Abstract
The Ventral Tegmental Area (VTA) is an important brain area for progesterone (P4)'s effects to facilitate female sexual behavior of rodents. We investigated the importance of dopaminergic neurons in the VTA, and two dopaminergic projection sites, the Nucleus Accumbens (NAc), and Caudate Nucleus of the Striatum (CN), in modulating P4-facilitated sex and motor behavior. Ovariectomized (ovx) rats and hamsters, administered estradiol benzoate (10 μg) and P4 (0, 50, 100, 200, or 500 μg), were tested for motor behavior in a chamber that automatically records horizontal beam breaks, and for sexual behavior in response to a sexually-experienced male. Animals were tested once a week until each P4 dosage was received; animals then had bilateral 6-hydroxydopamine (6-OHDA) or sham lesions to the VTA, NAc, or CN and were re-tested at each P4 dosage on subsequent weeks. Fixed brains were stained with cresyl violet and processed for dopamine transporter (DAT) immunoreactivity. The number of cresyl violet stained cells was significantly lower in all 6-OHDA infusion sites compared to non-6-OHDA infusion sites of rats and hamsters. Also, in rats, the number of DAT-immunoreactive neurons was lower in all 6-OHDA infusion sites compared to non-6-OHDA infusion sites. In rats, 6-OHDA but not sham, lesions to the VTA, NAc, or CN produced P4-dependent increases in lordosis quotients and resulted in modest increases in motor behavior. In hamsters, 6-OHDA, but not sham, lesions to the VTA, NAc, or CN produced P4-dependent increases in total lordosis durations and produced modest decreases in motor behavior. This suggests that the dopaminergic output neurons of midbrain VTA may play an important role in modulation of P4-facilitated sexual lordosis among rodents.
Keywords: Sexual receptivity, Female sexual behavior, Ventral Tegmental Area, Nucleus Accumbens, Caudate Nucleus, Striatum, Mesolimbic, Dopamine, Non-genomic, Progestin
1. Introduction
When estradiol benzoate (EB) and progesterone (P4) are subcutaneously, sequentially administered to ovariectomized (ovx) female rats and hamsters, proceptive (hopping, darting, ear wiggling in rats; lateral displacement in hamsters) and receptive (refractory lordosis in rats; lordosis with tonic immobility in hamsters) behaviors are observed akin to those of gonadally-intact, proestrous females [1,2]. The effects of centrally-administered progestogens can depend upon the brain region to which progestogens are applied and the species. Infusion of P4 to either the Ventromedial Hypothalamus (VMH) [3,4], the Ventral Tegmental Area (VTA) [5], or both sites simultaneously, produces proestrous-like behavior in EB-primed rats [6], whereas simultaneous application of P4 to both the VMH and the VTA is required for facilitation of lordosis in EB-primed hamsters [7,8]. Lesions to the VMH decrease frequency of lordosis among ovx, hormone-primed rats or hamsters [9,10]. However, lesions to the VTA produce different effects on lordosis of rats and hamsters. Nonspecific lesions to the VTA can decrease lordosis responses of rats [11]. Yet, 6-OHDA lesions have been reported to affect neither proceptive, nor lordosis, behavior of female rats [12]. In hamsters, lesions to the VTA can interfere with lordosis [13], although this lesion effect is not seen under all conditions [14]. The different effects that VTA lesions have on lordosis of rats and hamsters may be related to the divergent motor characteristics associated with the manifestation of lordosis in these species. Together, these studies suggest that P4's actions in the VMH predominately facilitate initiation of receptivity in both rats and hamsters; while, in the VTA, P4 may modulate lordosis intensity in rats, and maintenance of the prolonged lordosis stance in hamsters.
We, and others, have investigated the effects and mechanisms by which P4 facilitates lordosis of rodents. In the VMH, P4 can have actions through estradiol-induced intracellular progestin receptors (PRs) that act as ligand-dependent nuclear transcription factors [15–17]. Infusions of PR antagonists or anti-sense oligonucleotides to the VMH attenuate lordosis of rats and hamsters, but infusions of these same substances to the VTA do not inhibit lordosis [18,19]. VTA-targeted implants of P4 conjugated to bovine serum albumin (BSA), which does not cross cell membranes, and free P4, which penetrates cell membranes freely, are equally effective at facilitating lordosis of female rodents when applied to the VTA, but only free P4, and not P4 conjugates, facilitate lordosis when applied to the VMH [20–22]. In addition, P4's 5α-reduced metabolite, 5α-pregnan-3α-ol-20-one (3α,5α-THP), which is devoid of affinity for PRs [23], but is a potent positive modulator of membrane bound γ-aminobutyric acid (GABA)/benzodiazepine receptor complexes (GBRs) [24,25], and which have been localized to the VMH and VTA [15,16], facilitates lordosis when applied to the VTA of rats and hamsters [26,27]. Infusions of negative modulators of GBRs (bicuculline, picrotoxin) to the VTA attenuate lordosis of cycling or hormone-primed rats and hamsters [28,29], whereas VTA administration of the GABAA agonist muscimol or positive GBR modulators (i.e. some progestogens), facilitates lordosis of cycling and hormone-primed rats and hamsters [26,27,30,31]. Although these data suggest that, in the VTA, progestogens may mediate sexual receptivity in part through allosteric, membrane actions at GBRs, 5α-reduced P4 metabolites may act more effectively on other brain areas [32].
In addition to GBRs in the VTA being an important substrate through which P4 may facilitate lordosis of rodents, there are also many dopamine (DA) cell bodies in the VTA that project their axons to the Nucleus Accumbens (NAc) and the Caudate Nucleus (CN) of the Striatum [33] and that may play a role in modulating female sexual behavior of rodents. Electrolytic lesions, or lesions made with the DA specific neurotoxin, 6-hydroxydopamine (6-OHDA), to the ventral noradrenergic bundle, inhibited estrogen and P4-facilitated receptivity of rats [34]. Extracellular DA is increased in the midbrain and ventral striatum of hamsters in response to intromissions or mating-relevant stimuli [35,36]. Increases in DA concentrations in the striatum and accumbens of ovx, hormone-primed rats are also seen with mating in which the female actively solicits contacts from the male [37]. Pharmacological manipulations of DA can also produce changes in the sexual behavior of rodents. For example, systemic administration of DA agonists, such as cocaine, apomorphine, or SKF38393 increases lordosis in EB-primed rats and DA antagonists, such as bromopride, SCH23390, sulpiride, or raclopride decreases lordosis of rats, and appetitive conditioning associated with sexual receptivity in hamsters [38–44]. Some of these effects of dopaminergic drugs on sexual behavior may occur in part through actions in the VTA or on its DA projection areas. In support of this, VTA infusions of DA blockers (e.g. SCH23390 and anti-sense oligonucleotides for the D1- and D5-DA receptor subtypes) attenuate lordosis of rats or hamsters in behavioral estrus [17]. As well, intravenous P4 or SKF38393 to EB-primed rats, concurrent with exposure to a stimulus male, increases neuronal firing in the VTA and this effect can be blocked by SCH23390 [41]. Together, these studies imply that midbrain DA neurons play an important role in regulating female sexual behavior in rodents.
