Abstract
Objectives:
Cardioprotective activity of alcoholic extract of Saraca indica (SI) bark was investigated against cyclophosphamide induced cardiotoxicity.
Materials and Methods:
Cardiotoxicity was induced in Wistar rats by administering cyclophosphamide (200 mg/kg, i.p.) single injection on first day of experimental period. Saraca indica (200 and 400 mg/kg, p.o.) was administered immediately after administration of cyclophosphamide on first day and daily for 10 days. The general observations and mortality were measured.
Results:
Cyclophosphamide administration significantly (p < 0.05) increased lipid peroxidation (LPO) and decreased the levels of antioxidant markers such as reduced glutathione (GSH), superoxide dismutase (SOD) and catalase (CAT). Cyclophosphamide elevated the levels of biomarker enzymes like creatine kinase (CK), creatine kinase isoenzyme MB (CK-MB), lactate dehydrogenase (LDH), aspartate transaminase (AST), alanine transaminase (ALT) and alkaline phosphatase (ALP). Further, the cyclophosphamide treated rats showed changes in electrocardiogram (ECG) along with increased levels of cholesterol and triglycerides. Treatment with Saraca indica significantly (p < 0.05) reversed the status of cardiac biomarkers, ECG, oxidative enzymes and lipid profile in cyclophosphamide induced cardiotoxicity. Potential cardioprotective effect of Saraca indica was supported by histopathological examination that reduced severity of cellular damage of the myocardium.
Conclusion:
The biochemical, ECG and histopathology reports support the cardioprotective effect of Saraca indica which could be attributed to antioxidant activity.
KEY WORDS: Cardioprotective, cyclophosphamide, ECG, free radicals, Saraca indica Linn
Introduction
Cyclophosphamide (CP) is widely used as an antineoplastic and immunosuppressant agent. It is used for the treatment of chronic and acute leukemias, multiple myeloma, lymphomas, rheumatic arthritis and in preparation for bone marrow transplantation.[1] Although it has tumor selectivity and wide spectrum of clinical uses, CP is known to cause multiple organ toxicity.[2] High dose of CP can cause an acute type of cardiotoxicity within 10 days of its administration which presents as a combination of symptoms and signs of myopericarditis leading to fatal complications such as congestive heart failure, arrhythmias, cardiac tamponade and myocardial depression.[1]
Cyclophosphamide rapidly impairs cellular respiration and also damages the inner mitochondrial membrane of heart leading to the permeability of calcium ions mediated by oxidative stress.[3] Cyclophosphamide induced cardiotoxicity has been implicated to increase the generation of superoxide radicals and hydrogen peroxide. These reactive oxygen species (ROS) damage the heart by exceeding the oxygen radical detoxifying capacity of cardiac mitochondria.[4] Therefore, the antioxidant therapy may be useful in the management of CP induced cardiotoxicity.
Saraca indica (SI) is useful as heart tonic, hypoglycemic agent,[5] in treatment of abdominal pain, tumors, AIDS, inflammation,[6] and cancer.[7] Saraca indica contains phytochemical constituents like flavonoids, (−)-epicatechin, epiafzelechin-(4β→8)-epicatechin and procyanidin B2, n-octacosenol, β-sitosterol, glycoside, tannins and saponins.[8] Protective effect of epicatechin on myocardial ischemia-reperfusion injury .[9] There is paucity of scientific evidence available proving the cardioprotective activity of Saraca indica Linn. Hence, the present study was designed to evaluate the cardioprotective activity of ethanol extract Saraca indica.
Materials and Methods
Preparation of Saraca Indica Extract
The bark of SI was collected from the Kulgi national park, Dandeli, Karnataka which was identified and authenticated by a botanist. The bark was cut into small pieces and shade dried. It was then coarsely powdered and extracted using ethanol (90%) in a soxhlet extractor, concentrated in reduced pressure and stored in desiccators.
Animals
Male Wistar albino rats (150–200 g) were used. They were housed in clean polypropylene cages under standard conditions of temperature (25 ± 2 °C) and 12 h light/12 h dark cycle and fed with standard diet (Gold Mohur, Lipton India Ltd.) and water ad libitum. Experimental protocol was reviewed and approved by the Institutional Animal Ethics Committee.
