Abstract
Background:
Edible mushrooms have been used as flavorful foods and as health nutritional supplements for several centuries. A number of bioactive molecules have been identified in numerous mushroom species
Objective:
To evaluate the analgesic and anti-inflammatory potential of Oyster Mushroom Pleurotus florida using various experimental models in Wistar rats.
Materials and Methods:
Acute toxicity studies were performed whereby dose of 250 mg/ kg and 500 mg/kg was selected for present study, Analgesic activity was determined using hot plate method, tail flick method, acetic acid induced writhing and formalin induced pain in rats, while carrageenan was used to induce inflammation and anti-inflammatory studies were performed.
Results:
HEE showed significant (P < 0.01) analgesic and anti-inflammatory response against all experimental models.
Conclusion:
These studies conclude that Pleurotus florida possesses analgesic and anti- inflammatory potential which might be due to presence of myochemicals like flavonoids, phenolics and polysaccharides.
KEY WORDS: Analgesic, anti-inflammatory, Pleurotus florida
Introduction
Pharmaceuticals from natural origin have been used by man since time immortal for a variety of purpose including the treatment of pain. Opium has dictated its use to treat pain from prehistoric era. With the development of ultramodern techniques, isolation and utilizations of phytochemicals has turned out the domain of disease. In the field of pain management, a constant research based on natural pharmacophores and its interaction with analgesic targets, has lead to search of many potential therapeutic agents.[1]
Edible mushrooms have been used as flavorful foods and as health nutritional supplements for several centuries. For the Chinese, some mushrooms are especially regarded as medical substances to increase health and longevity. Nevertheless, systemic studies of its bio-function were not performed until the last third of this past century when biochemical technology for dissecting these traditional medicinal mushrooms and isolating their most active anticancer constituents became available. A number of bioactive molecules have been identified in numerous mushroom species.[2]
Macrofungi are considered as an important food source and as folk remedy since Greek and Roman days.[3] Dioscorides was one to recognize the medicinal potential of mushrooms. Mummified Iceman, 5300 year-old Otzi, was found to carry Piptoporus betulinus (Bull.) P. Karst. and Fomes fomentarius (L.) J.J. Kickx. Basidiomycetes have been widely studied over the past thirty years in terms of their polysaccharide composition and therapeutic application.[4]
Pleurotus has been regarded as an edible mushroom for many years.[5,6] P. florida possess antioxidant, immunostimulator, antitumor and anti-inflammatory activities.[7,8] Recently, Ganeshpurkar et al., reported in vitro anticataract potential of P. florida.[6] P. florida is also a rich source of phenolics and flavonoids that are responsible for anthelmintic potential.[9] Mushrooms are also known to contain anti-inflammatory activity. American Oyster Mushroom possesses antinoceceptive activity.[10] However, no such activity is reported for P. florida. Therefore the aim of current work was to evaluate the analgesic and anti-inflammatory activity of Oyster Mushroom P. florida.
Materials and Methods
Preparation of Extract
The mushroom basiciocarp were provided as gift sample from Mycology Research Laboratory, Rani Durgavati University, Jabalpur (M.P.). The voucher specimen (HDBJ#43) was deposited in Mycology Research Laboratory, Rani Durgavati University, Jabalpur (M.P.). Mushrooms were dried in shade, coarsely powered and used for preparation of extracts. The powder was extracted with ethanol:water (1:1) by stirring for 48 h and filtered through Whatman No. 4 filter paper. The residue was then extracted with two additional 200 ml portions of ethanol: water (1:1) as described above. The combined extract (HEE) was then evaporated at 40°C to dryness, and stored at 4°C for further use.
Phytochemical Screening
Phytochemical screening of the extract was done as per the standard method.[11]
Animals
Male albino rats of Wistar strain (150-200 g), maintained under standard conditions (27 ± 2°C; relative humidity 60 ± 5 %, light dark cycle of 12 hrs) and fed with standard pellet diet and water ad libidum were used for present study. All the experimental protocols were duly approved by Institutional Animal Ethics Committee, Kelaniketan Polytechnic College, Jabalpur (M.P.), India.
Drugs
Indomethacin (Sun Pharma), pethidine sodium (Neon Labs), naloxone (Samarth Pharma), metoclopramide (Lupin Pharma) and diclofenac (Torrent) were purchased from respective companies.
Acute Toxicity Studies
HEE of P. florida was studied for acute oral toxicity as per revised OECD guidelines No. 423.[12] The extract was devoid of any toxicity in rats when given in doses up to 5000 mg/kg by oral route. Hence 250 and 500 mg/kg doses of extract were used for the study.
