Abstract
Objective:
Aged garlic extract (AGE) has been proven to exhibit antioxidant, hypolipidemic, hypoglycemic and antidiabetic properties. However, its effect on diabetic nephropathy was unexplored. Therefore, the present study was designed to investigate the renoprotective effect of AGE in streptozotocin-induced diabetic rats.
Materials and Methods:
Albino Wistar rats were induced with diabetes by a single intraperitoneal injection of 45 mg/kg b.w. of streptozotocin. Commercially available AGE was supplemented orally at a dose of 500 mg/kg body weight/day. Aminoguanidine, which has been proven to be an anti-glycation agent was used as positive control and was supplemented at a dose of 1 g/L in drinking water. The serum and urinary biochemical parameters were analyzed in all the groups and at the end of 12 weeks follow up, the renal histological examination were performed using H & E and PAS staining.
Results:
The diabetic rats showed a significant change in the urine (P < 0.001) and serum (P < 0.01) constituents such as albumin, creatinine, urea nitrogen and glycated hemoglobin. In addition, the serum lipid profile of the diabetic rats were altered significantly (P < 0.05) compared to that of the control rats. However, the diabetic rats supplemented with aged garlic extract restored all these biochemical changes. The efficacy of the extract was substantiated by the histopathological changes in the kidney.
Conclusion:
From our results, we conclude that aged garlic extract has the ability to ameliorate kidney damage in diabetic rats and the renoprotective effect of AGE may be attributed to its anti-glycation and hypolipidemic activities.
KEY WORDS: Aged garlic extract, anti-glycation, diabetes, diabetic nephropathy, hypolipidemic, renoprotective
Introduction
The mortality in diabetics is accounted by the complications such as nephropathy, neuropathy and retinopathy. Approximately 30% of the patients with diabetes progress to diabetic nephropathy (DNP) and develop an end stage renal disease.[1]
In diabetic patients, there is an increase in the level of type - IV collagen with the concomitant decrease in the level of laminin and heparan sulphate, thus affecting the pore size and selectivity, causing kidney damage. The kidney damage in DNP is manifested histologically by the thickening of the glomerular basement membrane, mesangial matrix expansion, macrophage infiltration, podocyte loss and tubular epithelial degeneration.[2] Though various pathophysiological mechanisms have been predicted till date on DNP, the formation of advanced glycation end products induced by hyperglycemia and dyslipidemia are considered to play the vital role in the progression of DNP.[3] So, in search of new potential treatment strategies against DNP, interest has been focused on therapeutic interventions targeting hyperglycemia and dyslipidemia.
Garlic, a culinary herb with tremendous medicinal properties has been used historically for the treatment of diseases associated with ageing. Commercial and non-commercial preparations of garlic such as garlic powder, garlic oil, aged garlic extract (AGE), water and oil extract of garlic have been used as health supplements. Of all the aforementioned garlic preparations, AGE is found to have the maximum pharmacological effects.[4] AGE is found to exhibit antioxidant properties both in vitro and in vivo.[5] It possesses anti- thrombotic effect[6] and it has the ability to protect against coronary heart diseases. Epidemiological studies have revealed that garlic consumption has decreased the incidence of certain forms of cancer including stomach, colon and laryngeal cancers.[7] AGE has been found to increase the resistance of LDL to oxidation in vitro, thus inhibiting lipoprotein modifications.[8] Garlic has the ability to reduce the plasma lipid and cholesterol level, and the lipid lowering effect is accompanied by the decreased activities of lipogenic and cholesterogenic enzymes.[9] AGE has also been proven to have hypoglycemic and hypotensive properties.[10] Hence, our study was aimed to explore the anti-glycation and hypolipidemic properties of commercially available aged garlic extract and demonstrate its renoprotective effect in experimentally induced diabetic rats.
Materials and Methods
Chemicals and Reagents
Kyolic® Aged garlic extract was purchased from Wakunaga of America Co., Ltd., USA. Aminoguanidine and streptozotocin (STZ) were procured from Sigma Aldrich, India. The kits for albumin, urea, triglycerides, total cholesterol and HDL-Cholesterol were purchased from Span Diagnostics Ltd., Gujarat, India. The glycated hemoglobin kit was purchased from Euro Diagnostics Ltd., Chennai. All the other reagents and chemicals were obtained from Sisco Research Laboratories Ltd., India.