Experimental Treatment
Animals divided into five groups of six animals each. Group I served as normal control and administered 1 ml per kg body weight (p.o.) of 0.5 % carboxymethyl cellulose (CMC) daily for 10 days. Group 2 served as cyclophosphamide control where animals were administered with a single dose of CP (200 mg/kg, i.p.) on the first day of experimental period. Group 3 animals were treated with ethanol extract of Saraca indica at (200 mg/kg p.o.) alone for 10 days. Group 4 animals received single dose of CP (200 mg/kg i.p.) on first day followed by the administration of ethanol extract (200 mg/kg) of SI continuously for 10 days. Group 5 animals were daily administered SI ethanol extract (400 mg/kg) of for 10 days immediately after a single dose of CP (200 mg/kg i.p.) on first day.
Oxidative Marker Enzymes Assay
Animals were sacrificed 24 h after the last dose by mild ether anesthesia. Blood was collected for the separation of serum and analysed for creatine kinase (CK), creatine kinase isoenzyme MB (CK-MB), lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotrasferase (ALT) and alkaline phosphatase (ALP) by using commercial kits by ERBA diagnostics Mannheim GmbH. The heart tissue was dissected out immediately, washed with ice cold saline and used for the determination of lipid peroxidation (LPO),[10] reduced glutathione (GSH),[11] superoxide dismutase (SOD)[12] and catalase (CAT).[13]
Lipid Profile Assay
Serum cholesterol and triglyceride were analyzed by using commercial kits by ERBA diagnostics Mannheim GmbH.
ECG Pattern
Animals were anesthetized one day before sacrifice and ECG was recorded using Biopac MP-35 model system.
Histopathological Investigations
The heart tissue was dissected and fixed in 10% formalin. The paraffin sections were prepared and stained with haematoxylin and eosin for the examination using the light microscope.
Statistical Analysis
The data was expressed as the mean ± SEM. One-way analysis of variance (ANOVA) was used for comparisons between different groups followed by Dunnett's-t test. Data was computed for statistical analysis using Graph Pad Prism 5 Software and p < 0.05 was considered to be significant.
Results
Protective effects of Saraca indica (SI) against cyclophosphamide (CP) was established by observing the general behaviour, body weight, heart weight, relative heart weight, cardiac biomarker enzymes, endogenous antioxidants, ECG recording and cardiac histopathology.
General observations: During the treatment period, CP treated animals developed a pink tinge, alopecia and some dental abnormalities. These rats also had red exudates around the eyes and nose along with soft watery faces. These changes were less in Saraca indica treated groups.
Body weight, heart weight and relative heart weight: Protective effects of SI against CP induced changes in the body weight, heart weight and heart to body weight ratio is shown in Table 1. CP treatment significantly (p < 0.001) decreased the body weight, increased the heart rate and heart to body ratio as compared to normal rats. Treatment with SI did not produce any effect on its own. SI produced a significant dose dependant protection against the CP induced changes (p < 0.01 and p < 0.001) by increase in the body weight and significantly (p < 0.001) decreased the heart weight and relative heart weight at 400 mg/kg.
Table 1.
Effect of Saraca indica (SI) on body weight, heart weight and relative heart weight in cyclophosphamide (CP) induced cardiotoxicity in rats
Antioxidant status and lipid profile: Cyclophosphamide has significantly enhanced the levels of CPK, CK-MB, LDH, AST, ALT and ALP when compared with normal rats [Table 2]. Saraca indica (SI) treated rats significantly (p < 0.05 and p < 0.001 for 200 and 400 mg/kg) decreased the CPK, CK-MB, LDH, AST, ALT and ALP in dose dependent fashion compared to CP treated group. The level of MDA was significantly (p < 0.001) increased in CP treated rats compared with normal control. Rats treated with 200 mg/kg (p < 0.01) and 400 mg/kg (p < 0.001) of SI ethanol extract significantly decreased the level of lipid peroxidation (LPO) when compared with CP treated group. A significant (p < 0.001) decrease in non-enzymatic antioxidant, reduced glutathione (GSH) was observed in rats treated with CP compared to normal control. Treatment with 200 (p < 0.05) and 400 mg/kg (p < 0.001) of ethanol extract of SI significantly elevated the GSH, catalase (CAT), superoxide dismutase (SOD) levels when compared to CP treated group. Saraca indica (200 and 400 mg/kg) significantly decreased cholesterol and triglyceride levels as compared to CP group [Table 3].