In addition to evidence that mesolimbic DA neurons may play a modulatory role on sexual behavior of female rodents, DA cell bodies in the midbrain may also play a role in regulating motor behavior. Various components of sexual behavior require motor activity. Lordosis and proceptive behaviors of rats (hopping and darting) and hamsters (lateral displacement) require both forward locomotion and/or intermittent immobility. Indeed, proestrous female rats show increases in motor activity [45–50] and have higher extracellular DA concentrations in the striatum than do non-receptive female or male rats [51]. Pharmacological manipulations with DA agonists, such as apomorphine [52], and antagonists, such as clozapine [53], respectively increase and decrease, locomotion of rats in part through actions in the VTA and its projection sites [54,55].
Mesolimbic DA neurons may be an important substrate for mediating P4-facilitated sex and/or motor behavior. To test this hypothesis, 6-OHDA was infused in the VTA, or its projection sites (NAc or CN), to determine whether loss of DA in these regions alter sexual and/or motor behaviors of rodents. Effects in both rats and hamsters were examined due to their divergent dependence on P4 in the VTA for sexual behavior and their differential motor requirements for the expression of lordosis.
2. Methods
These methods were pre-approved by the Institutional Animal Care and Use Committee.
2.1. Subjects and housing
Sexually-naïve, female Long Evans rats (N=31; Taconic Farms, Germantown, NY) and LVG hamsters (N=38; Harlan, Indianapolis, IN ), approximately 55 days of age at the beginning of the experiment, were housed in separate temperature controlled rooms (21±1 °C) in the laboratory animal care facility and maintained on a 12/12h (rats) or a 14/10h (hamsters) reverse light cycle (lights off 0800) with access to Purina Rat Chow and tap water in their home cages. One week, prior to the commencement of this study, all animals were ovariectomized.
2.2. Sexual behavior and motor activity pre-test
Rats and hamsters were SC injected with 10 μg EB in 0.2cm3 sesame oil at hour 0 and P4 (0, 50, 100, 200, or 500 μg) at hour 44. Although lower dosages of estrogen can be used to facilitate receptivity of rats, and using lower concentrations of estrogens may help to parse out contributions of P4 [56,57], hamsters require 10 μg EB and P4 to prime them for sexual receptivity. As such, estrogen and P4 dosing were based upon that which would be effective in both species. Between hours 46 and 47, animals were placed in a dimly-lit Digiscan Optical Activity Monitor (39×39×30 cm; Accuscan Instruments, Columbus, OH) for 5min and the number of beam breaks that occurred was mechanically recorded. The animals were then placed in a dimly-lit Plexiglas aquarium (61×32×27 cm) for 10min with a sexually-vigorous male. There was no habituation to test chambers. For rats, the frequency of lordosis (Lordosis Quotient; LQ) and intensity of lordosis (Lordosis Rating; LR) were recorded by an observer. For hamsters, the total lordosis duration (TLD) and mean lordosis duration (MLD) were recorded [58]. Behavioral tests were conducted once a week, for 5 weeks, at each of the P4 levels prior to (pre-tests) and following (post-tests) bilateral sham and 6-OHDA lesions. To control for possible order effects, animals were randomly assigned to a P4 regimen, at which they began testing, based upon a Latin Square grid assignment. Animals then progressed though the other, weekly P4 regimen in a linear fashion.
2.3. 6-OHDA lesions
Following 5 weeks of pre-testing, animals were randomly assigned to receive bilateral 6-OHDA lesions. 6-OHDA is toxic to noradrenergic and dopaminergic cells, in a relatively selective fashion, and can be applied either to the cell body or terminals of these cells to produce a lesion [59]. As such, 6-OHDA was applied to either the VTA (rats n=7; hamsters n=9), NAc (rats n=7; hamsters n=9), or CN (rats n=7; hamsters n=9). Control sham lesions were applied to either the VTA (rats n=4; hamsters n=4), NAc (rats n=3; hamsters n=4), or CN (rats n=3; hamsters n=3). Fewer control animals were utilized as there was no indication of differences between them, such that data from control groups were combined. Briefly, rats were anesthetized with Rompun (12 mg/kg; Bayer Corp., Shawnee Mission, KS) and Ketaset (80 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA), placed in a stereotaxic apparatus and infused with 4 μg of 6-OHDA base (Sigma Chemical Co., St. Louis, MO) or 1 μl saline (over a 1min period) via a Hamilton microsyringe (needle outer diameter 0.25 mm; Reno, NV) at one of the following coordinates: VTA, AP-5.3, ML ±0.4, DV –7.0; NAc, AP +1.7, ML ±2.0, DV –4.0; or CN, AP +1.0, ML ±2.4, DV –4.0 [60,61]. Hamsters were anesthetized with Nembutal (75 mg/kg; Abbott Laboratories, North Chicago, IL), placed in a stereotaxic apparatus and infused with 4 μg of 6-OHDA base (Sigma Chemical Co., St. Louis, MO) or saline vehicle (1 μl) over a 1min period, via a Hamilton syringe (outer diameter 0.25 mm) at one of the following coordinates: VTA, AP-2.8, ML ±0.3, DV –7.8; NAc, AP +0.8, ML ±1.6, DV –3.5; or CN, AP +0.3, ML ±2.0, DV –3.5 [62,63]. To allow for maximum absorption of the infusate, the syringe was left in place for 2min post-infusion.
