Abstract
The characterization of human stem cells for the usability in regenerative medicine is particularly based on investigations regarding their differentiation potential in vivo. In this regard, the chicken embryo model represents an ideal model organism. However, the access to the chicken embryo is only achievable by windowing the eggshell resulting in limited visibility and accessibility in subsequent experiments. On the contrary, ex ovo-culture systems avoid such negative side effects. Here, we present an improved ex ovo-cultivation method enabling the embryos to survive 13 days in vitro. Optimized cultivation of chicken embryos resulted in a normal development regarding their size and weight. Our ex ovo-approach closely resembles the development of chicken embryos in ovo, as demonstrated by properly developed nervous system, bones, and cartilage at expected time points. Finally, we investigated the usability of our method for trans-species transplantation of adult stem cells by injecting human neural crest-derived stem cells into late Hamburger and Hamilton stages (HH26–HH28/E5—E6) of ex ovo-incubated embryos. We demonstrated the integration of human cells allowing experimentally easy investigation of the differentiation potential in the proper developmental context. Taken together, this ex ovo-method supports the prolonged cultivation of properly developing chicken embryos enabling integration studies of xenografted mammalian stem cells at late developmental stages.
1. Introduction
The chicken is a well-studied and cost-efficient model organism profiting from a great potential of in vivo manipulation techniques. As early as the 5th century B.C. Hippocrates and later on in the 4th century B.C. Aristotle studied embryonic development using chicken embryos. More than 2000 years later, in 1951, Hamburger and Hamilton classified the developmental stages of the chicken embryo in 46 HH stages [1] allowing temporally defined manipulations in developing embryos.
Using this kind of age-classification several in ovo experiments such as investigations on neural crest cells (NCCs) and their migratory behavior in the avian embryos were performed [2]. In this regard, stem cells obtained from different animals or even of human origin can be characterized for their potential neural crest ancestry. In a recent study, we transplanted human inferior turbinate stem cells (ITSCs) into early chicken embryos (HH15–HH18) [3]. The injected ITSCs migrated laterally forming chains, a characteristic hallmark of neural crest cells. In other studies by Soundararajan et al. and Son et al., motor neurons derived from embryonic stem cells as well as induced motor neurons reprogrammed from mouse and human fibroblasts were shown to integrate after transplantation into the chicken neural tube [4, 5].
For the investigation of developing chicken embryos, Auerbach and coworkers designed a method allowing long-term cultivation of chicken embryos in an ex ovo-setup [6]. In 1989, the containment for ex ovo-cultivation was improved concerning the short-term survival using a plastic cup covering the developing embryo with a petri dish [7]. This method permits easy access to the embryo as well as to the blood vessels of the chorioallantoic membrane (CAM). Besides the observation of the development, ex ovo-cultivated chicken embryos can be used for the investigation of toxicity of different substances in a vertebrate model. In this context, a shell-less cultivation method was used to observe the influence of nicotine and cigarette smoke in developing chicken embryos [8]. In addition, the effects of acute glucose toxicity could be assessed in shell-less chicken embryo cultures [9].
Recently, Yalcin and colleagues described an ex ovo-cultivation method of chicken embryos, which is suitable for microsurgical and imaging applications [10]. However, eggs were incubated for 72 hours previous to the transfer into the ex ovo-setup, and if cultivated beyond embryonic day (E) 7, crushed eggshell was added to achieve HH38, correlating with E12.
Here, we describe an inexpensive reusable shell-less cultivation method for chicken embryos in a broadly available containment. Using a defined amount of water and ground eggshell, we demonstrate for the first time the survival of ex ovo-cultivated embryos for at least 13 days up to E15 and HH stage 41. Moreover, in contrast to methods previously described, the herein presented method supports xenografts into late stages of ex ovo-cultivated chicken embryos.
2. Results
2.1. Ex Ovo-Cultivated Chicken Embryos Reveal Normal Morphological Development for 13 Days In Vitro
For the herein described ex ovo-cultivation method, egg contents of chicken eggs preincubated for 48 hours were gently transferred into a readily prepared shell-less containment, as demonstrated schematically in Figure 1. Development and morphology were compared to “normal stages of the chicken embryo” described by Hamburger and Hamilton [1]. As shown in Figure 2, embryos were easily detectable at E4 revealing normal development. During incubation, the yolk expanded on the support film of the ex ovo-containment, and the blood vessels started to span smoothly over the yolk. Furthermore, continuous observation showed normal morphological development of embryos up to E15, correlating with HH stage 41.