Table 2.
Effect of Saraca indica (SI) on cardiac marker enzymes CK, CK-MB, LDH, AST, ALT and ALP in cyclophosphamide (CP) induced cardiotoxicity in rats

Table 3.
Effect of Saraca indica (SI) on LPO, GSH, catalase, SOD, cholesterol and triglyceride levels in cyclophosphamide (CP) induced cardiotoxicity in rats
ECG pattern— ECG pattern showed P wave inversion, prolongation of QT and PR interval, decrease in heart rate and R wave amplitude in CP induced cardiotoxicity. Treatment with SI corrected the CP induced change in ECG to normal [Table 4 and Figure 1].
Table 4.
Effect of Saraca indica (SI) on ECG pattern in cyclophosphamide (CP) induced cardiac damage in rats

Figure 1.

Effect of Saraca indica (SI) on electrocardiographic patterns in cyclophosphamide (CP) induced cardiotoxicity in (a) normal (b1) CP treated (P wave inversion) (b2) CP treated (decreased BPM with P wave inversion (c) SI treated (d) CP+SI (200 mg/kg) treated and (e) CP+SI (400 mg/kg) treated rats
Histopathological observations—Control rats showed appearance of enlarged, swollen mitochondria and vacuoles. CP produced massive change in the myocardium showing a varying degree of vacuolar changes in the cardiac muscle fibers mainly in the form of degeneration of myocardial tissue, vacuolization of the cardiomyocytes, infiltration of inflammatory cells and myofibrillar loss. Saraca indica, provided dose dependant protection against CP induced myocardial damage [Figure 2].
Figure 2.
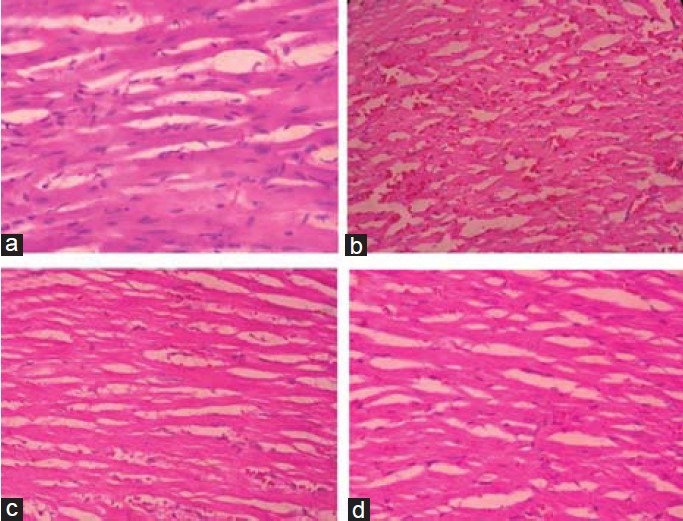
Histopathological evaluation of heart tissue in (a) normal (b) CP treated (c) CP+SI (200mg/kg) treated and (d) CP+SI (400mg/ kg) treated rats
Discussion
The CP treatment increased heart weight and decreased body weight in rats. The significant increase in mortality rate, decrease body and heart weight in the CP-treated group indicates that the general metabolic functions of the animals are in deranged and demonstrates the toxicity of CP as reported previously.[14] Increase in the heart weight might be due to increased oedema, vascular hemorrhage and/or extensive necrosis of cardiac muscle fibres followed by invasion of damaged tissues with inflammatory cells.[15] Treatment with Saraca indica showed dose dependent decrease in heart weight, relative heart weight and increase in body weight compared to CP group indicating cardioprotective effect.