Experimental Design
Eight weeks old male albino Wistar rats weighing 200-250 g were used for the study. They were maintained under standard laboratory conditions (22 ± 3 °C, 12-h light/dark cycle) supplied with standard pellet food and water given ad libitium in VIT Animal house, Vellore. The animals were cared in accordance with the guidelines provided by the CPCSEA and the Institutional Animal Ethics Committee approved the entire study (Approval no.VIT/ IAEC /IV/ 031/ 2011).
Induction of Diabetes
Streptozotocin (STZ), at a dose of 45 mg/kg body weight dissolved in citrate buffer, was injected intraperitoneally to induce diabetes. The animals were fasted for 16hrs before the STZ injection, and after the injection 5% sucrose was supplemented for 24hrs in order to prevent the animals from fatal hypoglycemia. One week after STZ injection, blood glucose level was checked using the glucometer. The animals with a blood glucose level of more than 300 mg/dl were considered diabetic and included in the study.
The animals were segregated into four groups with six animals each. The groups included were control rats (Con), STZ induced diabetic rats (Dia), diabetic rats treated with aminoguanidine (Dia+AMG) 1 g/L dissolved in drinking water, diabetic rats supplemented orally with AGE (Dia+AGE) at a dose of 500 mg/kg body weight. Based on the investigation done by Omotoso et al., 2011, 500 mg/kg body weight/day of AGE was considered as the optimum dosage and was used in our study.[11] The treatment was started after two weeks of STZ injection, once the animals recovered from mild nephrotoxic effects of STZ and it was continued for twelve weeks from STZ injection.
Determination of Glycated Hemoglobin Content
Glycated hemoglobin level was measured by ion-exchange resin method as described in the product insert of Euro Diagnostics Ltd., Chennai. Briefly, hemolysate was prepared by lysing 50 μl of blood with lysing reagent and the obtained hemolysate was added to the ion exchange resin tube. The tubes were vortexed after inserting the resin separator into it, such that, the rubber sleeve is 1 cm above the resin suspension. Finally, the resin separator was pushed in completely to remove the supernatant and its absorbance was measured at 415 nm against distilled water to obtain ΔGHb. 20 μl of haemolysate was added to 5 ml of distilled water to measure the ΔTotal Hb at 415 nm. The percentage glycated hemoglobin level was calculated using the formula, GHb% = (ΔGHb/ΔTotal Hb) × 4.61 (assay factor).
Biochemical Analyses
Measurement of Serum and Urinary Albumin
Albumin content was quantified by bromocresol green method using the kit procured from the Span Diagnostics Ltd., Gujarat, India. Briefly, 1 ml of reagent was added to ten microlitre of the sample and absorbance was measured after 1 min incubation. The anionic dye - bromocresol green binds to albumin present in the sample, forming a green colored complex. The absorbance measured was compared with the standard and the albumin content was expressed in g/dl.
Quantification of Serum and Urinary Creatinine
Creatinine content was measured by Jaffe method followed by Farrell and Bailey, 1990.[12] Briefly, 2 μl of sample was added to 240 μl of working reagent and the mixture was incubated at room temperature for 30 min to measure the absorbance at 505 nm. 20 μl of 30% acetic acid was added to the wells and further incubated for 10 min. The absorbance was again read at 505 nm and the difference in absorbance was noted. The concentration of creatinine was then calculated by comparing with the standard graph.
Measurement of Blood and Urine Urea Nitrogen
Urea nitrogen content was quantified by using the kit procured from the Span Diagnostics Ltd., Gujarat, India. Briefly, 10 μl of diluted urine (1: 20 v/v) or serum was added to the reagents and incubated in boiling water bath for 10 min. Urea present in the sample reacted with diacetylmonoxime in the presence of thiosemicarbazide to form a purple colored complex, which was measured at 525 nm. The absorbance was compared with that of the standard and the urea nitrogen content was calculated.