2.4. Histology
Animals were deeply anesthetized, intracardially injected with 0.15cm3 of 1000 units Heparin (Sigma Chemical Co., St. Louis, MO), subsequently exsanguinated with 0.9% saline, and then perfused with 4% paraformaldehyde. Brains were kept overnight in individual vials filled with 4% paraformaldehyde at 4 °C and then transferred to 30% sucrose–phosphate buffer solution (PBS) and stored at 4 °C until slicing. Brains were frozen at –20 °C and sliced at 40 μm. Every 10th section (rats) and every 5th section (hamsters) was mounted on a gelatin coated slide for cresyl violet staining while all other sections were placed in PBS with sodium azide and stored at 4 °C until used for immunocytochemistry. Rat and hamster brain sections that had been mounted on slides were stained with cresyl violet (Sigma Chemical Co., St. Louis, MO). The number of neurons in the VTA, NAc and CN of each animal were counted under a light microscope at 400×. Cells were counted on sections corresponding to Paxinos and Watson's [63] stereotaxic atlas figures 39 (VTA), 11 (NAc), and 14 (CN) or the corresponding sites and sections for hamsters [62]. To ensure that the same surface area was counted for each CNS site, a reticle was placed directly over the designated CNS site and the number of darkly-stained neurons within the same-size area of the reticle was counted. Comparisons were made between 6-OHDA-lesioned and 6-OHDA-non-lesioned sites, as well as, between 6-OHDA-lesioned and sham-lesioned animals.
Immunocytochemistry procedures for the DA transporter protein (DAT) were performed on free-floating sections of rat and hamster brain tissue. NB: Data are not reported for hamsters because the antibody for DAT was not effective in hamster tissue. Sections were put through a series of 3–10min PBS washes, soaked twice in 0.3% H2O2 for 20min each and then rinsed 5 times for 5min each in PBS. Sections were incubated for 45min at 23 °C in 10% normal goat serum (NGS) in PBS (Vector Laboratories, Burlingame, CA), followed by another incubation for 20min at 23 °C in 2% NGS in PBS, to decrease non-specific binding. Sections were then incubated for 48h at 4 °C in the DAT primary antibody in BSA (concentration 1:500; Chemicon, Temecula, CA). Post-incubation, sections were rinsed twice for 10min each in 2% NGS in PBS and then incubated at 23 °C for 60min in biotinylated secondary antibody (Vector Laboratories, Burlingame, CA). Sections were washed in PBS once for 10min and incubated at 23 °C for 30min in avidin–biotin solution (Vector Laboratories, Burlingame, CA). Sections were stained with a peroxidase staining kit for diaminobenzidine (Vector Laboratories, Burlingame, CA), washed in distilled H2O and PBS and mounted on gelatin coated slides. Once mounted, sections were placed under a hood and allowed to dry for 72h before cover slipping. The number of darkly-stained, DAT-immunoreactive, fibers or cell bodies, within the border of a reticle placed over the VTA, NAc and CN of the striatum for each rat was counted under a light microscope at 400×. Comparisons were made between 6-OHDA-lesioned and 6-OHDA-non-lesioned sites. Comparisons of DAT immunoreactivity were not made between sections from 6-OHDA-lesioned and sham-lesioned rats. Instead, the non-lesioned areas of the 6-OHDA treated tissue sections were used as control sections to compare DAT immunoreactivity in 6-OHDA infused and non-infused CNS sites. This was done because the majority of cells in these regions are non-dopaminergic and differences between lesioned and non-lesioned animals may have been in part due to degeneration.
2.5. Statistical analyses
2.5.1. Behavioral results
Mixed between- (infusion type, CNS site) and within- (P4 dosage) subject analyses of variance (ANOVAs) were performed on the post-test behavioral data and revealed main effects of infusion type. Hence, follow-up mixed between- (CNS site) and within- (pre-test vs. post-test, P4 dosage) ANOVAs analyzed separately the behavioral data resulting from 6-OHDA and control infusions. There were no differences in the patterns of results produced for the quantitative (LQ; TLD) and qualitative (LR; MLD) measures of lordosis in rats or hamsters, consequently, only quantitative data are reported and shown.
2.5.2. Histological results
For cresyl violet stained rat and hamster sections mixed between-(infusion type, CNS site) and within- (CNS site) subject ANOVAs were utilized and revealed an interaction between these variables. Subsequently, one-way ANOVAs were employed to compare cell counts of 6-OHDA-lesioned and non-6-OHDA-lesioned sites. To compare the number of DAT-immunoreactive neurons, a mixed between- (CNS lesion site) and within- (CNS cell count site) subject ANOVA was utilized (because saline-infused rat tissues were not processed for immunocytochemistry). This was followed by one-way ANOVAs to compare across treatment groups within the same site. These analyses compared the number of DAT-immunoreactive cells of rats infused with 6-OHDA to the VTA, NAc or CN. Where appropriate, ANOVAs were followed by post-hoc Fisher Least Significant Difference comparisons to ascertain group differences. Results of analyses were considered statistically significant when the p value was less than 0.05.
3. Results
3.1. Rats
Rat brain sections stained with cresyl violet revealed that 6-OHDA, but not saline, administration to the VTA, NAc or CN resulted in cell loss only in the targeted areas (F (4,50)=17.283, p≤0.0001). Rats infused with 6-OHDA to the VTA had significantly fewer cresyl violet stained cells in the VTA (18.3±2.2) than did rats infused with 6-OHDA to either the NAc (34.3±2.4) or CN (34.1±2.2) or saline infusions to the VTA (33.7±1.4; F (3,27)=14.709, p≤0.0001). Rats that received 6-OHDA to the NAc had significantly fewer cresyl violet stained cells in the NAc (16.3±0.7) than did rats with 6-OHDA infused to either the VTA (41.1±2.0), the CN (30.4±2.3) or saline infusions to the NAc (33.5±3.9) F (3,27)=11.341, p≤0.0001). Finally, rats that had 6-OHDA infused to the CN had significantly fewer cresyl violet stained cells in the CN (14.7±0.7) than did rats with 6-OHDA applied to either the VTA (27.8±1.2) or NAc (27.6±1.7) or saline infusions to the CN (27.6±2.0; F (3,27)=14.452, p≤0.0001).
The number of DAT-immunoreactive cells was significantly less in 6-OHDA administered VTA, NAc or CN compared to non-6-OHDA infused control sites (F (4,28)=14.510, p≤0.0001). The brains of rats with 6-OHDA infusions to the VTA had significantly fewer darkly-stained, DAT-immunoreactive neurons in the VTA (6.0±1.6) compared to the VTA of rats which were infused with 6-OHDA to the NAc (12.8±1.8) or CN (14.7 ±1.6; F (2,18)=12.326, p≤0.0004). 6-OHDA to the NAc produced significantly fewer DAT-immunoreactive, darkly-stained neurons in the NAc (12.7±2.1) than were seen in the NAc of rats with 6-OHDA infused to either the VTA (19.5±1.8) or CN (20.7±1.1;F (2,14)= 6.306, p≤0.0112). Lastly, rats with 6-OHDA infused to the CN had significantly fewer DAT-immunoreactive, darkly-stained neurons in the CN (9.3±0.4) than were seen in the CN of rats infused with 6-OHDA to the VTA (18.5±1.5) or NAc (13.0±1.0); F (2,15)=19.631, p≤0.0001).
