission intensity (72). A direct causal relationship between P. falciparum infection and atypical MBC expansion remains to be established; however, differential expansion of atypical MBCs has recently been observed in age-matched children living under similar conditions in rural Kenya with the exception of P. falciparum exposure (74), suggesting that P. falciparum infection per se may drive atypical MBC expansion. Intriguingly, recent work by Muellenback et al (75) showed at the single cell level that P. falciparum exposure is associated with the acquisition of atypical MBCs that produce broadly neutralizing Abs specific for P. falciparum blood stage antigens; and furthermore, that atypical MBCs, unlike classical MBCs, show signs of active Ab secretion, although spontaneous Ab secretion was not noted in other studies (68, 71). Compared to classical MBCs, atypical MBCs also differed in their IgG gene repertoire, suggesting that they develop from different precursors (75).
P. falciparum infections could also dysregulate helper T cell responses. Interestingly, a recent study showed that PD-1, a marker of T cell exhaustion, is upregulated on CD4+ T cells in children following P. falciparum infection (76). Although the functional significance of this observation in human remains to be determined, Butler et al. reported that P.yoelii infection in mice induces phenotypic and functional CD4+ T cell exhaustion, and that in vivo blockade of the PD-1 ligand PD-L1 and the inhibitory receptor LAG-3 restored CD4+ T cell function, amplified the number of TFH and GC B cells and plasmablasts, increased Ab levels and accelerated the clearance of blood-stage parasites (76). To understand the functional link between putative P. falciparum-specific CD4+ T cell exhaustion and Ab responses in humans will require tools that allow for the detection of P. falciparum-specific T cells, including MHC tetramer technology that has proved to be effective in identifying pathogen-specific T cells by flow cytometry.
Whether malaria-associated B cell dysregulation is driven by direct interactions between P. falciparum products and B cells or indirectly through systemic immune activation is unclear. To our knowledge the only published example of a direct P. falciparum-B cell interaction comes from in vitro studies which implicate the cysteine-rich interdomain regions 1α (CIDR1α) of PfEMP1 as a T cell-independent polyclonal B cell activator and Ig binding protein (77). The discovery of direct molecular interactions between P. falciparum and the human immune system has been accelerated by systematic protein interaction screening approaches (78). For example, a yeast two-hybrid approach identified an interaction between P. falciparum merozoite surface protein 1 (MSP1) and the pro-inflammatory protein S100P (79); and an avidity-based extracellular interaction screen identified basigin (CD147) as a host receptor essential for RBC invasion via the merozoite protein PfRh5 (80). Intriguingly, basigin is also expressed on B and T cells, as well as DCs, monocytes and macrophage, raising the possibility that basigin-PfRh5 interactions may modulate host responses during P. falciparum infection.
It is also possible that systemic mediators of B cell differentiation and survival (36), such as B-cell activating factor (BAFF), APRIL, IL-4, and IL-21 are modulated during P. falciparum infection. A recent study of Kenyan children (81) reported that acute malaria is associated with elevated plasma levels of BAFF and transient down-regulation of BAFF receptor expression on B cells. But interestingly, higher levels of BAFF-receptor expression on B cells were associated with more durable P. falciparum-specific IgG responses, suggesting that dysregulated BAFF-R expression may contribute to inefficient P. falciparum-specific LLPC responses. It has also been suggested (39) that binding of iRBCs to bone marrow stromal cells (82) may disrupt PC survival signals in bone marrow niches.
Chronic exposure to pathogen-associated molecular patterns (PAMPs) could possibly result in tolerance of pattern recognition receptors (PRRs) expressed on B cells and DCs which play a critical role in enhancing B cell responses (83) (84). Although few P. falciparum-derived PAMPs have been identified to date (85–88), recent clinical trials demonstrate that the TLR9 agonist CPG markedly enhances the IgG and MBC response to P. falciparum blood-stage vaccine candidates in malaria-naïve adults (89), but not in adults chronically exposed to P. falciparum in endemic areas (90). Further studies are needed to define how P. falciparum exposure modulates the function of PRRs and how this might influence homologous and heterologous LLPC and MBC responses.