Quantification of Serum Iipid Profile
The serum lipid parameters such as triglycerides, total cholesterol, high density lipoprotein (HDL) cholesterol were estimated by the enzymatic GPO-PAP method, CHOD-PAP method, and PEG-CHOD-PAP methods respectively using the commercial kit procured from the Span Diagnostics Ltd., Gujarat, India. The low density lipoprotein (LDL) cholesterol and the atherogenic index (AI) were estimated based on the Friedewald equation.[13]
LDL- cholesterol = Total cholesterol – (Triglycerides/5 – HDL cholesterol)
AI = (Total cholesterol – HDL cholesterol)/ HDL cholesterol
Histopathological Examination
Two animals from each group were sacrificed at the end of 12 weeks from STZ injection, by anaesthetizing them with diethyl ether. Kidneys were excised carefully without any damage and stored in 10% neutral buffered formalin, after washing with phosphate buffer saline (PBS). The kidneys were then processed and embedded in paraffin. Four micron sections were cut and stained with haematoxylin and eosin and periodic acid Schiff base for histopathological observations. The sections were then analyzed for the degree of tubular and glomerular damage. Glomerular damage index (GDI) was calculated from 0 to 4 on the basis of the degree of glomerulosclerosis, mesangiolysis and mesangial expansion. 80 to 100 glomeruli from renal cortex were observed for each sections and GDI was obtained by averaging the scores from counted glomeruli.[14]
Statistical Analysis
The data were analyzed on Graph Pad Prism 5.01 software and expressed as mean ± S.D (n = 4). Statistical analysis was performed by One-way ANOVA followed by Dunnet's test to compare the diseased and treated groups. The statistical difference between the normal and diseased was analyzed by Un-paired t-test. The results were considered statistically significant, if p < 0.05.
Results
Effect of AGE on Body Weight and Urine Volume
The change in the body weight of the rats in all the four groups is shown in Table 1. Control rats showed a slight increase in the body weight throughout the study period, but induction of diabetes significantly decreased the body weight (p < 0.001) of the animals in the diabetic group. Though there was a mild improvement in the body weight of the animals that were treated with aminoguanidine, AGE supplementation decreased the body weight of the animals. Our results coincided with the earlier finding that the supplementation of AGE resulted in weight loss in diabetic rats.[15]
Table 1.
Metabolic parameters and serum biochemistry in experimental STZ treated diabetic rats
In general, the volume of urine excreted by a normal adult rat remains the same. However, the diabetic rats showed a predominant increase in the urine volume (p < 0.001), because of the osmotic imbalance between the body fluids and cellular contents, induced by hyperglycemia. After treatment with aminoguanidine and AGE, the urine volume was significantly decreased (p < 0.001) and at the end of the study, it was very close to normal [Table 1].
Effect of AGE on Blood Glucose and Glycated Hemoglobin Content
In diabetic rats, the blood glucose level was significantly high (~ 600 mg/dl) throughout the study period (p < 0.001). The diabetic rats that were treated with aminoguanidine showed a significant decrease in the blood glucose level, but the AGE treatment elicited no significant difference in the blood glucose level [Table 1]. Our results are highly contradictory to the results obtained by Saravanan et al.[10] who demonstrated the anti-diabetic effect of S-allyl cysteine, a major constituent of AGE. Though AGE supplementation showed less effect on the blood glucose level, its effect on the glycated hemoglobin content was significant (p < 0.05) and was comparable with that of the aminoguanidine supplemented animals. This effect may be attributed to the anti-glycation activity of AGE.[16]
Effect of AGE on The Biomarker of DNP
Diabetic nephropathy is being diagnosed clinically by an increase in the level of albumin in urine (albuminuria), accompanied by a decrease in its level in serum. In our study, the diabetic rats showed a significant increase in the albumin content in urine (p < 0.001), with a significant decrease in the level of albumin in serum (p < 0.001). This confirmed that the rats that were induced with diabetes have progressed to DNP. After treatment with AGE, there was a significant decrease in the albumin level in urine (p < 0.01), which was similar to that observed in aminoguanidine supplemented animals [Table 2]. At the end of the study, the albumin level of the diabetic rats supplemented with AGE was brought back to normal, which proved that AGE has the potential to attenuate DNP.
Table 2.
