Abstract
TNF-α is a highly pleiotropic cytokine and plays an important role in regulating HIV-1 replication. It may compromise the integrity of the blood-brain-barrier and, thus, may contribute to the neurotoxicity of HIV-1-infection. Both intravenous drug abuse (IDU) and HIV infection can increase TNF-α activity, but little information is available on the effects of a combination of these factors on TNF-α. We investigated plasma TNF-α levels and mRNA in the peripheral monocytes of 166 men and women in three groups: HIV-1-positive IDUs, HIV-1-negative IDUs, and HIV-negative non-IDU control participants. HIV-1-positive IDUs had higher TNF-α levels than HIV-1-negative IDUs who, in turn, had higher levels than controls. TNF-α mRNA expression in peripheral monocytes was significantly increased in both HIV-1-positive and negative IDUs compared to controls. These findings show that the effects of HIV infection and intravenous drug use may be additive in increasing TNF-α levels. Given the multiple effects of TNF-α in HIV infection, additional investigation of its role is needed.
Keywords: HIV-1, TNF-α, cognition, drug abuse, immune function, dementia
Introduction
Injecting drug use (IDU) is a major risk factor for contracting human immunodeficiency virus type 1 (HIV-1), infection and accounts for almost one third of all cases of HIV-infection (Balode et al. 2004; Donahoe and Vlahov 1998). Research suggests that IDU and HIV-1 infection intertwine to bring about increased viral replication, immune suppression, and neurotoxicity (Abdala et al. 2003; Fernandez et al. 2001; Nath et al. 2002). Although neurodegeneration is one of the major complications in HIV-1 infection (Wang et al. 2004; Nath et al. 2002), its mechanisms are not fully understood. One proposed pathway for the neurodegenerative processes seen in HIV-1 infection implicates proinflammatory cytokines that are elevated among HIV-1-infected individuals.
Tumor necrosis factor-alpha (TNF-α) is one of the most important proinflammatory cytokines. It is primarily produced in the peripheral cells including activated monocytes or macrophages (Beutler and Cerami 1989). TNF-α levels are elevated in persons with HIV-1 infection (Molina et al. 1989; Roux-Lombard et al. 1989; Fauci 1996), and this elevation may have important implications for the course of HIV infection (Lawn et al. 2001). Earlier reports demonstrated that TNF-α stimulated HIV replication in a variety of cells and that its mRNA levels were higher in HIV-seropositive compared to uninfected brain tissue (Tyor et al. 1992; Wesselingh et al. 1993; Brown et al. 1994; Biswas et al. 1994; Weissman et al. 1994). Moreover, increased secretion of TNF-α and other proinflammatory cytokines has been reported in monocytes isolated from peripheral blood (Merrill et al. 1989; Nakajima et al. 1989; Wahl et al. 1989) and cultured cells from HIV-positive individuals (Breen et al. 1990; Fauci 1996; Molina et al. 1989; Roux-Lombard et al. 1989). TNF-α alone or in synergy with other cytokines may upregulate HIV replication and production in host cells (Fauci 1996; Poli 1999).
TNF-α has also been related to cognitive function in several populations, supporting its possible role in neurodegeneration. The possible role of TNF-α in HIV-related cognitive decline was recognized some time ago (Matsuyama et al. 1991). TNF-α levels are related to cognitive decline in HIV-infected individuals (Rostasy et al. 2005; Sevigny et al. 2004, 2007). A possible link between TNF-α and cognition may be through proinflammatory cytokines’ ability relation to depression (Pucak & Kaplin 2005) and the inhibition of hippocampal neurogenesis in depression (Henn & Vollmayr 2004; Sapolsky 2004). Since substance use has clear and complex effects on cognitive function (Ersche et al. 2006; Verdejo-García & Pérez-García 2007), the effects of drugs on cognition may also be mediated by proinflammatory cytokines such as TNF-α, further demonstrating the importance of TNF-α in understanding the relations among HIV infection, drug use, and inflammatory cytokines.
Research has also shown that TNF-α levels are increased by exposure to commonly used drugs of abuse including opioids (Roy et al. 1998) and methamphetamine (Nakajima et al. 2004). Cocaine use may decrease production of TNF-α (Irwin et al. 2007) although HIV-1-infected cocaine users have been found to have elevated levels of TNF-α (Letendre et al. 1999). IDU is a major risk factor for HIV-1 infection, and it is, thus, possible that IDU may affect the course of HIV infection through its effects on cytokine production. Little information is available, however, on TNF-α levels in HIV-positive injecting drug users. This study investigated plasma TNF-α levels and its peripheral monocyte mRNA expression in three groups: HIV-positive IDUs, HIV-negative IDUs, and HIV-negative nondrug using control participants. We hypothesized that levels of plasma TNF-α and monocyte TNF-α mRNA expression would be increased by both intravenous drug use and HIV infection.
Methods
Participants
Men and women within the age range of 18 to 50 years were enrolled for this study. All participants gave informed consent prior to initiation of the study, and all participants were paid for their participation. Potential participants were excluded from participation if they reported a history of head injury with loss of consciousness, learning disability, or a history of major psychiatric illness such as schizophrenia or bipolar disorder, hypertension, or diabetes mellitus. The study was conducted under a protocol approved by the University of Miami Human Subjects Research Office.