It is unclear whether Ab responses in the course of repeated P. falciparum infections are predominantly derived from newly recruited naïve B cells or cross-reactive MBC clones generated during prior infections (46) (i.e. clonal imprinting or original antigenic sin [OAS]). It useful to consider the case of influenza, which like P. falciparum, repeatedly infects much of the world’s population and exhibits extensive strain diversity. Work by Wrammert et al (91) suggests that plasmablasts producing broadly neutralizing Abs induced by pandemic H1N1 influenza are predominantly derived from activated MBCs specific for epitopes conserved in several influenza strains, consistent with OAS. Similar analyses of variable gene sequences from plasmablasts induced by human experimental (56) and natural P. falciparum infection, particularly serial infections with genetically distinct P. falciparum parasites, could define the degree to which OAS operates in malaria and how it relates to malaria susceptibility.
Conclusions
Although there appears to be more questions than answers as to how malaria immunity is acquired, it is nonetheless an exciting time in malaria research. Malaria is one of the few infectious diseases in which relatively small longitudinal cohort studies of natural infection are feasible. For example, in areas of intense seasonal P. falciparum transmission, where nearly all individuals are predictably exposed to P. falciparum each year, it is possible to analyze the human immune response before, during, and after P. falciparum infection and to prospectively study immune correlates of protection as well as the immunomodulatory effects of infection. Malaria is also one of the only infectious diseases for which experimental human infections are approved. These unique study populations coupled with the powerful tools made available through extraordinary advances in immunology and systems biology ought to provide a wealth of information on the impact of P. falciparum infections on the human immune system and on the acquisition of malaria immunity.
Acknowledgements
SP, SKP and PDC are supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
References
- 1.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 2.A Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Infants. N Engl J Med. 2012 doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 4.Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28:51–60. doi: 10.1111/j.1365-3024.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 5.Jelinek T, Schulte C, Behrens R, Grobusch MP, Coulaud JP, Bisoffi Z, Matteelli A, Clerinx J, Corachan M, Puente S, Gjorup I, Harms G, Kollaritsch H, Kotlowski A, Bjorkmann A, Delmont JP, Knobloch J, Nielsen LN, Cuadros J, Hatz C, Beran J, Schmid ML, Schulze M, Lopez-Velez R, Fleischer K, Kapaun A, McWhinney P, Kern P, Atougia J, Fry G, da Cunha S, Boecken G. Imported Falciparum malaria in Europe: sentinel surveillance data from the European network on surveillance of imported infectious diseases. Clin Infect Dis. 2002;34:572–576. doi: 10.1086/338235. [DOI] [PubMed] [Google Scholar]
- 6.Deloron P, Chougnet C. Is immunity to malaria really short-lived? Parasitol Today. 1992;8:375–378. doi: 10.1016/0169-4758(92)90174-z. [DOI] [PubMed] [Google Scholar]
- 7.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 8.McGregor IA, Carrington SP, Cohen S. Treatment of East African P. falciparum Malaria with West African Human γ-Globulin. Trans R Soc Trop Med Hyg. 1963;57:170–175. [Google Scholar]
- 9.Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, Foucault C, Chongsuphajaisiddhi T, Druilhe P. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg. 1991;45:297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- 10.Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beeson JG, Osier FH, Engwerda CR. Recent insights into humoral and cellular immune responses against malaria. Trends Parasitol. 2008;24:578–584. doi: 10.1016/j.pt.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Achtman AH, Bull PC, Stephens R, Langhorne J. Longevity of the immune response and memory to blood-stage malaria infection. Curr Top Microbiol Immunol. 2005;297:71–102. doi: 10.1007/3-540-29967-x_3. [DOI] [PubMed] [Google Scholar]
