Abstract
Background
With an incidence of 2 to 3 cases per 100 000 persons per year, Hodkgin’s lymphoma (HL) is rare, but nonetheless one of the most common cancers in young adults. Improved treatment has made HL curable even in advanced stages, but controversy still surrounds a number of issues in patient care. Current research focuses on the avoidance of long-term adverse effects and secondary malignancies.
Methods
We selectively searched MEDLINE, CENTRAL, and the Guideline International Network for publications about HL. Two experts independently screened the retrieved publications for pertinence and extracted data from potentially relevant meta-analyses, randomized controlled trials (RCTs), and cohort studies into evidence tables.
Results
32 key questions were answered with 160 recommendations on the basis of evidence from 43 RCTs, 21 meta-analyses, and 119 cohort studies. Patients in an early stage of HL should be treated with two cycles of ABVD followed by involved-field radiotherapy (IF-RT) at a dose of 20 Gy (5-year overall survival [OS]: 94%). Patients in an intermediate (early unfavorable) stage should be treated with two cycles of BEACOPP escalated followed by two cycles of ABVD and 30 Gy IF-RT (5-year OS: 97.2%). Patients in an advanced stage should be treated with six cycles of BEACOPP escalated, and the decision whether this should be followed by consolidating radiotherapy (30 Gy) should be based on the findings of positron-emission tomography (radiate in case of PET-positive residual tumor; 5-year OS: 95.3%). Depending on the treatment regimen, there may be adverse effects including infection, leukopenia, anemia, thrombocytopenia, secondary neoplasia, and fertility disorders.
Conclusion
Most questions in the treatment of HL can now be answered on the basis of sufficient evidence from the literature. This holds in particular for the potential benefit to be gained from PET, follow-up care, and lifestyle recommendations for patients.
Hodgkin’s lymphoma (HL) is a malignant disease of the lymphatic system that can affect other organs. In the industrial countries, the annual incidence of HL is two to three cases per 100 000 inhabitants (1). With regard to the age distribution, there are two peaks: one between 20 and 30 years, the other over 65 years. These days HL is regarded as a curable disease, because combined chemotherapy and radiotherapy achieves 5-year survival rates over 90% (2).
This is the first published S3 guideline giving evidence- and consensus-based recommendations for the diagnosis, treatment, and follow-up of HL. At the moment around 50% of all HL patients in Germany are being treated in clinical trials. Brilliant et al. showed that HL patients treated in the framework of clinical trials have an improved progression-free survival (Brilliant C, Terschueren C, Franklin J, et al.: Differences in survival rates for patients with Hodgkin lymphoma, who were treated inside versus outside therapy optimisation protocols in Germany. ASH Annual Meeting Abstracts 2007; 110 [2321]). The aim of this evidence-based guideline is to standardize and optimize diagnosis and treatment in order to provide a quality-assured treatment strategy for all adult HL patients at any stage, from disease onset through recurrence to follow-up.
Methods: Guideline conception and development
The leading medical society for this evidence-based guideline is the German Society of Hematology and Medical Oncology (Deutsche Gesellschaft für Hämatologie und Medizinische Onkologie, DGHO). The Cochrane Haematological Malignancies Group (CHMG) led the project coordination and was in charge of the evidence process. The guideline was prepared between November 2010 and March 2012 by an interdisciplinary group of medical experts and representatives from 19 professional associations, the German Hodgkin Study Group (GHSG), and the CHMG (eBox). At the kick-off meeting, key questions were formulated on previously defined areas (diagnosis/staging, first-line treatment, treatment of recurring disease, follow-up). The database of the Guideline International Network (www.g-i-n.net) was searched for relevant guidelines that could potentially be adapted. No evidence-based guidelines were found, so a librarian carried out a complex and specific systematic literature survey in MEDLINE and CENTRAL covering the period from 1980 to 1 December 2011. Two methodologists from the CHMG categorized the hits and extracted relevant data into evidence tables according to the clinical and methodological criteria of evidence-based medicine. They then assessed the quality of the evidence for each key question using a modified GRADE (Grading of Recommendations Assessment, Development and Evaluation) procedure (eTable 1). On the basis of these tables the working groups formulated draft texts and draft recommendations which went on to be approved at the consensus conference in a nominal group process involving the medical experts of all associations and societies.
eBOX. Participating medical societies, organizations and experts.