Effect of aged garlic extract on urine biochemistry in diabetic rats
Improvement of Creatinine Content after AGE Treatment
Any kidney damage is reflected by a decrease in the creatinine content in urine, and DNP is no exception. The diabetic rats showed a significant decrease in the level of creatinine in urine (p < 0.001) with a significant increase in serum (p < 0.001) which proved that the animals have encountered kidney damage. However, after treatment with AGE, the creatinine content increased significantly in urine (p < 0.05) with a significant decrease in serum (p < 0.01). This evinced that the AGE supplementation has improved the kidney damage in diabetic rats.
Improvement of Urea Nitrogen Content After AGE Treatment
The lack of glucose metabolism in diabetic patients enhances the protein catabolism, increasing the level of urea in blood. The blood urea nitrogen level is further aggravated by the kidney damage induced by hyperglycemia. Thus, in diabetic nephropathy condition, there was an increase in the level of blood urea nitrogen content with a decrease in its level in urine. The diabetic rats showed a significant increase (p < 0.001) in the blood urea nitrogen content and a significant decrease (p < 0.001) in the urine urea nitrogen content, which confirmed that the animals have progressed to diabetic nephropathy. However, the animals that are treated with AGE showed a significant decrease (p < 0.01) in the blood urea nitrogen content and a significant increase (p < 0.01) in the urine urea nitrogen content [Table 2], which was similar to that observed in aminoguanidine treated animals. This proves that AGE supplementation could be used as a nephroprotectant in diabetic animals.
Effect of AGE on Serum Lipid Profile
The changes in the lipid level of animals in all the four groups are indicated in Table 3. In diabetic animals, we observed a significant increase in the level of triglycerides (p < 0.01), total cholesterol (p < 0.01), and LDL-cholesterol (p < 0.01) with a significant decrease in the HDL-cholesterol level (p < 0.05). However, the diabetic animals that were supplemented with AGE showed a significant decrease in the level of triglycerides (p < 0.05), total cholesterol (p < 0.01), and LDL-cholesterol (p < 0.01) with an increase in the HDL-cholesterol level (p < 0.05). The serum lipid profile of the diabetic animals supplemented with AGE was highly comparable with that of the aminoguanidine treated animals.
Table 3.
Effect of aged garlic extract on lipid profile in diabetic rats
Effect of AGE on Kidney Histology
Figures 1 and 2 illustrate the changes in the kidney histology of the animals in all the four groups. The diabetic rats showed changes such as hypercellularity, glycosuria, and proteinuria in tubules with mild mesangial expansion and proliferation in the glomeruli. Prominent nodular glomerulosclerosis with glomerular basement membrane thickening was also observed. However, minimal changes were observed in the diabetic animals that were supplemented with AGE and aminoguanidine, which proves that AGE has the potential to ameliorate diabetic nephropathy [Figure 3].
Figure 1.
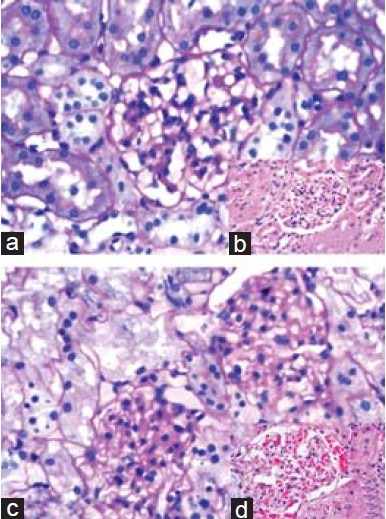
Histopathology images of kidney from control and diabetic rats. Kidney histology from control and diabetic rats. (a): control rats showing normal renal parenchyma, PAS, × 400; (b) (insert): control rats showing normal renal parenchyma, H and E, × 400; (c): diabetic rats showing mesangial expansion and proliferation with increase in the glomerular capillary thickening. Tubules showing evidence of glycosuria, PAS, × 400; (d) (insert): section showing nodular glomerulosclerosis and hypercellularity, H and E, × 400
Figure 2.