Cyclophosphamide is a cardiotoxic agent due to its debilitating actions leading to direct myocardial endothelial damage and destruction of myocardial cells. As a result, CK, CK-MB, LDH, ALT, AST and ALP are released into the blood stream and serve as the diagnostic markers of myocardial tissue damage.[16] The elevated levels of these enzymes are associated with certain types of heart damage such as myocardial infarction, myocarditis and heart failure. The administration of SI showed dose dependent reduction in CP induced elevated biomarkers of cardiac injury. This effect confirms that SI is responsible for restricting the leakage of biochemical markers due to its membrane stabilizing property. This action of SI could be attributable to its phytoconstituents such as β-sitosterol and epicatechin which are known to reduce the risk of heart failure.[17]
Cyclophosphamide induces free radicals, which may cause cellular cholesterol accumulation; (a) by increasing cholesterol biosynthesis and its esterification, (b) by decreasing cholesteryl ester hydrolysis and (c) by reducing cholesterol efflux.[18] Hence, elevation in cholesterol levels may be due to increase in biosynthesis and decrease in its utilization. Triacylglycerols are degraded by the lipoprotein lipase (LPL) to fatty acids.[19] The administration of SI showed dose dependent reduction in CP induced elevated serum cholesterol and triglyceride. Increase in the level of triglycerides could be due to the alterations in LPL activity. This reduction in levels of cholesterol and triglyceride confirms that the antihyperlipidemic action of SI is responsible to alleviate CP induced hyperlipidemic cardiomyopathy. Octacosanol, a phytoconstituent of SI has been reported for its lipid-lowering properties.[20] Antihyperlipidemic action of SI in CP induced hyperlipidemic cardiomyopathy may be due to presence of octacosanol and catechins.
Free radicals generated during treatment with CP causes membrane injury indicated by lipid peroxidation resulting in the loss of function and integrity of myocardial membrane. Our study revealed a significant decrease in lipid peroxidation by SI suggesting its protective effect which may be due to tannins and (-) epicatechin.[21] Reduced glutathione (GSH) is a major low molecular weight scavenger of free radicals in the cytoplasm and an important inhibitor of free radical generation mediated by lipid peroxidation. It is known to result in enhanced lipid peroxidation which causes increased glutathione consumption, as observed in our study. The administration of SI protected the myocytes against CP by decreasing their susceptibility to free radicals. SI restoration of GSH may be due to the presence of triterpenoids and (-) epicatechin. CP induced decline in both SOD and catalase activity along with GSH contents promotes the formation of OH- radicals, initiation and propagation of lipid peroxidation. The activities of antioxidant enzymes are in close relationship with the induction of lipid peroxidation, found in the present study. Administration of SI improved the antioxidant status and thereby prevented the damage to the heart.
Cyclophosphamide was observed to induce changes in ECG pattern such as decrease in the heart rate, P wave inversion, prolongation of QT interval, PR interval and decreased in R wave amplitude in this study. CP causes cardiac dysfunction through impaired mitochondrial metabolism.[22] These observations are in agreement with earlier report. Bradycardia observed in CP treated animals could be due to release of significant amount of acetylcholine (Ach) which is also linked with the genesis of myocardial damage.[23] Saraca indica 400 mg/kg treatment prevented CP induced bradycardia. CP treated group showed P wave inversion in the present study. CP administration is known to release significant amount of norepinephrine in myocardium which is linked with heart mitochondrial dysfunction.[23] Ischemia coupled with increased release of catecholamine can alter AV junctional rhythm.[24] Myocardial ischaemia and release of norepinephrine could be causing P wave inversion observed in present study. Treatment with SI decreased the event of P wave inversion indicating the protective effect against CP induced cardiotoxicity by cyclophosphamide.
Animals treated with CP showed decrease in R wave amplitude in present study. Cardiac rate and rhythm changes affect myocardial contractility and vice-versa. The decreased R wave amplitude indirectly indicate altered supply of oxygen to myocytes and affect energy consuming process leading to cell injury. These inhibitory mechanisms concomitant with delay in impulse conduction through AV bundle are known to cause cardiotoxicity. Animals treated with SI showed normalization of R wave amplitude which could be due to increase in the oxygen supply to the myocytes, thereby preventing myocardial injury. Treatment with CP prolonged the QT interval in the present study. CP is known to increase the cellular Na+ content and decreases in K+ content.[24] Hypokalemia may be associated with QT interval elongation. SI at the dose of 400 mg/kg decreased the elongated QT interval by restoring K+, thereby maintaining myocytes’ ion homeostasis which is essential for normal heart cell function. CP treated animals showed PR interval prolongation in present study. First degree of AV block is represented by delay along the conduction pathway (PR interval prolongation). The AV block may be due to change in parasympathetic tone and conduction system deformation. The occurrence of first degree of AV block was less in case of SI treated groups indicating the protective effect in CP induced cardiotoxicity.