Figure 1.
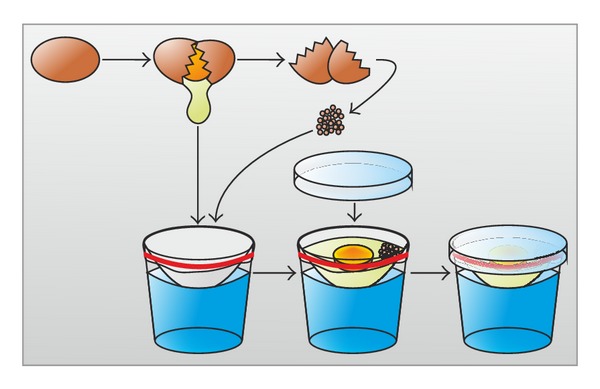
Schematic view of initial steps in ex ovo-cultivation of chicken embryos. Prior to transfer, chicken embryos were incubated at 37.8°C for 48 hours. Eggs were gently opened using a jigsaw. The indentation was expanded, and the contents were carefully transferred onto the support film. Ground eggshell of several eggs was added to the albumin and the experimental setup was covered using a bacterial dish.
Figure 2.
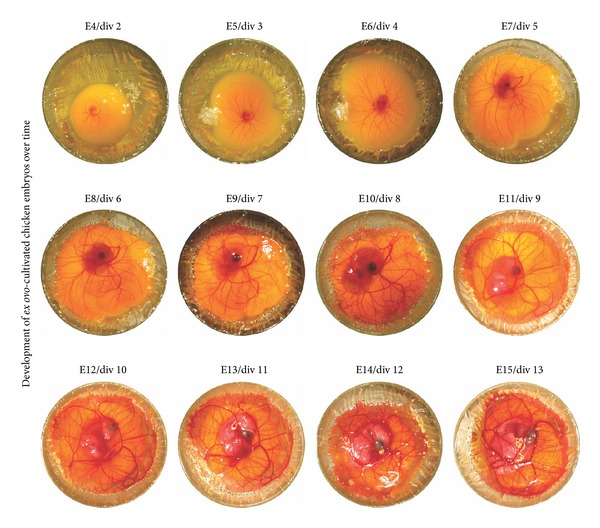
Ex ovo-cultivated chicken embryos show normal development over time. Starting with an easily visible chicken embryo at E4, morphological changes in the development of ex ovo-cultivated embryos are distinguishable. After the start of the ex ovo-cultivation, the embryo develops normally until E15 up to HH stage 41. The set of photos consist of a number of 4 different chicken embryos.
2.2. Ex Ovo-Cultivated Chicken Embryos Show Improved Survival without Significant Differences in Size and Weight
To investigate the size and weight, in ovo- and ex ovo-cultivated developing chicken embryos were sacrificed and compared at E10 as well as at E13 and E15 (Figure 3). Here, no significant differences in size were detectable between shell-less and traditionally cultivated chicken embryos. In addition, the embryos showed no significant differences in weight at E13 and E15 when incubated in a shell-less containment. We estimated the survival rate for embryos cultivated for 13 days in vitro (4 cohorts, 11 embryos each). Starting with easily visible chicken embryos, the survival rate was measured starting at E4 of ex ovo-cultivation. The addition of cell culture medium did not enhance the survival rate of chicken embryos (data not shown), which is contrary to the observations made by Auerbach and coworkers [6]. Importantly, cultivated in a humidified incubator at 37.8°C, more than 18% of the embryos were able to survive until E15.
Figure 3.