6-OHDA, but not sham, lesions to the VTA, NAc, or CN increased sexual receptivity on the post-tests of ovx, hormone-primed rats (F (1,25)=36.531, p≤0.0001). LQs were significantly greater on post-tests, after 6-OHDA lesions, compared to pre-tests (F (1,16)= 335.510, p≤0.0001) and as a function of increasing P4 dosage (F (4,64)=72.669, p≤0.0001). There was also an interaction between these variables for 6-OHDA-lesioned rats (F (4,64) = 20.998, p ≤ 0.0001) but not sham-lesioned rats (F (8,28) = 1.019, p≥0.4450). 6-OHDA lesions to the VTA or the NAc produced elevated LQs, compared to pre-lesion LQs when 0, 50, 100, or 200 μg P4 was administered (Fig. 1, first and second panels). At all P4 dosages, 6-OHDA lesions to the CN resulted in higher LQs (Fig. 1, third panel) compared to pre-lesion LQs at the same P4 dosage. The LQs of rats with sham lesions were not different across sites but did increase with P4 dosage (Fig. 1, fourth panel).
Fig. 1.
First panel represents LQ of pre- (open bars) and post 6-OHDA (black bars) VTA lesioned rats. Second panel represents LQ of pre- (open bars) and post 6-OHDA (black bars) NAc lesioned rats. Third panel represents LQ of pre 6-OHDA (open bars) and post 6-OHDA (black bars) CN lesioned rats. Fourth panel represents LQ of pre- (open bars) and post sham (striped bars) lesioned rats. Asterisk indicates significantly higher post 6-OHDA lesion than pre-lesion LQ at that P dosage (μg/rat).
There were no differences between the post-test horizontal crossings of rats with 6-OHDA (989.5±38.5) versus sham lesions (875.4±30.5) to the VTA, NAc, or CN (F (1,25)=1.346, p≥0.05). However, the number of beam breaks were significantly greater on post-tests, after 6-OHDA lesions (989.5±38.5), compared to pre-tests (805.1±32.3; F (1,18)=12.548, p≤0.003; data not shown).
3.2. Hamsters
Hamster brain sections stained with cresyl violet revealed that 6-OHDA, but not saline, administration to the VTA, NAc or CN resulted in cell loss only in the infusion sites (F (4,62)=9.311, p≤0.0001). Hamsters infused with 6-OHDA to the VTA had significantly fewer cresyl violet stained cells in the VTA (9.4±1.0) than did hamsters infused with 6-OHDA to either the NAc (17.8±1.5) or CN (21.4±0.9) or saline control infusions to the VTA (41.4±1.0; F (3,33)=161.823, p≤0.0001). Hamsters that had 6-OHDA to the NAc had significantly fewer cresyl violet stained cells in the NAc (13.7±1.3) than hamsters with 6-OHDA infused to either the VTA (22.2±1.7), the CN (22.6±0.9) or saline control infusions to the NAc (34.4±2.5; F (3,33)= 22.425, p≤0.0001). Finally, hamsters that had 6-OHDA infused to the CN had significantly fewer cresyl violet stained cells in the CN (8.9±0.6) than hamsters with 6-OHDA applied to either the VTA (19.7±1.1) or NAc (21.7±0.8) or saline control infusions to the CN (34.4±1.5; F (3,33)=87.996, p≤0.0001).
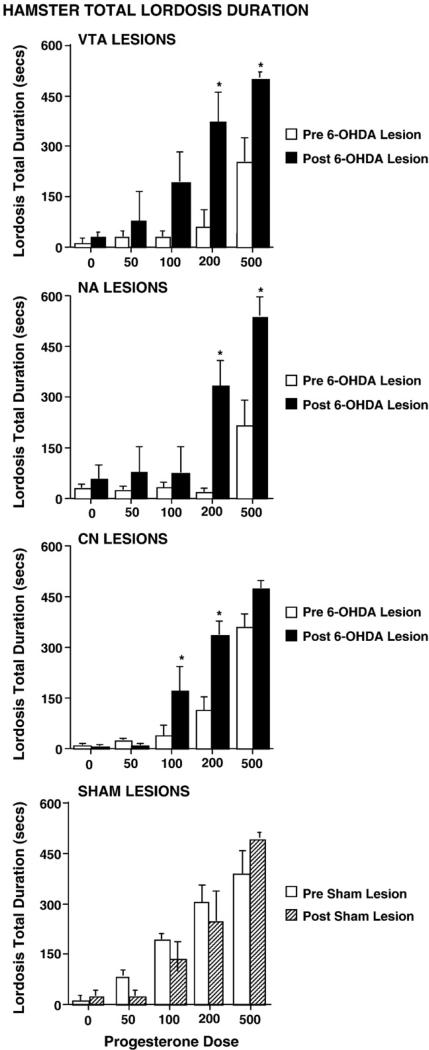
6-OHDA, but not sham, lesions to the VTA, NAc, or CN increased TLDs on the post-tests of hamsters (F (1,31)=5.776, p≤0.02). TLDs were significantly greater on post-tests, after 6-OHDA lesions, compared to pre-tests (F (1,23)=61.665, p≤0.0001) and as a function of increasing P4 dosage (F (4,92)=59.092, p≤0.0001). There was also an interaction between these variables for effects on TLDs of 6-OHDA-lesioned hamsters (F (4,92)=14.915, p≤0.0001) but not for sham-lesioned hamsters (F (4,32)=0.875, p≥0.4898). 6-OHDA lesions to the VTA or the NAc produced elevated TLDs, compared to pre-lesion TLDs when 200 or 500 μg P4 was administered (Fig. 2, first and second panels). At the 100 and 200 μg P4 dosages, 6-OHDA lesions to the CN resulted in higher TLDs (Fig. 2, third panel) compared to pre-lesion TLDs at the same P4 dosage. The TLDs of hamsters with sham lesions were not different across sites but did increase with P4 dosage (Fig. 2, fourth panel).
Fig. 2.
First panel represents TLD of pre- (open bars) and post 6-OHDA (black bars) VTA lesioned hamsters. Second panel represents TLD of pre- (open bars) and post 6-OHDA (black bars) NAc lesioned hamsters. Third panel represents TLD of pre- (open bars) and post 6-OHDA (black bars) CN lesioned hamsters. Fourth panel represents TLD of pre-(open bars) and post sham (striped bars) lesioned hamsters. Asterisk indicates significantly longer post 6-OHDA lesion than pre-lesion TLD at that P dosage (μg/rat).