-
Participating medical societies and organizations
German Society for Hematology/Oncology (leading) (1)
Society of Psychosocial Oncology (PSO) of the German Cancer Society (2)
Working Group for Medical Oncology 1. (AIO) of the German Cancer Society (3)
Working Group of Gynecological Oncology (AGO) of the German Cancer Society (4)
Working Group of Radiation Oncology (ARO) of the German Cancer Society (5)
Association of Scientific Medical Societies in Germany (AWMF) (6)
Cochrane Haematological Malignancies Group (CHMG) (7)
German Association for Endoscopy and Imaging Procedures (DGE-BV) (8)
German Society of Internal Medicine (DGIM) (9)
German Society of Nuclear Medicine (DGN) (10)
German Society of Pathology (DGP) (11)
German Society of Radiation Oncology (12)
German Society of Ultrasound in Medicine (DEGUM) (13)
German Hodgkin Study Group (GHSG) (14)
German Cancer Society (DKG) (15**)
German Cancer Aid (16)
German Leukemia and Lymphoma Aid (DLH) (17)
German Society of Radiology (DRG) (18)
Conference of Oncologic Nursing and Paedriatic Nursing Care (KOK) of the German Cancer Society (19)
Competence Network Malignant Lymphomas (KML) (20)
German Guideline Program in Oncology (GGPO) (21)
-
Participating experts
Prof. Dr. med. Gerald Antoch, Universitätsklinikum Düsseldorf, Institut für Diagnostische und Interventionelle Radiologie (18*)
PD Dr. med. Ana Barreiros, (8*), Universitätsmedizin der Johannes Gutenberg-Universität Mainz, I. Medizinischen Klinik und Poliklinik
Dr. med. Karolin Behringer, (14), Uniklinik Köln, Klinik I für Innere Medizin
Dr. med. Boris Böll, (14), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. med. Peter Borchmann (1*, 14), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. med. Andreas Buck, (10*), Universitätsklinikum Würzburg, Klinik und Poliklinik für Nuklearmedizin
Prof. Dr. med. Markus Dietlein, Uniklinik Köln, Klinik und Poliklinik für Nuklearmedizin
Prof. Dr. med. Hans-Theodor Eich, (12*), Universitätsklinikum Münster, Klinik für Strahlentherapie & Radioonkologie
Dr. med. Dennis A. Eichenauer, (14), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. med. Andreas Engert, (1*, 7, 14), Uniklinik Köln, Klinik I für Innere Medizin
Dr. Markus Follmann MPH MSc, (15, 21), Leitlinienprogramm Onkologie
Michael Fuchs, (14), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. med. Christian Goerg, (13), Deutsche Gesellschaft für Ultraschall in der Medizin
Dr. med. Teresa Halbsguth, (14), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Dr. h. c. Martin-Leo Hansmann, (11*), Universitätsklinikum Frankfurt, Senckenbergisches Institut für Pathologie
PD Dr. med. Marcus Hentrich, Städtisches Klinikum München, Klinik für Hämatologie, Onkologie und Palliativmedizin
Prof. Dr. med. Michael Herold, Deutsche Gesellschaft für Hämatologie und Onkologie e.V. (1)
Dr. med. Michael Hocke, Deutsche Gesellschaft für Endoskopie und Bildgebende Verfahren (8)
Dr. med. Ulrike Holtkamp, (17*), Deutsche Leukämie- und Lymphomhilfe
Prof. Dr. med. Jens Huober, (4*), Kantonspital St. Gallen
Dr. rer. medic. Patrick Jahn, (19*), Martin-Luther-Universtität Halle Wittenberg, Institut für Gesundheits- und - Pflegewissenschaft
Dr. med. Beate Klimm, (14), Uniklinik Köln, Klinik I für Innere Medizin
PD Dr. med. Carsten Kobe, Uniklinik Köln, Klinik und Poliklinik für Nuklearmedizin
Prof. Dr. Ina Kopp, (21, 6), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften
Dr. med. Jan Kriz, Universitätsklinikum Münster, Klinik für Strahlentherapie Universität Münster
Prof. Dr. med. Reinhard Kubale, (13*), Gemeinschaftspraxis für Radiologie und Nuklearmedizin
Dr. Horst Müller, (14), Uniklinik Köln, German Hodgkin Study Group Studienzentrale
Prof. Dr. med. Rolf-Peter Müller, (12), Uniklinik Köln, Klinik und Poliklinik für Strahlentherapie
Prof. Dr. med. Ralph Naumann, (3*, 9*), StiftungsKlinikum Mittelrhein GmbH, Klinik für Innere Medizin
Michaela Rancea, (7), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. med. Andreas Rosenwald (11), Universitätsklinikum Würzburg
PD Dr. med. Jens Ulrich Rüffer, (2*), Deutsche Fatigue Gesellschaft e.V.