Drug use inclusion and exclusion criteria
Men and women in IDU groups were required to have used injected drugs, i.e., heroin and/or cocaine, at least six times in a 1-year period. All participants were interviewed using the Structured Clinical Interview for DSM-IV Axis I Diagnosis through which a diagnosis of dependence or abuse on a particular substance was made. To qualify for the study as a control participant, the individual could have no current or recent past substance dependence; however, individuals with past substance abuse diagnoses were not excluded if the substance abuse had been in remission for at least 2 years. Individuals with a current substance abuse or dependence diagnosis who did not meet the criteria for injection drug use were excluded from participation. All participants also completed a comprehensive lifetime drug use interview that detailed information about drug use patterns with all forms of cocaine, opiates including heroin, marijuana, anxiolytics, and amphetamines. All IDU participants were required to have abstained from drugs and/or alcohol for at least 12 h prior to the study. This was verified by self-report and urine toxicology screens. If a participant was found to be acutely intoxicated, he or she was rescheduled for evaluation at a later date.
HIV-1 infection inclusion and exclusion criteria
HIV-1-positive participants were required to bring evidence of their serostatus to the study. Additionally, their peripheral plasma viral load was determined using polymerase chain reaction (PCR) amplicor method (Roche Diagnostics; at the Clinical Immunology Laboratory in the Department of Medicine, the University of Miami School of Medicine). HIV-1-positive participants were free of any AIDS-defining infections at the time of the study. Verification of HIV-1 seronegative status was not done as part of this study.
Cold pressor stress
The cold pressor challenge was performed between 8:00 a.m. and 11:00 a.m. On arrival, an indwelling venous catheter for drawing blood was placed in the antecubital vein of each participant. After 20 min of rest in a reclined position, a 9-ml sample of blood was drawn in a tube containing EDTA to determine participants’ baseline level of TNF-α. The cold pressor challenge was then administered. Participants placed their entire hand in an ice–water mixture (three parts ice and one part water) for 2 min. Following completion of the cold pressor challenge, three additional blood samples were collected at 15, 30, and 50 min after baseline.
Laboratory procedures
Plasma isolation
Blood was collected in tubes with EDTA added as an anticoagulant. Samples were centrifuged at 1,000×g within 30 min and stored at −20°C until analyzed.
Plasma TNF-α protein analysis
Levels of plasma TNF-α (pg/ml) were quantified using a commercially available ELISA kit (R&D Systems, Minneapolis, MN, USA). Briefly, 50μl of assay diluent were added to wells containing 200μl of standard or sample. The mixture was incubated at room temperature for 2 h. Wells were washed three times in an ELISA washer; 200μl of conjugated antibody were added to each well and samples were then incubated for 2 h at room temperature. Washing was then repeated, and 200μl of substrate solution was added to each well and incubated further for 20 min at room temperature. Fifty microliters of stop solution were added to each well and read at 450 nm within 30 min. Correction for OD at 540 nm was applied. The minimum detectable concentration of TNF-α by this method is 4.4 pg/ml.
Monocyte isolation
Monocytes were separated from 10 ml of whole blood, using the Mono–Poly resolving medium, Ficoll-Hypaque gradient (ICN Biochemicals) and washed twice with sterile phosphate-buffered saline. Monocyte pellets were stored in RNA-later (Ambion) solution at −80°C.
RNA isolation and TNF-α real-time RT-PCR
Total RNA was extracted from monocytes using a commercially available kit, and TNF-α mRNA expression was determined using Real-time RT-PCR. RNA was extracted from the cells using the RNeasy kit (Qiagen). Transcription and amplification was carried out using Real-time PCR (Model ABI Prism 7000 system, Applied Biosystems). One microgram of total RNA was reverse transcribed using a high capacity kit (Applied Biosystems) in a total volume of 20μl. Reverse transcription was carried out at 25°C for 10 min and at 37°C for 120 min. Transcribed cDNA was amplified using commercially available predeveloped primer pairs and Taqman probes (Applied Biosystems). Amplification was carried out at 50 for 2 min then at 95°C for 10 min. Forty-five amplification cycles were then completed at 95°C for 15 s/cycle. Concentration of each sample was calculated from the threshold cycle (Ct) and normalized according to the 18s rRNA (as a housekeeping gene) levels. A standard curve for the gene studied was generated by preparing serial dilution of the cDNA sample obtained from the monocytes isolated from the blood.
Data analyses
Data were examined for outliers and influential data points via analyses of means and standard deviations with a cutoff value of ±4 SD from the mean. In those cases where removal of the outlying data point resulted in a meaningful change in the results, the outlying data point was excluded from the analyses. Potential covariates evaluated in this study were: age, ethnicity, body mass index, education, gender, and drug use characteristics including duration of use of cocaine and heroin. In order to evaluate whether cocaine and heroin had divergent effects on TNF-α, an indicator variable for persons who used one but not the other substance was created and included in models. The effects of viral load and any antiretroviral treatment were also included as potentially important covariates.
To test the hypothesis that the three study groups would differ in plasma TNF-α levels, a mixed effects repeated measures model was created using SAS PROC MIXED (SAS, Inc., Cary, NC, USA). Random effects included in the model allowed an evaluation of individual differences in baseline TNF-α (intercept) as well as of differences in change over time (slope). Fixed effects included potential confounding variables as well as group membership. To assess group differences in mRNA levels, only a random intercept model was evaluated as mRNA was measured on only one occasion. This approach has the considerable advantages of allowing for individual variability in baseline variables and change over time and a more precise determination of the relation of group-level variables to outcomes of interest (Finucane et al. 2007).