There were differences between the post-test horizontal crossings (beam breaks) of hamsters with 6-OHDA versus sham lesions to the VTA, NAc, or CN (F (1,31)=18.553, p≤0.0064). This is attributable to the 6-OHDA-lesioned hamsters have a smaller number of beam breaks (1120.8±26.9) compared to sham-lesioned hamsters (1380.8±40.9). However, the number of beam breaks were not significantly greater on post-tests, after 6-OHDA lesions (1120.8±26.9), compared to pre-tests (1070.2±18.4; F (1,23)=2.414, p≥0.1339; data not shown).
4. Discussion
The hypothesis that the integrity of the mesolimbic DA system is important for regulating sexual and motor behavior in rats and hamsters was supported. 6-OHDA, but not saline, infusions to the VTA, NAc or CN produced increases in lordosis and changes in motor behavior of rats and hamsters. In support: 1) Infusions of 6-OHDA, but not saline, successfully lesioned targeted areas as 6-OHDA infused areas had fewer cresyl violet stained cells compared to non-6-OHDA infusion sites and sham-lesioned areas. 2) The number of DAT-immunoreactive neurons was significantly lower at all 6-OHDA-lesioned compared to non-6-OHDA-lesioned sites of rats, indicating DA containing cells were, in fact, depleted. 3) The 6-OHDA lesions produced increases in lordosis of rats and hamsters evidenced by lesioned rats having significantly greater LQ scores and lesioned hamsters having greater TLDs compared to sham-lesioned control animals. Significant increases in lordosis were also seen in comparisons of pre- and post- 6-OHDA lesion LQ scores and TLDs. 4) In rats, 6-OHDA lesions to the VTA, NAc and CN produced small, but statistically significant, increases in motor behavior that were independent of P4 dosage. In hamsters, 6-OHDA lesions to the VTA, NAc or CN produced decreases in motor behavior compared to that of sham-lesioned animals. In sum, 6-OHDA lesions enhanced lordosis of rats and hamsters concomitant with increases in motor behavior of rats and decreases in motor behavior of hamsters.
The current data are consistent with previous research showing depletions of midbrain catecholamines alter sexual and/or motor behavior of rats. Rats that received bilateral intraventricular injections of 6-OHDA exhibited increased lordosis frequency and intensity and these effects were further augmented by central administration of the catecholamine synthesis inhibitor α-methyl-p-tyrosine [64]. Additionally, intraventricular 6-OHDA infusions prolong the average duration of lordosis in rats in the presence and in the absence of P4 [47]. Electrical stimulation of the VTA inhibits lordosis of EB-primed, ovx rats [60]. In sum, the present and previous work, suggest a role for midbrain DA to modulate sexual behavior of female rodents.
This study is the first to show that 6-OHDA lesions to DA containing cell bodies in the VTA, or its affected forebrain structures (NAc and CN), enhance P4-facilitated lordosis of both rats and hamsters and concurrent, modest, changes in motor behavior. Like the observed increases in sexual behavior, the modest changes in motor activity seen in this study show manipulations of midbrain DA results in some alterations in motor behavior. Increases and decreases in locomotion of rats can be produced through actions in the VTA and its projection sites [54,55] by administration of the DA agonists apomorphine [52] and the DA antagonist clozapine [53], respectively. The inclusion of rats and hamsters in these findings is important because of their divergent dependence on VTA progestogens for the expression of lordosis. Progestogen- and DA-facilitated female rodent sexual behaviors are closely linked with motor behavior. Proceptive behaviors require active motor patterns with forward locomotion while lordosis is a reflexive posture that demands immobility. Prior to intracerebroventricular 6-OHDA lesions, rats engage in less lordosis and more motor-dependent proceptive behaviors [64]. However, after icv lesioning rats engage in more lordosis, a posture requiring immobility, and less hopping and darting, activities that demand locomotion [64]. This suggests that DA actions in the midbrain may be involved in responding to stimuli that require motor functioning, consequently lesions to this pathway may result in changes in motor behavior that increase the individual's ability to respond to motor stimuli.
It may seem counterintuitive that brain lesions facilitate sexual behavior in female rodents; however, 6-OHDA lesions substantially and permanently reduce the availability of DA [42,65] to both the D1 and D2 receptors, which are respectively excitatory and inhibitory in their ability to alter cAMP [66]. It may be that D1 receptors augment P4-induced sexual behaviors in female rodents and D2 receptors attenuate female rodent sexual behavior. Evidence for this notion includes: the D1 agonist SKF38393 intravenously or centrally administered to the third ventricle increases lordosis of rats [40,41]. Intravenous [19,46] or VTA infusions of D1 antagonist SCH23390 and central administration of D1 anti-sense oliogonucleotides [19] attenuate lordosis of ovx, hormone-primed or behavioral estrous rats and hamsters. Manipulations that increase the availability of DA to D2 receptors decrease sexual behavior in female rodents. Central administration of the monoamine oxidase-B inhibitor pargyline, which increases DA concentrations by preventing its degradation, decreases lordosis in female rats and the amount of lordosis inhibition, is positively correlated with increases in DA levels [38]. In addition, centrally-administered D2 agonist, quinpirole, had no stimulatory effect on lordosis of EB-primed ovx rats [67]. In sum, these data support the notion that dopaminergic neurons in the mesolimbic pathway are included in the modulation of P4-induced lordosis in female rodents and suggest that D1 and D2 receptor activation may exert opposing effects on the lordosis response.
Although the present findings are consistent and extend previous research, the observed increases in rat and hamster sexual behavior following 6-OHDA lesions should be considered with caution. First, this was a partially within-subject design, such that practice effects of repeatedly testing experimental, female rats and hamsters, may have influenced the results. As well, increases in sexual vigor of stimulus males, over the course of the testing paradigm, could have influenced the results. However, then significant increases in receptivity would have been observed in the sham-lesioned animals that underwent the same testing paradigm, and this was not the case. Second, damage arising from 6-OHDA lesions outside of the VTA, NAc, or CN may have produced the observed increases in sexual behavior. Indeed, intracerebroventricular (icv) infusions of 6-OHDA, which produce 85% depletion of caudate DA or 53% depletion of midbrain DA, produce dramatic increases in the intensity and frequency of lordosis responses of rats [46,47,65]. These aforementioned findings are congruous with the present results that demonstrate that cell loss circumscribed to the targeted mesolimbic DA system produce increases in lordosis responses. It is possible that if other types of lesions, or lesions to other CNS sites, were examined, different behavioral effects may have been observed. For example, adult female ovx rats that had been neonatally decorticated display less lordosis than controls following treatment with either low levels of EB or EB plus P4 [48]. Additionally, electrolytic lesions of A-10 DA containing cell bodies, or the ventral midbrain, produce marked decreases in lordosis of ovx, hormone-primed rats and hamsters [11,13]. Furthermore, there is a large body of evidence that strongly links modulation of female rodent sexual behavior with the DA mesolimbic system [36,68–70]. Hormones may alter effects of norepinephrine and/or DA on stress responses, arousal, and/or prefrontal cortex-dependent cognitive function [71–73]. Thus, actions at these diverse substrates may account for some of the heterogeneity in effects observed with these various CNS lesions.