Prof. Dr. med. Heinz Schmidberger, (5*), Universitätsmedizin der Johannes Gutenberg-Universität Mainz, Klinik und - Poliklinik für Radioonkologie und Strahlentherapie
Bettina Schmidtke, (7), Universitätsklinik Köln, Klinik I für Innere Medizin
Dr. med. Nicole Skoetz, (7), Universitätsklinik Köln, Klinik I für Innere Medizin
PD Dr. med. Jörg Stattaus, (18), Universitätsklinikum Essen, Klinik für Radiologie und Nuklearmedizin
Indra Thielen, (14), Universitätsklinik Köln, Klinik I für Innere Medizin
Dr. med. Bastian von Tresckow, (14), Universitätsklinik Köln, Klinik I für Innere Medizin
Diana Wongso, (14), Universitätsklinik Köln, Klinik I für Innere Medizin
Dr. med. Christoph Wyen, (14), Universitätsklinik Köln, Klinik I für Innere Medizin
The numbers in parentheses show which professional associations or organizations the experts represented.
*Delegate with voting rights in the nominal group process
**The German Cancer Society performed a moderator function in the nominal group process
The affiliations relate to the time of completion of the guideline
eTable. Recommendation grades and quality of evidence.
| Recommendation grades | ||
| Recommendation level | Description | Syntax |
| A | Strong recommendation | Strongly recommended |
| B | Recommendation | Should |
| 0 | Recommendation open | May |
| Quality of evidence | ||
| Evidence level | Definition | Symbol |
| High quality | We are very confident that the true effect lies close to that of the estimate of the effect. | + + + + |
| Moderate quality | The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. | + + + – |
| Low quality | The true effect may be substantially different from the estimate of the effect. | + + – – |
| Very low quality | The true effect is likely to be substantially different from the estimate of the effect. | + – – – |
According to a standardized methodological procedure, quality assurance stakeholders and representatives of the guideline group derived 12 quality indicators from the strong recommendations. These are recorded in national cancer registries, so the disease-specific and guideline-compliant care of adult HL patients can be evaluated.
Results
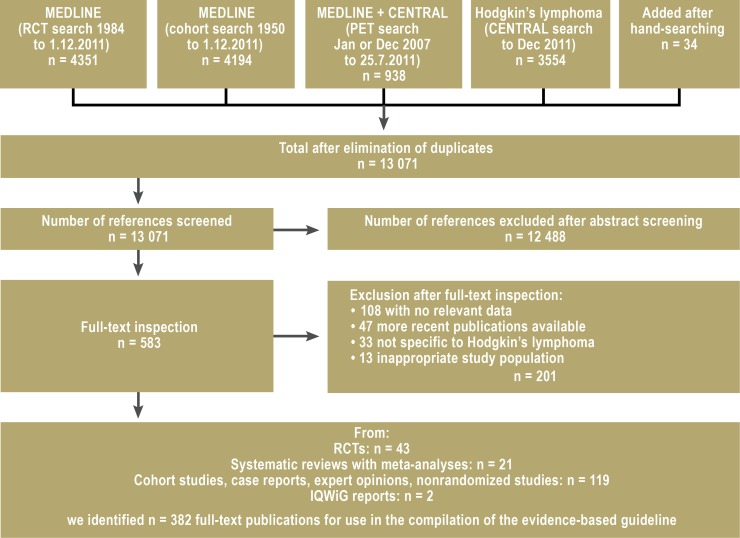
The systematic literature survey revealed 13 071 potentially relevant references, out of which 43 randomized controlled trials, 21 systematic reviews with meta-analyses, and 119 cohort studies were identified for use in answering the 32 key questions in 160 recommendations ( Figure).
Figure.
Flow diagram of literature survey
IQWiG, Institute for Quality and Efficiency in Health Care; RCT, randomized controlled trial
The long version of the guideline, the short version, and the guideline report are available (in German) at www.awmf.org and http://leitlinienprogramm-onkologie.de/Leitlinien.7.0.html. Publication of the patient guideline is planned for summer 2013.
Diagnosis/staging
It is recommended to conduct a histological diagnosis and biopsy for every swollen lymph node of unclear cause that persists longer than 4 weeks or shows distinct progression (expert consensus). It is recommended that staging is based on the Ann Arbor classification (Box), taking account of precisely defined risk factors (expert consensus). If involvement of the lymph nodes might affect the decision on the best treatment, PET-positive lymph nodes should be subjected to histological examination (expert consensus). The primary histological diagnosis should be confirmed by a reference pathologist (expert consensus).
Box. Staging according to the Ann Arbor classification.
