Abstract
There are sex and hormonal differences in response to cocaine that have been demonstrated in people and animal models. Cocaine can alter secretion of progestogens, such as progesterone (P), and its neuroactive metabolite, 5α-pregnan-3α-ol-20-one (3α,5α-THP). However, little research has been done on the neuroendocrine effects in the initiation phase of cocaine use. We hypothesize that some sex/hormonal differences in initiation phase responses to cocaine may be related to formation of progestogens. To investigate the role of progestogens in sex differences in response to acute cocaine, male and female rats in the high (proestrous) or low (diestrous) progestogen phase of the estrous cycle were administered cocaine (0, 5, 10, or 20 mg/kg, IP). We examined cocaine's acute neuroendocrine effects on P and 3α,5α-THP levels, as well as its effects on acute psychomotor stimulation, anxiety, and sexual behaviors. Among rats that had P and/or 3α,5α-THP levels increased in response to cocaine, enhanced acute psychomotor stimulation was observed. Results suggest that cocaine produces U-shaped curves for progestogens, and anxiety-like behaviors. Male rats were less susceptible to these effects of cocaine than were proestrous or diestrous female rats. However, cocaine's disruption of sexual behaviors was similar among males and proestrous females. These data suggest a complex interaction between hormonal milieu and the neuroendocrine and behavioral effects of cocaine.
Keywords: Estrous cycle, Psychomotor stimulation, Anxiety, Sexual behavior, Progesterone, Allopregnanolone
Introduction
There is a growing literature that suggests sex- and gender-dependent factors may influence the experience of illicit drugs during various stages of drug addiction. With cocaine, in particular, there are observable gender differences in peripheral and interoceptive effects wherein women tend to have less cerebral perfusions and neurovascular responses (Kaufman et al., 2001), as well as, less appetite suppression, and fewer changes in blood pressure, than do men (Lynch et al., 2008). Furthermore, women, compared to men, tend to report less euphoria, more anxiety, and stronger cue-induced cravings, and are more likely to relapse (Kosten et al., 1993; Lynch et al., 2002; Lukas et al., 1996). In addition, these behaviors have been replicated in rat models, wherein female rats are typically more sensitive to some behavioral effects of cocaine, such as psychomotor sensitization and conditioned cue response, compared to males (Becker et al., 2001; Carroll et al., 2004; Hu and Becker, 2003; Fuchs et al., 2005). In self-administration paradigms, female rats make more responses for cocaine during acquisition, extinction and reinstatement (Lynch and Carroll, 2000; Kosten and Zhang, 2008; Lynch, 2008), and administer higher dosages, than do males (Roberts et al., 1989). Thus, there are salient sex and gender differences in cocaine use and response.
The evidence of gender and sex differences in cocaine response suggests a role for hormonal milieu, in influencing peripheral and subjective effects of cocaine. During the luteal (high hormone) phase of the menstrual cycle, women who use cocaine report attenuated subjective response and less desire to smoke cocaine than do women in the lower hormone (follicular) phase of the menstrual cycle (Evans et al., 2002; Sofuoglu et al., 1999, 2002). These data are congruent with physiological measures, in that women in the luteal phase have less cardiovascular responses to drug cues (Sinha et al., 2007; Turner and de Wit, 2006). Thus, these data suggest that endogenous changes in hormones may influence responses to cocaine among females.
An important question is how progesterone (P) might have effects on cocaine response. P through actions of its metabolite, 5α-pregnan-3α-ol-20-one (3α,5α-THP), has modulatory effects on sexual and anti-anxiety behaviors in rodents (Frye et al., 2007, 2009; Engin and Treit, 2007). 3α,5α-THP increases during periods of natural reward, such as during sexual receptivity, and can be increased further with mating. Lordosis responses are also attenuated by inhibiting 3α,5α-THP formation (Frye et al., 2008, 2009; Petralia et al., 2005). Administration of 3α,5α-THP conditions a place preference (Finn et al., 1997; Frye et al., 1998; Russo et al., 2003), and rats will preferentially drink 3α,5α-THP over water (Sinnott et al., 2002). As such, we became interested in the role of 3α,5α-THP in mediating some of cocaine's acute behavioral effects. We previously observed that cocaine administration to male (5 mg/kg) and female (20 mg/kg) Sprague–Dawley rats increase concentrations of P and its metabolite, 3α,5α-THP, in serum, hippocampus, and striatum concomitant with psychomotor stimulation (Frye, 2007; Quiñones-Jenab et al., 2008). Thus, progestogens may mediate some aspects of acute behavioral response to cocaine.
Few studies have looked at cocaine's neuroendocrine effects during the initiation, or first drug experience, phase. Developing research on sex differences in drug response indicates a role for hormonal milieu in the initiation phase of drug abuse. There is evidence that cocaine has stimulant effects, and may alter anxiety and sexual behaviors; which are particularly sensitive to progestogen levels. We hypothesized that if cocaine's effects are in fact, mediated in part by progestogens, then natural variations in progestogen milieu may alter cocaine's acute effects, such that low and stable levels will produce less stimulant effects, produce anxiolytic responses, and less aberrant sexual behavior. To assess this, we acutely administered 0, 5, 10, or 20 mg/kg IP cocaine to male, diestrous female, and proestrous female rats. We examined stimulant effects, anti-anxiety effects, and sexual effects, of cocaine. Our behavioral measures of interest were chosen as they are differentially sensitive to progestogens. There are previously observed psychomotor differences between hormonal groups. Anxiety behaviors are both categorical and continuous in nature, providing a range of responses. Sexual behaviors have threshold effects, wherein, for females, a certain threshold value of progestogens must be present for sexual behaviors to occur, and are thereafter continuous. Levels of P and 3α,5α-THP were used as a measure of cocaine's neuroendocrine effects.
Methods
These methods were pre-approved by The Institutional Care and Use Committee at The University at Albany-SUNY and studies were conducted in compliance with ethical guidelines defined by The National Institute of Health and the Society for Neuroscience.
Animals
Subjects were female (n=82, n=9–11/group) and male (n=47, n=11–12/group), inbred, Long–Evans rats, raised on-site in the rat vivarium of the Life Sciences Research Building at the University at Albany-SUNY Building (original stock from Charles River Laboratories, Raleigh, Wilmington NC). Rats were ~60 days and housed 4/cage in a humidity- (50±5%) and temperature- (21±1°) controlled room on a reversed L–D light cycle (lights off at 0800 h) with water and Purina Rat Chow provided ad libitum.