- 13.Struik SS, Riley EM. Does malaria suffer from lack of memory? Immunol Rev. 2004;201:268–290. doi: 10.1111/j.0105-2896.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 14.Takala SL, Plowe CV. Genetic diversity and malaria vaccine design, testing and efficacy: preventing and overcoming 'vaccine resistant malaria'. Parasite Immunol. 2009;31:560–573. doi: 10.1111/j.1365-3024.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scherf A, Lopez-Rubio JJ, Riviere L. Antigenic variation in Plasmodium falciparum. Annu Rev Microbiol. 2008;62:445–470. doi: 10.1146/annurev.micro.61.080706.093134. [DOI] [PubMed] [Google Scholar]
- 16.Chan JA, Howell KB, Reiling L, Ataide R, Mackintosh CL, Fowkes FJ, Petter M, Chesson JM, Langer C, Warimwe GM, Duffy MF, Rogerson SJ, Bull PC, Cowman AF, Marsh K, Beeson JG. Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J Clin Invest. 2012 doi: 10.1172/JCI62182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beeson JG, Brown GV. Pathogenesis of Plasmodium falciparum malaria: the roles of parasite adhesion and antigenic variation. Cell Mol Life Sci. 2002;59:258–271. doi: 10.1007/s00018-002-8421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cham GK, Turner L, Kurtis JD, Mutabingwa T, Fried M, Jensen AT, Lavstsen T, Hviid L, Duffy PE, Theander TG. Hierarchical, domain type-specific acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 in Tanzanian children. Infect Immun. 2010;78:4653–4659. doi: 10.1128/IAI.00593-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bull PC, Lowe BS, Kortok M, Molyneux CS, Newbold CI, Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med. 1998;4:358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leech JH, Barnwell JW, Miller LH, Howard RJ. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J Exp Med. 1984;159:1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giha HA, Staalsoe T, Dodoo D, Roper C, Satti GM, Arnot DE, Hviid L, Theander TG. Antibodies to variable Plasmodium falciparum-infected erythrocyte surface antigens are associated with protection from novel malaria infections. Immunol Lett. 2000;71:117–126. doi: 10.1016/s0165-2478(99)00173-x. [DOI] [PubMed] [Google Scholar]
- 22.Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 23.Mackintosh CL, Mwangi T, Kinyanjui SM, Mosobo M, Pinches R, Williams TN, Newbold CI, Marsh K. Failure to respond to the surface of Plasmodium falciparum infected erythrocytes predicts susceptibility to clinical malaria amongst African children. Int J Parasitol. 2008;38:1445–1454. doi: 10.1016/j.ijpara.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguiar JC, Albrecht GR, Cegielski P, Greenwood BM, Jensen JB, Lallinger G, Martinez A, McGregor IA, Minjas JN, Neequaye J, Patarroyo ME, Sherwood JA, Howard RJ. Agglutination of Plasmodium falciparum-infected erythrocytes from east and west African isolates by human sera from distant geographic regions. Am J Trop Med Hyg. 1992;47:621–632. doi: 10.4269/ajtmh.1992.47.621. [DOI] [PubMed] [Google Scholar]
- 25.Bull PC, Lowe BS, Kortok M, Marsh K. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect Immun. 1999;67:733–739. doi: 10.1128/iai.67.2.733-739.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen AT, Magistrado P, Sharp S, Joergensen L, Lavstsen T, Chiucchiuini A, Salanti A, Vestergaard LS, Lusingu JP, Hermsen R, Sauerwein R, Christensen J, Nielsen MA, Hviid L, Sutherland C, Staalsoe T, Theander TG. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J Exp Med. 2004;199:1179–1190. doi: 10.1084/jem.20040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rottmann M, Lavstsen T, Mugasa JP, Kaestli M, Jensen AT, Muller D, Theander T, Beck HP. Differential expression of var gene groups is associated with morbidity caused by Plasmodium falciparum infection in Tanzanian children. Infect Immun. 2006;74:3904–3911. doi: 10.1128/IAI.02073-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsh K, Howard RJ. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science. 1986;231:150–153. doi: 10.1126/science.2417315. [DOI] [PubMed] [Google Scholar]
- 29.Bull PC, Lowe BS, Kaleli N, Njuga F, Kortok M, Ross A, Ndungu F, Snow RW, Marsh K. Plasmodium falciparum infections are associated with agglutinating antibodies to parasite-infected erythrocyte surface antigens among healthy Kenyan children. J Infect Dis. 2002;185:1688–1691. doi: 10.1086/340420. [DOI] [PubMed] [Google Scholar]