-
Stage I
Disease confined to a single lymph node region
or
a single localized area of disease outside the lymphatic system
-
Stage II
Disease affecting two or more lymph node regions on the same side of the diaphragm
or
localized disease outside the lymphatic system and disease affecting a lymph node region, both on the same side of the diaphragm
-
Stage III
Spread to both sides of the diaphragm, with involvement of two or more lymph node regions and/or organs outside the lymphatic system
-
Stage IV
Nonlocalized, diffuse, or disseminated disease affecting one or more extralymphatic organs with or without involvement of lymphatic tissue
Suffix A: no B-symptoms present
Suffix B: B-symptoms present (temperature >38°C and/or night sweats and/or weight loss)
Participation in clinical studies
It has been shown that HL patients treated in clinical trials experienced improved progression-free survival compared to patients treated outside therapy optimizing protocols (Brilliant C, Terschueren C, Franklin J, et al.: Differences in survival rates for patients with Hodgkin lymphoma, who were treated inside versus outside therapy optimisation protocols in Germany. ASH Annual Meeting Abstracts 2007; 110 [2321]). Moreover, clinical trials lead to continuous quality improvement and progress in treatment. There is overwhelming consensus that patients should be treated within clinical trials unless they fail to meet the inclusion criteria (expert consensus).
Treatment of early stage HL
For patients with early-stage HL, combined chemotherapy and radiotherapy (combined modality treatment) is strongly recommended (recommendation grade: A), which has the potential to achieve high primary tumor control with low treatment-related toxicity (3– 5). The recommended regimen for combined modality treatment is two cycles of ABVD (doxorubicin, bleomycin, vinblastine, DTIC = dacarbazine) (recommendation grade: A) followed by involved-field radiotherapy (IF-RT) with a radiation dose of 20 Gy (5-year overall survival rate: 94%) (recommendation grade: A) (3, 4, 6). Of the patients treated with this chemotherapy regimen, 14.9% developed leukopenia and 1.7% contracted infections (World Health Organization (WHO) grade III/IV) (3). Final evaluation of the GHSG HD10 trial showed noninferiority of 20 Gy irradiation to the hitherto usual 30 Gy, with a statistically significantly lower toxicity rate (2.8% WHO grade III/IV acute toxicity) (3).
Treatment of intermediate stage HL
It is strongly recommended that patients in the intermediate stage of HL should receive a combination of chemotherapy followed by IF-RT with a total dose of 30 Gy (recommendation grade for combined therapy: A; recommendation grade for 30 Gy: B) (7, 8). On the basis of the final evaluation of the HD14 trial, which showed statistically significant superiority of two cycles of BEACOPP escalated (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) followed by two cycles of ABVD over four cycles of ABVD (5-year overall survival: 97.2% versus 96.8%), with comparable toxicity (WHO grade III/IV: thrombopenia 21.9%, leukopenia 79%, infections 7.3%, nervous system 3.2%), patients up to 60 years old in intermediate stages of HL should be treated with two cycles of BEACOPP escalated and two cycles of ABVD (recommendation grade: B) (7).
Treatment of advanced stage HL
For patients up to the age of 60 with advanced HL, treatment with six cycles of BEACOPP escalated is strongly recommended (recommendation grade: A) (9, 10). The following WHO grade IV toxicities resulted from six cycles of BEACOPP escalated: anemia 11.4%, thrombopenia 32.8%, leukopenia 79%, nervous system 0.4% (9, 10). After chemotherapy, consolidation radiotherapy of PET-positive residual mass ≥2.5 cm is strongly recommended (recommendation grade: A) (2, 11). A positive finding should be irradiated with 30 Gy (recommendation grade: A) (12). This strategy has achieved a 5-year overall survival rate of 95.3%, and none of those patients experienced any radiotherapy-related WHO grade IV toxicities.
Role of PET in decisions on treatment
For advanced stage HL, it has been established that radiotherapy of PET-negative residual mass after chemotherapy can be dispensed with (2). Ongoing randomized controlled trials have yet to yield results for the early and intermediate stages. Between the cycles of chemotherapy the individual response of advanced-stage patients may be assessed by PET (recommendation grade: 0) (13, 14). The treating physician may, however, have special reasons for requesting interim PET as an aid to therapeutic decision-making in patients who are not participating in trials, e.g., in the event of toxicity or unusual developments in individual cases.
Treatment of subgroups
Patients over 60 years of age should never be treated with BEACOPP because of its high acute toxicity (expert consensus) (15). Therefore, patients over 60 with intermediate HL should receive four cycles of ABVD followed by 30 Gy IF-RT (expert consensus). Patients over 60 with HL in the advanced stage may be treated with six to eight cycles of polychemotherapy (e.g., ABVD or PVAG [prednisone, vinblastine, adriamycin, gemcitabine]) followed by local 30-Gy radiotherapy of large residual masses (>1.5 cm) (expert consensus).