Evaluation of estrous cycle phase
Estrous cycle phase of female rats was assessed by collection of vaginal epithelium daily between 9 am and 10 am and evaluation of the cell cytology using a light microscope per previous methods (Frye et al., 2000). Vaginal cytology was evaluated for the presence of nucleated cells (indicative of proestrous cycle phase) or leukocytic cells (characteristics of diestrous cycle phase). Rats were also vaginally masked and screened for lordosis in response to sexually-vigorous males. Rats with proestrous cytology, which demonstrated lordosis in response to male mounting, and had previously met this criteria 4 to 5 days prior, comprised the proestrous group. Likewise, only rats with diestrous cytology that did not demonstrate lordosis in response to male mounting and had been in proestrus 2–3 days prior were considered in this phase.
Drug condition
Cocaine was diluted in 0.9% saline immediately before each intraperitoneal (IP) injection. Males and females received 5, 10, or 20 mg/kg of cocaine or saline control. All chemicals were purchased from Sigma Scientific (Saint Louis, MO). Previous studies have suggested that specific doses of cocaine will exert psychomotor and neuroendocrine responses in rat (Frantz et al., 2007; Hu and Becker, 2003; Quiñones-Jenab et al., 2008; Todtenkopf and Carlezon, 2006). There is evidence that cycle condition does not affect cocaine metabolism, and as such metabolism was not measured (Evans and Foltin, 2006b; Mendelson et al., 1999).
Procedure
Estrous cycle was assessed daily in female rats between 0900 and 1000 h, while male rats were handled. Cocaine dose subgroups were assigned randomly to rats identified as proestrous, diestrous, or males. On the testing day, female rats were screened for receptivity (1300 h). Subjects were counterbalanced for testing day effects. Rats were injected IP with cocaine or saline, and open field behavior was recorded for 30min (1300 and 1600 h). After open field, rats were assessed in a sex-specific paced mating paradigm for 15min. Immediately following sex testing, rats were sacrificed via rapid decapitation; brain and serum were collected for radioimmunoassay (RIA).
Behavioral testing
All data were collected using the automated ANY-Maze data collection program (Stoelting Co., Wheat Dale, IL) with videocapture data, which is saved on a secure server. Rearing and mating behaviors were also hand-scored, and time was kept with stopwatches.
Open field
Open field analyses was completed in a chamber (37.5×75×30 cm) with a 50-square grid floor (5×10 squares) with an overhead light illuminating the central squares. The number of peripheral (26) and central (24) squares entered was recorded during a 30-minute test period. The amount of time spent in the central squares is used as an index of anti-anxiety behavior.
Paced mating
Paced mating tests were conducted in concordance with previously reported procedures (Erskine, 1985; Frye and Erskine, 1990) in a chamber (37.5×75×30 cm), which was equally divided by a clear divider with a small (5 cm in diameter) hole in the bottom center. This divider allows females a free access to control the frequency of their sexual encounters, as female rats can fit through this hole while the stimulus males cannot. Females were placed in the side of the chamber opposite the stimulus male and behaviorally tested for 15min, or an ejaculatory series. Sexual behaviors (lordosis ratings) were quantified by frequency of lordosis in response to these contacts (Hardy and DeBold, 1972). Male mating was conducted in this apparatus per previously defined methods (Frye and Rhodes, 2006a,b). Male mating behaviors were analyzed on a 0–3 scale, with 0 indicating no engagement in sexual behavior. Males received a score of 1 for every engagement in anogenital contact, a score of 2 for every mount, and a score of 3 for every intromission or ejaculation. Mean scores were used to compare male sexual behavior to female sexual behavior via the 0–3 scale described by Hardy and DeBold (1972) to rate lordosis of female rodents.
Tissue collection
Immediately following the completion of testing, each rat had trunk blood and whole brains collected and stored at –80 °C for later measurement of brain and/or circulating 3α,5α-THP, and P by RIA (described below). Immediately prior to measurement of steroids, diencephalon, cortex, hippocampus, and midbrain were grossly dissected.
Measurement of steroid hormones
Progestogen levels were measured by RIA using a modified version of previously reported methods (Frye et al., 1996, 1998; Frye and Bayon, 1999). Steroid was extracted from brain regions with MeOH in chromatography separation on Sepak cartridges. Samples were reconstituted to a volume of 100 μl per assay. Progestogen assays were incubated overnight at 4 °C. To separate bound and free steroid, dextran-coated charcoal was added and samples were incubated for 10 min then centrifuged for 10 min at 3000 g. Supernatant was decanted into a glass vial containing 5 ml of scintillation cocktail. Using the logit–log method (Rodbard and Hutt, 1974), interpolation of the standards, and correction for recovery with ‘AssayZap’ interpolation software published by Biosoft (1994), sample tube concentrations were calculated. The inter- and intra-assay reliability coefficients were: for P, 0.10 and 0.07, and for 3α,5α-THP, 0.12 and 0.15.
Statistical analyses
Behavioral data were analyzed using two-way analyses of variance (ANOVAs) with hormone status (proestrous, diestrous, and male) and cocaine condition (saline, 5, 10, and 20 mg/kg) as between-subject variables. Simple regression analyses were used to determine the variance present in each measured behavior, such that steroid hormones were indicated as the predictors, and behaviors were indicated as the measurements. Where appropriate, Fisher's Least Significant Difference post-hoc tests were used to elucidate group differences. The alpha level for statistical significance was p≤0.05. Endocrine data in the four brain regions were assessed via ANOVAs followed by Bonferroni corrections for multiple comparisons in which the alpha level for statistical significance was p≤0.01. Trend level statistics were reported when p≤0.10.
Results
Endocrine measures
Progesterone
Results indicate a main effect of hormone condition to influence P levels in the cortex [F(2,109)=8.37, p<0.01], hippocampus [F(2,109)=11.80, p<0.01], and midbrain [F(2,109)=8.08, p<0.01]. Results also indicate a main effect of cocaine condition to influence P levels in the diencephalon [F(3,109)=14.77, p<0.01], cortex [F(3,109)=17.25, p<0.01], hippocampus [F(3,109)=19.83, p<0.01], and midbrain [F(3,109)=13.13, p<0.01]. These data show increased P levels among female rats compared to male rats, such that higher dosages of cocaine increased, and lower dosages of cocaine decreased P levels across brain regions (Table 1).
