Abstract
Objectives:
Terlipressin improves renal function in hepatorenal syndrome (HRS) is a known fact. However the reason for lack of its long-term survival benefits despite improvement in renal function remains unclear. The aim of this study was to analyze the survival benefits of terlipressin in HRS and to address the issue of non-responder state to terlipressin.
Materials and Methods:
Electronic databases and relevant articles were searched for all types of studies related to HRS and use of terlipressin in HRS. Reduction in all-cause mortality rate was the primary outcome measure. Reduction in mortality rate due to HRS and other causes of death were also analyzed.
Results:
With total 377 patients analyzed from eight eligible studies; terlipressin reduced all-cause mortality rate by 15% (Risk Difference: -0.15%, 95% CI:-0.26 to -0.03). Reduction in the mortality rate due to HRS at three months was 9% (Risk Difference:-0.09%, 95% CI:-0.18 to 0.00).
Conclusion:
Terlipressin has long term survival benefits perhaps at least up to three months but only with HRS as a cause of death not for other causes of death. Benefits and role of antioxidants like N- Acetylcysteine (NAC) in non-responder patients’ needs to be studied further. Long-term use of low dose terlipressin (<4mg/d) plus albumin and addition of antioxidant NAC to this regimen may help in improving both HRS reversal rate and survival rate in non-responders to terlipressin.
KEY WORDS: Hepatorenal syndrome, terlipressin, albumin, N-Acetylcysteine
Introduction
As defined by international ascites club, Hepatorenal syndrome (HRS) is a ‘potentially reversible syndrome occurring in patients with cirrhosis, ascites and liver failure. It is characterized by impaired renal failure, marked alterations in the cardiovascular function and over-activity of the endogenous vasoactive systems. Marked vasoconstriction in the kidney causes low GFR, whereas in the systemic circulation, there is decreased vascular resistance due to splanchnic and peripheral arterial vasodilatation. A similar syndrome can also occur in acute liver failure and acute alcoholic hepatitis.’[1] Diagnosis of this condition is essentially based on presence of revised International Ascites Club framed diagnostic criteria [Table 1].[1] Chances of occurrence of HRS in patients with cirrhosis of liver is 18 and 39% at one and five years respectively.[2] Based on clinical features and prognosis HRS is classified into two types. Type 1 HRS is the most common and severe variety, characterized by acute onset and rapid progression with mean survival period of two weeks after the onset of renal failure. On the other hand type 2 HRS has slow progression and comparatively better prognosis with mean survival period of six months.[1–3] Although liver transplantation remains the final and treatment of choice for type 1 HRS; pharmacological treatment acts as a bridging therapy to transplantation.[2] Use of vasoconstrictors like terlipressin and plasma expander albumin in HRS was a breakthrough pharmacological intervention. Combination of terlipressin and albumin is found to be most efficacious in improving renal function in these patients.[3] Recently, the focus on HRS has shifted towards the issues of non-responders to terlipressin and long term survival benefits of terlipressin in HRS.[4,5] Meta-analysis by Gludd et al. 2010, with mortality as primary outcome measure reports 20% reduction in mortality (Relative risk (RR): 0.80; 95% CI: 0.66-0.97) by terlipressin. However their long term survival benefit in these patients is either inconclusive or insignificant.[4]
Table 1.
Revised International Ascites Club Diagnostic criteria for Hepatorenal syndrome

There are seven meta-analysis and two systematic reviews conducted on the efficacy of terlipressin, except for meta-analysis by Gludd et al. 2010, others have mainly focused on the renal function improvement or HRS reversal efficacy of terlipressin.[2,4] With an aim to address the issues of non-responders to terlipressin and long term survival benefits of terlipressin, present meta-analysis differs from meta-analysis by Gludd et al. 2010, in that we specifically analyzed survival benefits of terlipressin against individual causes of death apart from analyzing overall survival benefits against all causes of death. In addition, we also addressed the issue of treating the non-responder state to terlipressin.
Materials and Methods
Eligibility Criteria
Studies with any type of study designs, irrespective of type of HRS comparing treatment of terlipressin (with or without albumin/other drugs) with control group (with or without albumin/other drugs) on patients of either sex aged above18 years and publishing mortality data at the end of study period or follow up were eligible for inclusion.
Search Methodology
Two authors independently conducted electronic data search for the relevant articles in MEDLINE, Cochrane library The Cochrane Register for Controlled trials and SCIRUS with MeSH terms ‘Terlipressin’ and ‘Hepatorenal syndrome’. Electronic data search was also conducted in certain specific journal websites like Journal of Hepatology, Hepatology, Gastroentorolgy, American Journal of Gastroenterology, Digestive Disease Science, etc. Manual search of bibliographies of all the meta-analysis, published trials and other relevant articles was also carried out. We limited our search to those studies published up to July 2011in English literature.