Nodular lymphocyte-predominant HL (NLPHL) accounts for around 5% of all HL. It is strongly recommended that patients with stage 1A NLPHL without clinical risk factors are treated exclusively by 30 Gy IF-RT without chemotherapy (overall survival 100% after median follow-up of 17 months) (expert consensus) (16). Patients with all other stages of NLPHL should be treated as for classic HL (expert consensus).
Treatment of recurrences
After first-line treatment, 15% to 20% of patients experience a primary progression or relapse (17). The treatment recommendations for this group of patients are as follows: For patients up to the age of 60 with relapsed HL it is strongly recommended to administer high-dose chemotherapy with autologous stem-cell transplantation (3-year rate of freedom from treatment failure: 55% with stem-cell transplantation versus 34% without stem-cell transplantation, p = 0.019) (recommendation grade: A) (18). There is currently no international standard for salvage therapy, but two cycles of intensive DHAP (dexamethasone, high-dose cytosine arabinoside [Ara-C], cisplatin) should be administered, based on an effective response rate of 89% and a relatively low complication rate of 48% WHO grade III/IV toxicity (recommendation grade: B) (19).
Before transplantation, it is strongly recommended that patients receive conditioning with BEAM (BCNU [carmustine], etoposide, Ara-C [cytarabine], melphalan) (recommendation grade: A) (18, 20). High-risk patients, especially those in whom reinduction chemotherapy has not achieved complete remission, may be treated with double transplantation, with BEAM as the first and TAM (total body irradiation, Ara-C [cytarabine], melphalan) or BAM (busulfan, Ara-C [cytarabine], melphalan) as the second conditioning scheme (recommendation grade: 0) (21). This strategy is based on evaluation of a nonrandomized study.
Myeloablative allogeneic stem-cell transplantation has shown high treatment-associated mortality coupled with unsatisfactory disease control. Therefore, it is strongly recommended not to treat patients with refractory or recurring HL with allogeneic transplantation using myeloablative conditioning (recommendation grade: A) (22). In the event of recurrence after autologous stem-cell transplantation, patients in good general health may receive reduced-dose (nonmyeloablative) conditioning followed by allogeneic stem-cell transplantation (recommendation grade: 0) (13). Alternatively, late recurrence after autologous transplantation may be treated with a second autologous transplantation (expert consensus) (23). It is strongly recommended that patients in whom no transplantation is possible receive palliative antibody therapy with brentuximab vedotin, chemotherapy, or irradiation. This applies particularly to patients with multiple recurrences (recommendation grade: A) (24). It is strongly advisable for these patients to be included in clinical trials.
There are no prospective data on the use of PET before autologous transplantation; the data that have been published are contradictory. Positive PET before a planned autologous transplantation does not justify either abandonment of the transplantation strategy or extension of the salvage therapy with sequential high-dose treatment or a switch to allogeneic transplantation (recommendation grade: B) (25). There are also no prospective data on the importance of PET after transplantation, but PET may be conducted in patients with refractory or recurring HL to determine the remission status after autologous transplantation (recommendation grade: 0) (26). The current state of knowledge does not allow conclusive assessment of the role of PET in recurrent HL; further research is urgently required.
Behavior during and after Hodgkin’s lymphoma
There is strong consensus that complementary and alternative medicine should be discussed with the patient (expert consensus). It is strongly recommended to avoid mistletoe preparations because of their immunomodulatory effect and the associated insufficient calculability of risks (expert consensus). Additionally, it is strongly recommended that patients do not smoke because of the increased risk of secondary tumors, especially bronchial carcinoma (expert consensus). The superiority of exercise for lymphoma patients, with regard to physical function, general quality of life, and fitness, was demonstrated in a randomized trial. Patients should be motivated to engage in physical exercise during and after treatment if possible (recommendation grade: B) (27).
Hodgkin’s lymphoma principally affects young patients who may still want children. The risk of treatment-related infertility thus plays a significant role for this group. It is strongly recommended to adapt fertility-preserving measures in female patients according to the stage of disease, because the treatment-related gonadotoxicity depends on the administered dose (recommendation grade: A). Administration of GnRH analogs, cryopreservation of fertilized or unfertilized ova, and the freezing of ovarian tissue may be offered as ways of protecting fertility (recommendation grade: 0) (28, 29). For male patients at any stage of HL, it is strongly recommended to provide information about cryopreservation of sperm before treatment begins. If no sperm can be harvested from the ejaculate, it is strongly recommended to extract sperm from testicular tissue (recommendation grade: A) (30).
The substances used to treat HL may have harmful effects on the development of an unborn child. It is therefore strongly recommended that women at childbearing age use effective methods (double protection with, for example, estrogen- and gestagen-containing contraceptives: “pill” and condom) of preventing pregnancy during treatment. Men should use effective means to avoid procreation (expert consensus).