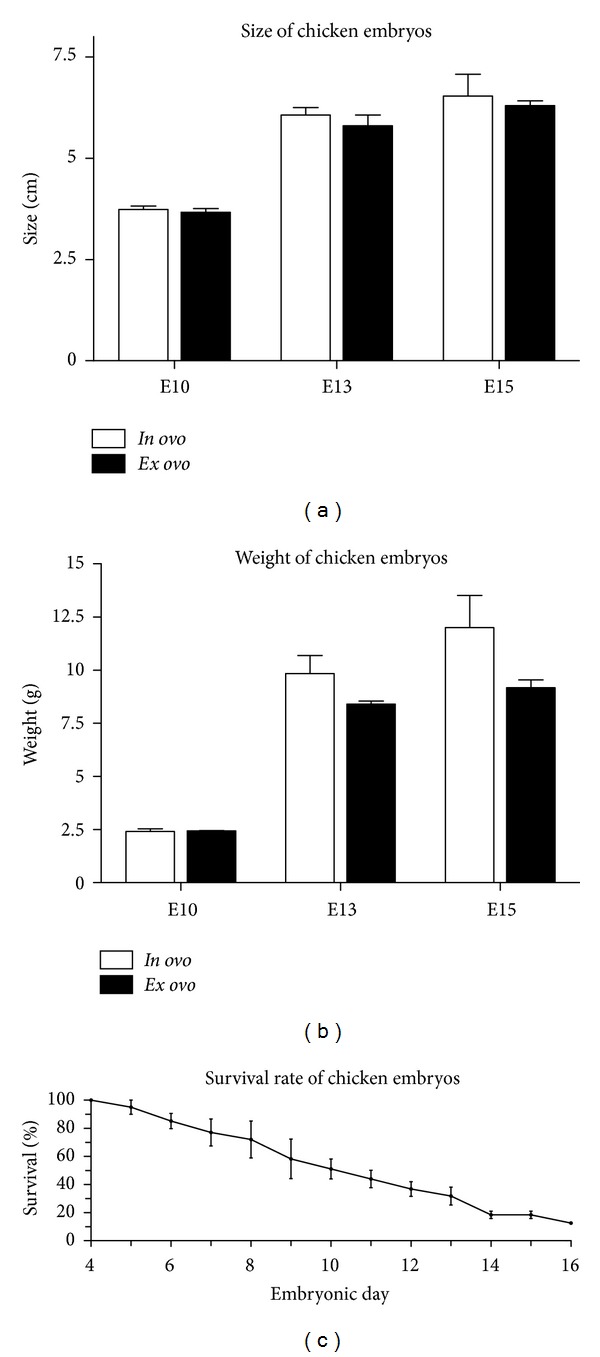
Statistical analysis of ex ovo-cultivated chicken embryos. (a) Comparison of size of in ovo- and ex ovo-cultivated chicken embryos on days E10, E13, and E15. No significant differences in size were detectable between shell-less and traditionally cultivated embryos. Error bar indicates standard error of the mean (SEM), n = 3. (b) In ovo- and ex ovo- cultivated chicken embryos were investigated regarding their size on days E10, E13, and E15 of incubation. Shell-less cultivated embryos revealed slightly decreased weight at E15 of incubation. Error bar indicates SEM, n = 3. (c) Survival rate of ex ovo-cultivated chicken embryos over time. After 48 hours of traditional incubation, egg contents were transferred into a shell-less containment and incubated for at least 13 days in vitro. Starting with easily visible embryos at E4 (4 independent cohorts, 11 embryos each), survival rate was determined for ex ovo-cultivated chicken embryos up to E16, by visual inspection of vital signs (heartbeat and movement) each day. Dead embryos were removed from the incubator. At E16 one living embryo was observed.
2.3. Chondrogenesis, Osteogenesis, and Myelination of Nerves Were Not Impaired by Ex Ovo-Cultivation
Development of in ovo- and ex ovo-cultivated chicken embryos was compared at given time points regarding chondrogenesis, osteogenesis, and myelination of the optic nerves. At E5, the vertebrae of chicken embryos started to undergo chondrification [11]. In contrast to E5 embryos, which did not show specific staining for cartilage, E10 chicken embryos were positive for Alcian blue staining suggesting that cartilage and bone tissue of the chicken embryo started to chondrify at this point of time (Figure 4). At E13 and E15 of development, no differences in chondrification between in ovo- and ex ovo-cultivated chicken embryos were observed. Regarding osteogenesis, chicken embryos at E5 showed no specific staining as expected (Figure 4). At E10, the wings, skull, and ribs began to ossify. However, in the respective tissues no specific staining for bone was observed in any of the analyzed chicken embryos. In contrast, at E13, ex ovo- and in ovo-cultivated chicken embryos showed distinct staining for bone at comparable amounts. Up to E15, ex ovo-cultivated embryos showed normal osteogenesis in comparison to in ovo-cultivated chicken embryos.