Data Extraction
Published data independently extracted by two authors was considered for the analysis. Final data was prepared after achieving consensus between two authors. In analyzing overall survival benefits against all causes of death, published data at the end of study period or maximum days of follow-up was extracted for the analysis. For analyzing survival benefits against individual causes of death, data on causes of death due to HRS and other causes were extracted. Corresponding authors of studies with the unpublished or unclear data on the individual causes of death were requested for the same through electronic mail.
Outcome Measures
Difference in the mortality rate due to all causes of death was the primary outcome measure. In addition, differences in the mortality rates of deaths due to individual causes of death and overall mortality rate at different time periods were also estimated. Additional subgroup analysis to estimate the influence of various factors like percentage of patients with alcoholic liver disease (ALD) as cause of cirrhosis, dose of terlipressin used (>4mg/d and<4mg/d); type of interventions used (terlipressin with vs without albumin; terlipressin vs placebo; terlipressin vs noradrenaline), blinding and quality score of study (studies of low quality vs high quality) was also conducted.
Statistical Methods
Overall survival benefit against all causes of mortality was analyzed by calculating the Risk Difference (RD) in the mortality rate between the terlipressin group (with or without albumin/other drugs) and control group (with or without albumin/other drugs). Differences in the mortality rate due to individual causes of death between terlipressin group and control group were analyzed by fixed effect model as the numbers of studies analyzed were only two. In analyzing overall survival benefits, both random effects model using Der Simonian and Laird method and fixed effect model by Mantel-Haenszel method were used for analysis. Separate analysis of only randomized studies was also conducted. Considering the possibility of heterogeneity between the studies; random effects model results were used for analysis and discussion. Sensitivity analysis for the robustness of the results was done by comparing the results of random effect model with fixed effect model. Cochrane Q test for heterogeneity and I2 test were used for analyzing inter-trial heterogeneity. A chi square test with P value <0.10 and I2 test value >50% was considered as an indicator of significant heterogeneity. RevMan software version 4.3.2 by Cochrane collaboration was used for statistical analysis of the data.
Quality Evaluation and Publication Bias
Studies with full text article were subjected to structured review for quality evaluation as described by Nancy et al.[6] The un-blinded quality assessments of published information were independently performed by two co-authors and then a consensus was achieved on the final score. Publication bias was analyzed by funnel plot method.