Follow-up
Close monitoring for early detection of recurrence is particularly important in the first 5 years after the end of treatment, because 90% of all recurrences fall within this period. Patients in partial remission should be re-evaluated with computed tomography (CT) 3 months after final staging (expert consensus). Asymptomatic patients in complete remission with no clinical signs of recurrence should not routinely undergo CT (recommendation grade: B) (31). If the clinical or imaging findings indicate a possible recurrence, the diagnostic procedures should be the same as for a first diagnosis (expert consensus). Since the chemotherapy and radiotherapy administered for the treatment of HL can both cause long-term organ damage, the follow-up should include screening for organotoxicity. Symptoms of cardiac disease (recommendation grade: A), hypo- or hyperthyroidism (recommendation grade: A), and pulmonary disease (recommendation grade: B) should always be recorded. Studies have found an up to 7.6-fold risk of myocardial infarction. Thyroid function disorders are also frequent and may be found in up to 80% of patients, depending on study conditions, the form of treatment, and the type of test used. Pulmonary disease is detected in up to 25% of patients (32). Secondary neoplasms are a relevant morbidity factor in long-term survivors of HL. The risk of breast cancer in women treated by irradiation before the age of 30 is increased to a level higher than that in 50-year-old women in the general population (33). In view of the latency period, regular screening should begin 8 years after primary treatment (expert consensus). It should be noted that no long-term data on the outcome of the current treatment regimens are yet available.
Conclusion
This evidence-based guideline compiles, for the first time, evidence- and consensus-based operational recommendations for the diagnosis, treatment, and follow-up of Hodgkin’s lymphoma in adults.
The intention of the published guideline is to ensure that all patients are diagnosed and treated in timely fashion according to the very latest research findings, particularly those who are not taking part in clinical trials. With the aid of these freely available treatment recommendations all patients can receive optimized, individually adapted care.
One limitation of the guideline is that high-quality randomized controlled trials and meta-analyses could not be identified for all key questions of this guideline. Particularly for some critical issues such as the additional benefit of PET, advice on how patients should behave during and after treatment, and questions regarding follow-up, recommendations were formulated on the basis of nonrandomized trials and expert opinions. These aspects will be reanalyzed in the course of the planned update of the guideline in 2 years, in the hope that more high-quality evidence will have emerged in the meantime.
Glossary.
-
Allogeneic stem-cell transplantation
Stem-cell transplantation from a (related or unrelated) donor
-
Autologous stem-cell transplantation
Transplantation of the patient’s own stem cells
-
Reduced-dose conditioning
In reduced-dose/nonmyeloablative conditioning, reduced-toxicity treatment (chemotherapy, possibly combined with radiotherapy) is given before stem-cell transplantation
-
Escalated
Intensified form of conventional treatment (e.g., BEACOPP escalated: BEACOPP with higher doses than usual)
-
GRADE system
A classification enabling standardized assessment of the quality of a body of evidence which takes into account, among other factors, the clinical relevance of the outcome parameter, the extent of the effect, and the transferability of the study results to the target group of patients and the German health care system
-
Involved-field radiotherapy
Irradiation of the involved lymph nodes
-
Consolidating radiotherapy
Complementary radiotherapy after chemotherapy
-
Cryopreservation
Storage of fertilized or unfertilized cells in liquid nitrogen
-
Myeloablative conditioning
Destruction of the recipient’s hematopoietic stem cells by means of chemotherapy and/or irradiation (before allogeneic stem-cell transplantation)
-
PET (positron emission tomography)
A nuclear medicine imaging procedure used to help decide whether and how to modify treatment
-
Remission
Response to therapy
-
Salvage therapy
Renewed intensive treatment with curative intent in patients with tumor recurrence
Acknowledgments
Translated from the original German by David Roseveare.
The guideline process was funded and methodologically supported by the German Guideline Program in Oncology (grant No: 109230)—a joint initiative by the Association of Scientific Medical Societies in Germany, the German Cancer Society, and the German Cancer Aid.
Footnotes
Conflict of interest statement
Prof. Engert is a member of the advisory boards of Millennium, Takeda, and Seattle Genetics. He has received research funding from Millennium, Takeda, Roche, and Amgen. From Takeda and Millennium he has received honoraria for acting as a reviewer on the topic covered in this guideline, reimbursement of congress participation fees and travel and accommodation costs, and honoraria for preparation of scientific educational events. Moreover, he has received fees for conducting commissioned clinical studies from Millennium and Takeda and funding from Millennium and Novartis for a research project of his own devising.
