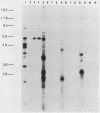
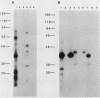
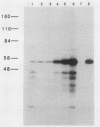
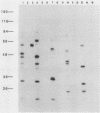
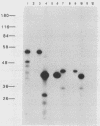
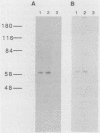
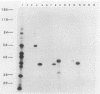
Abstract
It has previously been demonstrated that the simian virus 40 large T antigen and adenovirus E1A proteins can form complexes with the retinoblastoma susceptibility gene product (RB). We studied the ability of these proteins to bind to mutant RB proteins in vitro. A region of RB spanning residues 379 to 792 was found to be both necessary and sufficient for binding to T or E1A. Furthermore, this region of RB contains sufficient structural information to mimic wild-type RB in its ability to distinguish between wild-type T and the transformation-defective T mutant K1. The results of competition experiments with peptide analogs of the RB-binding sequence in T suggest that this region of RB makes direct contact with a short colinear region of T, i.e., residues 102 to 115, previously implicated in both transformation and RB binding.
Full text
PDF

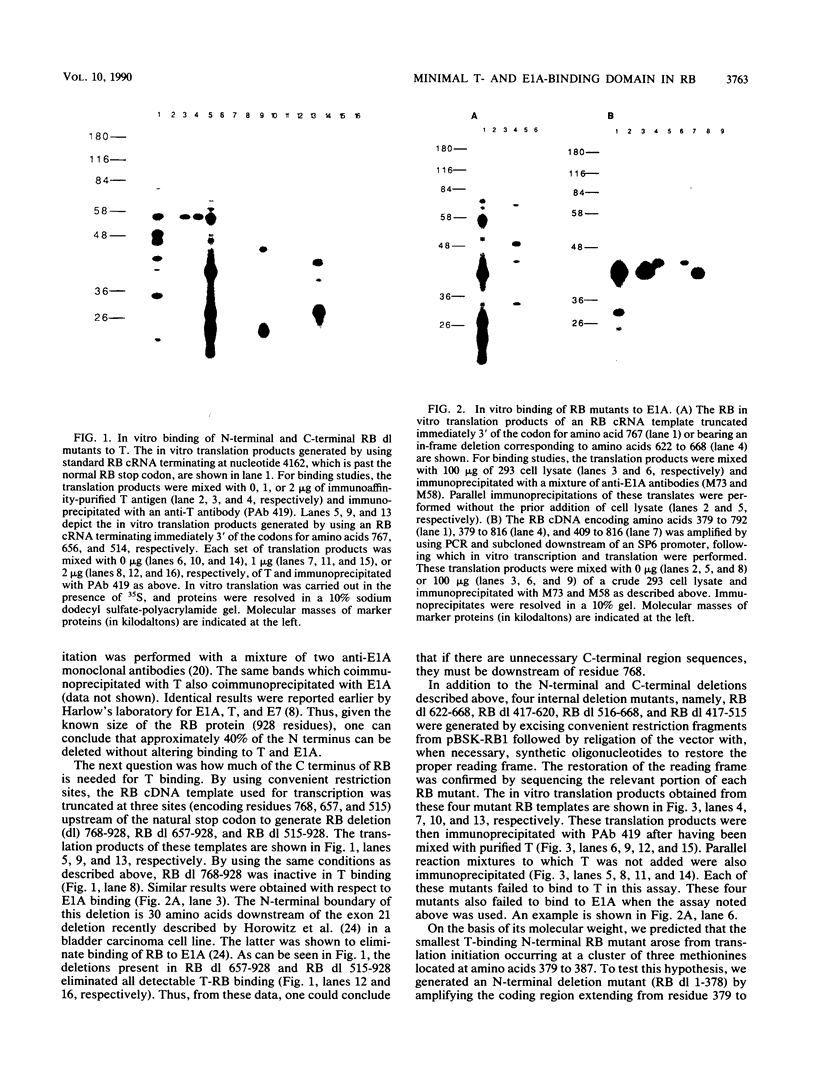




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedict W. F., Murphree A. L., Banerjee A., Spina C. A., Sparkes M. C., Sparkes R. S. Patient with 13 chromosome deletion: evidence that the retinoblastoma gene is a recessive cancer gene. Science. 1983 Feb 25;219(4587):973–975. doi: 10.1126/science.6336308. [DOI] [PubMed] [Google Scholar]
- Bernards R., Shackleford G. M., Schackleford G. M., Gerber M. R., Horowitz J. M., Friend S. H., Schartl M., Bogenmann E., Rapaport J. M., McGee T. Structure and expression of the murine retinoblastoma gene and characterization of its encoded protein. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6474–6478. doi: 10.1073/pnas.86.17.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Lynch D., Furukawa Y., Griffin J., Piwnica-Worms H., Huang C. M., Livingston D. M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989 Sep 22;58(6):1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Cavenee W., White R., Rapaport J. M., Petersen R., Albert D. M., Bruns G. A. Homozygosity of chromosome 13 in retinoblastoma. N Engl J Med. 1984 Mar 1;310(9):550–553. doi: 10.1056/NEJM198403013100902. [DOI] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Ludlow J. W., Marsilio E., DeCaprio J. A., Millikan R. C., Cheng S. H., Paucha E., Livingston D. M. An N-terminal transformation-governing sequence of SV40 large T antigen contributes to the binding of both p110Rb and a second cellular protein, p120. Cell. 1989 Jul 28;58(2):257–267. doi: 10.1016/0092-8674(89)90840-4. [DOI] [PubMed] [Google Scholar]
- Figge J., Smith T. F. Cell-division sequence motif. Nature. 1988 Jul 14;334(6178):109–109. doi: 10.1038/334109a0. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Dryja T. P., Weinberg R. A. Oncogenes and tumor-suppressing genes. N Engl J Med. 1988 Mar 10;318(10):618–622. doi: 10.1056/NEJM198803103181007. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Horowitz J. M., Gerber M. R., Wang X. F., Bogenmann E., Li F. P., Weinberg R. A. Deletions of a DNA sequence in retinoblastomas and mesenchymal tumors: organization of the sequence and its encoded protein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9059–9063. doi: 10.1073/pnas.84.24.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Godbout R., Dryja T. P., Squire J., Gallie B. L., Phillips R. A. Somatic inactivation of genes on chromosome 13 is a common event in retinoblastoma. Nature. 1983 Aug 4;304(5925):451–453. doi: 10.1038/304451a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Green M. R. When the products of oncogenes and anti-oncogenes meet. Cell. 1989 Jan 13;56(1):1–3. doi: 10.1016/0092-8674(89)90975-6. [DOI] [PubMed] [Google Scholar]