- 30.Kinyanjui SM, Mwangi T, Bull PC, Newbold CI, Marsh K. Protection against clinical malaria by heterologous immunoglobulin G antibodies against malaria-infected erythrocyte variant surface antigens requires interaction with asymptomatic infections. J Infect Dis. 2004;190:1527–1533. doi: 10.1086/424675. [DOI] [PubMed] [Google Scholar]
- 31.Ofori MF, Dodoo D, Staalsoe T, Kurtzhals JA, Koram K, Theander TG, Akanmori BD, Hviid L. Malaria-induced acquisition of antibodies to Plasmodium falciparum variant surface antigens. Infect Immun. 2002;70:2982–2988. doi: 10.1128/IAI.70.6.2982-2988.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giha HA, Staalsoe T, Dodoo D, Elhassan IM, Roper C, Satti GM, Arnot DE, Hviid L, Theander TG. Overlapping antigenic repertoires of variant antigens expressed on the surface of erythrocytes infected by Plasmodium falciparum. Parasitology. 1999;119(Pt 1):7–17. doi: 10.1017/s0031182099004485. [DOI] [PubMed] [Google Scholar]
- 33.Giha HA, Staalsoe T, Dodoo D, Elhassan IM, Roper C, Satti GM, Arnot DE, Theander TG, Hviid L. Nine-year longitudinal study of antibodies to variant antigens on the surface of Plasmodium falciparum-infected erythrocytes. Infect Immun. 1999;67:4092–4098. doi: 10.1128/iai.67.8.4092-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manske M, Miotto O, Campino S, Auburn S, Almagro-Garcia J, Maslen G, O'Brien J, Djimde A, Doumbo O, Zongo I, Ouedraogo JB, Michon P, Mueller I, Siba P, Nzila A, Borrmann S, Kiara SM, Marsh K, Jiang H, Su XZ, Amaratunga C, Fairhurst R, Socheat D, Nosten F, Imwong M, White NJ, Sanders M, Anastasi E, Alcock D, Drury E, Oyola S, Quail MA, Turner DJ, Ruano-Rubio V, Jyothi D, Amenga-Etego L, Hubbart C, Jeffreys A, Rowlands K, Sutherland C, Roper C, Mangano V, Modiano D, Tan JC, Ferdig MT, Amambua-Ngwa A, Conway DJ, Takala-Harrison S, Plowe CV, Rayner JC, Rockett KA, Clark TG, Newbold CI, Berriman M, MacInnis B, Kwiatkowski DP. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature. 2012;487:375–379. doi: 10.1038/nature11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 36.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11:681–688. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- 37.Good-Jacobson KL, Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: the influence of germinal center interactions and dynamics. J Immunol. 2010;185:3117–3125. doi: 10.4049/jimmunol.1001155. [DOI] [PubMed] [Google Scholar]
- 38.Amanna IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010;236:125–138. doi: 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinyanjui SM, Conway DJ, Lanar DE, Marsh K. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar J. 2007;6:82. doi: 10.1186/1475-2875-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fruh K, Doumbo O, Muller HM, Koita O, McBride J, Crisanti A, Toure Y, Bujard H. Human antibody response to the major merozoite surface antigen of Plasmodium falciparum is strain specific and short-lived. Infect Immun. 1991;59:1319–1324. doi: 10.1128/iai.59.4.1319-1324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller HM, Fruh K, von Brunn A, Esposito F, Lombardi S, Crisanti A, Bujard H. Development of the human immune response against the major surface protein (gp190) of Plasmodium falciparum. Infect Immun. 1989;57:3765–3769. doi: 10.1128/iai.57.12.3765-3769.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fonjungo PN, Elhassan IM, Cavanagh DR, Theander TG, Hviid L, Roper C, Arnot DE, McBride JS. A longitudinal study of human antibody responses to Plasmodium falciparum rhoptry-associated protein 1 in a region of seasonal and unstable malaria transmission. Infect Immun. 1999;67:2975–2985. doi: 10.1128/iai.67.6.2975-2985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss GE, Traore B, Kayentao K, Ongoiba A, Doumbo S, Doumtabe D, Kone Y, Dia S, Guindo A, Traore A, Huang CY, Miura K, Mircetic M, Li S, Baughman A, Narum DL, Miller LH, Doumbo OK, Pierce SK, Crompton PD. The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000912. e1000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akpogheneta OJ, Duah NO, Tetteh KK, Dunyo S, Lanar DE, Pinder M, Conway DJ. Duration of naturally acquired antibody responses to blood stage Plasmodium falciparum is age dependent and antigen specific. Infect Immun. 2008 doi: 10.1128/IAI.01333-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cavanagh DR, Elhassan IM, Roper C, Robinson VJ, Giha H, Holder AA, Hviid L, Theander TG, Arnot DE, McBride JS. A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein-1 in an area of unstable malaria in Sudan. J Immunol. 1998;161:347–359. [PubMed] [Google Scholar]