Dr. von Tresckow has received honoraria for acting as a consultant from Novartis Pharma GmbH. From Takeda Pharma he has received reimbursement of congress participation fees, travel, and accommodation costs and honoraria for preparation of scientific educational events.
Ms Rancea, Dr. Halbsguth, Dr. Behringer, and Dr. Skoetz declare that no conflict of interest exists.
References
- 1.Diehl V, Thomas RK, Re D. Part II: Hodgkin’s lymphoma—diagnosis and treatment. Lancet Oncol. 2004;5:19–26. doi: 10.1016/s1470-2045(03)01320-2. [DOI] [PubMed] [Google Scholar]
- 2.Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379:1791–1799. doi: 10.1016/S0140-6736(11)61940-5. [DOI] [PubMed] [Google Scholar]
- 3.Engert A, Plutschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363:640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 4.Engert A, Franklin J, Eich HT, et al. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended-field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin’s lymphoma: final results of the GHSG HD7 trial. J Clin Oncol. 2007;25:3495–3502. doi: 10.1200/JCO.2006.07.0482. [DOI] [PubMed] [Google Scholar]
- 5.Noordijk EM, Carde P, Dupouy N, et al. Combined-modality therapy for clinical stage I or II Hodgkin’s lymphoma: long-term results of the European Organisation for Research and Treatment of Cancer H7 randomized controlled trials. J Clin Oncol. 2006;24:3128–3135. doi: 10.1200/JCO.2005.05.2746. [DOI] [PubMed] [Google Scholar]
- 6.Ferme C, Eghbali H, Meerwaldt JH, et al. Chemotherapy plus involved-field radiation in early-stage Hodgkin’s disease. N Engl J Med. 2007;357:1916–1927. doi: 10.1056/NEJMoa064601. [DOI] [PubMed] [Google Scholar]
- 7.von Tresckow B, Plutschow A, Fuchs M, et al. Dose-Intensification in Early Unfavorable Hodgkin’s Lymphoma: Final Analysis of the German Hodgkin Study Group HD14 Trial. J Clin Oncol. 2012;30 M:907–913. doi: 10.1200/JCO.2011.38.5807. [DOI] [PubMed] [Google Scholar]
- 8.Eich HT, Diehl V, Gorgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28:4199–4206. doi: 10.1200/JCO.2010.29.8018. [DOI] [PubMed] [Google Scholar]
- 9.Bauer K, Skoetz N, Monsef I, Engert A, Brillant C. Comparison of chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for patients with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database Syst Rev. 2011;8 doi: 10.1002/14651858.CD007941.pub2. CD007941. [DOI] [PubMed] [Google Scholar]
- 10.Engert A, Haverkamp H, Kobe C, et al. Reduced intensity of chemotherapy and PET-giuded radiotherapy in patients with advanced stage Hodgkin lymphoma: an open-label, randomised phase 3 trial. Lancet. 2012;379:1791–1799. doi: 10.1016/S0140-6736(11)61940-5. [DOI] [PubMed] [Google Scholar]
- 11.Kobe C, Dietlein M, Franklin J, et al. Positron emission tomography has a high negative predictive value for progression or early relapse for patients with residual disease after first-line chemotherapy in advanced-stage Hodgkin lymphoma. Blood. 2008;112:3989–3994. doi: 10.1182/blood-2008-06-155820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borchmann P, Haverkamp H, Diehl V, et al. Eight Cycles of Escalated-Dose BEACOPP Compared With Four Cycles of Escalated-Dose BEACOPP Followed by Four Cycles of Baseline-Dose BEACOPP With or Without Radiotherapy in Patients With Advanced-Stage Hodgkin’s Lymphoma: Final Analysis of the HD12 Trial of the German Hodgkin Study Group. J Clin Oncol. 2011;29:4234–4242. doi: 10.1200/JCO.2010.33.9549. [DOI] [PubMed] [Google Scholar]
- 13.IQWiG. Institute for Quality + Efficiency in Health Care. 2009. Positronen-Emissions-Tomographie (PET) bei malignen Lymphomen. [Google Scholar]
- 14.Terasawa T, Nagai H. Current clinical evidence on interim fluorine-18 fluorodeoxy glucose positron emission tomography for advanced-stage Hodgkin lymphoma and diffuse large B-cell lymphoma to predict treatment outcomes. Leuk Lymphoma. 2009;50:1750–1752. doi: 10.3109/10428190903308080. [DOI] [PubMed] [Google Scholar]
- 15.Engert A, Ballova V, Haverkamp H, et al. Hodgkin’s lymphoma in elderly patients: a comprehensive retrospective analysis from the German Hodgkin’s Study Group. J Clin Oncol. 2005;23:5052–5060. doi: 10.1200/JCO.2005.11.080. [DOI] [PubMed] [Google Scholar]