The cardiac muscle fibers were found to be of uniform size, shape and configurations with no inflammatory cell infiltrates. CP produced massive change in the myocardium showing a varying degree of vacuolar changes in the cardiac muscle fibers mainly in the form of degeneration of myocardial tissue, vacuolization of the cardiomyocytes, infiltration of inflammatory cells and myofibrill loss. Treatment with SI effectively inhibits CP induced cardiac damage by reversal of infiltration of inflammatory cells and fragmentation of myofibrils. Epicatechin present in SI may increase the integrity of cardiac muscle by vasodilatation and restore the antioxidant status.[25] Phytosterols present in SI such as β-sitosterol exerts antioxidant and cardioprotective properties.[26] Flavonoids, triterpenoids and tannins are also reported as potent antioxidants and/or cardioprotectives.[27] These phytoconstituents along with others in SI may be providing the protective effect against CP induced cardiotoxicity.
Conclusion
CP treatment causes pronounced oxidative stress and tissue damage in the heart. Administration of SI extract protects the CP induced cardiotoxicity in dose dependent manner. Biochemical, ECG and histopathological studies confirm the cardioprotective role of Saraca indica.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Goldberg MA, Antin JH, Guinan EC, Rappeport JM. Cyclophosphamide cardiotoxicity: An analysis of dosing as a risk factor. Blood. 1986;68:1114–8. [PubMed] [Google Scholar]
- 2.Desouza CA, Santini G, Marino G, Nati S, Congiu AM, Vigorito AC, et al. Amifostine (WR-2721), a cytoprotective agent during high-dose cyclophosphamide treatment of non-hodgkin's lymphomas: A phase II study. Braz J Med Boil Res. 2000;33:791–8. doi: 10.1590/s0100-879x2000000700009. [DOI] [PubMed] [Google Scholar]
- 3.Souid AK, Tacka KA, Galvan KA, Penefsky HS. Immediate effects of anticancer drugs on mitochondrial oxygen consumption. Biochem Pharmacol. 2003;66:977–87. doi: 10.1016/s0006-2952(03)00418-0. [DOI] [PubMed] [Google Scholar]
- 4.Mythili Y, Sudharsan PT, Varalakshmi P. dl-α-lipoic acid ameliorates cyclophosphamide induced cardiac mitochondrial injury. Toxicology. 2005;215:108–14. doi: 10.1016/j.tox.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Preethi F, Jennifer F, Pricilla K. Hypoglycemic activity of Saraca indica Linn barks. J Pharm Res. 2010;3:491–3. [Google Scholar]
- 6.Shelar DB, Shirote PJ, Naikwade NS. Anti-inflammatory activity and brine shrimps leathality test of Saraca Indica Linn leaves extract. J Pharm Res. 2010;3:2004–6. [Google Scholar]
- 7.Cibin TR, Devi DG, Abraham A. Chemoprevention of Skin Cancer by the flavonoid fraction of Saraca asoka. Phytother Res. 2010;24:666–72. doi: 10.1002/ptr.2950. [DOI] [PubMed] [Google Scholar]
- 8.Pradhan P, Joseph L, Gupta V, Chulet R, Arya H, Verma R, Bajpai A. Saraca asoca (Ashoka): A Review. J Chem Pharm Res. 2009;1:62–71. [Google Scholar]