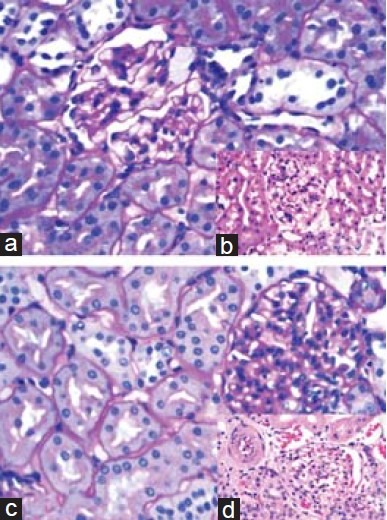
Histopathology images of kidney from diabetic rats supplemented with aminoguanidine and aged garlic extract. Kidney histology from diabetic rats supplemented with aminoguanidine (a, b) and garlic extract (c, d). (a): section showing mild mesangial proliferation with normal glomerular capillary basement membrane thickening. Tubules and interstitium are within normal limits, PAS, × 400. (b) (insert): section showing mild mesangial proliferation with no mesangial nodules. Tubules and interstitium are within normal limits, HandE, × 400. (c, d (insert)): section showing glomerulus with normal cellularity and membrane thickness. Tubules, interstitium and blood vessels are within normal limits, H and E, PAS respectively, × 400
Figure 3.
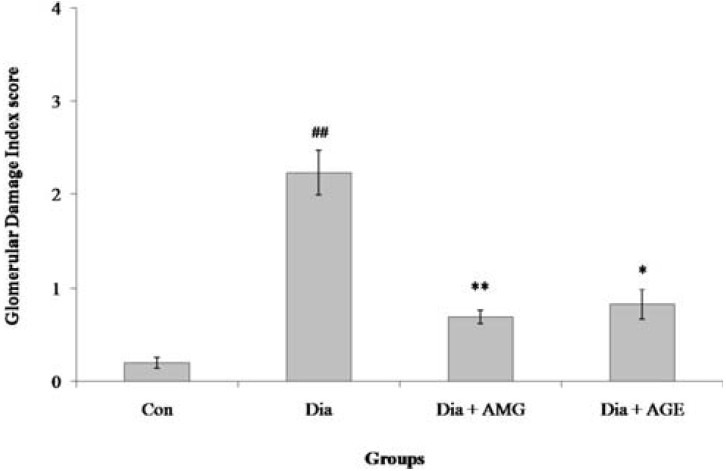
Semiquantitative morphometric analysis of glomerular damage index. Figure summarizes the glomerular damage index (GDI) of animals in all the four groups. GDI was calculated on PAS stained sections at a magnification of ×400 with a scoring system of 0 to 4. 0 represents no lesion; 1+ represents sclerosis of <25% of the glomerulus; 2+ represents sclerosis of 25-50% of the glomerulus; 3+ represents 50-75% of the glomerulus and 4+ represents sclerosis of >75% of the glomerulus. Values represent the mean ± standard deviation (n= 4) of the samples. Significant difference between Con and Dia groups: #p< 0.05; ##p< 0.01; ###p< 0.001. Significant difference between Dia and Dia+AMG, Dia and Dia+AGE groups: *p< 0.05; ** p< 0.01; ***p< 0.001
Discussion
This study explored the effect of Kyolic® Aged garlic extract on DNP induced Wistar rats. The formation of advanced glycation end products induced by hyperglycemia and hyperlipidemia are considered to play the major role in causing DNP.[3] So any therapeutic intervention targeting this would be a better treatment for DNP. This study has exploited the potential of AGE in attenuating DNP.
Various pharmacological effects of AGE have been identified that include its antioxidant,[5] anti-thrombotic,[6] anti-cancer,[16] hypoglycemic,[10] hypolipidemic and hypocholesterolemic activities.[17] Since AGE constitutes a mixture of phytochemicals, it is not surprising that it has tremendous biological effects. Though the active principle responsible for these activities is not exactly known, reports from various studies show that these activities are pertained to S-allyl cysteine, a major constituent of AGE.[10] Apart from this, the other beneficial constituents of AGE are allin, γ-glutamyl cysteine and S-allyl mercaptocysteine. The most common side effect of AGE supplementation is weight loss[15] which was observed in our study.