Table 1.
Concentrations of progesterone and 3α,5α-THP in diencephalon, cortex, hippocampus, and midbrain of diestrous female, proestrous female, and male rats administered 0, 5, 10, or 20 mg/kg intraperitoneal cocaine (n = 9–12/group).
| Diestrous |
Proestrous |
Male |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cocaine dose (mg/kg) | 0 | 5 | 10 | 20 | 0 | 5 | 10 | 20 | 0 | 5 | 10 | 20 |
| Progesterone concentration (ng/g±SEM) | ||||||||||||
| Diencephalon | 6.8±1.7 | 5.6±1.8 | 7.8±1.8 | 16.8±1.9* | 12.3±2.8 | 8.9±2.5 | 7.8±1.9* | 18.3±2.3* | 7.8±1.4 | 3.9±1.7* | 4.7±0.6* | 9.3±1.1 |
| Cortex | 3.5±0.9 | 3.7±1.1 | 3.9±1.0 | 8.3±1.1 | 5.9±1.3 | 3.2±0.6* | 2.9±0.4* | 8.1±0.8* | 3.1±0.6 | 1.3±0.2 | 1.9±0.2 | 5.0±0.7* |
| Hippocampus | 4.1±1.0 | 3.8±1.1 | 3.7±0.7 | 9.5±0.9* | 6.9±1.5 | 3.4±0.5* | 3.0±0.4* | 9.1±1.0* | 3.1±0.6 | 1.3±0.2* | 2.3±0.3 | 4.8±0.5* |
| Midbrain | 8.7±2.4 | 10.5±3.4 | 13.5±4.1 | 23.0±3.2* | 14.9±3.4 | 8.0±1.3* | 8.5±1.9* | 22.6±2.8* | 9.6±1.8 | 3.3±0.5* | 7.8±0.9 | 17.0±2.7* |
| Serum | 5.1±0.4 | 4.5±0.9 | 6.1±0.2* | 2.5±0.7* | 6.2±0.6 | 4.1±1.1* | 6.2±0.2 | 4.7±1.0* | 4.9±0.6 | 6.0±0.2* | 6.4±0.3* | 3.0±0.8* |
| 3α,5α-THP (ng/g ± SEM) | ||||||||||||
| Diencephalon | 21.9±3.7 | 13.5±2.7* | 6.5±1.9* | 27.2±6.2 | 40.6±13.9 | 9.7±4.5* | 10.5±2.4* | 33.0±5.3 | 7.5±2.7 | 4.4±0.5* | 10.5±2.4 | 33.0±5.3* |
| Cortex | 22.1±6.3 | 14.7±3.0 | 9.0±2.8* | 29.4±5.9 | 32.6±7.5 | 22.8±9.1 | 14.0±3.4* | 38.3±8.4 | 7.4±2.2 | 5.0±1.0 | 2.9±0.4* | 17.3±2.6* |
| Hippocampus | 16.0±3.0 | 16.8±3.0 | 6.6±1.7* | 22.3±4.0 | 43.3±10 | 17.3±4.5* | 16.0±6.4* | 43.8±10.3 | 10.7±4.6 | 8.6±1.6 | 5.7±0.8 | 38.9±8.1* |
| Midbrain | 32.3±6.0 | 46.5±12.8 | 19.6±6.6* | 43.3±7.2 | 57.1±15.3 | 35.1±13.9 | 24.1±7.8* | 89.0±23.0 | 28.2±15.2 | 12.7±2.3 | 13.1±2.8 | 43.7±7.6 |
| Serum | 1.6+0.2 | 2.6+0.5* | 1.6+0.5 | 1.8+0.5 | 2.6+0.4 | 4.5+0.1* | 0.5+0.1* | 0.8+0.3* | 1.9+0.3 | 3.2+0.4* | 0.8+0.3* | 2.8+0.3* |
indicates significant difference compared to vehicle, p<0.05.
There was a trend for an interaction between hormone/sex and cocaine conditions [F(6,109)=1.83, p<0.10] where the high dose of cocaine produced greater increases, compared to vehicle, in hippocampus P among females, compared to males (Table 1). Serum analyses indicate that there was a main effect of cocaine condition to alter P levels in serum [F(3,109)=10.675, p<0.05] where low doses of cocaine increased, and high levels of cocaine decreased, compared to vehicle, P levels across hormone conditions (Table 1).
3α,5α-THP
Results indicate a main effect of hormone condition to influence 3α,5α-THP levels in the diencephalon [F(2,109)=5.06, p<0.01], cortex [F(2,109)=13.90, p<0.01], hippocampus [F(2,109)=8.74, p<0.01], and midbrain [F(2,109)=6.86, p<0.01]. Results also indicate a main effect of cocaine condition to influence 3α,5α-THP levels in the diencephalon [F(3,109) =6.01, p<0.01], cortex [F(3,109)=8.53, p<0.01], hippocampus [F(3,109) = 8.80, p<0.01], and midbrain [F(3,109)=10.32, p<0.01] in response to cocaine administration. These data were such that females had higher neuroendocrine 3α,5α-THP compared to males, but the highest dose of cocaine increased, whereas the lower doses decreased, 3α,5α-THP levels in brain, across all groups. In serum, there was an interaction between hormone condition and drug condition to influence 3α,5α-THP levels [F(6,109)= 6.198, p<0.05], such that high doses of cocaine did not significantly alter serum 3α,5α-THP, whereas moderate doses significantly decreased, and low doses significant increased, serum 3α,5α-THP (Table 1). Serum 3α,5α-THP levels did not predict behavioral, or neuroendocrine outcomes.
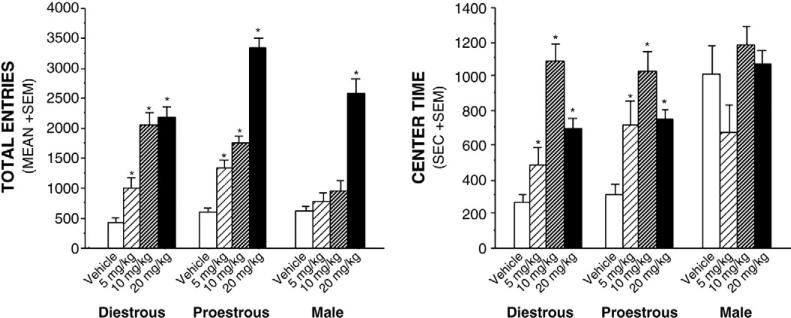
Psychomotor behavior
Hormone/sex and cocaine condition interacted to increase total entries in the open field [F(6,117)=2.56, p≤0.05] (Fig. 1, left), such that female rats had increased total entries in response to all cocaine doses, whereas males required the high dose (20 mg/kg) to produce a motor response. Increased P levels in the diencephalon, cortex and midbrain, and decreased serum P (Table 1), predicted a significant amount of variance (8–23%; Table 2) in total entries among cocaine administered rats.