- Harbour J. W., Lai S. L., Whang-Peng J., Gazdar A. F., Minna J. D., Kaye F. J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Franza B. R., Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985 Sep;55(3):533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H. The analysis of malignancy by cell fusion: the position in 1988. Cancer Res. 1988 Jun 15;48(12):3302–3306. [PubMed] [Google Scholar]
- Hong F. D., Huang H. J., To H., Young L. J., Oro A., Bookstein R., Lee E. Y., Lee W. H. Structure of the human retinoblastoma gene. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5502–5506. doi: 10.1073/pnas.86.14.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Yandell D. W., Park S. H., Canning S., Whyte P., Buchkovich K., Harlow E., Weinberg R. A., Dryja T. P. Point mutational inactivation of the retinoblastoma antioncogene. Science. 1989 Feb 17;243(4893):937–940. doi: 10.1126/science.2521957. [DOI] [PubMed] [Google Scholar]
- Hu Q. J., Dyson N., Harlow E. The regions of the retinoblastoma protein needed for binding to adenovirus E1A or SV40 large T antigen are common sites for mutations. EMBO J. 1990 Apr;9(4):1147–1155. doi: 10.1002/j.1460-2075.1990.tb08221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Smith A. E. In vitro mutagenesis of a putative DNA binding domain of SV40 large-T. Virology. 1984 Nov;139(1):109–137. doi: 10.1016/0042-6822(84)90334-9. [DOI] [PubMed] [Google Scholar]
- Klein G. The approaching era of the tumor suppressor genes. Science. 1987 Dec 11;238(4833):1539–1545. doi: 10.1126/science.3317834. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. Leucine zippers of fos, jun and GCN4 dictate dimerization specificity and thereby control DNA binding. Nature. 1989 Aug 17;340(6234):568–571. doi: 10.1038/340568a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987 Aug 20;196(4):947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., To H., Shew J. Y., Bookstein R., Scully P., Lee W. H. Inactivation of the retinoblastoma susceptibility gene in human breast cancers. Science. 1988 Jul 8;241(4862):218–221. doi: 10.1126/science.3388033. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Shew J. Y., Hong F. D., Sery T. W., Donoso L. A., Young L. J., Bookstein R., Lee E. Y. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987 Oct 15;329(6140):642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- Ludlow J. W., DeCaprio J. A., Huang C. M., Lee W. H., Paucha E., Livingston D. M. SV40 large T antigen binds preferentially to an underphosphorylated member of the retinoblastoma susceptibility gene product family. Cell. 1989 Jan 13;56(1):57–65. doi: 10.1016/0092-8674(89)90983-5. [DOI] [PubMed] [Google Scholar]
- Ludlow J. W., Shon J., Pipas J. M., Livingston D. M., DeCaprio J. A. The retinoblastoma susceptibility gene product undergoes cell cycle-dependent dephosphorylation and binding to and release from SV40 large T. Cell. 1990 Feb 9;60(3):387–396. doi: 10.1016/0092-8674(90)90590-b. [DOI] [PubMed] [Google Scholar]