Results
Study search results
Figure 1 shows study attrition diagram and number of trials included in meta-analysis. Eight randomized controlled trials and one retrospective study met eligibility criteria and were included under analysis. Of the total 12 prospective randomized controlled trials conducted on the role of terlipressin in HRS, five studies were excluded.[7–18] The reason for excluding study by Hedengue et al. was that it did not had mortality data at the end of 2 days treatment.[14] Study by Srivastava et al. was of 5 days duration and had no mortality data published.[15] Study by Angeli et al. was excluded because terlipressin was the test drug in both groups; testing terlipressin given as continuous intravenous infusion versus terlipressin given as intravenous boluses.[16] Similarly study by Romero et al. compared terlipressin in both the groups differentiated by presence or absence of active infection.[17] Study by Yang et al. was published in Chinese literature.[18] Though our criterion was to exclude studies published in other languages we also made a separate analysis after including this study. Data from the remaining 7 randomized studies were extracted and included in the final analysis.[7–13] Among the non-randomized studies, retrospective cohort study by Danalinglu et al. met the eligibility criteria and was included for analysis.[19]
Figure 1.
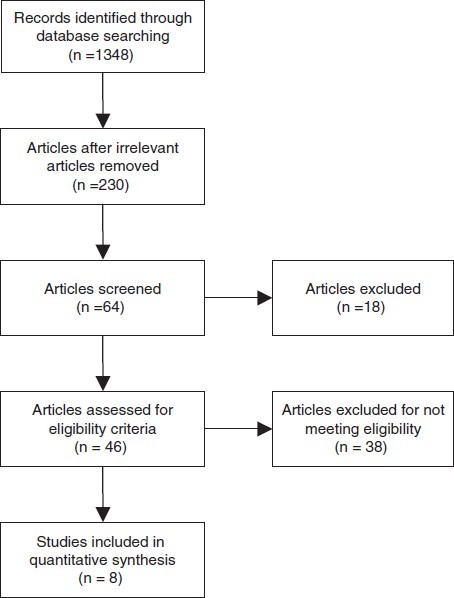
Flow chart showing study attrition diagram and number of trials included in meta-analysis
Characteristics of Included Studies
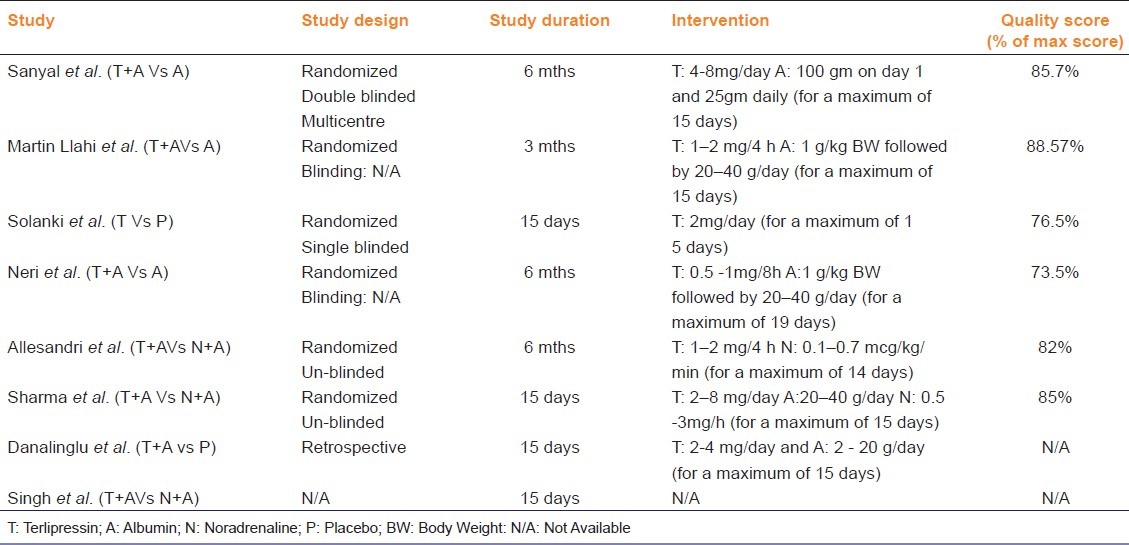
From six of eight eligible studies, baseline demographic and clinical features of the patients included in each study were available as shown in Tables 2 and 3.[7–12] There is a wide variation in the total number of patients included in each study and their demographic and clinical features. In all the studies mortality data at the end of either treatment period or follow-up period was available. With regard to interventions, albumin was used in most of the studies at various doses and different regimens. In all included studies terlipressin was administered intermittently, except in Singh et al. where information was not available. The dose and duration of treatment; study design and results of quality evaluation are presented in Table 4. No significant publication bias was observed in funnel plot method when all studies were included in analysis.
Table 2.
Baseline demographic and clinical features of included studies
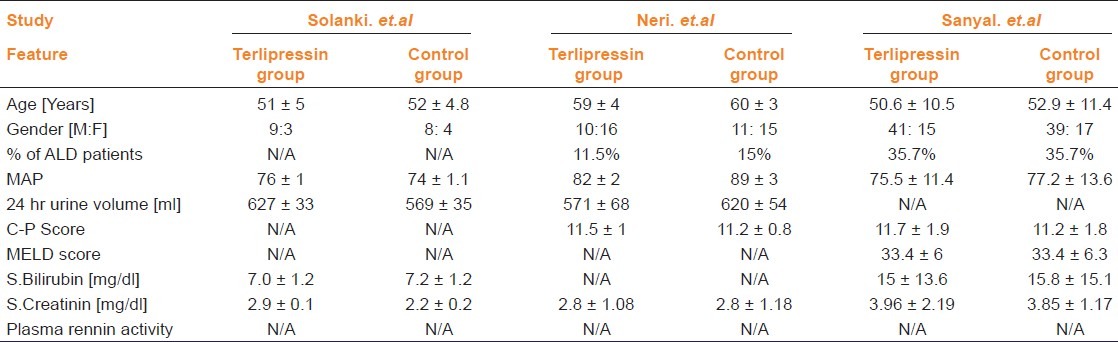
Table 3.
(continued) Baseline demographic and clinical features of individual studies
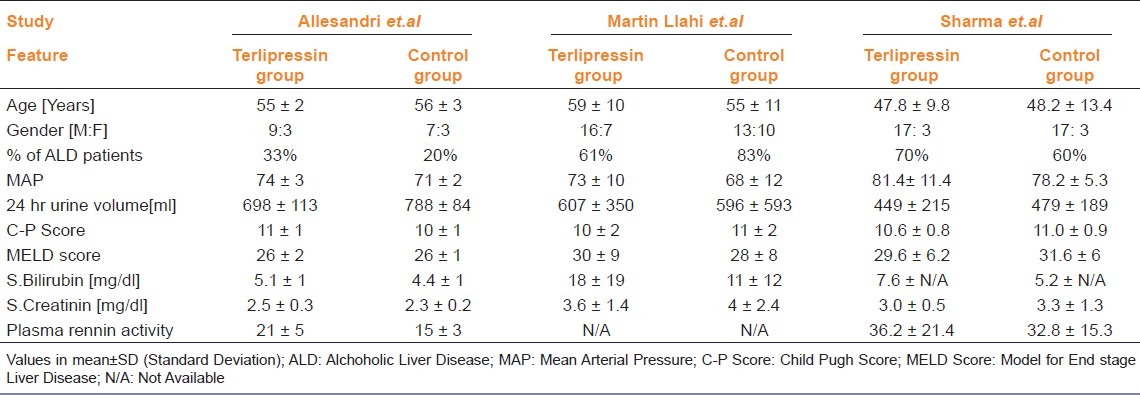
Table 4.