In sum, the current findings support the notion that DA and P4 circuitry converges in the VTA, NAc and CN to orchestrate the production of sexual behavior in female rats and hamsters; however, the exact roles of the different DA receptor subtypes remain to be fully elucidated. The mesolimbic DA pathway is an important substrate of behavior; consequently it is valuable to ascertain the characteristics of neuronal loss in this system as well as any regulatory role that progestogens may play.
Acknowledgments
This research was supported by grants from NIMH (MH06769801), NSF (IBN03-16083) and The Parkinson's Foundation.
References
- 1.Feder HH. Hormones and sexual behavior. Annu Rev Psychol. 1984;35:165–200. doi: 10.1146/annurev.ps.35.020184.001121. [DOI] [PubMed] [Google Scholar]
- 2.Tiefer L. Gonadal hormones and mating behavior in the adult golden hamster. Horm Behav. 1970;1:189–202. [Google Scholar]
- 3.Rubin BS, Barfield RJ. Progesterone in the ventromedial hypothalamus facilitates estrous behavior in ovariectomized, estrogen-primed rats. Endocrin. 1983;113:797–804. doi: 10.1210/endo-113-2-797. [DOI] [PubMed] [Google Scholar]
- 4.Rubin BS, Barfield RJ. Induction of estrous behavior in ovariectomized rats by sequential replacement of estrogen and progesterone to the ventromedial hypothalamus. Neuroendocrin. 1983;37:218–24. doi: 10.1159/000123546. [DOI] [PubMed] [Google Scholar]
- 5.Pleim ET, Baumann J, Barfield RJ. A contributory role for midbrain progesterone in the facilitation of female sexual behavior in rats. Horm Behav. 1991;25:19–28. doi: 10.1016/0018-506x(91)90036-h. [DOI] [PubMed] [Google Scholar]
- 6.Frye CA, Gardiner SG. Progestins can have a membrane-mediated action in rat midbrain for facilitation of sexual receptivity. Horm Behav. 1996;30:682–91. doi: 10.1006/hbeh.1996.0069. [DOI] [PubMed] [Google Scholar]
- 7.DeBold JF, Malsbury CW. Facilitation of sexual receptivity by hypothalamic and midbrain implants of progesterone in female hamsters. Physiol Behav. 1989;46:655–60. doi: 10.1016/0031-9384(89)90347-8. [DOI] [PubMed] [Google Scholar]
- 8.Pleim ET, Lisciotto CA, DeBold JF. Facilitation of sexual receptivity in hamsters by simultaneous progesterone implants in the VMH and the ventral mesencephalon. Horm Behav. 1990;24:139–51. doi: 10.1016/0018-506x(90)90001-e. [DOI] [PubMed] [Google Scholar]
- 9.Malsbury CW, Kow LM, Pfaff DW. Effects of medial hypothalamic lesions on the lordosis response and other behaviors in female golden hamsters. Physiol Behav. 1977;19:223–37. doi: 10.1016/0031-9384(77)90331-6. [DOI] [PubMed] [Google Scholar]
- 10.Mathews D, Donovan KM, Hollingsworth EM, Hutson VB, Overstreet CT. Permanent deficits in lordosis behavior in female rats with lesions of the ventromedial nucleus of the hypothalamus. Exp Neurol. 1983;79:714–41. doi: 10.1016/0014-4886(83)90035-3. [DOI] [PubMed] [Google Scholar]
- 11.Herndon JG., Jr Effects of midbrain lesions on female sexual behavior in the rat. Physiol Behav. 1976;17:143–8. doi: 10.1016/0031-9384(76)90281-x. [DOI] [PubMed] [Google Scholar]
- 12.Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav Neurosci. 1991;105:588–98. doi: 10.1037//0735-7044.105.4.588. [DOI] [PubMed] [Google Scholar]
- 13.Lisciotto CA, DeBold JF. Ventral tegmental lesions impair sexual receptivity in female hamsters. Brain Res Bull. 1992;26:877–83. doi: 10.1016/0361-9230(91)90252-f. [DOI] [PubMed] [Google Scholar]
- 14.Floody OR, DeBold JF. Effects of midbrain lesions on lordosis and ultrasound production. Physiol Behav. 2004;82:791–804. doi: 10.1016/j.physbeh.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Frye CA, Walf AA. Membrane actions of progestins at dopamine type 1-like and GABAA receptors involve downstream signal transduction pathways. Steroids. 2008;73:906–13. doi: 10.1016/j.steroids.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3a-hydroxy-5apregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience. 2006;138:1007–14. doi: 10.1016/j.neuroscience.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etgen AM. Progestin receptors and the activation of female reproductive behavior: a critical review. Horm Behav. 1984;18:411–30. doi: 10.1016/0018-506x(84)90027-8. [DOI] [PubMed] [Google Scholar]
- 18.Frye CA, Murphy RE, Platek SM. Anti-sense oligonucleotides, for progestin receptors in the VMH and glutamic acid decarboxylase in the VTA, attenuate progesterone-induced lordosis in hamsters and rats. Behav Brain Res. 2000;115:55–64. doi: 10.1016/s0166-4328(00)00242-4. [DOI] [PubMed] [Google Scholar]
- 19.Frye CA, Vongher JM. GABAA, D1, and D5, but not progestin receptor, antagonist and anti-sense oligonucleotide infusions to the ventral tegmental area of cycling rats and hamsters attenuate lordosis. Behav Brain Res. 1999;103:23–34. doi: 10.1016/s0166-4328(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 20.Frye CA, DeBold JF. P-3-BSA but not P-11-BSA implants in the VTA rapidly facilitate receptivity in hamsters after progesterone priming to the VMH. Behav Brain Res. 1992;53:167–75. doi: 10.1016/s0166-4328(05)80276-1. [DOI] [PubMed] [Google Scholar]
- 21.Frye CA, Gardiner SG. Progestins can have a membrane-mediated action in rat midbrain for facilitation of sexual receptivity. Horm Behav. 1996;30:682–91. doi: 10.1006/hbeh.1996.0069. [DOI] [PubMed] [Google Scholar]
- 22.Frye CA, Mermelstein PG, DeBold JF. Evidence of a nongenomic action of progestins on sexual receptivity in hamster ventral tegmental area but not hypothalamus. Brain Res. 1992;578:87–93. doi: 10.1016/0006-8993(92)90233-y. [DOI] [PubMed] [Google Scholar]