- 16.Nogová L, Reineke T, Eich HT, et al. Extended field radiotherapy, combined modality treatment or involved field radiotherapy for patients with stage IA lymphocyte-predominant Hodgkin’s lymphoma: a retrospective analysis from the German Hodgkin Study Group (GHSG) Ann Oncol. 2005;16:1683–1687. doi: 10.1093/annonc/mdi323. [DOI] [PubMed] [Google Scholar]
- 17.von Tresckow B, Engert A. The role of autologous transplantation in Hodgkin lymphoma. Curr Hematol Malig Rep. 2011;6:172–179. doi: 10.1007/s11899-011-0091-0. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359:2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 19.Josting A, Rudolph C, Reiser M, et al. Time-intensified dexamethasone/cisplatin/cytarabine: an effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin’s disease. Ann Oncol. 2002;13:1628–1635. doi: 10.1093/annonc/mdf221. [DOI] [PubMed] [Google Scholar]
- 20.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–1054. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 21.Morschhauser F, Brice P, Ferme C, et al. Risk-adapted salvage treatment with single or tandem autologous stem-cell transplantation for first relapse/refractory Hodgkin’s lymphoma: results of the prospective multicenter H96 trial by the GELA/SFGM study group. J Clin Oncol. 2008;26:5980–5987. doi: 10.1200/JCO.2007.15.5887. [DOI] [PubMed] [Google Scholar]
- 22.Akpek G, Ambinder RF, Piantadosi S, et al. Long-term results of blood and marrow transplantation for Hodgkin’s lymphoma. J Clin Oncol. 2001;19:4314–4321. doi: 10.1200/JCO.2001.19.23.4314. [DOI] [PubMed] [Google Scholar]
- 23.Smith SM, van Besien K, Carreras J, et al. Second autologous stem cell transplantation for relapsed lymphoma after a prior autologous transplant. Biol Blood Marrow Transplant. 2008;14:904–912. doi: 10.1016/j.bbmt.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruffer JU, Ballova V, Glossmann J, et al. BEACOPP and COPP/ABVD as salvage treatment after primary extended field radiation therapy of early stage Hodgkins disease - results of the German Hodgkin Study Group. Leuk Lymphoma. 2005;46:1561–1567. doi: 10.1080/10428190500178167. [DOI] [PubMed] [Google Scholar]
- 25.Moskowitz AJ, Yahalom J, Kewalramani T, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. 2010;116:4934–4937. doi: 10.1182/blood-2010-05-282756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer J, Goggins T, Broadwater G, et al. Early post transplant (F-18) 2-fluoro-2-deoxyglucose positron emission tomography does not predict outcome for patients undergoing auto-SCT in non-Hodgkin and Hodgkin lymphoma. Bone Marrow Transplant. 2011;46:847–851. doi: 10.1038/bmt.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Courneya KS, Sellar CM, Stevinson C, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27:4605–4612. doi: 10.1200/JCO.2008.20.0634. [DOI] [PubMed] [Google Scholar]
- 28.Muller A, Keller K, Wacker J, et al. Retransplantation of cryopreserved ovarian tissue: the first live birth in Germany. Dtsch Arztebl Int. 2012;109(1-2):8–13. doi: 10.3238/arztebl.2012.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Wolff M, Dian D. Fertility preservation in women with malignant tumors and gonadotoxic treatments. Dtsch Arztebl Int. 2012;109(12):220–226. doi: 10.3238/arztebl.2012.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsiao W, Stahl PJ, Osterberg EC, et al. Successful treatment of postchemotherapy azoospermia with microsurgical testicular sperm extraction: the Weill Cornell experience. J Clin Oncol. 2011;29:1607–1611. doi: 10.1200/JCO.2010.33.7808. [DOI] [PubMed] [Google Scholar]
- 31.Dryver ET, Jernstrom H, Tompkins K, Buckstein R, Imrie KR. Follow-up of patients with Hodgkin’s disease following curative treatment: the routine CT scan is of little value. Br J Cancer. 2003;89:482–486. doi: 10.1038/sj.bjc.6601052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng AK, Li S, Neuberg D, et al. A prospective study of pulmonary function in Hodgkin’s lymphoma patients. Ann Oncol. 2008;19:1754–1758. doi: 10.1093/annonc/mdn284. [DOI] [PubMed] [Google Scholar]
- 33.Hodgson DC, Koh ES, Tran TH, et al. Individualized estimates of second cancer risks after contemporary radiation therapy for Hodgkin lymphoma. Cancer. 2007;110:2576–2586. doi: 10.1002/cncr.23081. [DOI] [PubMed] [Google Scholar]