Fig. 1.
Left panel depicts total entries in the open field of diestrous female, proestrous female and male rats (n=9–12/group) administered cocaine (0, 5, 10, or 20 mg/kg, IP). Right panel depicts time spent in the center of the open field among diestrous female, proestrous female, and male rats (n=9–12/group) administered cocaine (0, 5, 10, or 20 mg/kg, IP). * indicates significant differences from respective vehicle-administered control group.
Table 2.
Regression relationships between progesterone (P) and/or 3α,5α-THP in specific brain regions, and behavioral observations, in cocaine administered rats. Positive relationships are denoted by an up arrow, and negative by a down arrow.
| Total entries | |||
|---|---|---|---|
| Regression effect | Regression slope | Variance explained | |
| Diencephalon | ↑p, ↑Total entries | β= 53.03, t(83)=4.356* | R2 = 0.19, r(83)=0.43, p≤ 0.01* |
| Hippocampus | ↑P, ↑Total entries | β=167.5, t(83)=4.932* | R2=0.23, r(83)=0.47, p≤0.01* |
| Cortex | ↑P, ↑Total entries | β=158.4, t(83) =4.281* | R2=0.18, r(83)=0.43, p≤0.01* |
| Midbrain | ↑P, ↑Total entries | β=75.4, t(83)=4.394* | R2=0.19, r(83)=0.44, p≤0.01* |
| Serum | ↓P, ↑Total entries | β=–146.2, t(83)=–2.763* | R2=0.09, r(83)=0.29, p≤0.01* |
| Time spent in the center Cortex | ↓3α,5α-THP, ↑Center time | β=–5.3, t(83)=–2.637* | R2=0.08, r(83)=0.28, p≤0.01* |
indicates significant, p< 0.05.
Anxiety behavior
Hormone/sex and cocaine condition interacted to increase time spent in the brightly-lit center of the open field [F(6,117)=2.78, p≤0.05], such that females display lesser anxiety-like behaviors when administered cocaine, whereas males did not significantly differ from vehicle. In addition, males displayed significantly less while females administered the dose of 10 mg/kg cocaine displayed male-typical anti-anxiety behaviors (Fig. 1, Right). Decreased 3α,5α-THP in cortex (Table 2), predicted a small, but significant, amount of variance (8%; Table 2) in time spent in the center of the open field by rats administered cocaine.
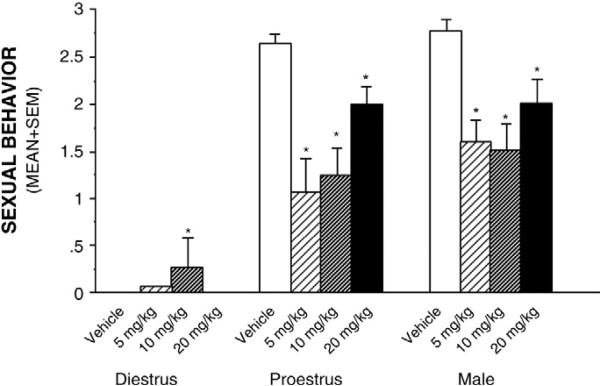
Sexual behaviors
Hormone/sex and cocaine condition interacted to decrease receptivity in naturally receptive females, and male rats, and increase receptivity in naturally non-receptive female rats [F(6,109)=4.53, p≤0.05], such that males administered cocaine, compared to vehicle, intromitted and mounted less [F(3, 43)=7.31, p≤0.05] (Fig. 2). Levels of 3α,5α-THP in midbrain (Table 1) predicted a significant amount of variance in female lordosis [R2=0.16, F(1,23)=4.378], among vehicle-administered, but not cocaine administered, rats.
Fig. 2.
Sexual behaviors of diestrous female, proestrous female and male rats (n=9–12/group) administered cocaine (0, 5, 10, or 20 mg/kg, IP). * indicates significant differences from respective vehicle-administered control group.
Discussion
The present data supported our hypothesis that natural variations in progestogen milieu altered the dose-dependent effects of cocaine to influence the neuroendocrine response, and psychomotor, affective and reproductive behaviors of rats. Our results indicate that cocaine produced U-shaped curves for some, but not all measures. First, cocaine significantly altered neural P and 3α,5α-THP levels, in a U-shaped manner. Second, brain P accounted for 18–23% of variance in psychomotor behaviors, which were dose-dependently increased. Third, although all cocaine doses decreased anxiety-like behavior compared to vehicle; among cocaine administered rats, low dosages of cocaine decreased, and high dosages of cocaine increased, anxiety-like behaviors and progestogen levels. Fourth, sexual behaviors were dysregulated among rats administered cocaine. Fifth, the neuroendocrine and behavioral effects of cocaine were hormone/sex dependent. Administration of low doses of cocaine (5 or 10 mg/kg) significantly reduced concentrations of P in diencephalon, cortex, hippocampus, and midbrain of proestrous female and male rats. 3α,5α-THP levels were also reduced by low doses of cocaine in these brain regions among all rats. The high dose of cocaine (20 mg/kg) enhanced P concentrations among rats, and enhanced 3α,5α-THP among male rats. Behavior was altered concomitantly with cortical, and midbrain P explaining a significant amount of variance in psychomotor behavior among cocaine administered rats. Cortical 3α,5α-THP accounted for a small, but significant, amount of variance present in center time among cocaine administered rats. A significant amount of variance in lordosis was explained by 3α,5α-THP levels in midbrain among vehicle-administered, but not cocaine administered, females. Thus, these data provide evidence that endogenous hormonal milieu may mitigate some of the neuroendocrine and behavioral effects observed in the acute cocaine response.