Figure 4.
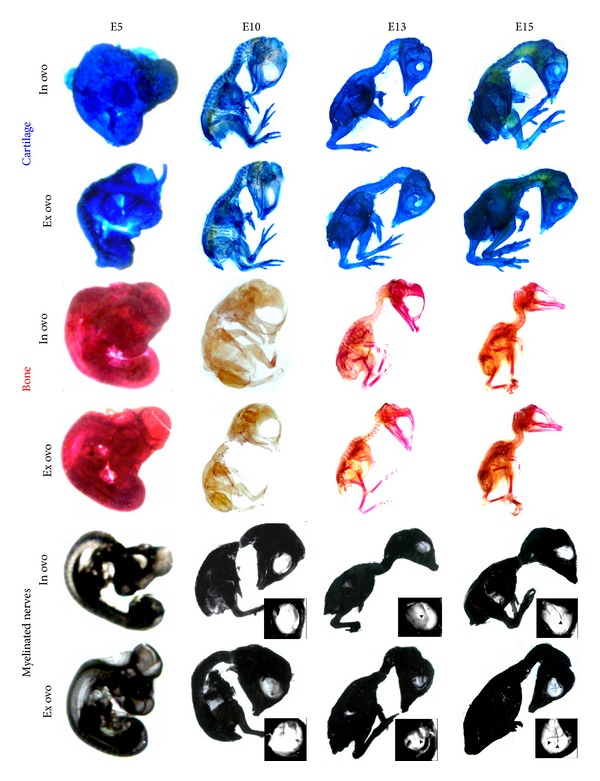
Histochemical stainings of cartilage, bone, and myelinated nerves of chicken embryos revealed normal chondrogenesis, osteogenesis, and myelination of the nerves. Chicken embryos cultivated either in ovo or ex ovo were sacrificed at E5, E10, E13, and E15 and subsequently fixed using 4% PFA. Afterwards, specimen was skinned and eviscerated followed by staining at 37°C overnight. Destaining in a graded series of ethanol was followed by clearing in 1 : 2 BABB/ethanol, 2 : 1 BABB/ethanol, and 100% BABB (1 : 2 benzyl alcohol/benzyl benzoate). Upper panel: 0.3% Alcian Blue staining solution in 70% ethanol and addition of 5% acetic acid for cartilage at E5, E10, E13, and E15 of in ovo- and ex ovo-cultivated chicken embryos. Middle panel: comparison of osteogenesis at E5, E10, E13, and E15 using 0.1% Alizarin Red S staining in 95% ethanol. Lower panel: staining of lipids using 5% saturated Sudan Black B in 70% ethanol indicates myelination of the optic nerves in E13 and E15. Arrowheads show the ending of optic nerves in the blowup.
We applied Sudan Black B to specifically stain lipid-rich myelinated nerves within ex ovo-cultivated chicken embryos [12–14]. Focusing on the myelination of the optic nerves the orbital cavity was investigated at E10, E13, and E15. Chicken embryos at E10 did not show specific staining for myelinated optic nerves. However, in E13, chicken embryos staining of the optic nerve could be observed (Figure 4). A more distinct staining of the optic nerve at E15 indicated advanced myelination.
2.4. Xenografted ITSCs Integrate in the Basal Layer of the Epidermis of Ex Ovo-Cultivated Chicken Embryos
For xenografts into developing chicken embryos, ITSCs were virally transduced using lentivirus harboring the lacZ-gene leading to a deep blue color of the cell nuclei after β-galactosidase staining. Using the here-described ex ovo-cultivation method, labeled ITSCs were injected into developing chicken embryos as late as HH stages 26 to 28, correlating with E5 to E6. An adequate time span of up to 4 days allowed proper integration and differentiation of xenografted adult human stem cells. Subsequently, manipulated chicken embryos were sacrificed followed by fixation and staining for lacZ-positive ITSCs. Tissue containing lacZ-positive stem cells was sectioned and stained using specific antibodies. LacZ-positive ITSCs injected into lesioned developing chicken embryos remained positive for the neural crest stem cell-marker nestin after 4 days, as demonstrated in Figure 5. Furthermore, xenografted ITSCs showed expression of the ectodermal marker β-III-tubulin suggesting partial phenotypic switch towards ectodermal lineage in vivo. Interestingly, transplanted ITSCs also showed expression of the basal cell-marker cytokeratin 14 (CK14) pointing towards a basal cell-like differentiation. This observation was underlined by bright field microscopy showing integration of ITSCs into the basal cell layer of the epidermis.