Results
Participants in this study included 166 men and women in three study groups: HIV + IDUs (N = 56), HIV – IDUs (N = 59) and HIV-nondrug using control participants (N = 51). Of these 166 men and women, TNF-α gene expression assay for mRNA was conducted in 69 participants (HIV-positive IDUs (N = 30), HIV-negative IDUs (N = 19), and HIV-negative nondrug using control participants (N = 20)).
Demographic characteristics and levels of TNF-α and TNF-α mRNA of the participants are presented in Table 1.
Table 1.
Characteristics of study participants
| Controls | HIV− IDUs | HIV+ IDUs | Statistic | df | p Value | ||
|---|---|---|---|---|---|---|---|
| Age in Yearsa | M (SD) | 32.2 (9.1) | 38.7 (6.4) | 39.1 (5.5) | F = 15.58 | 2, 162 | <0.001 |
| Years of Educationb | M (SD) | 13.7 (2.1) | 11.7 (2.0) | 12.1 (2.0) | F = 13.92 | 2, 161 | <0.001 |
| BMIc | M (SD) | 27.7 (5.9) | 24.8 (4.6) | 26.1 (5.3) | F = 4.37 | 2, 158 | 0.016 |
| Log Baseline TNF-αd | M (SD) | 1.49 (.30) | 1.99 (.33) | 2.45 (.26) | F = 140.48 | 2,163 | <0.001 |
| Log TNF-α at 15 minutes | M (SD) | 1.41 (.49) | 2.00 (.31) | 2.44 (.25) | F = 109.96 | 2,164 | <0.001 |
| Log TNF-α at 30 minutes | M (SD) | 1.48 (.26) | 1.93 (.36) | 2.43 (.27) | F = 128.04 | 2,162 | <0.001 |
| Log TNF-α at 50 minutes | M (SD) | 1.45 (.49) | 2.00 (.30) | 2.39 (.28) | F = 88.47 | 2,163 | <0.001 |
| Log TNF-α mRNAe | M (SD) | 22.81 (1.71) | 24.46 (.99) | 24.82 (.98) | F = 16.50 | 2, 66 | <0.001 |
| Genderf | Male | 23 | 44 | 36 | χ2=10.27 | 2 | 0.006 |
| Female | 28 | 15 | 20 | ||||
| Ethnicity | White | 10 | 20 | 4 | χ2=23.31 | 4 | <0.000 |
| Black | 24 | 21 | 43 | ||||
| Hispanic | 17 | 18 | 9 |
Comparisons for continuous variables were completed with one-way analysis of variance with Scheffé post hoc comparisons. Controls were significantly younger than either IDU group (p<0.001 for both comparisons)
Controls reported significantly more years of education than did either of the other two groups (p<0.001 for both comparisons)
BMI was significantly higher in controls compared to HIV− IDUs (p=0.016)
All Scheffé between-group comparisons for TNF-α were statistically significant (all p<0.001)
Scheffé between-group comparisons for TNF-α mRNA showed a statistically significant difference between controls and HIV− IDUs (p<0.001) but not for the difference between HIV− IDUs and HIV+ IDUs
Comparisons of categorical variables were completed using Pearson chi-square statistics
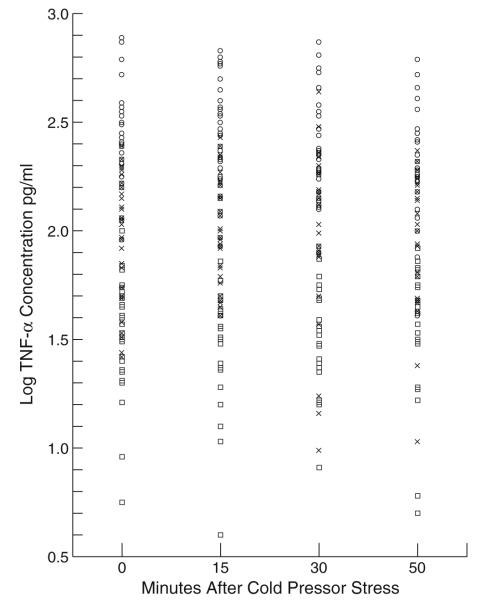
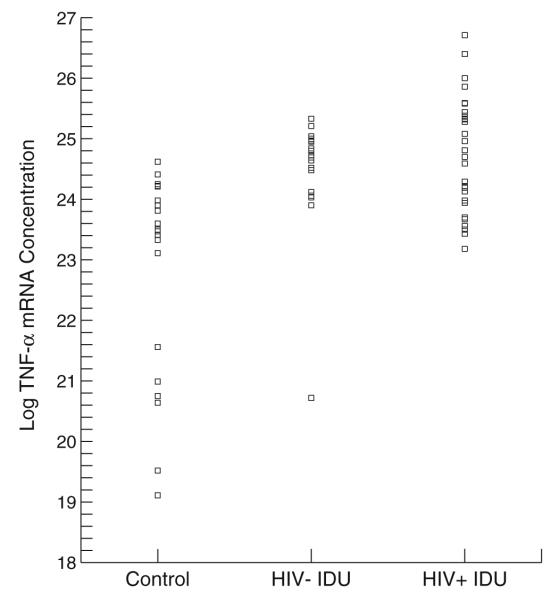
The groups differed significantly in age, years of education, and body mass index. The groups differed in their gender and ethnic compositions as well. Simple one-way analyses of variance showed group differences at each time point for TNF-α and overall group differences for mRNA, but it should be noted that these analyses are not corrected for participants’ drug use characteristics and demographic differences (for which, see Tables 3, 4, 5, and 6). Figures 1 and 2 present scatter plots of TNF-α levels for each group at each sampling time and for baseline TNF-α mRNA values, respectively.