Congruent with previous findings, steroid hormones can act to alter cocaine-induced psychomotor stimulation. Female rats had stimulant responses to all doses of cocaine, whereas males required the 20 mg/kg dose to exhibit any effect, compared to vehicle. In addition, rats in a state of high endogenous progestogen production (proestrus) had increased psychomotor stimulation in response to low doses of cocaine compared to rats in a low state of endogenous progestogen production (diestrous and male). Other laboratories have also reported that female rats have increased locomotor responses to acute cocaine administration, compared to males (Festa and Quinones-Jenab, 2004; Hu and Becker, 2003; Lopez-Ojeda et al., 2008). However, when we administered the high dose of 20 mg/kg, male rats experienced a significant increase in psychomotor behaviors, suggesting that males require a higher dose of cocaine to experience cocaine's stimulant effects. Serum level analyses indicate an inverted U-shape relationship between progestogen levels and stimulant effects of cocaine. Extirpation and replacement studies report that females administered progestogens and estrogens have increased psychomotor stimulation compared to ovariectomized females not administered hormones (Niyomchai et al., 2006; Perrotti et al., 2001). In addition, sex differences in stimulant effects of cocaine have been observed, wherein females tend to have a greater response than do males (Walker et al., 2001a,b). These data suggest that there may be salient sex differences in the stimulant effects of cocaine which may be dose-dependent.
Interestingly, there are many findings that progestogens and estrogens can act antagonistically on psychomotor responding with exogenous estrogen enhancing and progesterone reducing response to cocaine (Hu and Becker, 2003; Niyomchai et al., 2006; Perrotti et al., 2001; Sell et al., 2000). In this investigation, we find that P (diencephalon, cortex, and midbrain) positively correlated, whereas serum P negatively correlated, to cocaine's psychomotor effects on rats. Regression analyses indicate that these effects, in brain, were driven by proestrous and male rats, as well as rats administered cocaine, but not vehicle. Diestrous rats, however, did not display differences in progesterone when administered low doses of cocaine compared to vehicle. These data suggest that endogenously increased progestogens may contribute to psychomotor stimulation, but the involvement of estrogen cannot be ruled out as the largest enhancement was observed in proestrous rats, which typically have considerably higher circulating estrogen levels than do diestrous or male rats (Beyer et al., 2007; Blaustein, 2008). In addition, these data suggest that there may be differing actions of central and peripheral progestogens for cocaine's stimulant effects, which may account for some of the dose and duration dependent differences previously observed. Thus, these data extend previous findings that progestogens may underlie, in part, enhancement of psychomotor response to cocaine.
There is evidence that P and/or its metabolites have biphasic effects on anxiety-like behaviors. P administration has been shown to decrease anxiety-like behaviors (Bitran et al., 2000; Frye and Walf, 2004; Toufexis et al., 2004), prevent anxiogenic effects of drug stimuli (Jain et al., 2005), and treat withdrawal symptoms (Schweizer et al., 1995). While P has positive anxiety-modulating effects, chronic exposure to P (Devall et al., 2009; Gallo and Smith, 1993), and high or unstable P levels (van Wingen et al., 2008), can induce anxiogenic behavior. 3α,5α-THP has similar effects as P to reduce anxiety when administered (Engin and Treit, 2007; Frye and Walf, 2004; Picazo and Fernandez-Guasti, 1995; Reddy et al., 2005; Reddy and Kulkarni, 1997), but may also have an inverted U-shape curve where high levels of 3α,5α-THP can induce negative effect (Andreen, et al. 2009). In addition, women administered P can differentially respond based on dose and 3α,5α-THP synthesis (Andreen et al., 2006), and women administered high doses may experience neuropsychiatric side effects (Elwan et al., 1973). In women who experience PMDD, indicative of hormone sensitivity, administration of P or 3α,5α-THP can result in more mood disturbances (Andreen et al., 2009). These data add to evidence which suggests that P and 3α,5α-THP may have biphasic actions, based on whether or not the subject has an endogenously high, or has recently been exposed to, high levels of progestogens (van Wingen et al., 2008). These effects may be due to 3α,5α-THP's ability to activate both inhibitory (GABA) and excitatory (NMDAR and D1-like) receptors (Frye et al., 2006, 2004). The data reported herein demonstrate that high progestogens, such as that seen in proestrous rats, can contribute susceptibility to anxiolytic effects of cocaine, depending on dose; whereas, low progestogen levels, such as that seen in male rats, may inhibit some effects of cocaine at lower doses. Thus, these findings imply that differential effects of P and/or 3α,5α-THP may account for some of the neuroendocrine or behavioral endpoints observed in anxiety response to cocaine.
In addition to the psychostimulant and anxiety effects of cocaine, we assessed its effects on sexual behavior of male and female rats. Sexual behaviors are affected by drugs of abuse in humans and in rodents (Holder et al., 2009; Lopez et al., 2009; Pallonen et al., 2008). Under non-manipulated circumstances, male and female proestrous rats are expected to have high sexual behaviors, whereas female diestrous rats are expected to have low sexual behaviors (Beyer et al., 2007; Blaustein, 2008; Pfaus et al., 1999). In intact studies, this presents ceiling effects for males and proestrous females, and floor effects for diestrous female rats. In the present study, regression analyses reveal that among vehicle-administered females, midbrain 3α,5α-THP levels explain 23% of variance in lordosis, whereas in cocaine administered females, midbrain 3α,5α-THP levels explain a reduced amount, only 4%, of variance in lordosis. These data suggest that cocaine produces sexual behavior deviant from what is expected of rats in these hormonal conditions.