Figure 5.
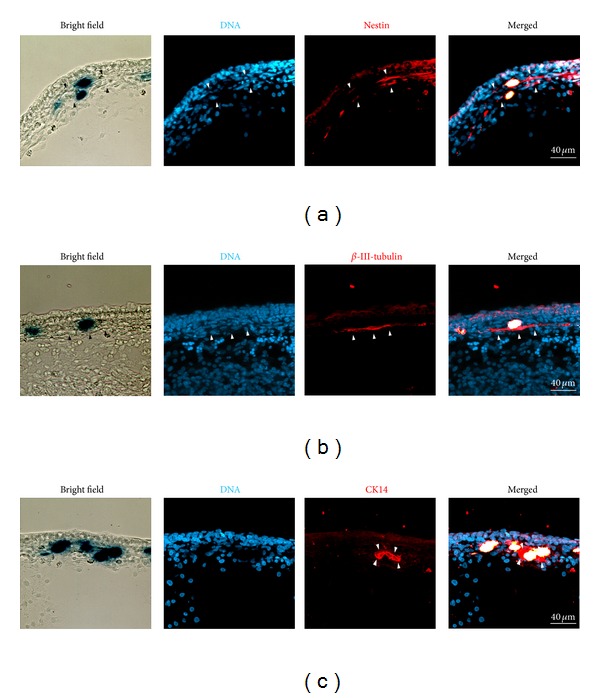
Immunohistochemical analysis of xenografted ITSCs. (a) Cryosections of embryonic chicken tissue harboring transplanted, human lacZ-positive cells. Xenografted integrated ITSCs revealed typical nestin expression (arrowheads). (b) Integrated ITSCs were positive for β-III-tubulin (arrowheads). (c) Integration of ITSCs in the basal layer of the epidermis was underlined by expression of CK14 (arrowheads) of xenografted cells. Transplantation experiments were performed as quadruplicate with consistent results. Representative results are shown.
Taken together, virally transduced ITSCs expressing the lacZ-gene were able to integrate into late stages of the developing chicken embryos after xenografting in ex ovo-cultivated chicken embryos.
3. Discussion
The herein described ex ovo-cultivation system allows survival of chicken embryos for up to embryonic day 15 and microsurgical transplantation of human NCSCs into the developing embryo at late stages (E5-7). Cultivated chicken embryos showed normal development, as demonstrated by proper osteogenesis, chondrogenesis, and myelination of nerves, as well as no significant differences to the in ovo-approach regarding their size and weight.
Its cost efficiency make the chicken embryo ideal for investigation and manipulation of development processes using variety of experimental methods. However, most of the cultivation methods deal only with early stages of development since in ovo-experiments of late developmental stages are restricted by the necessity of windowing the shell as well as by strong vascularization and presence of membranes [15].
To investigate late developmental stages of chicken embryos, the shell-less or ex ovo-cultivation was established and subsequently improved regarding the short-term survival of chicken embryos [6, 7]. In 1999, Brooks and coworkers studied angiogenesis in 10-days-old chicken embryos by using shell-less culture systems [16]. Moreover, shell-less chicken embryo cultures were used to investigate functional importance of N-cadherin in the developing chicken limb by application of monoclonal N-cadherin-specific antibodies [17].