Table 3.
Mixed effect model for TNF-α levels
| Fixed effects | Numerator df | Denominator df | F | p value |
|---|---|---|---|---|
| Time | 3 | 248 | 1.35 | 0.26 |
| BMI | 1 | 134 | 0.00 | 0.98 |
| Age | 1 | 134 | 0.53 | 0.47 |
| Ethnicity | 2 | 134 | 0.88 | 0.42 |
| Gender | 1 | 134 | 3.27 | 0.07 |
| Education | 1 | 134 | 0.08 | 0.77 |
| Cocaine Use Intensity (grams/wk) | 1 | 134 | 0.43 | 0.51 |
| Length of Cocaine Abstinence | 1 | 134 | 2.45 | 0.12 |
| Heroin Use Intensity (injections/wk) | 1 | 134 | 1.49 | 0.22 |
| Length of Heroin Abstinence | 1 | 134 | 0.17 | 0.69 |
| Viral Load | 1 | 134 | 0.01 | 0.92 |
| ARV Treatment | 1 | 134 | 0.01 | 0.92 |
| Cocaine User Only | 1 | 134 | 0.63 | 0.43 |
| Heroin User Only | 1 | 134 | 0.52 | 0.47 |
| HIV/IDU Status | 1 | 142 | 99.30 | <0.001 |
Table 4.
Model-corrected mean TNF-α values
| Group | Estimate | Standard error |
|---|---|---|
| Controls | 1.60a | 0.09 |
| HIV− IDUs | 1.92a | 0.07 |
| HIV+ IDUs | 2.50a | 0.08 |
| Men | 2.06b | 0.05 |
| Women | 1.95b | 0.06 |
| Cocaine and heroin users | 1.97c | 0.05 |
| Cocaine and not heroin user (n=94) | 2.03c | 0.07 |
| Heroin and not cocaine user (n=30) | 2.04c | 0.09 |
All comparisons between model-adjusted mean values for participant groups were significantly different (p<0.001)
Difference between men and women was significantly different (p=0.04)
Differences between individuals who reported using cocaine but not heroin and those reporting heroin use but not cocaine and all other individuals were not significant (cocaine only vs. others, p=0.43; heroin only vs. others, p=0.47). The majority of participants reported polysubstance use
Table 5.
Mixed effect model for TNF-α mRNA
| Fixed effects | Numerator df | Denominator df | F | p Value |
|---|---|---|---|---|
| BMI | 1 | 46 | 0.04 | 0.84 |
| Age | 1 | 46 | 2.98 | 0.09 |
| Ethnicity | 2 | 46 | 3.17 | 0.05 |
| Gender | 1 | 46 | 0.36 | 0.55 |
| Education | 1 | 46 | 0.47 | 0.50 |
| Cocaine Use Intensity (grams/wk) | 1 | 46 | 0.91 | 0.34 |
| Length of Cocaine Abstinence | 1 | 46 | 3.94 | 0.05 |
| Heroin Use Intensity (injections/wk) | 1 | 46 | 1.38 | 0.25 |
| Length of Heroin Abstinence | 1 | 46 | 0.62 | 0.44 |
| Viral Load | 1 | 46 | 6.47 | 0.01 |
| ARV Treatment | 1 | 46 | 2.62 | 0.11 |
| Cocaine User Only | 1 | 46 | 0.75 | 0.39 |
| Heroin User Only | 1 | 46 | 0.08 | 0.79 |
| HIV/IDU Status | 2 | 59 | 5.30 | 0.009 |
Table 6.
Model-corrected mean log TNF-α mRNA values
| Group | Estimate | Standard error |
|---|---|---|
| Participant groupsa | ||
| Controls | 22.32 | 0.65 |
| HIV− IDUs | 24.74 | 0.42 |
| HIV+ IDUs | 24.75 | 0.49 |
| Ethnic groupsb | ||
| Whites | 24.47 | 0.45 |
| Blacks | 24.21 | 0.40 |
| Hispanics | 23.13 | 0.51 |
Comparisons between controls and HIV− IDUs was significant (t (46)=−3.10, p=0.003); between controls and HIV+ IDUs was significant (t (46)=−3.08, p=0.004); between HIV− and HIV+ IDUs was not significant (t (46)=−0.01, p=0.99)
Comparisons between values for ethnic groups; between whites and blacks not significant (t (46)=.52, p=0.61); whites and Hispanics (t (46)=2.30, p=0.03); blacks and Hispanics (t (46)=2.21, p=0.03)
Fig. 1.
TNF-α levels for each group at each sampling point. Squares=controls; crosses=HIV negative IDUs; circles=HIV positive IDUs
Fig. 2.
TNF-α mRNA for each group
Comparisons of self-reported drug use histories and length of abstinence are presented in Table 2. HIV + participants reported significantly longer abstinence from anxiolytics and heroin but significantly shorter abstinence from cocaine compared to HIV− participants.
Table 2.