- 46.Taylor RR, Egan A, McGuinness D, Jepson A, Adair R, Drakely C, Riley E. Selective recognition of malaria antigens by human serum antibodies is not genetically determined but demonstrates some features of clonal imprinting. Int Immunol. 1996;8:905–915. doi: 10.1093/intimm/8.6.905. [DOI] [PubMed] [Google Scholar]
- 47.Ramasamy R, Nagendran K, Ramasamy MS. Antibodies to epitopes on merozoite and sporozoite surface antigens as serologic markers of malaria transmission: studies at a site in the dry zone of Sri Lanka. Am J Trop Med Hyg. 1994;50:537–547. doi: 10.4269/ajtmh.1994.50.537. [DOI] [PubMed] [Google Scholar]
- 48.John CC, Ouma JH, Sumba PO, Hollingdale MR, Kazura JW, King CL. Lymphocyte proliferation and antibody responses to Plasmodium falciparum liver-stage antigen-1 in a highland area of Kenya with seasonal variation in malaria transmission. Am J Trop Med Hyg. 2002;66:372–378. doi: 10.4269/ajtmh.2002.66.372. [DOI] [PubMed] [Google Scholar]
- 49.Brown AE, Webster HK, Lyon JA, Thomas AW, Permpanich B, Gross M. Characterization of naturally acquired antibody responses to a recombinant fragment from the N-terminus of Plasmodium falciparum glycoprotein 195. Am J Trop Med Hyg. 1991;45:567–573. doi: 10.4269/ajtmh.1991.45.567. [DOI] [PubMed] [Google Scholar]
- 50.Riley EM, Morris-Jones S, Blackman MJ, Greenwood BM, Holder AA. A longitudinal study of naturally acquired cellular and humoral immune responses to a merozoite surface protein (MSP1) of Plasmodium falciparum in an area of seasonal malaria transmission. Parasite Immunol. 1993;15:513–524. doi: 10.1111/j.1365-3024.1993.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 51.Wipasa J, Suphavilai C, Okell LC, Cook J, Corran PH, Thaikla K, Liewsaree W, Riley EM, Hafalla JC. Long-lived antibody and B Cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000770. e1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baird JK, Jones TR, Danudirgo EW, Annis BA, Bangs MJ, Basri H, Purnomo, Masbar S. Age-dependent acquired protection against Plasmodium falciparum in people having two years exposure to hyperendemic malaria. Am J Trop Med Hyg. 1991;45:65–76. doi: 10.4269/ajtmh.1991.45.65. [DOI] [PubMed] [Google Scholar]
- 53.Baird JK, Purnomo, Basri H, Bangs MJ, Andersen EM, Jones TR, Masbar S, Harjosuwarno S, Subianto B, Arbani PR. Age-specific prevalence of Plasmodium falciparum among six populations with limited histories of exposure to endemic malaria. Am J Trop Med Hyg. 1993;49:707–719. doi: 10.4269/ajtmh.1993.49.707. [DOI] [PubMed] [Google Scholar]
- 54.Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. J Clin Invest. 2010;120:4168–4178. doi: 10.1172/JCI44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crompton PD, Kayala MA, Traore B, Kayentao K, Ongoiba A, Weiss GE, Molina DM, Burk CR, Waisberg M, Jasinskas A, Tan X, Doumbo S, Doumtabe D, Kone Y, Narum DL, Liang X, Doumbo OK, Miller LH, Doolan DL, Baldi P, Felgner PL, Pierce SK. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci U S A. 2010;107:6958–6963. doi: 10.1073/pnas.1001323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sauerwein RW, Roestenberg M, Moorthy VS. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol. 2011;11:57–64. doi: 10.1038/nri2902. [DOI] [PubMed] [Google Scholar]
- 57.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 58.Doolan DL. Plasmodium immunomics. Int J Parasitol. 2011;41:3–20. doi: 10.1016/j.ijpara.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tran TM, Samal B, Kirkness E, Crompton PD. Systems immunology of human malaria. Trends Parasitol. 2012;28:248–257. doi: 10.1016/j.pt.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ndungu FM, Olotu A, Mwacharo J, Nyonda M, Apfeld J, Mramba LK, Fegan GW, Bejon P, Marsh K. Memory B cells are a more reliable archive for historical antimalarial responses than plasma antibodies in no-longer exposed children. Proc Natl Acad Sci U S A. 2012;109:8247–8252. doi: 10.1073/pnas.1200472109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nogaro SI, Hafalla JC, Walther B, Remarque EJ, Tetteh KK, Conway DJ, Riley EM, Walther M. The breadth, but not the magnitude, of circulating memory B cell responses to P. falciparum increases with age/exposure in an area of low transmission. PLoS One. 2011;6:e25582. doi: 10.1371/journal.pone.0025582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dorfman JR, Bejon P, Ndungu FM, Langhorne J, Kortok MM, Lowe BS, Mwangi TW, Williams TN, Marsh K. B cell memory to 3 Plasmodium falciparum blood-stage antigens in a malaria-endemic area. J Infect Dis. 2005;191:1623–1630. doi: 10.1086/429671. [DOI] [PubMed] [Google Scholar]