S-allyl cysteine (SAC) which is a major component of AGE has been proven to be an effective antidiabetic agent.[15] However, in our study, we did not observe any hypoglycemic effect for AGE. Though, there is no hypoglycemic effect in AGE, its anti-glycation effect is noteworthy. The glycated hemoglobin content of the diabetic animals that were supplemented with AGE decreased significantly compared to that of the diabetic rats. We presume that the supplementation of AGE would have prevented amadori product formation, which subsequently decreases free radical generation for protein oxidation.
AGE supplementation has significantly reduced the level of triglycerides (p < 0.05), total cholesterol (p < 0.01), and LDL-cholesterol (p < 0.01) with a small increase (p < 0.05) in the HDL-cholesterol level [Table 3]. This proved the hypolipidemic activity of AGE, which was supported by several other independent investigations.[18,19] The lipid lowering effect of AGE may be attributed to the di-allyl, di-sulphide, tri-sulphide compounds of garlic which have the ability to inhibit the activity of HMG-CoA reductase.[20] In a few other studies, it was found that the organosulphur compounds present in garlic have the ability to reduce the lipoprotein modification in vitro and in vivo. Since TGF-β induced by lipoprotein modification also plays a major role in the pathogenesis of DNP,[21] the role of AGE in inhibiting lipoprotein modification might be important along with its hypolipidemic effect in attenuating DNP.
AGE has been proven to inhibit the expression of CD36 on human macrophages in vitro. CD36 is a receptor protein that uptakes the glycated proteins and modified lipoproteins, thus mediating the apoptosis of proximal tubular epithelial cells.[22] Since CD36 has been predicted to play a direct role in causing tubular damage in diabetic patients, inhibition of CD36 expression might also contribute to the protective effect of AGE on DNP. However, further research is required to validate our presumption, which is underway.
In diabetic patients, when there is any damage to the glomerulus or the tubules, the proteins that have to be retained in the blood leak out in the urine. Since albumin is the most common protein present in the blood, it is released easily in the urine and is used as a forerunner for DNP.[23] Further, the kidney damage is reflected by an increase in the level of metabolic wastes such as urea and creatinine in the blood. In our study, the biochemical analyses showed evidence for albuminuria and kidney damage, which is reflected by the altered level of creatinine and urea nitrogen in both blood and urine of diabetic rats. This proved that the diabetic rats have progressed to diabetic nephropathy. However, the diabetic rats that were treated with AGE showed a significant decrease in albuminuria (p < 0.01) with a significant increase in urinary urea (p < 0.01) and creatinine content (p < 0.05). This proved that the AGE supplementation has ameliorated kidney damage in diabetic rats, thus attenuating DNP.
In accordance with the results obtained in biochemical analyses, the histological analysis in diabetic rats supplemented with AGE showed mild mesangial expansion with no change in the glomerular basement membrane. Further, the tubules, interstitium and blood vessels were within normal limits, which revealed the efficacy of AGE in treating DNP. Several investigators have proven the renoprotective effect of AGE on nephrectomised[24–26] and nephrotoxic rat models.[27–29] By virtue of its anti-oxidant property, AGE was able to render renoprotection in these models by attenuating oxidative and nitrosative stress. So it was speculated that the renoprotective effect of AGE might be due to its antioxidant property. However, we found that, apart from antioxidant effect, anti-glycation and hyolipidemic activity of AGE also plays a role in attenuating DNP.
Conclusion
In conclusion, our results suggest that AGE supplementation has the ability to ameliorate kidney damage, thus attenuating DNP in Wistar rats. The biochemical results were validated by our histological findings that the diabetic rats supplemented with AGE showed minimal changes, compared to that of the untreated diabetic rats. The protective effect of AGE on DNP may be attributed to its anti-glycation, hypolipidemic effects. The compound responsible for this attenuating effect might be S-allyl cysteine, which is a major constituent of AGE. SAC has been proven to inhibit the expression of CD36 along with its antioxidant and anti-glycation properties. Further research is required to determine the active principle responsible for the attenuating effect of AGE on DNP.
Acknowledgment
Shiju TM is thankful to Council of Scientific and Industrial Research (CSIR), New Delhi, India for the financial assistance in the form of Senior Research Fellow. Authors are thankful to the VIT University for their financial support for this entire study. Authors are also thankful to Mr. N. S. Prasanthkumar, Assistant Professor, School of Social Sciences and Language, VIT University for his help in grammatical and typographical corrections.