Drug use characteristics of HIV− and HIV+ IDUs
| HIV− IDUs |
HIV+ IDUs |
t | df a | p Value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Length of time used (weeks) | |||||||
| Anxiolytics | 99.24 | 98.16 | 126.60 | 230.72 | −.68 | 46.69 | .50 |
| Marijuana | 314.58 | 310.95 | 433.03 | 453.29 | −1.51 | 82.38 | .13 |
| Heroin | 289.95 | 322.08 | 278.20 | 397.07 | 0.17 | 103 | .87 |
| Cocaine | 225.61 | 325.36 | 287.77 | 305.15 | −.94 | 89 | .35 |
| Crack cocaine | 241.80 | 334.99 | 336.40 | 349.43 | −1.30 | 86 | .20 |
| Length of abstinence (weeks) | |||||||
| Anxiolytics | 721.00 | 953.67 | 1431.83 | 906.96 | −3.45 | 83 | <0.001 |
| Marijuana | 354.60 | 545.99 | 363.84 | 657.02 | −0.07 | 91 | .94 |
| Heroin | 193.01 | 572.61 | 836.07 | 1072.73 | −3.75 | 71.37 | <0.001 |
| Cocaine | 645.40 | 975.80 | 224.54 | 505.31 | 2.67 | 72.66 | .01 |
Degrees of freedom vary across tests due to some questions not being relevant to some participants (i.e., never having used the substance and, thus, not reporting either use or abstinence). For comparisons in which group variances differed, a correction to degrees of freedom was applied
The hypothesis of group differences in TNF-α levels was evaluated using a mixed effects repeated measures model that allowed individuals’ baseline level of TNF-α and the time course of their response to cold pressor stress to vary. Results are presented in Tables 3 and 4. Controls had the lowest levels of TNF-α, while HIV− and HIV+ IDUs had progressively higher levels. All between-group comparisons were statistically significant. Men had model-corrected values that were larger than those for women. TNF-α levels did not change over time after participants experienced cold pressor stress (i.e., we found a nonsignificant effect for time; see Tables 3 and 4).
Analyses of group differences in mRNA levels were completed using a similar model but with only a random intercept as mRNA levels were measured on only one occasion (at baseline). Both IDU groups had higher levels of mRNA compared to controls. Length of abstinence from cocaine and viral load were positively related to mRNA levels (Tables 5 and 6).
Discussion
In this study, we assessed the TNF-α levels in intravenous drug users who were either infected or not infected with HIV-1 and compared their levels to those of uninfected and nondrug using controls. All experienced cold pressor stress as part of their participation in a larger study of neurohormonal changes in HIV− and HIV+ individuals. We found that participants’ levels of TNF-α reflected the effects of both intravenous drug use and HIV infection, with those with both characteristics having the highest levels of TNF-α. TNF-α mRNA was similarly elevated in both IDU groups, although in these comparisons the difference between levels in HIV− and HIV+ individuals was not statistically significant.
Earlier studies from our group showed that elevated TNF-α levels might be related to neurodegeneration (Kumar et al. 2002, 2003). Other investigators have shown that HIV-1 infection is characterized by a significant increase in the levels of proinflammatory cytokines, predominantly secreted by activated monocytes that are storehouses of the virus (Breen 2002). As the equilibrium between Th1 and Th2 cytokines is disturbed in HIV-1 infection, this imbalance may lead to clinical disease progression related to increased Th1 activity. TNF-α can be considered a Th1 cytokine, has been associated with increased HIV-1 replication, and is highly neurotoxic (Nakajima et al. 2004). Variability in virus strains’ ability to elicit TNF-α increases may be related to the different disease courses causes by these strains (Khanna et al. 2000).
The role of TNF-α in HIV-related cognitive decline was recognized long ago (Matsuyama et al. 1991). High levels of TNF-α are associated with compromise of the blood-brain barrier (Brabers & Nottet 2006;Vandamme et al. 2004; Pu et al. 2003). With disruption in the blood-brain barrier, HIV-containing monocytes can enter the brain and may infect microglia (Pu et al. 2003, Annunziata 2003). Earlier reports demonstrate that both HIV-infected monocytes and microglia secrete large amounts of TNF-α (Sippy et al. 1995). TNF-α is associated with cognitive decline and mortality in HIV-infected individuals (Rostasy et al. 2005; Sevigny et al. 2004, 2007). Several lines of evidence, thus, show that level of TNF-α is associated with severity and progression of HIV-related diseases, including cognitive impairment (Brabers & Nottet 2006).
The molecular mechanism for upregulation of HIV expression by proinflammatory cytokines is best characterized for TNF-α. TNF-α activates NF-κB, a transcription factor that is sequestered in its active form in the cytoplasm of cells (Fauci 1996; Poli 1999). Once activated, NF-κB translocates to the nucleus, binds near the transcription start site of HIV (located near the long terminal repeat sequences of the viral genome), and initiates and/or enhances HIV expression and viral production. HIV infection itself increases levels of TNF-α which can in turn affect viral replication (Leghmari et al. 2008).
Although data on TNF-α levels are available for HIV+ individuals, no readily identifiable study has compared TNF-α levels in HIV+ and HIV− IDUs. The study presented here demonstrates differences in TNF-α levels and TNF-α mRNA across these groups and in comparison to controls. TNF-α levels were increased in HIV− IDUs and were still higher in HIV+ IDUs. This suggests that the effects of HIV infection on TNF-α production are increased relative to drug use alone. This finding is, thus, significant for understanding the role of proinflammatory cytokines in the progression of neurodegeneration and neurocognitive deficits in individuals affected by both conditions.