- 63.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 64.Clark EH, Silva CJ, Weiss GE, Li S, Padilla C, Crompton PD, Hernandez JN, Branch OH. Plasmodium falciparum malaria in the Peruvian Amazon, a region of low transmission, is associated with immunologic memory. Infect Immun. 2012;80:1583–1592. doi: 10.1128/IAI.05961-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakkas LI, Boulbou M, Kyriakou D, Makri I, Sinani C, Germenis A, Stathakis N. Immunological features of visceral leishmaniasis may mimic systemic lupus erythematosus. Clin Biochem. 2008;41:65–68. doi: 10.1016/j.clinbiochem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 66.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sansonno L, Tucci FA, Sansonno S, Lauletta G, Troiani L, Sansonno D. B cells and HCV: an infection model of autoimmunity. Autoimmun Rev. 2009;9:93–94. doi: 10.1016/j.autrev.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 68.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O'Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Charles ED, Green RM, Marukian S, Talal AH, Lake-Bakaar GV, Jacobson IM, Rice CM, Dustin LB. Clonal expansion of immunoglobulin M+CD27 + B cells in HCV-associated mixed cryoglobulinemia. Blood. 2008;111:1344–1356. doi: 10.1182/blood-2007-07-101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ehrhardt GR, Hsu JT, Gartland L, Leu CM, Zhang S, Davis RS, Cooper MD. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J Exp Med. 2005;202:783–791. doi: 10.1084/jem.20050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, Kayentao K, Ongoiba A, Doumbo OK, Pierce SK. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol. 2009;183:2176–2182. doi: 10.4049/jimmunol.0901297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weiss GE, Clark EH, Li S, Traore B, Kayentao K, Ongoiba A, Hernandez JN, Doumbo OK, Pierce SK, Branch OH, Crompton PD. A Positive Correlation between Atypical Memory B Cells and Plasmodium falciparum Transmission Intensity in Cross-Sectional Studies in Peru and Mali. PLoS One. 2011;6:e15983. doi: 10.1371/journal.pone.0015983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Portugal S, Doumtabe D, Traore B, Miller LH, Troye-Blomberg M, Doumbo OK, Dolo A, Pierce SK, Crompton PD. B cell analysis of ethnic groups in Mali with differential susceptibility to malaria. Malar J. 2012;11:162. doi: 10.1186/1475-2875-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Illingworth J, Butler NS, Roetynck S, Mwacharo J, Pierce SK, Bejon P, Crompton PD, Marsh K, Ndungu FM. Chronic Exposure to Plasmodium falciparum Is Associated with Phenotypic Evidence of B and T Cell Exhaustion. J Immunol. 2012 doi: 10.4049/jimmunol.1202438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muellenbeck MF, Ueberheide B, Amulic B, Epp A, Fenyo D, Busse CE, Esen M, Theisen M, Mordmuller B, Wardemann H. Atypical and classical memory B cells produce Plasmodium falciparum neutralizing antibodies. J Exp Med. 2013 doi: 10.1084/jem.20121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2011;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Donati D, Mok B, Chene A, Xu H, Thangarajh M, Glas R, Chen Q, Wahlgren M, Bejarano MT. Increased B cell survival and preferential activation of the memory compartment by a malaria polyclonal B cell activator. J Immunol. 2006;177:3035–3044. doi: 10.4049/jimmunol.177.5.3035. [DOI] [PubMed] [Google Scholar]