Nevertheless, these studies described ex ovo-cultivation at early stages of developing chicken embryos, particularly, at age prior to or at day 3 of incubation. Hamamichi and Nishigori as well as Datar and Bhonde used late stages of ex ovo-cultivated chicken embryonic development to examine the influence of nicotine in E7 embryos and effects of acute glucose toxicity, respectively [8, 9]. Recently, Leong and coworkers applied a chorioallantoic membrane (CAM) assay to investigate cell migration and metastatic growth of cancer cells in ex ovo-cultivated chicken embryo systems [18]. However, none of the studies applied microsurgical applications to the chicken embryo itself. Although Dhole and colleagues already reported an injection method into the vitreous of the eye of late stage ex ovo-cultivated chicken, the authors did not investigate behavior and survival of injected cells [19]. Apart from this, the survival rate of chicken embryos was postulated to be over 50% after transfer, but no time-dependent statistics were made. More recently, Yalcin and colleagues presented an ex ovo-culture system allowing the cultivation of chicken embryos up to HH stage 38, correlating with E12 [10]. Despite using microsurgical methods, those were only applied to early stages of developing chicken embryos without statistically analyzing the survival.
Extending these promising findings, the herein described ex ovo-cultivation method allows cultivation of chicken embryos starting at 48 hours (E2) of incubation up to E15 correlating with HH stage 41 and beyond. Interestingly, the survival rate of chicken embryos decreases over time below 18.4% for E15 embryos indicating absence of important factors for embryonic development in ex ovo-cultures. Remarkably, only one embryo survived the ex ovo-incubation until E16 indicating that E15 represents the latest possible time point of investigation.
We further describe for the first time the utilization of microsurgical applications for experiments on late stages of ex ovo-cultivated chicken embryos at E5 and later. In this context, lacZ-positive ITSCs were stained for the ITSC-marker nestin after transplantation and integration into developing chicken embryos. This observation is consistent with expression in their endogenous niche of the human inferior turbinate [3, 20].
Although early stages of developing chicken embryos are used for the characterization of stem cells such as in case of chain migration of NCSCs, differentiated tissues of chicken embryos cultivated for a prolonged time more closely resemble late stages of development of an organism as in case of differentiated tissue of limbs. Therefore, information about the differentiation potential in already developed tissue such as bones, cartilage, the nervous system, or skin can be obtained.
Taken together, we describe an ex ovo-cultivation method suitable for long-term cultivation and investigation of chicken embryos. In a developmental context, normal growth was shown with regard to weight and size as well as cartilage, bone, and myelinated nerves of embryos. Moreover, the injection of human neural crest-derived ITSCs was performed to investigate the usability of the herein-described ex ovo-method with respect to microsurgical applications. Here, our method allows the use of late developmental stages of ex ovo-cultivated chicken embryos for microsurgical transplantation of stem cell xenografts.
4. Experimental Procedures
4.1. Materials
-
Reagents:
-
(1)distilled water,
-
(2)70% ethanol,
-
(3)ground eggshell.
-
(1)
-
Chicken eggs
-
fertilized nonincubated chicken eggs were obtained from a local supplier (Brüterei “Brormann”, Rheda-Wiedenbrück, Germany).
-
4.1.1. Equipment
-
Ex ovo-cultivation:
-
(1)glasses (GODIS, Art-nr: 800.921.09, IKEA, 7 3/4 oz. 23 cl),
-
(2)cling film,
-
(3)elastic bands,
-
(4)scalpel or razor blade,
-
(5)sterile bacterial petri dishes,
-
(6)incubator.
-
(1)
4.2. Methods
4.2.1. Precultivation-Steps
Incubate fertilized chicken eggs for 48 h at 37.8°C prior to ex ovo-cultivation. Caution: see Note 1.
Warm a humidified incubator to 37.8°C. Tip: see Note 2.
Use ground eggshell as a source of calcium nutrition for the chicken embryo to efficiently prolong the survival of embryos. Caution: see Note 3 tip: see Note 4.
-
Prepare surrogate shell as follows:
- (1)
-
(2)Preparation of support film: place a quadratic piece of cling film on top of the glass. Carefully lower the film manually until an area of 4 cm-5 cm in diameter is in contact with the surface of the water. Caution: see Note 7.
-
(3)Fix the support film with an elastic band on the glass.
-
(4)Cut off excess cling film with a scalpel or razor blade.
-
(5)Place one side of a sterile bacterial petri dish as a lid on top of the glass. Critical: see Note 8.