Other studies have shown increases in TNF-α levels (Tyor et al. 1992), associated mRNA (Wesselingh et al. 1993) and receptors (Sippy et al. 1995) in the brains of HIV-1-infected individuals. A relation between TNF-α and HIV-associated cognitive deficits has also been reported (Sippy et al. 1995) and we have previously shown that cognitive functioning in HIV + IDUs may be affected by the neurohormonal response to stress (Ownby et al. 2006). The study reported here evaluated the relation between the TNF-α mRNA and intravenous drug use and HIV infection. We found that mRNA levels were elevated in both groups of IDUs. Although the model-corrected level of mRNA was higher in IDUs with HIV infection than in those without, the difference was not statistically significant. It is, thus not clear whether the incremental effect of HIV infection on TNF-α levels is related to increased TNF-α production or to some other factor.
Several limitations of this study should be noted. This study did not include a group of participants who were HIV+ but not intravenous drug users. While this inclusion would have been desirable, given the context in which the study was completed inclusion of a fourth group of participants was not possible. Another issue that may affect study results is the divergent characteristics of the groups of participants, who varied with respect to age, education, body mass index, and drug use severity and abstinence. Although these issues are statistically controlled in data analyses, between-group differences may have affected results in ways that cannot be predicted. It should also be noted cold pressor stress did not significantly affect TNF-α levels over the sampling intervals used in this study. This is likely due to the time frame in which samples were collected, which was based on interest in plasma catecholamines and cortisol in the parent study. In fact, data on the levels of catecholamines and cortisol showed a clear and robust effect of cold pressor stress that we have reported elsewhere (Ownby et al. 2006). The apparent lack of effect on TNF-α is, thus, likely the result of the time course of sampling.
These findings, thus, show that levels of TNF-α are increased both by intravenous drug use and HIV-1 infection. Since intravenous drug use is an important risk behavior related to HIV infection, it is important to note that drug use itself may affect immune system factors that may be neurotoxic and have been related to cognitive function. The additional finding that HIV-1 infection may be an additive factor in increasing TNF-α and, thus, in increasing risk for cognitive impairment underscores the importance of addressing both issues in future research. Additional research on the effects of intravenous drug use and HIV infection on immune factors and cognitive functioning is, thus, indicated.
Acknowledgments
This study was supported by grant R01 DA12792 and DA13550 to Dr. Mahendra Kumar.
Contributor Information
Raymond L. Ownby, Nova Southeastern University, 804 SE 14th Street, Fort Lauderdale, FL 33316, USA
Adarsh M. Kumar, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Miami, FL 33136, USA
J. Benny Fernandez, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Miami, FL 33136, USA.
Irina Moleon-Borodowsky, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Miami, FL 33136, USA.
Louis Gonzalez, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Miami, FL 33136, USA.
Seth Eisdorfer, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Miami, FL 33136, USA.
Drenna Waldrop-Valverde, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Miami, FL 33136, USA.
Mahendra Kumar, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Miami, FL 33136, USA.
References
- Abdala N, Carney JM, Durante AJ, Klimov N, Ostrovski D, Somlai AM, Kozlov A, Heimer R. Estimating the prevalence of syringe-borne and sexually transmitted diseases among injection drug users in St Petersburg, Russia. Int J STD AIDS. 2003;14:697–703. doi: 10.1258/095646203322387965. doi:10.1258/095646203322387965. [DOI] [PubMed] [Google Scholar]
- Annunziata P. Blood-brain barrier changes during invasion of the central nervous system by HIV-1. Old and new insights into the mechanism. J Neurol. 2003;250:901–906. doi: 10.1007/s00415-003-1159-0. doi:10.1007/s00415-003-1159-0. [DOI] [PubMed] [Google Scholar]
- Balode D, Ferdats A, Dievberna I, Viksna L, Rozentale B, Kolupajeva T, Konicheva V, Leitner T. Rapid epidemic spread of HIV type 1 subtype A1 among intravenous drug users in Latvia and slower spread of subtype B among other risk groups. AIDS Res Hum Retroviruses. 2004;20:245–249. doi: 10.1089/088922204773004978. doi:10.1089/088922204773004978. [DOI] [PubMed] [Google Scholar]
- Beutler B, Cerami A. The biology of cachectin/TNF-a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Biswas P, Poli G, Orenstein JM, Fauci AS. Cytokine-mediated induction of human immunodeficiency virus (HIV) expression and cell death in chronically infected U1 cells: do tumor necrosis factor alpha and gamma interferon selectively kill HIV-infected cells? J Virol. 1994;68:2598–2604. doi: 10.1128/jvi.68.4.2598-2604.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabers NA, Nottet HS. Role of the pro-inflammatory cytokines TNF-alpha and IL-1 beta in HIV-associated dementia. Eur J Clin Invest. 2006;36:447–458. doi: 10.1111/j.1365-2362.2006.01657.x. doi:10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- Breen EC. Pro- and anti-inflammatory cytokines in human immunodeficiency virus infection and acquired immunodeficiency syndrome. Pharmacol Ther. 2002;95:295–304. doi: 10.1016/s0163-7258(02)00263-2. doi:10.1016/S0163-7258(02) 00263-2. [DOI] [PubMed] [Google Scholar]