Analgesic Activity
Analgesic activity of HEE of P. florida was determined by following methods
Eddy's Hot Plate Test
The animals were divided into four groups of five animals each. Group I served as control. Group II served as standard and were injected pethidine sodium (4 mg/kg, ip). Group III and IV were treated orally with HEE of 250 and 500 mg/kg body weight, respectively. The animals were individually placed on the hot plate maintained at 55°C, one hour after their respective treatments. The response time was noted as the time at which animals reacted to the pain stimulus either by paw licking or jump response, whichever appeared first. The cut off time for the reaction was 15 seconds.[13] Further to investigate the effect of naloxone on rats along with HEE, animal were divided into two groups of five animals each. First group received only HEE (500 mg/kg, po) while other group received Naloxone (1 mg/kg) and HEE (1.5 mg/kg, po). Effect of metoclopramide (1.5 mg/ kg) on rats was studied similarly as in case of noloxone. In both cases, after 30 min animals form both groups were subjected for trial on hot plate and change in response was observed.
Tail Flick Test
The animals were divided into four groups of five animals each. Group I served as control. Group II served as standard and were injected diclofenac sodium (9 mg/kg) intraperitonially. Group III and IV were treated orally with HEE of 250 and 500 mg/kg body weight respectively. After one hour, the tip of tail was dipped up to 5 cm into hot water maintained at 58°C. The response time was noted as the sudden withdrawal of the tail from the hot water. Cut off time of 10 seconds was maintained to avoid damage to the tail for all groups. The time required for flicking of the tail, was recorded, to assess response to noxious stimulus.[14]
Acetic Acid-Induced Writhing Response
To evaluate the analgesic effects of the plant extract, the method described by Dharmasiri et al.,[15] was followed with slight modifications. Different groups of five rats each received orally normal saline solution (2 ml/kg) (i.e. control), indomethacin (10 mg/kg), or HEE (250 and 500 mg/kg). Thirty minutes later, 0.7% acetic acid (10 ml/kg) solution was injected intraperitoneally to all the animals in different groups. The number of writhes (abdominal constrictions) occurring between 5 and 20 min after acetic acid injection was counted. A significant reduction of writhes in tested animals compared to those in the control group was considered as an antinociceptic response.
Formalin Test
Pain was induced by injecting 0.05 ml of 2.5% formalin (40% formaldehyde) in distilled water in the subplantar of the right hindpaw. Rats (five per group) were given HEE (250 and 500 mg/ kg, p.o.), indomethacin (10 mg/kg, ip), pethidine (4 mg/kg, ip), and distilled water (p.o.) 30 min prior to injecting formalin. These rats were individually placed in a transparent plexiglass cage (25 × 15 × 15 cm) observation chamber. The amount of time spent licking and biting the injected paw was indicative of pain and was recorded in 0-5 min/0-300 s (first phase) and 20-60 min /1200-3600s (second phase).[16]
Anti-Inflammatory Activity
The anti-inflammatory activity of the extracts was determined as reported elsewhere.[17] The rats were divided into four groups of five rats each. The control group received 1% (v/v) Tween 80 in water, p.o. at a dose of 10 ml/kg. The positive control group was treated orally with the standard drug, indomethacin (10 mg/kg). HEE was administered to the other groups in doses of 250 and 500 mg/kg. All the suspensions were administered 30 min before the induction of oedema by administering 0.1 ml of 1% w/v carrageenan in saline. The degree of paw oedema of all the groups was measured using a plethysmometer at 30, 60, 120, 180 and 240 min after the administration of carrageenan to each group.
Evaluation of Sedative Activity
Ten rats were randomly divided into two equal groups (n = 5) and were fasted for 12 h. The rats in Group I were orally administered 2.5 ml of the 500 mg/kg freeze-dried suspension of HEE and those in Group II were orally administered 2.5 ml normal saline. Each of these rats were then placed on a rat hole-board apparatus[18] and observed for 7.5 min. During this period, the number of head dips, rears, locomotor activity, and number of fecal boluses expelled were counted.
Rotarod Test
The effect of compounds on locomotor performance was tested on the rotarod apparatus as described previously.[19] Twenty-four hours before the experiments, all animals were trained in the rotarod (3.7 cm in diameter, 16 r.p.m) until they could remain in the apparatus for 60 s without falling. On the day of the experiment, rats were treated with diazepam (10 mg/kg, i.p) and tested in the rotarod from 0.5-1 h after their administration. Same procedure was followed with Group II animals (n = 5) treated with HEE (500 mg/kg). The latency to fall and the number of fall from the apparatus were recorded with a stopwatch for up to 240 s.