Given these data, interactions between cocaine-dose and hormone variability have implications for hormone action in drug exposure, response, and addiction. As this experiment involved an acute-administration paradigm, it is most applicable to the initiation stage of cocaine abuse. As with our results, in humans it is found that there are sex differences in the effects of acute exposure to cocaine (Lukas et al., 1996). As first time exposure experiences are highly influential on continued use, it is important to investigate the substrates that are involved in susceptibility to extended use. Previous data has linked exogenous hormones with cocaine response in conditioned place preference (Russo et al., 2008, 2003), stereotypic behaviors, (Festa and Quinones-Jenab, 2004; Frye, 2007; Quiñones-Jenab et al., 1999; Russo et al., 2003; Walker et al., 2001a,b), as well as sex/gender differences in cocaine-induced psychomotor stimulation (Crombag et al., 1999; Quiñones-Jenab et al., 1999), affective response (Evans and Foltin, 2006a,b; Kosten et al., 1993; Lynch et al., 2002; Mendelson, 1996), and sexual behaviors (Cocores et al., 1988; Horvath et al., 2007; Kendirci et al., 2007). Previous studies also provide evidence that there are differential effects of cocaine depending on dose magnitude, timing, frequency, and duration of exposure (Calogero et al., 1989; de Jong et al., 2009; Fontana and Commissaris, 1989; Goeders, 2002; Muller et al., 2008; Yap and Miczek, 2007), and women in high-P phases of their hormone cycle experience greater cardiovascular changes and peripheral response, as well as increased anxiety, than do women in low-P phases, or men, in response to cocaine administration (Ambrose-Lanci et al., 2009; Evans et al., 2002; Kaufman et al., 2001; Lukas et al., 1996). The current investigation supports these findings and extends them, suggesting a biphasic relationship among cocaine administered females, between cocaine and behavior in which low doses of cocaine correspond to decreased progestogens, and decreased anxiety, whereas in high doses the opposite is found. Progestogen exposure prior to challenge with cocaine may influence the behavioral and neuroendocrine responses to the drug. Some of these effects may be due in part to central modulation of 3α,5α-THP which can act in hypothalamus to attenuate neuroendocrine responses to a stressor and restore homeostasis (Patchev et al., 1994, 1996). Given that these effects are observed in the present study on an acute-administration basis, the long-term implications for stress response and allostasis of the organism are of great interest. Perturbation of homeostatic and allostatic regulation are proposed to underlie the pathology of addiction (reviewed in Koob, 2009; Le Moal, 2009). Limitations of the present study are the U-shaped curves, and differing patterns of disruption among behaviors, which make it difficult to discern relationships among behavioral outcomes. Indeed, changes in progestogen levels and anxiety behavior may partly underlie observed disruption of sexual behavior. In order to further examine these phenomena, future investigations will seek to examine interactions of endogenous progestogens and cocaine response in a chronic paradigm, such as conditioned place preference and/or self-administration.
References
- Ambrose-Lanci LM, Sterling RC, Weinstein SP, Van Bockstaele EJ. The influence of intake urinalysis, psychopathology measures, and menstrual cycle phase on treatment compliance. Am. J. Addict. 2009;18:167–172. doi: 10.1080/10550490902772710. [DOI] [PubMed] [Google Scholar]
- Andreen L, Sundstrom-Poromaa I, Bixo M, Nyberg S, Backstrom T. Allopregnanolone concentration and mood—a bimodal association in postmenopausal women treated with oral progesterone. Psychopharmacology (Berl.) 2006;187:209–221. doi: 10.1007/s00213-006-0417-0. [DOI] [PubMed] [Google Scholar]
- Andreen L, Nyberg S, Turkmen S, van Wingen G, Fenandez G, Backstrom T. Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABA(A) modulators. Psychoneuroendocrinology. 2009;34:1121–1132. doi: 10.1016/j.psyneuen.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann. N.Y. Acad. Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Beyer C, Hoffman KL, Gonzalez-Flores O. Neuroendocrine regulation of estrous behavior in the rabbit: similarities and differences with the rat. Horm. Behav. 2007;52:2–11. doi: 10.1016/j.yhbeh.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Bitran D, Klibansky DA, Marin GA. The neurosteroid pregnanolone prevents the anxiogenic-like effect of inescapable shock in the rat. Psychopharmacology (Berl.) 2000;151:31–37. doi: 10.1007/s002130000472. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Neuroendocrine regulation of feminine sexual behavior: lessons from rodent models and thoughts about humans. Annu. Rev. Psychol. 2008;59:93–118. doi: 10.1146/annurev.psych.59.103006.093556. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Gallucci WT, Kling MA, Chrousos GP, Gold PW. Cocaine stimulates rat hypothalamic corticotrophin-releasing hormone secretion in vitro. Brain Res. 1989;505:7–11. doi: 10.1016/0006-8993(89)90109-1. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol. Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Cocores JA, Miller NS, Pottash AC, Gold MS. Sexual dysfunction in abusers of cocaine and alcohol. Am. J. Drug Alcohol Abuse. 1988;14:169–173. doi: 10.3109/00952999809001544. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Mueller H, Browman KE, Badiani A, Robinson TE. A comparison of two behavioral measures of psychomotor activation following intravenous amphetamine or cocaine: dose- and sensitization-dependent changes. Behav. Pharmacol. 1999;10:205–213. doi: 10.1097/00008877-199903000-00009. [DOI] [PubMed] [Google Scholar]
- Devall AJ, Liu ZW, Lovick TA. Hyperalgesia in the setting of anxiety: sex differences and effects of the oestrous cycle in Wistar rats. Psychoneuroendocrinology. 2009;34:587–596. doi: 10.1016/j.psyneuen.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Elwan O, Madkour O, Kamal I, Abdallah M. Steroid contraceptives: neuropsychiatric and electroencephalographic complications. J. Int. Med. Res. 1973;1:534–547. doi: 10.1177/030006057300100605. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. The anxiolytic-like effects of allopregnanolone vary as a function of intracerebral microinfusion site: the amygdala, medial prefrontal cortex, or hippocampus. Behav. Pharmacol. 2007;18:461–470. doi: 10.1097/FBP.0b013e3282d28f6f. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Effects of paced coital stimulation on estrus duration in intact cycling rats and ovariectomized and ovariectomized–adrenalectomized hormone-primed rats. Behav. Neurosci. 1985;99:151–161. doi: 10.1037//0735-7044.99.1.151. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006a;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Pharmacokinetics of repeated doses of intravenous cocaine across the menstrual cycle in Rhesus monkeys. Phamacol. Biochem. Behav. 2006b;83:56–66. doi: 10.1016/j.pbb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle. Psychopharmacology (Berl.) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm. Behav. 2004;446:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Finn DA, Phillips TJ, Okorn DM, Chester JA, Cunningham CL. Rewarding effect of the neuroactive steroid 3 alpha-hydroxy-5 alpha-pregnan-20-one in mice. Pharmacol. Biochem. Behav. 1997;56:261–264. doi: 10.1016/s0091-3057(96)00218-3. [DOI] [PubMed] [Google Scholar]