- Breen EC, Rezai AR, Nakajima K, Beall GN, Mitsuyasu RT, Hirano T, Kishimoto T, Martinez-Maza O. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990;144:480–484. [PubMed] [Google Scholar]
- Brown CC, Poli G, Lubaki N, St Louis M, Davachi F, Musey L, Manzila T, Kovacs A, Quinn TC, Fauci AS. Elevated levels of tumor necrosis factor-alpha in Zairian neonate plasmas: implications for perinatal infection with the human immunodeficiency virus. J Infect Dis. 1994;169:975–980. doi: 10.1093/infdis/169.5.975. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infection to AIDS. J Neuroimmunol. 1998;83:77–87. doi: 10.1016/s0165-5728(97)00224-5. doi:10.1016/S0165-5728(97) 00224-5. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory functions associated with amphetamine and opiate dependence. Neuropsychopharmacol. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. doi:10.1038/sj.npp. 1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci AS. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. doi:10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- Fernandez DM, Gomez MA, Mayor AM, Gomez O, Hunter RF. Survival of AIDS according to injecting drug use among Puerto Rican AIDS patients. Cell Mol Biol Noisy-le-grand. 2001;47:1121–1127. [PubMed] [Google Scholar]
- Finucane MM, Samet JH, Horton NJ. Translational methods in biostatistics: Linear mixed effect regression models of alcohol consumption and HIV disease progression over time. Epidemiol Perspect Innov. 2007;4:8. doi: 10.1186/1742-5573-4-8. doi:10.1186/1742-5573-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn FA, Vollmayr B. Neurogenesis and depression: etiology or epiphenomenon? Biol Psychiatry. 2004;56:146–150. doi: 10.1016/j.biopsych.2004.04.011. doi:10.1016/j.biopsych.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmos L, Wang M, Walladares EM, Motivala SJ, Fong T, Newton T, Butch A, Olmstead R, Cole SW. Cocaine dependence and acute cocaine induce decreases of monocytes proinflammatory cytokine expression across the diurnal period: Autonomic mechanisms. J Pharmacol Exp Ther. 2007;320:507–515. doi: 10.1124/jpet.106.112797. doi:10.1124/jpet.106.112797. [DOI] [PubMed] [Google Scholar]
- Khanna KV, Yu XF, Ford DH, Lee R, Hildreth JK, Markham RB. Differences among HIV-1 variants in their ability to elicit secretion of TNF-alpha. J Immunol. 2000;164:1408–1415. doi: 10.4049/jimmunol.164.3.1408. [DOI] [PubMed] [Google Scholar]
- Kumar M, Kumar AM, Waldrop D, Antoni MH, Eisdorfer C. HIV-1 infection and its impact on the HPA axis, cytokines, and cognition. Stress. 2003;6:167–172. doi: 10.1080/10253890310001605376. doi:10.1080/10253890310001605376. [DOI] [PubMed] [Google Scholar]
- Kumar M, Kumar AM, Waldrop D, Antoni MH, Schneiderman N, Eisdorfer C. The HPA axis in HIV-1 infection. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S89–S93. doi: 10.1097/00126334-200210012-00010. [DOI] [PubMed] [Google Scholar]
- Lawn SD, Butera ST, Folks TM. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1infection. Clin Microbiol Rev. 2001;14:753–777. doi: 10.1128/CMR.14.4.753-777.2001. doi:10.1128/CMR.14.4.753-777.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leghmari K, Contreras X, Moureau C, Bahraoui E. HIV-1 TAT protein induces TNF-alpha and IL-10 production by human macrophages: Differential implications of PKC-beta and –delta isozymes and MAP kinases ERK 1-2 and p38. Cell Immunol. 2008 doi: 10.1016/j.cellimm.2008.06.011. in press. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Durand RJ, McCutchan JA. Cerebrospinal fluid tumor necrosis factor (TNF)-alpha and soluble TNF receptors levels in cocaine users and patients with HIV-associated dementia [Abstract]. Paper presented at the Conference on Retroviruses and Opportunistic Infections; Chicago IL. 1999. Available on-line: http://gateway.nlm.nih.gov/MeetingAbstracts/ma?f=102188887.html. [Google Scholar]
- Matsuyama T, Kobayashi N, Yamamoto N. Cytokines and HIV infection: Is AIDS a tumor necrosis factor disease? AIDS. 1991;5:1405–1417. doi: 10.1097/00002030-199112000-00001. doi:10.1097/00002030-199112000-00001. [DOI] [PubMed] [Google Scholar]
- Merrill JE, Koyanagi Y, Chen IS. Interleukin-1 and tumor necrosis factor alpha can be induced from mononuclear phagocytes by human immunodeficiency virus type 1 binding to the CD4 receptor. J Virol. 1989;63:4404–4408. doi: 10.1128/jvi.63.10.4404-4408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JM, Scadden DT, Byrn R, Dinarello CA, Groopman JE. Production of tumor necrosis factor alpha and interleukin 1 beta by monocytic cells infected with human immunodeficiency virus. J Clin Invest. 1989;84:733–737. doi: 10.1172/JCI114230. doi:10.1172/JCI114230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Yamada K, Nagai T, Uchiyama T, et al. Role of tumor necrosis factor-alpha in methampetamine-induced drug dependence and neurotoxicity. J Neurosci. 2004;24:2212–2225. doi: 10.1523/JNEUROSCI.4847-03.2004. doi:10.1523/JNEUROSCI.4847-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Martinez-Maza O, Hirano T, Breen EC, Nishanian PG, Salazar-Gonzalez JF, Fahey JL, Kishimoto T. Induction of IL-6 (B cell stimulatory factor-2/IFN-beta 2) production by HIV. J Immunol. 1989;142:531–536. [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S62–S69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Ownby RL, Waldrop-Valverde D, Kumar A, Mack A, Fernandez JB, González L, González P, Kumar M. Cortisol mediates HIV-1 cognitive deficits among injecting drug abusers. Am J Infect Dis. 2006;2:74–79. [Google Scholar]