- 78.Bushell KM, Sollner C, Schuster-Boeckler B, Bateman A, Wright GJ. Large-scale screening for novel low-affinity extracellular protein interactions. Genome Res. 2008;18:622–630. doi: 10.1101/gr.7187808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Waisberg M, Cerqueira GC, Yager SB, Francischetti IM, Lu J, Gera N, Srinivasan P, Miura K, Rada B, Lukszo J, Barbian KD, Leto TL, Porcella SF, Narum DL, El-Sayed N, Miller LH, Pierce SK. Plasmodium falciparum merozoite surface protein 1 blocks the proinflammatory protein S100P. Proc Natl Acad Sci U S A. 2012;109:5429–5434. doi: 10.1073/pnas.1202689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, Uchikawa M, Mboup S, Ndir O, Kwiatkowski DP, Duraisingh MT, Rayner JC, Wright GJ. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nduati E, Gwela A, Karanja H, Mugyenyi C, Langhorne J, Marsh K, Urban BC. The plasma concentration of the B cell activating factor is increased in children with acute malaria. J Infect Dis. 2011;204:962–970. doi: 10.1093/infdis/jir438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rogers NJ, Hall BS, Obiero J, Targett GA, Sutherland CJ. A model for sequestration of the transmission stages of Plasmodium falciparum: adhesion of gametocyte-infected erythrocytes to human bone marrow cells. Infect Immun. 2000;68:3455–3462. doi: 10.1128/iai.68.6.3455-3462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manicassamy S, Pulendran B. Modulation of adaptive immunity with Toll-like receptors. Semin Immunol. 2009;21:185–193. doi: 10.1016/j.smim.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boutlis CS, Yeo TW, Anstey NM. Malaria tolerance--for whom the cell tolls? Trends Parasitol. 2006;22:371–377. doi: 10.1016/j.pt.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shio MT, Eisenbarth SC, Savaria M, Vinet AF, Bellemare MJ, Harder KW, Sutterwala FS, Bohle DS, Descoteaux A, Flavell RA, Olivier M. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000559. e1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krishnegowda G, Hajjar AM, Zhu J, Douglass EJ, Uematsu S, Akira S, Woods AS, Gowda DC. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem. 2005;280:8606–8616. doi: 10.1074/jbc.M413541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parroche P, Lauw FN, Goutagny N, Latz E, Monks BG, Visintin A, Halmen KA, Lamphier M, Olivier M, Bartholomeu DC, Gazzinelli RT, Golenbock DT. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A. 2007;104:1919–1924. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sharma S, DeOliveira RB, Kalantari P, Parroche P, Goutagny N, Jiang Z, Chan J, Bartholomeu DC, Lauw F, Hall JP, Barber GN, Gazzinelli RT, Fitzgerald KA, Golenbock DT. Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity. 2011;35:194–207. doi: 10.1016/j.immuni.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Crompton PD, Mircetic M, Weiss G, Baughman A, Huang CY, Topham DJ, Treanor JJ, Sanz I, Lee FE, Durbin AP, Miura K, Narum DL, Ellis RD, Malkin E, Mullen GE, Miller LH, Martin LB, Pierce SK. The TLR9 ligand CpG promotes the acquisition of Plasmodium falciparum-specific memory B cells in malaria-naive individuals. J Immunol. 2009;182:3318–3326. doi: 10.4049/jimmunol.0803596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Traore B, Kone Y, Doumbo S, Doumtabe D, Traore A, Crompton PD, Mircetic M, Huang CY, Kayentao K, Dicko A, Sagara I, Ellis RD, Miura K, Guindo A, Miller LH, Doumbo OK, Pierce SK. The TLR9 agonist CpG fails to enhance the acquisition of Plasmodium falciparum-specific memory B cells in semi-immune adults in Mali. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng NY, Lee JH, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O'Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Urban BC, Hien TT, Day NP, Phu NH, Roberts R, Pongponratn E, Jones M, Mai NT, Bethell D, Turner GD, Ferguson D, White NJ, Roberts DJ. Fatal Plasmodium falciparum malaria causes specific patterns of splenic architectural disorganization. Infect Immun. 2005;73:1986–1994. doi: 10.1128/IAI.73.4.1986-1994.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ibison F, Olotu A, Muema DM, Mwacharo J, Ohuma E, Kimani D, Marsh K, Bejon P, Ndungu FM. Lack of Avidity Maturation of Merozoite Antigen-Specific Antibodies with Increasing Exposure to Plasmodium falciparum amongst Children and Adults Exposed to Endemic Malaria in Kenya. PLoS One. 2012;7:e52939. doi: 10.1371/journal.pone.0052939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 95.Asito AS, Moormann AM, Kiprotich C, Ng'ang'a ZW, Ploutz-Snyder R, Rochford R. Alterations on peripheral B cell subsets following an acute uncomplicated clinical malaria infection in children. Malar J. 2008;7:238. doi: 10.1186/1475-2875-7-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abele DC, Tobie JE, Hill GJ, Contacos PG, Evans CB. Alterations in Serum Proteins and 19s Antibody Production during the Course of Induced Malarial Infections in Man. Am J Trop Med Hyg. 1965;14:191–197. doi: 10.4269/ajtmh.1965.14.191. [DOI] [PubMed] [Google Scholar]