- Fontana DJ, Commissaris RL. Effects of cocaine on conflict behavior in the rat. Life Sci. 1989;45:819–827. doi: 10.1016/0024-3205(89)90175-6. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, O'Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Frye CA. Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol. Biochem. Behav. 2007;86:209–219. doi: 10.1016/j.pbb.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3alpha, 5alpha-THP and 3alpha-diol. J. Neuroendocrinol. 1999;11:839–847. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE, Pursnani NK, Purdy RH. The neurosteroids, progesterone and 3alpha, 5alpha-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998;808:72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- Frye CA, Erskine MS. Influence of time of mating and paced copulation on induction of pseudopregnancy in cyclic female rats. J. Reprod. Fertil. 1990;90:375–385. doi: 10.1530/jrf.0.0900375. [DOI] [PubMed] [Google Scholar]
- Frye CA, McCormick CM, Coopersmith C, Erskine MS. Effects of paced and non-paced mating stimulation on plasma progesterone, 3 alpha-diol and cort. Psychoneuroendocrinology. 1996;21:431–439. doi: 10.1016/0306-4530(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti anxiety, nor social behavior, increases 5alpha-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007;133:663–674. doi: 10.1530/rep.1.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME. Exploratory, anti-anxiety, social, and sexual behaviors of rats in behavioral estrus is attenuated with inhibition of 3alpha, 5alpha-THP formation in the midbrain ventral tegmental area. Behav. Brain Res. 2008;193:269–276. doi: 10.1016/j.bbr.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME. Increasing 3alpha, 5alpha-THP following inhibition of neurosteroid biosynthesis in the ventral tegmental area reinstates anti-anxiety, social and sexual behavior of naturally receptive rats. Reproduction. 2009;137:119–128. doi: 10.1530/REP-08-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrus cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol. Biochem. Behav. 2000;67:1–10. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Progestin concentrations are increased following paced mating in midbrain, hippocampus, diencephalon, and cortex of rats in behavioral estrus, but only in midbrain of diestrous rats. Neuroendocrinology. 2006a;83:336–347. doi: 10.1159/000096051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Infusions of 5-alpha-pregnan-3-alpha-ol-20-one (3alpha, 5alpha-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3alpha, 5alpha-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J. Neuroendocrinol. 2006b;18:960–975. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behav. Neurosci. 2004;118:306–313. doi: 10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Petralia SM. In the ventral tegmental area, progestins have actions at D1 receptors for lordosis of hamsters and rats that involve GABAA receptors. Horm. Behav. 2006;50:332–337. doi: 10.1016/j.yhbeh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Sumida K. Progestins’ actions in the VTA to facilitate lordosis involve dopamine-like type 1 and 2 receptors. Phamacol. Biochem. Behav. 2004;78:405–418. doi: 10.1016/j.pbb.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl.) 2005;179:662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Gallo MA, Smith SS. Progesterone withdrawal decreases latency to and increases duration of electrified prod burial: a possible rat model of PMS anxiety. Pharmacol. Biochem. Behav. 1993;46:897–904. doi: 10.1016/0091-3057(93)90219-j. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J. Pharmacol. Exp. Ther. 2002;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Hardy DF, DeBold JF. Effects of coital stimulation upon behavior of the female rat. J. Comp. Physiol. Psychol. 1972;78:400–408. doi: 10.1037/h0032536. [DOI] [PubMed] [Google Scholar]
- Holder MK, Hadjimarkou MM, Zup SL, Blutstein T, Benham RS, McCarthy MM, Mong JA. Methamphetamine facilitates female sexual behavior and enhances neuronal activation in the medial amygdala and ventromedial nucleus of the hypothalamus. Psychoneuroendocrinology (Electronic publication) 2009 doi: 10.1016/j.psyneuen.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath KJ, Calsyn DA, Terry C, Cotton A. Erectile dysfunction medication use among men seeking substance abuse treatment. J. Addict. Dis. 2007;26:7–13. doi: 10.1300/J069v26n04_02. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J. Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain NS, Hirani K, Chopde CT. Reversal of caffeine-induced anxiety by neurosteroid 3-alpha-hydroxy-5-alpha-pregnane-20-one in rats. Neuropharmacology. 2005;48:627–638. doi: 10.1016/j.neuropharm.2004.11.016. [DOI] [PubMed] [Google Scholar]
- de Jong IE, Steenbergen PJ, de Kloet ER. Behavioral sensitization to cocaine: cooperation between glucocorticoids and epinephrine. Psychopharmacology (Berl.) 2009;204:693–703. doi: 10.1007/s00213-009-1498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MJ, Levin JM, Maas LC, Kukes TJ, Villafuerte RA, Dostal K, Lukas SE, Mendelson JH, Cohen BM, Renshaw PF. Cocaine-induced cerebral vasoconstriction differs as a function of sex and menstrual cycle phase. Biol. Psychiatry. 2001;49:774–781. doi: 10.1016/s0006-3223(00)01091-x. [DOI] [PubMed] [Google Scholar]
- Kendirci M, Pradhan L, Trost L, Gur S, Chandra S, Agrawal KC, Hellstrom WJ. Peripheral mechanisms of erectile dysfunction in a rat model of chronic cocaine use. Eur. Urol. 2007;52:555–563. doi: 10.1016/j.eururo.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Koob G. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. Subst. Abuse Treat. 1993;10:63–66. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY. Sex differences in non-reinforced responding for cocaine. Am. J. Drug Alcohol Abuse. 2008;34:472–488. doi: 10.1080/00952990802082206. [DOI] [PubMed] [Google Scholar]
- Le Moal M. Drug Abuse: vulnerability and transition to addiction. Pharmacopsychiatry. 2009;42:S42–S55. doi: 10.1055/s-0029-1216355. [DOI] [PubMed] [Google Scholar]