- 23.Iswari S, Colas AE, Karavolas HJ. Binding of 5α-dihydroprogesterone and other progestins to female rat anterior pituitary nuclear extracts. Steroids. 1986;47:189–203. doi: 10.1016/0039-128x(86)90088-7. [DOI] [PubMed] [Google Scholar]
- 24.Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure–activity relationships for steroid interaction with the γ-aminobutyric acid A receptor complex. J Pharmacol Exp Ther. 1987;241:346–53. [PubMed] [Google Scholar]
- 25.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–7. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 26.Frye CA, DeBold JF. 3α-OH-DHP and 5α-THDOC implants to the ventral tegmental area facilitate sexual receptivity in hamsters after progesterone priming to the ventromedial hypothalamus. Brain Res. 1993;612:130–7. doi: 10.1016/0006-8993(93)91653-a. [DOI] [PubMed] [Google Scholar]
- 27.Frye CA, Vongher JM. Progestins’ rapid facilitation of lordosis when applied to the ventral tegmentum corresponds to efficacy at enhancing GABAA receptor activity. J Neuroendocrinol. 1999;11:829–38. doi: 10.1046/j.1365-2826.1999.00367.x. [DOI] [PubMed] [Google Scholar]
- 28.Frye CA. Inhibition of 5α-reductase enzyme in the VMH and VTA attenuates progesterone-induced sexual behavior in rats and hamsters. J Endocrinol Invest. 2001;24:399–407. [PubMed] [Google Scholar]
- 29.Frye CA, Mermelstein PG, DeBold JF. Bicuculline infused into the hamster ventral tegmentum inhibits, while sodium valproate facilitates, sexual receptivity. Pharmacol Biochem Behav. 1993;46:1–8. doi: 10.1016/0091-3057(93)90308-g. [DOI] [PubMed] [Google Scholar]
- 30.Frye CA. The role of neurosteroids and non-genomic effects on progestins in the ventral tegmental area in mediating sexual receptivity of rodents. Horm Behav. 2001;40:226–33. doi: 10.1006/hbeh.2001.1674. [DOI] [PubMed] [Google Scholar]
- 31.Frye CA, DeBold JF. Muscimol facilitates sexual receptivity in hamsters when infused to the ventral tegmentum. Pharmacol Biochem Behav. 1992;42:879–87. doi: 10.1016/0091-3057(92)90044-g. [DOI] [PubMed] [Google Scholar]
- 32.Beyer C, González-Mariscal G, Eguíbar JR, Gómora P. Lordosis facilitation in estrogen primed rats by intrabrain injection of pregnanes. Pharmacol Biochem Behav. 1988;31:919–26. doi: 10.1016/0091-3057(88)90405-4. [DOI] [PubMed] [Google Scholar]
- 33.Fallon JH, Loughlin SE. Substantia nigra. In: Paxinos G, editor. The rat nervous system. 2nd Edition Academic Press; New York: 1995. pp. 215–37. [Google Scholar]
- 34.Hansen S, Stanfield EJ, Everitt BJ. The role of ventral bundle noradrenergic neurones in sensory components of sexual behaviour and coitus-induced pseudopregnancy. Nature. 1980;10(286):52–154. doi: 10.1038/286152a0. [DOI] [PubMed] [Google Scholar]
- 35.Kohlert JG, Rowe RK, Meisel RL. Intromissive stimulation from the male increases extracellular dopamine release from fluoro-gold-identified neurons within the midbrain of female hamsters. Horm Behav. 1997;32:143–54. doi: 10.1006/hbeh.1997.1415. [DOI] [PubMed] [Google Scholar]
- 36.Meisel RL, Camp DM, Robinson TE. A microdialysis of ventral striatal dopamine during sexual behavior in female Syrian hamsters. Behav Brain Res. 1993;55:151–7. doi: 10.1016/0166-4328(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 37.Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behav Neurosci. 1995;109:354–65. doi: 10.1037//0735-7044.109.2.354. [DOI] [PubMed] [Google Scholar]
- 38.Allen DL, Renner KJ, Luine VN. Pargyline-induced increase in serotonin levels: correlation with inhibition of lordosis in rats. Pharmacol Biochem Behav. 1993;45:837–41. doi: 10.1016/0091-3057(93)90129-h. [DOI] [PubMed] [Google Scholar]
- 39.Apostolakis EM, Garai J, Clark JH, O'Malley BW. In vivo regulation of central nervous system progesterone receptors: cocaine induces steroid-dependent behavior through dopamine transporter modulation of D5 receptors in rats. Mol Endocrinol. 1996;10:595–604. doi: 10.1210/mend.10.12.8961269. [DOI] [PubMed] [Google Scholar]
- 40.Hamburger-Bar R, Rigter H. Apomorphine facilitation of sexual behavior in female rats. Eur J Pharmacol. 1975;32:357–60. doi: 10.1016/0014-2999(75)90304-0. [DOI] [PubMed] [Google Scholar]
- 41.Frye CA, Bayon LE, Vongher J. Intravenous progesterone elicits a more rapid induction of lordosis in rats than does SKF38393. Psychobiology. 2000;28:99–109. [Google Scholar]
- 42.Felicio LF, Nasello AG. Effect of acute bromopride treatment on rat prolactin levels and sexual behavior. Braz J Med Biol Res. 1989;22:1011–4. [PubMed] [Google Scholar]
- 43.Meisel RL, Joppa MA, Rowe RK. Dopamine receptor antagonists attenuate conditioned place preference following sexual behavior in female Syrian hamsters. Eur J Pharmacol. 1996;309:21–4. doi: 10.1016/0014-2999(96)00389-5. [DOI] [PubMed] [Google Scholar]
- 44.Apostolakis EM, Garai J, Fox C, Smith CL, Watson SJ, Clark JH, O'Malley BW. Dopaminergic regulation of progesterone receptors: brain D5 dopamine receptors mediate induction of lordosis by D1-like agonists in rats. J Neurosci. 1996;16:4823–34. doi: 10.1523/JNEUROSCI.16-16-04823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Becker JB, Cha JH. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989;35:117–25. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- 46.Breese GR, Traylor TD. Effect of 6-hydroxydopamine on brain norepinephrine and dopamine: evidence for selective degeneration of catecholamine neurons. J Pharmacol Exp Ther. 1970;174:413–20. [PMC free article] [PubMed] [Google Scholar]