Characteristics of individual studies and interventions used
Results of outcome measure
Analysis of overall mortality rate reduction after analyzing all studies irrespective of the number of days of study period or follow up period was 15% (RD: -0.15; 95% CI: -0.26 to -0.03) [Figure 2]. Among the overall mortality rate at the end of 15 days; there was 26% (RD:-0.26; 95% CI:-0.43 to -0.09) reduction in mortality rate due to treatment with terlipressin. However, as observed in the study by Gludd et al. 2010, the survival benefits of terlipressin were not sustained at or beyond 30 days. Analysis of RCTs alone and exclusion of study by Yang et al. published in Chinese literature; did not have significant impact on the results of mortality rate. Results of overall survival benefits by random effects model (-15%) appear to be robust as they didn’t differ significantly from fixed effect model (-13%). Interestingly, though we expected significant inter-trial heterogeneity there was evidence of only mild inter-trial heterogeneity with Chi square p-value and I2 test value for heterogeneity being 0.14 and 35.7% respectively.
Figure 2.
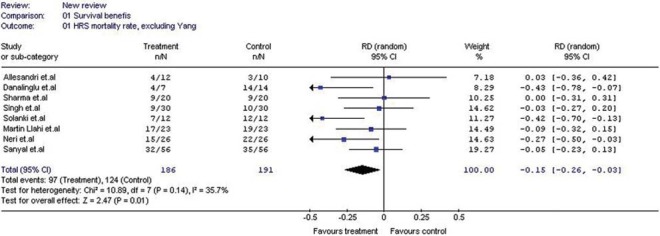
Forest plot showing the risk difference in mortality rate due to all causes of death
In subgroup analysis of survival benefits of studies with <50% of patients with ALD as cause of cirrhosis (RD: -0.12; 95% CI: -0.28 to 0.05) vs >50% of patients with ALD as cause of cirrhosis (RD: -0.05; 95% CI: -0.24 to 0.13); those comparing terlipressin plus albumin vs noradrenaline plus albumin (RD: -0.01; 95% CI:-0.18 to 0.16) or terlipressin plus albumin vs albumin alone (RD: -0.12; 95% CI: -0.25 to 0.01) and those where dose of terlipressin was >4mg/d (RD: -0.05; 95% CI: -0.17 to 0.08) showed no statistically significant survival benefits favoring terlipressin unlike the studies comparing terlipressin versus placebo (RD: -0.42; 95% CI: -0.65 to -0.20) and those studies where dose of terlipressin was <4mg/d (RD: -0.35; 95% CI: -0.51 to -0.19). Similarly no survival benefits were observed on subgroup analysis of studies with high quality score (>85%) (RD: -0.05; 95% CI: -0.18 to 0.08) or studies which are randomized but un-blinded (RD: -0.12; 95% CI: -0.25 to 0.02) or randomized and blinded (double or single) (RD: -0.22; 95% CI: -0.58 to 0.15) unlike the studies with low quality score (RD: -0.25; 95% CI: -0.51 to -0.03) or low quality and non-randomized studies (RD: -0.22; 95% CI: -0.40 to -0.05).
Published data on individual causes of death in terlipressin group and control group were available only from two studies.[7,10]
It was either not available or unclear from other studies. We failed to get the data from the corresponding authors of these studies even after contact through electronic mail. However, analysis of available data from two studies with the data on causes of death in individual patients at the end of 3months revealed statistically significant 9% reduction in mortality rate due to HRS alone as cause of death (RD: -0.09; 95% CI: -0.18 to 0.00;) [Figure 3]. However, the decrease in mortality due to liver failure alone as cause of death was not significant (RD: -0.05; 95% CI: -0.18 to 0.08). Interestingly, reduction in mortality rate due to multi-organ failure alone as cause of death favored control group (RD: 0.10; 95% CI: 0.00 to 0.21).
Figure 3.
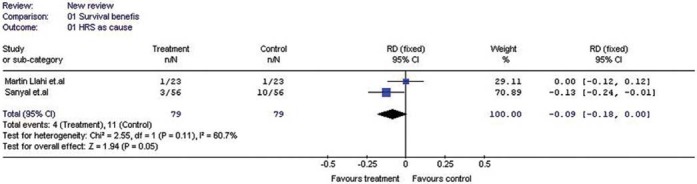
Forest plot showing the risk difference in the mortality rate due to HRS alone
Discussion
Analysis of data from two randomized studies where information on causes of death in individual patients were available revealed that the majority of patients treated with terlipressin died either because of multi-organ failure or sepsis or Systemic Inflammatory Response Syndrome (SIRS) or hepatic failure.[7,10] Reduction in the mortality rate due to HRS alone as cause of death even at three months was though low but significant. As terlipressin doesn’t reduce severity of underlying liver failure, its lack of survival benefits against liver failure as cause of death can be expected.[7] Interestingly, survival benefits against multi-organ failure as cause of death was favored in control group; raising the doubt of possibility of increased incidences of deaths due to multi-organ failure in terlipressin group. Lack of survival benefits of terlipressin and perhaps with other vasoconstrictor drugs in HRS despite improvement in renal function may be attributed due either to their failure to prevent deaths due to other causes of death or increased incidences of deaths due to multi-organ failure. This might have been responsible for inter-individual and inter-study variations observed with survival benefits of terlipressin.