Footnotes
Source(s) of Support: VIT University, Vellore
Conflicting Interest: None declared
References
- 1.American Diabetes Association. Nephropathy in diabetes. Diabetes Care. 2004;27:S79–83. doi: 10.2337/diacare.27.2007.s79. [DOI] [PubMed] [Google Scholar]
- 2.Viswanathan V. Prevention of Diabetic Nephropathy: A diabetologist's perspective. Indian J Nephrol. 2004;14:157–62. [Google Scholar]
- 3.Rosario RF, Prabhakar S. Lipids and diabetic nephropathy. Curr Diab Rep. 2006;6:455–62. doi: 10.1007/s11892-006-0079-7. [DOI] [PubMed] [Google Scholar]
- 4.Morihara N, Ide N, Weiss N. Aged garlic extract inhibits scavenger receptor CD36 expression and oxidized LDL cholesterol uptake on human macrophages in vitro. J Ethnopharmacol. 2001;12:711–6. doi: 10.1016/j.jep.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Moriguchi T, Takasugi N, Itakura Y. The effects of aged garlic extract on lipid peroxidation and the deformability of erythrocytes. J Nutr. 2001;131:1016S–9S. doi: 10.1093/jn/131.3.1016S. [DOI] [PubMed] [Google Scholar]
- 6.Fukao H, Yoshida H, Tazawa Y, Hada T. Antithrombotic effects of odorless garlic powder both in vitro and in vivo. Biosci Biotechnol Biochem. 2007;71:84–90. doi: 10.1271/bbb.60380. [DOI] [PubMed] [Google Scholar]
- 7.Fleischauer AT, Arab L. Garlic and cancer: A critical review of the epidemiologic literature. J Nutr. 2001;131:1032S. doi: 10.1093/jn/131.3.1032S. [DOI] [PubMed] [Google Scholar]
- 8.Lau BH. Suppression of LDL oxidation by garlic compounds is a possible mechanism of cardiovascular health benefit. Nutrition. 2006;136:765S–8. doi: 10.1093/jn/136.3.765S. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoodi M, Islami MR, Asadi Karam GR, Khaksari M, Sahebghadam Lotfi A, Hajizadeh MR, et al. Study of the effects of raw garlic consumption on the level of lipids and other blood biochemical factors in hyperlipidemic individuals. Pak J Pharm Sci. 2006;19:295–8. [PubMed] [Google Scholar]
- 10.Saravanan G, Ponmurugan P, Kumar GPS, Rajarajan T. Anti-diabetic properties of S-allylcysteine, a garlic component on streptozotocin induced diabetes in rats. J App Biomed. 2009;7:151–9. [Google Scholar]
- 11.Omotoso GO, Saibu SA, Akinlolu A, Kadir E. Effects of aqueous extract of garlic and vitamin C on the kidney of albino rats. Asian J Exp Biol Sci. 2011;2:455–61. [Google Scholar]
- 12.Farrell SC, Bailey MP. Measurement of creatinine in peritoneal dialysis fluid. Ann Clin Biochem. 1991;28:624–5. doi: 10.1177/000456329102800616. [DOI] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;226:499–502. [PubMed] [Google Scholar]
- 14.Raji L, Azar S, Keane W. Mesangial immune injury, hypertension and progressive glomerular damage in Dahl rats. Kidney Int. 1984;26:137–43. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- 15.Morbidoni L, Arterburn JM, Young V, Mullins D, Mulrow C, Lawrence V. Garlic: Its history and adverse effects. J Herb Pharmacother. 2001;1:63–83. [Google Scholar]
- 16.Yeh YY, Liu L. Cholesterol lowering effect of garlic extracts and organosulphur compounds: Human and animal studies. J Nutr. 2001;131:989S–93S. doi: 10.1093/jn/131.3.989S. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Jiao F, Wang QW, Wang J, Yang K, Hu RR et al. Aged black garlic extract induces inhibition of gastric cancer cell growth in vitro and in vivo. Mol Med Rep. 2012;5:66–72. doi: 10.3892/mmr.2011.588. [DOI] [PubMed] [Google Scholar]