Statistical Analysis
Data are given as mean ± standard error of mean. Data were analyzed with ANOVA where control group was compared with rest group. Significance was set at *p < 0.05, **p < 0.01, ***p < 0.001.
Results
Phytochemical Screening
Mushroom extract (HEE) obtained was yellow brown in color. Phytochemical screening revealed the presence of flavonoids, phenolics, carbohydrates, aminoacids and alkaloids.
Eddy's Hot Plate Test
The results of the hotplate test [Figure 1] revealed that the reaction time for the rats was significantly increased from the dose of 250 mg/kg (9.14 ± 0.14 s, P < 0.01) and above. This was somewhat dose dependent. Pethidine at a dose of 4 mg/kg significantly (9.94 ± 0.16 s, P < 0.01) increased the pain latency. Naloxone effectively blocked the effect of extract [Figure 2], unlike metoclopramide [Figure 3].
Figure 1.
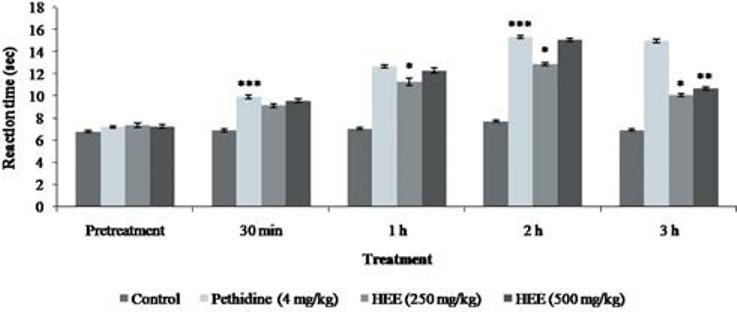
Effect of P. florida extract on hot plate activity in wistar rats. Results are given as mean ± SEM of five animals in each group. Control group compared with rest of the treated groups. Significance at *P < 0.05; **P < 0.01; ***P < 0.001
Figure 2.
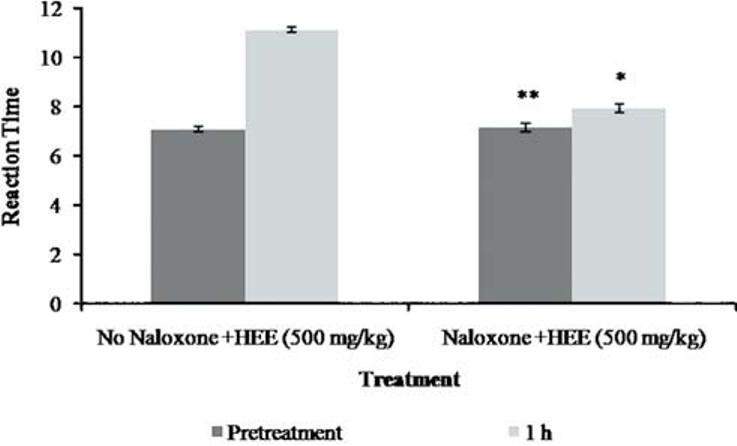
Effect of P. florida extract along with naloxone on hot plate activity in wistar rats. Results are given as mean ± SEM of five animals in each group. Control group compared with rest of the treated groups. Significance at *P < 0.05; **P < 0.01; ***P < 0.001
Figure 3.
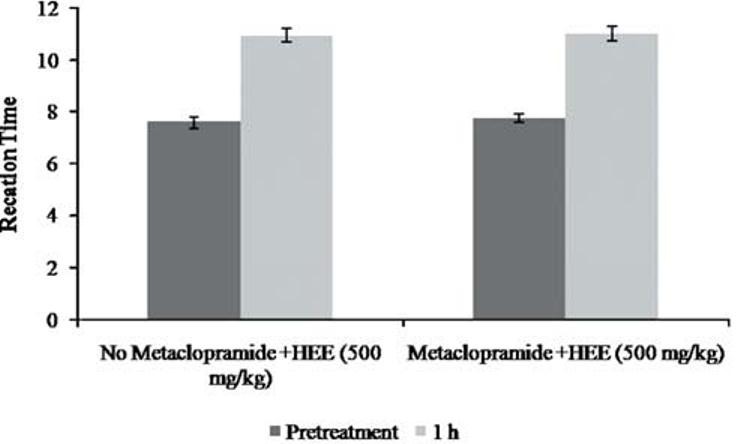
Effect of P. florida extract along with Metaclopramide on hot plate activity in wistar rats. Results are given as mean ± SEM of five animals in each group. Control group compared with rest of the treated groups. Significance at *P < 0.05; **P < 0.01; ***P < 0.001
Tail Flick Test
After a latency period of 1h following oral administration of the HEE at a dose of 250 mg/kg, there was significant reduction to painful sensation. The antinociceptive property of the extract at 500 mg/kg was profound at 2 h [Figure 4].