HRS reversal (decrease in serum creatinine< 1.5mg/dl) rate of terlipressin varies from 40 to 60%.[20] The factors found to be favoring the HRS reversal or survival benefits in most of the randomized studies were HRS of type 2, young age, low baseline Child Pugh Score, low baseline Model for End-Stage Liver Disease (MELD) score, low baseline serum bilirubin, low baseline serum creatinine, high baseline creatinine clearance, alcoholic liver disease as etiology and significant rise in Mean Arterial Pressure (MAP) following treatment with terlipressin. Majority of patients who respond are those with mild renal failure (baseline serum creatinine<5mg/dl) and liver failure (Child Pugh score <11).[1,5] And responder patients usually achieve a sufficient increase in MAP following treatment with terlipressin.[5]
Interestingly, patients who respond to terlipressin still die of HRS.[21] Reason why responders still die of HRS and how HRS can be reversed in non-responders needs to be explored.
An important factor that determines the responder and non-responder (failure achieve serum creatinine <1.5mg/dl after treatment) states is the baseline severity of renal failure. The reversal of HRS in patients with severe renal failure (baseline serum creatinine level >5mg/dl) is rare and early diagnosis of renal failure and immediate initiation of treatment with vasoconstrictors is advised.[5] Perhaps the reason could be onset of subclinical renal tubular damage induced by long-term ischemia of kidney leading to a state of functional irreversibility.[22] Failure of kidney to return from this point of irreversibility as a reason for non-responder state in patients with severe renal failure could be forwarded. Early treatment may thus prevent the onset of state of irreversibility or tubular damage and thus non-responder state to the vasoconstrictor drugs. However the question of treating non-responder patients who present with possible already set irreversible state remains to be answered. A pharmacological agent that needs to be explored to improve renal function in non-responders is the use of N-Acetylcysteine (NAC). NAC used alone in HRS has improved the renal function and survival rates.[23] Mechanism behind improvement in renal function by NAC remains unknown as no improvement in severity of liver failure or hemodynamic derangements were observed in the study. An indirect evidence for possible beneficial effects of NAC in non-responders to terlipressin comes from a case report using combination of nonspecific endothelin receptor antagonist bosentan with NAC.[24] Combination of these two drugs was found to be beneficial in improving renal function in a patient not responding to terlipressin. However whether the beneficial effects so observed was attributable to bosentan was doubtful. We are of the opinion that the beneficial effect so observed perhaps are attributable to NAC alone, not to bosentan as treatment with another non-specific endothelin receptor anatagonist tezosenton alone has not only failed to improve renal function, but also deteriorated the renal function.[25] In addition, by its ability to reduced renal oxidant stress, NAC is known to prevent renal tubular damage induced by radiocontrast agents.[26] Perhaps the same mechanism could have been responsible behind observed improvement in renal function with NAC in HRS. Role of oxidative stress in inducing both renal dysfunction and state of vascular non-reactivity in HRS and our concept of reversing them by antioxidants is also supported by few preliminary studies.[27–29]
As found in most of the randomized studies, incidences of HRS reversal and survival rate were also related to the severity of liver failure (Child Pugh score >11). Influence of severity of liver failure on both survival and HRS reversal rate has also been emphasized.[30] Bringing down the severity of liver failure in non-responder patients with CP score >11 may be essential in order to observe beneficial effects in this group of patients. Alternatively, decreasing the severity of liver failure and indirectly renal failure by this way may either help in making the patients suitable for surgical treatment with better outcome expected. Though liver transplantation treats the primary problem of hepatic failure, it may not be indicated or affordable by all patients. A pharmacological agent that decreases the severity of liver failure in patients with Child Pugh score >11 could be worth using in these patients. Such agent could again be NAC; though it has been found not to have any effect on severity of liver failure in HRS. Being antioxidant, NAC may exert indirect beneficial effects at least on deleterious manifestations of liver failure as does the albumin.[31] Unlike the possibilities of reversing renal failure, benefits of NAC in bringing the non-responsive state to responsive state by altering severity of liver failure in severe/irreversible end stage cirrhosis is doubtful. However in the background of increased oxidative stress in the onset of multi-organ failure associated with liver failure, the most common cause of death in HRS and possible increased incidences of deaths due to multi-organ failure associated with use of terlipressin, addition of antioxidant like NAC to therapeutic regimen of terlipressin and albumin seems justifiable.[32]