- McGee T. L., Yandell D. W., Dryja T. P. Structure and partial genomic sequence of the human retinoblastoma susceptibility gene. Gene. 1989 Aug 1;80(1):119–128. doi: 10.1016/0378-1119(89)90256-4. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Murphree A. L., Benedict W. F. Retinoblastoma: clues to human oncogenesis. Science. 1984 Mar 9;223(4640):1028–1033. doi: 10.1126/science.6320372. [DOI] [PubMed] [Google Scholar]
- Murphy C. I., Weiner B., Bikel I., Piwnica-Worms H., Bradley M. K., Livingston D. M. Purification and functional properties of simian virus 40 large and small T antigens overproduced in insect cells. J Virol. 1988 Aug;62(8):2951–2959. doi: 10.1128/jvi.62.8.2951-2959.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shew J. Y., Chen P. L., Bookstein R., Lee E. Y., Lee W. H. Deletion of a splice donor site ablates expression of the following exon and produces an unphosphorylated RB protein unable to bind SV40 T antigen. Cell Growth Differ. 1990 Jan;1(1):17–25. [PubMed] [Google Scholar]
- Shew J. Y., Lin B. T., Chen P. L., Tseng B. Y., Yang-Feng T. L., Lee W. H. C-terminal truncation of the retinoblastoma gene product leads to functional inactivation. Proc Natl Acad Sci U S A. 1990 Jan;87(1):6–10. doi: 10.1073/pnas.87.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanis V., Lane D. P. An immunoaffinity purification procedure for SV40 large T antigen. Virology. 1985 Jul 15;144(1):88–100. doi: 10.1016/0042-6822(85)90308-3. [DOI] [PubMed] [Google Scholar]
- Skuse G. R., Rowley P. T. Tumor suppressor genes and inherited predisposition to malignancy. Semin Oncol. 1989 Apr;16(2):128–137. [PubMed] [Google Scholar]
- Sparkes R. S., Murphree A. L., Lingua R. W., Sparkes M. C., Field L. L., Funderburk S. J., Benedict W. F. Gene for hereditary retinoblastoma assigned to human chromosome 13 by linkage to esterase D. Science. 1983 Feb 25;219(4587):971–973. doi: 10.1126/science.6823558. [DOI] [PubMed] [Google Scholar]
- T'Ang A., Varley J. M., Chakraborty S., Murphree A. L., Fung Y. K. Structural rearrangement of the retinoblastoma gene in human breast carcinoma. Science. 1988 Oct 14;242(4876):263–266. doi: 10.1126/science.3175651. [DOI] [PubMed] [Google Scholar]
- Varley J. M., Armour J., Swallow J. E., Jeffreys A. J., Ponder B. A., T'Ang A., Fung Y. K., Brammar W. J., Walker R. A. The retinoblastoma gene is frequently altered leading to loss of expression in primary breast tumours. Oncogene. 1989 Jun;4(6):725–729. [PubMed] [Google Scholar]
- Vinson C. R., Sigler P. B., McKnight S. L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989 Nov 17;246(4932):911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- Weichselbaum R. R., Beckett M., Diamond A. Some retinoblastomas, osteosarcomas, and soft tissue sarcomas may share a common etiology. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2106–2109. doi: 10.1073/pnas.85.7.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Yandell D. W., Campbell T. A., Dayton S. H., Petersen R., Walton D., Little J. B., McConkie-Rosell A., Buckley E. G., Dryja T. P. Oncogenic point mutations in the human retinoblastoma gene: their application to genetic counseling. N Engl J Med. 1989 Dec 21;321(25):1689–1695. doi: 10.1056/NEJM198912213212501. [DOI] [PubMed] [Google Scholar]
- Yokota J., Akiyama T., Fung Y. K., Benedict W. F., Namba Y., Hanaoka M., Wada M., Terasaki T., Shimosato Y., Sugimura T. Altered expression of the retinoblastoma (RB) gene in small-cell carcinoma of the lung. Oncogene. 1988 Oct;3(4):471–475. [PubMed] [Google Scholar]