- 47.Caggiula AR, Herndon JG, Jr, Scanlon R, Greenstone D, Bradshaw W, Sharp D. Dissociation of active from immobility components of sexual behavior in female rats by central 6-hydroxydopamine: implications for CA involvement in sexual behavior and sensorimotor responsiveness. Brain Res. 1979;172:505–20. doi: 10.1016/0006-8993(79)90582-1. [DOI] [PubMed] [Google Scholar]
- 48.Carter CS, Witt DM, Kolb B, Whishaw IQ. Neonatal decortication and adult female sexual behavior. Physiol Behav. 1983;29:763–6. doi: 10.1016/0031-9384(82)90254-2. [DOI] [PubMed] [Google Scholar]
- 49.Frye CA, Rhodes ME. Progesterone's 5α-reduced metabolite, 3, 5-THP, mediates lateral displacement of hamsters. Brain Res. 2005;1038:59–68. doi: 10.1016/j.brainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 50.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3, 5-THP. Pharmacol Biochem Behav. 2000;67:587–96. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 51.Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett. 1994;180:155–8. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 52.Geyer MA, Russo PV, Segal DS, Kuczenski R. Effects of apomorphine and amphetamine on patterns of locomotor and investigatory behavior in rats. Pharmacol Biochem Behav. 1987;28:393–9. doi: 10.1016/0091-3057(87)90460-6. [DOI] [PubMed] [Google Scholar]
- 53.Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Comparison of the effects of clozapine, haloperidol, chlorpromazine and d-amphetamine on performance on a time-constrained progressive ratio schedule and on locomotor behaviour in the rat. Psychopharmacol. 2000;152:47–54. doi: 10.1007/s002130000486. [DOI] [PubMed] [Google Scholar]
- 54.Olds ME. The response of non-dopamine neurons in substantia nigra and ventral tegmental area to amphetamine and apomorphine during hypermotility: the striatal influence. Brain Res. 1988;452:237–54. doi: 10.1016/0006-8993(88)90029-7. [DOI] [PubMed] [Google Scholar]
- 55.Steketee JD. Injection of SCH 23390 into the ventral tegmental area blocks the development of neurochemical but not behavioral sensitization to cocaine. Behav Pharmacol. 1998;9:69–76. [PubMed] [Google Scholar]
- 56.de Vries GJ, Södersten P. Sex differences in the brain: the relation between structure and function. Horm Behav. 2009;55:589–96. doi: 10.1016/j.yhbeh.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Södersten P. Sexual differentiation: do males differ from females in behavioral sensitivity to gonadal hormones? Prog Brain Res. 1984;61:257–70. doi: 10.1016/S0079-6123(08)64440-4. [DOI] [PubMed] [Google Scholar]
- 58.Noble RG. The sexual responses of the female hamster: a descriptive analysis. Physiol Behav. 1979;23:1001–5. doi: 10.1016/0031-9384(79)90288-9. [DOI] [PubMed] [Google Scholar]
- 59.Bové J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson's disease. NeuroRx. 2005;2:484–94. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hasegawa T, Takeo T, Akitsu H, Hoshina Y, Sakuma Y. Interruption of the lordosis reflex of female rats by ventral midbrain stimulation. Physiol Behav. 1991;50:1033–8. doi: 10.1016/0031-9384(91)90433-o. [DOI] [PubMed] [Google Scholar]
- 61.Havens MD, Rose JD. Impaired lordosis response in golden hamsters with lesions in the ventromedial midbrain. Exp Neurol. 1984;86:583–9. doi: 10.1016/0014-4886(84)90091-8. [DOI] [PubMed] [Google Scholar]
- 62.Malsbury CW. A schematic stereotaxic atlas of the golden hamster brain. Cat Sel Doc Psychol. 1977;7:67. [Google Scholar]
- 63.Paxinos G, Watson C. The rat brain in stereotaxic coordinates: compact. 3rd Edition Academic Press; San Diego: 1997. [Google Scholar]
- 64.Herndon JG, Jr, Caggiula AR, Sharp D, Ellis D, Redgate E. Selective enhancement of the lordotic component of female sexual behavior in rats following destruction of central catecholamine-containing systems. Brain Res. 1978;141:137–51. doi: 10.1016/0006-8993(78)90623-6. [DOI] [PubMed] [Google Scholar]
- 65.Uretsky NJ, Iversen LL. Effects of 6-hydroxydopamine on catecholamine-containing neurons in the rat brain. J Neurochem. 1970;17:269–78. doi: 10.1111/j.1471-4159.1970.tb02210.x. [DOI] [PubMed] [Google Scholar]
- 66.Kuhar MJ, Couceyro PR, Lambert PD. Catecholamines. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Basic neurochemistry: molecular, cellular and medical aspects. 6th Edition Lippincott-Raven Publishers; Philadelphia: 1999. pp. 243–62. [Google Scholar]
- 67.Mani SK, Allen JMC, Clark JH, Blaustein JD, O'Malley BW. Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science. 1994;265:1246–9. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- 68.Mogenson GL, Jones DL, Yim CY. From motivation to action: functional interface between the limbic and motor systems. Prog Neurobiol. 1989;14:69–79. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 69.Robbins TW, Cador M, Taylor JR, Everett BJ. Limbic–striatal interactions in reward-related processes. Neurosci Biobehav Rev. 1989;13:163–73. doi: 10.1016/s0149-7634(89)80025-9. [DOI] [PubMed] [Google Scholar]
- 70.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psycholog Rev. 1989;94:469–92. [PubMed] [Google Scholar]
- 71.Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–20. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 72.Berridge CW. Neural substrates of psychostimulant-induced arousal. Neuropsychpharmacology. 2006;31:2332–40. doi: 10.1038/sj.npp.1301159. [DOI] [PubMed] [Google Scholar]
- 73.Berridge CW, Stalnaker TA. Relationship between low-dose amphetamine-induced arousal and extracellular norepinephrine and dopamine levels within prefrontal cortex. Synapse. 2002;46:140–9. doi: 10.1002/syn.10131. [DOI] [PubMed] [Google Scholar]