- Lopez HH, Webb SA, Nash S. Cannabinoid receptor antagonism increases female sexual motivation. Pharmacol. Biochem. Behav. 2009;92:17–24. doi: 10.1016/j.pbb.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Lopez-Ojeda W, Welsh ML, Segarra AC. Estrous cyclicity and the locomotor response to acute and repeated cocaine administration. FASEB J. 2008;22:946.6. [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L, Mendelson JH. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology (Berl.) 1996;125:346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl.) 2008;197:237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl.) 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Kalayasiri R, Sughodhabirom A, Pittman B, Vladimir C, Morgan PT, Malison RT. Subjective responses and cardiovascular effects of self-administered cocaine in cocaine-abusing men and women. Addict. Biol. 2008;13:403–410. doi: 10.1111/j.1369-1600.2008.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl.) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Mendelson JH. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology (Berl.) 1996;125:346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 2008;21:294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Muller CP, Carey RJ, Wilkisz M, Schwenzner S, Jocham G, Huston JP, De Souza Silva MA. Acute anxiolytic effects of cocaine: the role of test latency and activity phase. Pharmacol. Biochem. Behav. 2008;89:218–226. doi: 10.1016/j.pbb.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Niyomchai T, Akhavan A, Festa ED, Lin SN, Lamm L, Foltz R, Quinones-Jenab V. Estrogen and progesterone affect cocaine pharmacokinetics in female rats. Brain Res. Bull. 2006;68:310–314. doi: 10.1016/j.brainresbull.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Pallonen UE, Williams ML, Timpson SC, Bowen A, Ross MW. Personal and partner measures in stages of consistent condom use among African–American heterosexual crack cocaine smokers. AIDS Care. 2008;20:205–213. doi: 10.1080/09540120701513669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev VK, Hassan AH, Holsboer DF, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharamcology. 1996;15:533–540. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Shoaib M, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62:265–271. doi: 10.1016/0306-4522(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Russo SJ, Fletcher H, Chin J, Webb T, Jenab S. Ovarian hormones modulate cocaine-induced locomotor and stereotypic activity. Ann. N.Y. Acad. Sci. 2001;937:202–216. doi: 10.1111/j.1749-6632.2001.tb03566.x. [DOI] [PubMed] [Google Scholar]
- Petralia SM, Jahagirdar V, Frye CA. Inhibiting biosynthesis and/or metabolism of progestins in the ventral tegmental area attenuates lordosis of rats in behavioural oestrus. J. Neuroendocrinol. 2005;17:545–552. doi: 10.1111/j.1365-2826.2005.01342.x. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Smith WJ, Coopersmith CB. Appetitive and consummatory sexual behaviors of female rats in bilevel chambers. I. A correlational and factor analysis and the effects of ovarian hormones. Horm. Behav. 1999;35:224–240. doi: 10.1006/hbeh.1999.1516. [DOI] [PubMed] [Google Scholar]
- Picazo O, Fernandez-Guasti A. Anti-anxiety effects of progesterone and some of its reduced metabolites: an evaluation using the burying behavior test. Brain Res. 1995;680:135–141. doi: 10.1016/0006-8993(95)00254-n. [DOI] [PubMed] [Google Scholar]
- Quiñones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ. Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behav. Brain Res. 1999;101:15–20. doi: 10.1016/s0166-4328(98)00073-4. [DOI] [PubMed] [Google Scholar]
- Quiñones-Jenab V, Minerly AC, Niyomchia T, Akahvan A, Jenab S, Frye C. Progesterone and allopregnanolone are induced by cocaine in serum and brain tissues of male and female rats. Pharmacol. Biochem. Behav. 2008;89:292–297. doi: 10.1016/j.pbb.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Differential anxiolytic effects of neurosteroids in the mirrored chamber behavior test in mice. Brain Res. 1997;752:61–71. doi: 10.1016/s0006-8993(96)01447-3. [DOI] [PubMed] [Google Scholar]
- Reddy DS, O'Malley BW, Rogawski MA. Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology. 2005;48:14–24. doi: 10.1016/j.neuropharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl.) 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Rodbard D, Hutt DM. Statistical analysis of radioimmunoassay and immunoradiometric assays: a generalized, weighted iterative, least squares method for logistic curve fitting. In: International Atomic Energy Agency, editor. Symposium on Radioimmunoassay and Related Procedures in Medicine. Uniput; New York: 1974. pp. 209–233. [Google Scholar]
- Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quiñones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120:523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Sun WL, Minerly ACE, Weierstall K, Nazarian A, Festa ED, Niyomchai T, Akhavan A, Luine V, Jenab S, Quinones-Jenab V. Progesterone attenuates cocaine-induced conditioned place preference in female rats. Brain Res. 2008;1189:229–235. doi: 10.1016/j.brainres.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Schweizer E, Case WG, Garcia-Espana F, Greenblatt DJ, Rickels K. Progesterone co-administration in patients discontinuing long-term benzodiazepine therapy: effects on withdrawal severity and taper outcome. Psychopharmacology (Berl.) 1995;117:424–429. doi: 10.1007/BF02246214. [DOI] [PubMed] [Google Scholar]
- Sell LA, Morris JS, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug Alcohol Depend. 2000;60:207–216. doi: 10.1016/s0376-8716(99)00158-1. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox H, Hong KI, Sofuoglu M, Morgan PT, Bergguist KT. Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: implications for relapse and susceptibility. Exp. Clin. Psychopharmacol. 2007;15:445–452. doi: 10.1037/1064-1297.15.5.445. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Mark GP, Finn DA. Reinforcing effects of the neurosteroid allopregnanolone in rats. Pharmacol. Biochem. Behav. 2002;72:923–929. doi: 10.1016/s0091-3057(02)00776-1. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol. Biochem. Behav. 2002;72:431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp. Clin. Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Carlezon WA., Jr. Contribution of drug doses and conditioning periods to psychomotor stimulant sensitization. Psychopharmacology (Berl.) 2006;185:451–458. doi: 10.1007/s00213-005-0259-1. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Davis C, Hammond A, Davis M. Progesterone attenuates corticotropin-releasing factor-enhanced but not fear-potentiated startle via the activity of its neuroactive metabolite, allopregnanolone. J. Neurosci. 2004;24:10280–10287. doi: 10.1523/JNEUROSCI.1386-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JM, de Wit H. Menstrual cycle phase and response to drugs of abuse in humans. Drug Alcohol Depend. 2006;84:1–13. doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Walker QD, Francis R, Cabassa J, Kuhn CM. Effect of ovarian hormones and estrous cycle on stimulation of the hypothalamo-pituitary-adrenal axis by cocaine. J. Pharmacol. Exp. Ther. 2001a;297:291–298. [PubMed] [Google Scholar]
- Walker QD, Cabassa J, Kaplan KA, Li ST, Haroon J, Spohr HA, Francis R, Kuhn CM. Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacology. 2001b;25:118–130. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Backstrom T, Buitelaar JK, Fernandez G. Progesterone selectively increases amygdala reactivity in women. Mol. Psychiatry. 2008;13:325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Miczek KA. Social defeat stress, sensitization, and intravenous cocaine self-administration in mice. Psychopharmacology (Berl.) 2007;192:261–723. doi: 10.1007/s00213-007-0712-4. [DOI] [PubMed] [Google Scholar]