-
(6)Add up to 5 mL cell culture medium such as DMEM high glucose with and without supplementation with penicillin and streptomycin (P/S) (5 mL/50 mg; PAA, Pasching, Austria), amphotericin B (amphoB) (5 mL/1.25 mg; PAA), L-glutamin (L-glu) (200 mM; Sigma-Aldrich), and 10% of fetal calf serum (FCS). Caution: see Note 9.
4.2.2. Transfer of Egg Contents and Ex Ovo-Culture
Sterilize eggshells with 70% ethanol and wipe with a paper towel.
Gently open eggs incubated for 48 h at 37.8°C laterally using a jigsaw (Figure 1). Caution: see Note 10.
Saw until a dent puncturing the eggshell appears. Tip: see Note 11.
Widen the dent this way up to 5 cm-6 cm laterally. Critical: see Note 12.
Place thumbs besides the dent and turn the egg dent-side down. Gently pull the two pieces of eggshell apart at the dent. Critical: see Note 13.
Let the egg contents gently flow onto the support film. Tip: see Note 14.
Carefully add about 1 g ground eggshell to besides the embryo. Caution: see Note 15
Cultivate chicken embryos within the surrogate shell and the bacterial dish on top at 37.8°C in a humidified incubator.
4.3. Notes
Note 1 —
Set a humidified incubator at temperatures between 37°C and 38°C; incubation of chicken eggs should not exceed 48 hours.
Note 2 —
Use autoclaved water containing 1 mM CuSO4 to prevent contamination.
Note 3 —
Sterilize the exterior of the eggshell with 70% ethanol and grind eggshell pieces to a fine powder.
Note 4 —
Prepare sufficient amounts of shell from several eggs at once and store remaining ground eggshell at −20°C for further setups.
Note 5 —
Autoclave glasses prior to use to prevent contamination.
Note 6 —
Use a defined amount of water; too much water may result in leakage of albumin; an insufficient amount of water may result in drop-related damage of the yolk as well as the embryo.
Note 7 —
Use sterile gloves to prevent contamination.
Note 8 —
Spray the support film with 70% ethanol for sterilization before placing the bacterial petri dish on top. Allow the ethanol to evaporate or remove it manually with a sterile paper towel prior to transfer of the egg contents.
Note 9 —
Addition of amphoB to the medium might result in decreased neurogenesis of chicken embryos.
Note 10 —
Sterilize jigsaw prior to use with 70% ethanol.
Note 11 —
Do not exert too much pressure on the shell. Simply slide the jigsaw back and forth until a dent appears.
Note 12 —
Avoid the leaking of egg white.
Note 13 —
While gently pulling the eggshells apart, hold the egg closely over the support film to avoid damage to the yolk and embryo.
Note 14 —
If the embryo is not located on top of the yolk, it will move there autonomously within 24 hours.
Note 15 —
Do not drop ground eggshell directly on the chicken embryo.
4.4. Variations
To achieve a prolonged survival Auerbach additives may be applied as follows:
add 5–10 mL tissue culture medium to the surrogate shell,
add 100–200 units/mL of gentamicin and mycostatin to the medium,
incubate chicken embryos in an incubator with 1%-2% CO2.
4.4.1. Addition of Ground Eggshell at E10
-
At E10, the yolk and blood vessels are fully spread in the surrogate shell/shell-less containment, though addition of ground eggshell on the CAM might provide better accessibility to the supplement.
Conflict of Interests
The authors declare no potential conflict of interests.
Acknowledgments
The excellent technical help of Angela Krahlemann-Köhler is gratefully acknowledged. This study was supported by the University of Bielefeld and by a Grant of the German Ministry of Research and Education (BMBF, Grant: 01GN1006A). The authors acknowledge the support for the Article Processing Charge by the Deutsche Forschungsgemeinschaft (DFG) and the Open Access Publication Funds of Bielefeld University Library.