Failure to achieve sufficient increase in MAP with terlipressin is another important determinant of non-responder state.[5] Achieving sufficient increase in MAP in these patients is quite challenging owing to the vascular non-reactivity induced by oxidant stress.[27–29] Considering the possible role of oxidants in altering vascular reactivity to vasoconstrictors in HRS, use of antioxidants to improve vascular reactivity is thus favored.[27–29] Short term use of terlipressin in HRS improves only circulatory dysfunction not renal function.[14] On the other hand, long term use of terlipressin may be associated with improved responder rate.[33] It is possible that long-term administration of vasoconstrictors may help in maintaining persistent and significant increase in MAP and there by maintain renal blood flow. Considering the lack of survival benefits with use of >4mg/d dose of terlipressin, prolonged treatment with low dose (<4mg/d) of terlipressin can thus be favored in non-responders for improving renal function. However considering the adverse effects of terlipressin, continuous monitoring for its cardiovascular adverse effects would become a major limitation for prolonged use. As NAC is found to prevent vasoconstricton related adverse effects of terlipressin, addition of NAC to long term regimen of terlipressin and albumin could also prove to be safe apart from synergistic in improving responder and survival rate.[34] Considering the high cost of terlipressin, its long term use may not be practical. Noradrenaline, another vasoconstrictor drug that has relatively similar adverse reaction profile and beneficial effects as terlipressin in improving renal function and survival rate, could be promising cost effective alternative for terlipressin.[11–13] Theoretically, noradrenaline has risk of decreasing blood flow to the liver.[12] In addition, as the data on the causes of death in individual patients treated with noradrenaline is not available, it will be inconclusive to state whether it decreases or increase incidences of other causes of death.
Idea of overcoming the three major determinants of non-responder state by addition of antioxidants like NAC to long term regimen of terlipressin plus albumin may prove to be beneficial in improving renal function and survival in non-responders. With regard to improving survival benefits with increasing responder rate; uncertainty still remains over the success of achieving improved survival benefits. As patients who respond to vasoconstrictor therapy still die of HRS, impact of improving responder rate by improving vascular reactivity and severity of renal failure perse needs to be studied further in randomized controlled trials. The significance of achieving survival benefits perhaps can also be blunted by the etiology of cirrhosis.[35] Hence, apart from lack of survival benefits of terlipressin against other causes of death and possible increased incidences of deaths due to multi-organ failure, discrepancies in the survival rate and HRS reversal rate observed with terlipressin could also have been significantly influenced by the etiology of cirrhosis. Considering the complex interplay of the different factors influencing the survival rate; we are of the opinion that the true survival benefits of terlipressin cannot be estimated even if required sample size for its estimation is achieved. A study with patient characteristics favorable for higher survival or HRS reversal can record high survival benefits and vice-versa.
We must admit that our study has certain drawbacks. We have restricted our search to published studies in English. Our conclusion that the terlipressin has survival benefit only with respect to HRS alone as cause of death and not against other causes of death is dependent only on data from two randomized studies. With total population of only 158 patients analyzed from these two studies; sample size is very low to make such a conclusion. Since the data on individual causes of death in other randomized studies were not available, future reviews may come up with an acceptable conclusion if these data become available. Opinion that NAC can be beneficial in reversing non-responder state to responder state is supported by preliminary or observational studies not controlled studies is another drawback. As most of the eligible studies included patients of both types of HRS, considering the comparatively better prognosis associated with type 2 HRS, an influence of including these patients on the results of survival and HRS reversal benefits cannot be ruled out.