- Poli G. Laureate ESCI award for excellence in clinical science 1999. Cytokines and the human immunodeficiency virus: from bench to bedside. European Society for Clinical Investigation. Eur J Clin Invest. 1999;29:723–732. doi: 10.1046/j.1365-2362.1999.00525.x. doi:10.1046/j.1365-2362.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- Pu H, Tian J, Flora G, Lee YW, Nath A, Hennig B, Toborek M. HIV-1 TAT protein upregulates inflammatory mediators and inducesmonocyte invasion into the brain. Mol Cell Neurosci. 2003;24:224–237. doi: 10.1016/s1044-7431(03)00171-4. doi:10.1016/S1044-7431(03) 00171-4. [DOI] [PubMed] [Google Scholar]
- Pucak ML, Kaplin AI. Unkind cytokines: Current evidence for the potential role of cytokines in immune-mediated depression. Int Rev Psychiatry. 2005;17:477–483. doi: 10.1080/02646830500381757. [DOI] [PubMed] [Google Scholar]
- Rostasy K, Monti L, Lipton SA, Kedreen JC, Gonzalez RG, Navia BA. HIV leukoencephalopathy and TNF-alpha expression in neurones. J Neurol Neurosurg Psychiatry. 2005;76:960–964. doi: 10.1136/jnnp.2004.036889. doi:10.1136/jnnp. 2004.036889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux-Lombard P, Modoux C, Cruchaud A, Dayer JM. Purified blood monocytes from HIV 1-infected patients produce high levels of TNF alpha and IL-1. Clin Immunol Immunopathol. 1989;50:374–384. doi: 10.1016/0090-1229(89)90144-x. doi:10.1016/0090-1229(89) 90144-X. [DOI] [PubMed] [Google Scholar]
- Roy S, Barke R, Loh HH. Mu-opioid receptor-knockout mice: Role of mu-opioid receptor on morphine mediated immune functions. Brain Res Mol Brain Res. 1998;61:190–194. doi: 10.1016/s0169-328x(98)00212-5. doi:10.1016/S0169-328X(98) 00212-5. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol Psychiatry. 2004;56:137–139. doi: 10.1016/j.biopsych.2004.04.012. doi:10.1016/j.biopsych.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Sevigny JJ, Albert SM, McDermott MP, McArthur JC, Sacktor N, Conant K, Schifitto G, Selnes OA, Stern Y, McClernon DR, Palumbo D, Kieburtz K, Riggs G, Epstein LG, Marder K. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63:2084–2090. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- Sevigny JJ, Albert SM, McDermott MP, McArthur JC, Sacktor N, Conant T, Selnes OA, Stern Y, McClernon DR, Palumbo D, Kieburtz K, Riggs G, Cohen B, Marder K, Epstein LG. An evaluation of neurocognitive status and markers of immune activation as predictors of time to death in advanced HIV infection. Arch Neurol. 2007;64:97–102. doi: 10.1001/archneur.64.1.97. doi:10.1001/archneur.64.1.97. [DOI] [PubMed] [Google Scholar]
- Sippy BD, Hofman FM, Wallach D, Hinton DR. Increased expression of tumor necrosis factor-alpha receptors in the brains of patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:511–521. doi:10.1097/00042560-199510050-00004. [PubMed] [Google Scholar]
- Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. doi:10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Vandamme W, Braet K, Cabooter L, Leybaert L. Tumour necrosis factor alpha inhibits purinergic calcium signalling in blood-brain barrier endothelial cells. J Neurochem. 2004;88:411–421. doi: 10.1046/j.1471-4159.2003.02163.x. [DOI] [PubMed] [Google Scholar]
- Wahl LM, Corcoran ML, Pyle SW, Arthur LO, Harel-Bellan A, Farrar WL. Human immunodeficiency virus glycoprotein (gp120) induction of monocyte arachidonic acid metabolites and interleukin 1. Proc Natl Acad Sci USA. 1989;86:621–625. doi: 10.1073/pnas.86.2.621. doi:10.1073/pnas.86.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Trillo-Pazos G, Kim SY, Canki M, Morgello S, Sharer LR, Gelbard HA, Su ZZ, Kang DC, Brooks AI, Fisher PB, Volsky DJ. Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: potential role in neuropathogenesis. J Neurovirol. 2004;10(Suppl 1):25–32. doi: 10.1080/753312749. [DOI] [PubMed] [Google Scholar]
- Weissman D, Poli G, Fauci AS. Interleukin 10 blocks HIV replication in macrophages by inhibiting the autocrine loop of tumor necrosis factor alpha and interleukin 6 induction of virus. AIDS Res Hum Retroviruses. 1994;10:1199–1206. doi: 10.1089/aid.1994.10.1199. [DOI] [PubMed] [Google Scholar]
- Wesselingh SL, Power C, Glass JD, Tyor WR, McArthur JC, Farber JM, Griffin JW, Griffin DE. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol. 1993;33:576–582. doi: 10.1002/ana.410330604. doi:10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Pérez-García M. Profile of executive deficits in cocaine and heroin polysubstance users: Common and differential effects on separate executive components. Psychopharmacol. 2007;190:517–530. doi: 10.1007/s00213-006-0632-8. doi:10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]