- 18.Stevinson C, Pittler MH, Ernst E. Garlic for treating hypercholesterolemia. A meta analysis of randamosid clinical trials. Ann Intern Med. 2000;133:420–9. doi: 10.7326/0003-4819-133-6-200009190-00009. [DOI] [PubMed] [Google Scholar]
- 19.Natural Standard Research Collaboration. An evidence based review of garlic and its hypolipidemic properties. Nat Med J. 2010;2:1–7. [Google Scholar]
- 20.Liu L, Yeh YY. Inhibition of cholesterol biosynthesis by organosulphur compounds derived from garlic. Lipids. 2000;35:197–203. doi: 10.1007/BF02664770. [DOI] [PubMed] [Google Scholar]
- 21.Nakhjavani M, Esteghamati A, Khalizadeh O, Asgarani F, Mansournia N, Abbasi M. Association of macroalbuminuria with oxidized LDL and TGF-b in type 2 diabetic patients: A case control study. Int Urol Nephrol. 2010;242:487–92. doi: 10.1007/s11255-009-9643-9. [DOI] [PubMed] [Google Scholar]
- 22.Susztak K, Ciccone E, McCue P, Sharma K, Bottinger EP. Multiple metabolic hits converge on CD36 as novel mediator of Tubular Epithelial Apoptosis in Diabetic nephropathy. PLoS Med. 2005;2:152–61. doi: 10.1371/journal.pmed.0020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caramori ML, Fioretto P, Mauer M. The need for early predictors of diabetic nephropathy risk: Is albumin excretion rate sufficient? Diabetes. 2000;49:1399–408. doi: 10.2337/diabetes.49.9.1399. [DOI] [PubMed] [Google Scholar]
- 24.Cruz C, Correa-Rotter R, Sanchez-Gonzalez DJ, Hernandez-Pando R, Maldonado PD, Martinez-Martinez CM, et al. Renoprotective and antihypertensive effects of S-allylcysteine in 5/6 nephrectomized rats. Am J Physiol Renal Physiol. 2007;293:F1691–8. doi: 10.1152/ajprenal.00235.2007. [DOI] [PubMed] [Google Scholar]
- 25.Bautista-Garcia P, Sanchez-Lozada LG, Cristobal-Garcia M, Tapia E, Soto V, Avila-Casado MC, et al. Chronic inhibition of NOS-2 ameliorates renal inhury, as well as COX-2 and TGF-beta 1 overexpression in 5/6 nephrectomized rats. Nephrol Dial Transplant. 2006;21:3074–81. doi: 10.1093/ndt/gfl444. [DOI] [PubMed] [Google Scholar]
- 26.Fujihara CK, Antunes GR, Mattar AL, Malheiros DM, Vieira JM, Jr, Zatz R. Chronic inhibition of nuclear factor-kappa B attenuates renal injury in the 5/6 renal ablation model. Am J Physiol Ren Physiol. 2007;292:F92–9. doi: 10.1152/ajprenal.00184.2006. [DOI] [PubMed] [Google Scholar]
- 27.Maldonado PD, Barrera D, Medina-Campos ON, Hernandez-Pando R, Ibarra-Rubio ME, Pedraza-Chaverri J. Aged garlic extract attenuates gentamicin induced renal damage and oxidative stress in rats. Lif Sci. 2003;73:2543–56. doi: 10.1016/s0024-3205(03)00609-x. [DOI] [PubMed] [Google Scholar]
- 28.Wongmekiat O, Thamprasert K. Investigating the protective effects of aged garlic extract on cyclosporine induced nephrotoxicity in rats. Fundam Clin Pharmacol. 2005;19:555–62. doi: 10.1111/j.1472-8206.2005.00361.x. [DOI] [PubMed] [Google Scholar]
- 29.Kabasakal L, Sehiril O, Cetinel S, Cikler E, Gedik N, Sener G. Protective effect of aqueous garlic extract against renal ischemia/reperfusion injury in rats. J Med Food. 2005;8:319–26. doi: 10.1089/jmf.2005.8.319. [DOI] [PubMed] [Google Scholar]