References
- 1.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Developmental Dynamics. 1992;195(4):231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- 2.Krispin S, Nitzan E, Kassem Y, Kalcheim C. Evidence for a dynamic spatiotemporal fate map and early fate restrictions of premigratory avian neural crest. Development. 2010;137(4):585–595. doi: 10.1242/dev.041509. [DOI] [PubMed] [Google Scholar]
- 3.Hauser S, Widera D, Qunneis F, et al. Isolation of novel multipotent neural crest-derived stem cells from adult human inferior turbinate. Stem Cells and Development. 2012;21:742–756. doi: 10.1089/scd.2011.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soundararajan P, Miles GB, Rubin LL, Brownstone RM, Rafuse VF. Motoneurons derived from embryonic stem cells express transcription factors and develop phenotypes characteristic of medial motor column neurons. Journal of Neuroscience. 2006;26(12):3256–3268. doi: 10.1523/JNEUROSCI.5537-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Son EY, Ichida JK, Wainger BJ, et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9(3):205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auerbach R, Kubai L, Knighton D, Folkman J. A simple procedure for the long term cultivation of chicken embryos. Developmental Biology. 1974;41(2):391–394. doi: 10.1016/0012-1606(74)90316-9. [DOI] [PubMed] [Google Scholar]
- 7.Jakobson AM, Hahnenberger R, Magnusson A. A simple method for shell-less cultivation of chick embryos. Pharmacology and Toxicology. 1989;64(2):193–195. doi: 10.1111/j.1600-0773.1989.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 8.Hamamichi S, Nishigori H. Establishment of a chick embryo shell-less culture system and its use to observe change in behavior caused by nicotine and substances from cigarette smoke. Toxicology Letters. 2001;119(2):95–102. doi: 10.1016/s0378-4274(00)00300-3. [DOI] [PubMed] [Google Scholar]
- 9.Datar S, Bhonde RR. Shell-less chick embryo culture as an alternative in vitro model to investigate glucose-induced malformations in mammalian embryos. The Review of Diabetic Studies. 2005;2:221–227. doi: 10.1900/RDS.2005.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yalcin HC, Shekhar A, Rane AA, Butcher JT. An ex-ovo chicken embryo culture system suitable for imaging and microsurgery applications. Journal of Visualized Experiments. 2010;44(article e2154) doi: 10.3791/2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellairs R, Osmond M. The Atlas of Chick Development. Boston, Mass, USA: Elsevier; 2005. [Google Scholar]
- 12.Filipski GT, Wilson MVH. Sudan black B as a nerve stain for whole cleared fishes. Copeia. 1984;1984:204–208. [Google Scholar]
- 13.Nishikawa KC. Staining amphibian peripheral-nerves with sudan black B: progressive versus regressive methods. Copeia. 1987;1987:489–491. [Google Scholar]
- 14.Meyers JJ, Herrel A, Nishikawa KC. Comparative study of the innervation patterns of the hyobranchial musculature in three iguanian lizards: sceloporus undulatus, Pseudotrapelus sinaitus, and Chamaeleo jacksonii. Anatomical Record. 2002;267(2):177–189. doi: 10.1002/ar.10096. [DOI] [PubMed] [Google Scholar]
- 15.Korn MJ, Cramer KS. Windowing chicken eggs for developmental studies. Journal of Visualized Experiments. 2007;(5, article e306) doi: 10.3791/306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks PC, Montgomery AM, Cheresh DA. Use of the 10-day-old chick embryo model for studying angiogenesis. Methods in Molecular Biology. 1999;129:257–269. doi: 10.1385/1-59259-249-X:257. [DOI] [PubMed] [Google Scholar]
- 17.Oberlender SA, Tuan RS. Application of functional blocking antibodies. N-cadherin and chick embryonic limb development. Methods in Molecular Biology. 2000;137:37–42. doi: 10.1385/1-59259-066-7:37. [DOI] [PubMed] [Google Scholar]
- 18.Leong HS, Chambers AF, Lewis JD. Assessing cancer cell migration and metastatic growth in vivo in the chick embryo using fluorescence intravital imaging. Methods in Molecular Biology. 2012;872:1–14. doi: 10.1007/978-1-61779-797-2_1. [DOI] [PubMed] [Google Scholar]
- 19.Dohle DS, Pasa SD, Gustmann S, et al. Chick ex ovo culture and ex ovo CAM assay: how it really works. Journal of Visualized Experiments. 2009;(article e1620) doi: 10.3791/1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minovi A, Witt M, Prescher A, et al. Expression and distribution of the intermediate filament protein nestin and other stem cell related molecules in the human olfactory epithelium. Histology and Histopathology. 2010;25(2):177–187. doi: 10.14670/HH-25.177. [DOI] [PubMed] [Google Scholar]