- 9.Katrina GY, Pam RT, Maraliz BH, Maria MR, Alexander CZ, Guillermo C, et al. Effects of (−)-Epicatechin on Myocardial Infarct Size and Left Ventricular Remodeling After Permanent Coronary Occlusion. J Am Coll Cardiol. 2010;55:2869–76. doi: 10.1016/j.jacc.2010.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang ZY, Hunt JY, Wolff SP. Detection of lipid hydroperoxides using the fox method. Anal Biochem. 1992;202:384–9. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- 11.Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 12.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 13.Sinha AK. Colorimetric assay of catalse. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 14.Tarek MK, Nermin AH, Ayat R. Cytoprotective effects of DL-alpha-lipoic acid or squalene on cyclophosphamide-induced oxidative injury: An experimental study on rat myocardium, testicles and urinary bladder. Food Chem Toxicol. 2010;48:2326–36. doi: 10.1016/j.fct.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 15.Baky NA, Al-Rasheed NM, Al-Rasheed NM, Zaghloul IY, Radwan MA. Alpha-lipoic acid and amlodipine ameliorate myocardial infarction induced by isoproterenol in rats. Int J Acad Res. 2009;1:68–77. [Google Scholar]
- 16.Shanmugarajan TS, Arunsunder M, Somasundaram I, Krishnakumar E, Sivaraman D, Ravichandiran V. Protective effect of Ficus hispida Linn. on cyclophosphamide provoked oxidative myocardial injury in rat model. Int J Pharmacol. 2008;1:1–10. [Google Scholar]
- 17.Rathee P, Rathee S, Rathee D. Quantitative estimation of (+)-Catechin in stem bark of Saraca asoka Linn using HPTLC. Scholars Research Library. Der Pharm Chem. 2010;2:306–14. [Google Scholar]
- 18.Gesquiere L, Loreau N, Minnich A, Davignon J, Blache D. Oxidative stress leads to cholesterol accumulation in vascular smooth muscle cells. Free Radic Biol Med. 1999;27:134–45. doi: 10.1016/s0891-5849(99)00055-6. [DOI] [PubMed] [Google Scholar]
- 19.Lespine A, Chap H, Perret B. Impaired secretion of heart lipoprotein lipase in cyclophosphamide-treated rabbit. Biochim Biophys Acta. 1997;1345:77–85. doi: 10.1016/s0005-2760(96)00167-1. [DOI] [PubMed] [Google Scholar]
- 20.Taylor JC, Rapport L, Lockwood GB. Octacosanol in human health. Nutrition. 2003;19:192–5. doi: 10.1016/s0899-9007(02)00869-9. [DOI] [PubMed] [Google Scholar]
- 21.Quine SD, Raghu PS. Effects of (-)- epicatechin, a flavonoid on lipid peroxidation and antioxidants in streptozotocin-induced diabetic liver, kidney and heart. Pharmacol Rep. 2005;57:610–5. [PubMed] [Google Scholar]
- 22.Hanaki Y, Sugiyama S, Akiyama N, Ozawa T. Role of the autonomic nervous system in cyclophosphamide-induced heart mitochondrial dysfunction in rats. Biochem Int. 1990;21:289–95. [PubMed] [Google Scholar]
- 23.Atlee JL. Protective cardiac dysrhythmias: Diagnosis and management. Anesthesiology. 1997;86:1397–424. doi: 10.1097/00000542-199706000-00026. [DOI] [PubMed] [Google Scholar]
- 24.Levine ES, Friedman HS, Griffith OW, Colvin OM, Raynor JH, Lieberman M. Cardiac cell toxicity induced by 4-hydroperoxycyclophosphamide is modulated by glutathione. Cardiovasc Res. 1993;27:1248–53. doi: 10.1093/cvr/27.7.1248. [DOI] [PubMed] [Google Scholar]
- 25.Corti R, Flammer AJ, Hollenberg NK, Luscher TF. Cocoa and cardiovascular health. Circulation. 2009;119:1433–41. doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida Y, Niki E. Antioxidant effects of phytosterol and its components. J Nutr Sci Vitaminol. 2003;49:277–80. doi: 10.3177/jnsv.49.277. [DOI] [PubMed] [Google Scholar]
- 27.Pawar RS, Bhutani KK. Effect of oleanane triterpenoids from Terminalia arjuna – a cardioprotective drug on the process of respiratory oxyburst. Phytomedicine. 2005;12:391–3. doi: 10.1016/j.phymed.2003.11.007. [DOI] [PubMed] [Google Scholar]