Conclusion
Terlipressin has even long term survival benefits perhaps at least up to three months; but only against HRS as a cause of death not for other causes of death. Benefits and role of antioxidants like NAC in non-responders needs to be studied further. Long-term use of low dose terlipressin (<4mg/d) plus albumin and addition of antioxidant NAC to this regimen may help in improving both HRS reversal rate and survival rate in non-responders to terlipressin.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Fernandez J, Arroyo V. Novel Definition of Hepatorenal Syndrome: Clinical Consequences. Front Gastrointest Res. 2011;28:122–9. [Google Scholar]
- 2.Tandon P, Bain VG, Tsuyuki RT, Klarenbach S. Systematic review: Renal and other clinically relevant outcomes in hepatorenal syndrome trials. Aliment Pharmacol Ther. 2007;25:1017–28. doi: 10.1111/j.1365-2036.2007.03303.x. [DOI] [PubMed] [Google Scholar]
- 3.Hani MW, Martin LM, Nasimul A, Thomas AG. Hepatorenal Syndrome: Pathophysiology and Management. Clin J Am Soc Nephrol. 2006;1:1066–79. doi: 10.2215/CJN.01340406. [DOI] [PubMed] [Google Scholar]
- 4.Gluud LL, Christensen K, Christensen E, Krag A. Systematic Review of Randomized Trials on Vasoconstrictor Drugs for Hepatorenal Syndrome. Hepatology. 2010;51:576–84. doi: 10.1002/hep.23286. [DOI] [PubMed] [Google Scholar]
- 5.Boyer TD, Sanyal AJ, Garcia-Tsao G, Blei A, Carl D, Bexon AS, et al. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: Relationship of serum creatinine to hemodynamics. J Hepatol. 2011;55:315–21. doi: 10.1016/j.jhep.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nancy GB, Robert AP. Meta-analysis: Neither quick nor easy. BMC Med Res Methodol. 2002;2:10. doi: 10.1186/1471-2288-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, et al. A Randomized, Prospective, Double-Blind, Placebo Controlled Trial of Terlipressin for Type 1 Hepatorenal Syndrome. Gastroenterology. 2008;134:1360–8. doi: 10.1053/j.gastro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solanki P, Chawla A, Garg R, Gupta R, Jain M, Sarin SK. Beneficial effects of terlipressin in hepatorenal syndrome: A prospective, randomized placebo-controlled clinical trial. J Gastroenterol Hepatol. 2003;18:152–6. doi: 10.1046/j.1440-1746.2003.02934.x. [DOI] [PubMed] [Google Scholar]
- 9.Neri S, Pulvirenti D, Malaguarnera M, Cosimo BM, Bertino G, Ignaccolo L, et al. Terlipressin and Albumin in Patients with Cirrhosis and Type I Hepatorenal Syndrome. Dig Dis Sci. 2008;53:830–5. doi: 10.1007/s10620-007-9919-9. [DOI] [PubMed] [Google Scholar]
- 10.Martín–Llahí M, Pepin M, Guevara M, Diaz F, Torre A, Monescillo A, et al. Terlipressin and Albumin vs Albumin in patients with cirrhosis and Hepatorenal Syndrome: A Randomized Study. Gastroenterology. 2008;134:1352–9. doi: 10.1053/j.gastro.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Sharma P, Kumar A, Shrama BC, Sarin SK. An Open Label, Pilot, randomized Controlled Trial of Noradrenaline versus Terlipressin in the Treatment of Type 1 Hepatorenal Syndrome and Predictors of Response. Am J Gastroenterol. 2008;3:1689–97. doi: 10.1111/j.1572-0241.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- 12.Alessandria C, Ottobrelli A, Debernardi-Venon W, Todros L, Torrani M, Cerenzia S, et al. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: A prospective,randomized, unblinded, pilot study. J Hepatol. 2007;47:499–505. doi: 10.1016/j.jhep.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Singh V, Ghosh S, Chawla Y, Singh B, Sharma N, Bhalla A, et al. Noradrenaline Versus Terlipressin in the Treatment of Hepatorenal Syndrome. Gastroenterology. 2011;140(Suppl 1):S–958. [Google Scholar]
- 14.Hadengue A, Gadano A, Moreau R, Giostra E, Durand F, Valla D, et al. Beneficial effects of the 2-day administration of terlipressin in patients with cirrhosis and hepatorenal syndrome. J Hepatol. 1998;29:565–70. doi: 10.1016/s0168-8278(98)80151-7. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava S, Madan K, Prakash S, Sreenivas V, Khanal SP, Acharya SK. A Randomized Controlled Trial of Terlipressin and Albumin Versus Albumin, Low Dose Dopamine and Frusemide in Hepatorenal Syndrome. J Clin Exp Hepatol. 2011;1(Suppl 1):23–4. doi: 10.1016/j.jceh.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angeli P, Fasolato S, Cavallin M, Trotta E, Maresio G, Callegaro A, et al. Terlipressin given as continuous intravenous infusion versus terlipressin given as intravenous boluses in the treatment of type 1 hepatorenalsyndrome (hrs) in patients with cirrhosis. J Hepatol. 2009;50(Suppl 1):S73. [Google Scholar]