- 97.Daniel-Ribeiro CT, Zanini G. Autoimmunity and malaria: what are they doing together? Acta Trop. 2000;76:205–221. doi: 10.1016/s0001-706x(00)00099-1. [DOI] [PubMed] [Google Scholar]
- 98.Regules JA, Cummings JF, Ockenhouse CF. The RTS,S vaccine candidate for malaria. Expert Rev Vaccines. 2011;10:589–599. doi: 10.1586/erv.11.57. [DOI] [PubMed] [Google Scholar]
- 99.Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, Schwenk R, Nielsen RA, Debebe Z, Pinelis E, Juompan L, Williams J, Dowler M, Stewart VA, Wirtz RA, Dubois MC, Lievens M, Cohen J, Ballou WR, Heppner DG., Jr Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200:337–346. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 100.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, Mandomando I, Spiessens B, Guinovart C, Espasa M, Bassat Q, Aide P, Ofori-Anyinam O, Navia MM, Corachan S, Ceuppens M, Dubois MC, Demoitie MA, Dubovsky F, Menendez C, Tornieporth N, Ballou WR, Thompson R, Cohen J. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–1420. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 101.Bejon P, Lusingu J, Olotu A, Leach A, Lievens M, Vekemans J, Mshamu S, Lang T, Gould J, Dubois MC, Demoitie MA, Stallaert JF, Vansadia P, Carter T, Njuguna P, Awuondo KO, Malabeja A, Abdul O, Gesase S, Mturi N, Drakeley CJ, Savarese B, Villafana T, Ballou WR, Cohen J, Riley EM, Lemnge MM, Marsh K, von Seidlein L. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359:2521–2532. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fowkes FJ, Richards JS, Simpson JA, Beeson JG. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000218. e1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Druilhe P, Khusmith S. Epidemiological correlation between levels of antibodies promoting merozoite phagocytosis of Plasmodium falciparum and malaria-immune status. Infect Immun. 1987;55:888–891. doi: 10.1128/iai.55.4.888-891.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stanley HA, Mayes JT, Cooper NR, Reese RT. Complement activation by the surface of Plasmodium falciparum infected erythrocytes. Mol Immunol. 1984;21:145–150. doi: 10.1016/0161-5890(84)90129-9. [DOI] [PubMed] [Google Scholar]
- 105.Crompton PD, Miura K, Traore B, Kayentao K, Ongoiba A, Weiss G, Doumbo S, Doumtabe D, Kone Y, Huang CY, Doumbo OK, Miller LH, Long CA, Pierce SK. In vitro Growth Inhibitory Activity and Malaria Risk in a Cohort Study in Mali. Infect Immun. 2009 doi: 10.1128/IAI.00960-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Claessens A, Adams Y, Ghumra A, Lindergard G, Buchan CC, Andisi C, Bull PC, Mok S, Gupta AP, Wang CW, Turner L, Arman M, Raza A, Bozdech Z, Rowe JA. A subset of group A-like var genes encodes the malaria parasite ligands for binding to human brain endothelial cells. Proc Natl Acad Sci U S A. 2012;109:E1772–E1781. doi: 10.1073/pnas.1120461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Ouattara A, Kone AK, Guindo AB, Traore K, Traore I, Kouriba B, Diallo DA, Diarra I, Daou M, Dolo A, Tolo Y, Sissoko MS, Niangaly A, Sissoko M, Takala-Harrison S, Lyke KE, Wu Y, Blackwelder WC, Godeaux O, Vekemans J, Dubois MC, Ballou WR, Cohen J, Thompson D, Dube T, Soisson L, Diggs CL, House B, Lanar DE, Dutta S, Heppner DG, Jr, Plowe CV. A field trial to assess a blood-stage malaria vaccine. N Engl J Med. 2011;365:1004–1013. doi: 10.1056/NEJMoa1008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 110.Douglas AD, Williams AR, Illingworth JJ, Kamuyu G, Biswas S, Goodman AL, Wyllie DH, Crosnier C, Miura K, Wright GJ, Long CA, Osier FH, Marsh K, Turner AV, Hill AV, Draper SJ. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat Commun. 2011;2:601. doi: 10.1038/ncomms1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007;7:105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 112.Carter R. Transmission blocking malaria vaccines. Vaccine. 2001;19:2309–2314. doi: 10.1016/s0264-410x(00)00521-1. [DOI] [PubMed] [Google Scholar]