- 17.Romero SM, Pascasio JM, Sousa JM, Cayuela A, Ferrer MT, Gómez-Navarro E, et al. Terlipressin and albumin in cirrhotic patients with hepatorenal syndrome type-1 with and without active infection. J Hepatol. 2008;48(Suppl 2):S123. [Google Scholar]
- 18.Yang YZ, Dan ZL, Liu NZ, Liu M. Efficacy of terlipressin in treatment of liver cirrhosis with hepatorenal syndrome. J Intern Intensive Med. 2001;7:123–5. [Google Scholar]
- 19.Danalioglu A, Cakaloglu Y, Karaca C, Aksoy N, Akyuz F, Ozdil S, et al. Terlipressin and albumin combination treatment in hepatorenal syndrome. Hepatogastroenterology. 2003;50:ccciii–cccv. [PubMed] [Google Scholar]
- 20.Angeli P. Terlipressin for Hepatorenal syndrome: Novel strategies and future perspectives. Front Gastrointest Res. 2011;28:189–97. [Google Scholar]
- 21.Muñoz LE, Alcalá EG, Cordero P, Martínez MA, Vázquez NY, Galindo S, et al. Reversal of hepatorenal syndrome in cirrhotic patients with terlipressin plus albumin. First experience in Mexico. Ann Hepatol. 2009;8:207–11. [PubMed] [Google Scholar]
- 22.Angeli P, Volpin R, Gerunda G, Craighero R, Roner P, Merenda R, et al. Reversal of Type 1 Hepatorenal Syndrome With the Administration of Midodrine and Octreotide. Hepatology. 1999;29:1690–7. doi: 10.1002/hep.510290629. [DOI] [PubMed] [Google Scholar]
- 23.Holt S, Goodier D, Marley R, Patch D, Burroughs A, Fernando B, et al. Improvement in renal function in hepatorenal syndrome with N-acetylcysteine. Lancet. 1999;353:294–5. doi: 10.1016/s0140-6736(05)74933-3. [DOI] [PubMed] [Google Scholar]
- 24.Gressner OA, Siluschek M, Lahme B, Gressner AM. Endothelin-receptor antagonist/Nacetylcysteine combination in type 1 hepatorenal syndrome. J Hepatol. 2009;50:1051–6. [Google Scholar]
- 25.Wong F, Moore K, Dingemanse J, Jalan R. Lack of Renal Improvement with Nonselective Endothelin Antagonism with Tezosentan in Type 2 Hepatorenal Syndrome. Hepatology. 2008;47:160–8. doi: 10.1002/hep.21940. [DOI] [PubMed] [Google Scholar]
- 26.Drager LF, Andrade L, Barros de Toledo JF, Laurindo FR, Cesar LA, Seguro AC. Renal effects of N-acetylcysteine in patients at risk of nephropathy: Decrease in oxidant stress mediated tubular injury. Nephrol Dial Transplant. 2004;19:1803–7. doi: 10.1093/ndt/gfh261. [DOI] [PubMed] [Google Scholar]
- 27.Alcaraz A, Iyu D, Atucha NM, García-Estan J, Ortiz MC. Vitamin E supplementation reverses renal altered vascular reactivity in chronic bile duct-ligated rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1486–93. doi: 10.1152/ajpregu.00309.2006. [DOI] [PubMed] [Google Scholar]
- 28.Ortiz MC, Manriquez CM, Nath KA, Lager DJ, Romero JC, Juncos LA. Vitamin E prevents renal dysfunction induced by experimental chronic bile duct ligation. Kidney Int. 2003;64:950–61. doi: 10.1046/j.1523-1755.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- 29.Bomzon A, Ljubuncie P. Oxidative stress and vascular smooth muscle cell function in liver disease. Pharmacol Ther. 2001;89:295–308. doi: 10.1016/s0163-7258(01)00129-2. [DOI] [PubMed] [Google Scholar]
- 30.Beate A, Julia Z, Karl AB, Jörg H, Tilman S, Michael S. Degree of hepatic dysfunction and improvement of renal function predict survival in patients with HRS type I: A retrospective analysis. Eur J Gastroenterol Hepatol. 2009;21:1428–32. doi: 10.1097/MEG.0b013e32832ec16a. [DOI] [PubMed] [Google Scholar]
- 31.Davis NA, Garcia R, Proven A, Jalan R. Albumin: Not just a plasma expander. In: Gerbes AL, editor. Ascites, Hyponatremia and Hepatorenal Syndrome: Progress in Treatment. Vol. 28. Basel: Karger; 2011. pp. 40–51. [Google Scholar]
- 32.Arroyo V, Fernandez J, Gines P. Pathogenesis and treatment of hepatorenal syndrome. Semin Liver Dis. 2008;28:81–95. doi: 10.1055/s-2008-1040323. [DOI] [PubMed] [Google Scholar]
- 33.Moreau R, Durand F, Poynard C, Duhamel C, Cervoni JP, Ichai P, et al. Terlipressin in Patients with Cirrhosis and Type 1 Hepatorenal Syndrome: A Retrospective Multicenter Study. Gastroenterology. 2002;122:923–30. doi: 10.1053/gast.2002.32364. [DOI] [PubMed] [Google Scholar]
- 34.Sen S, Mookerjee RP, Jalan R. Terlipressin-induced vasoconstriction reversed with N-acetylcysteine: A case for combined use in hepatorenal syndrome? Gastroenterology. 2002;123:2160–1. doi: 10.1053/gast.2002.37303. [DOI] [PubMed] [Google Scholar]
- 35.Martinez MO, Harlan S, Renuga V, D’ Souza S, Florescu MC. Hepatorenal Syndrome: Are We Missing Some Prognostic Factors? Dig Dis Sci. 2011 doi: 10.1007/s10620-011-1861-1. [In Press] [DOI] [PubMed] [Google Scholar]