Figure 4.
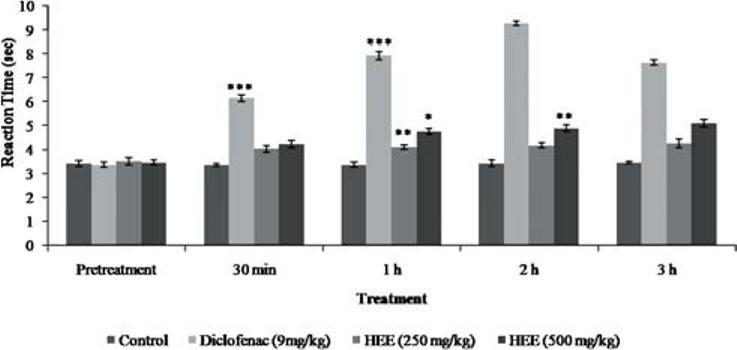
Effect of P. florida extract on tail flick response in wistar rats. Results are given as mean ± SEM of five animals in each group. Control group compared with rest of the treated groups. Significance at *P < 0.05; **P < 0.01; ***P < 0.001
Acetic Acid-Induced Writhing Response
The effect of the HEE on writhing response in rats is shown in Figure 5. HEE at dose 500 mg/kg p.o. caused an inhibition on the writhing response induced by acetic acid. The maximal inhibition of the nociceptive response was achieved at a dose of 500 mg/kg. A similar inhibition was observed with indomethacin
Figure 5.
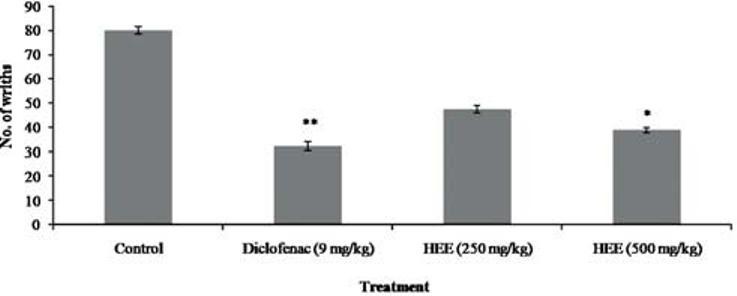
Effect of P. florida extract on acetic acid induced writhing in wistar rats. Results are given as mean ± SEM of five animals in each group. Control group compared with rest of the treated groups. Significance at *P < 0.05; **P < 0.01; ***P < 0.001
Formalin Test
Intraplantar injection of 2.5% formalin evoked a characteristic biphasic licking response. The duration of licking for the early phase (0-300 sec) was 75.2 ± 3.11 s and for the late phase (1200-3600 sec) was 855.2 ± 15.11 s in control groups. As shown in Figure 6, pretreatment (3600 sec) with different doses of HEE at the dose 500 mg/kg had significant effect against the duration of licking activity in the early and late phase.
Figure 6.
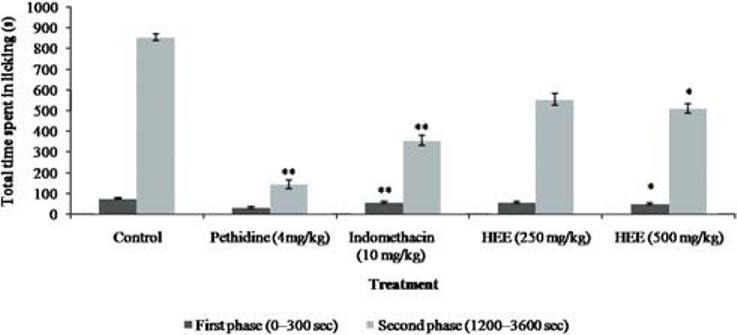
Effect of P. florida extract on formalin induced pain in wistar rats. Results are given as mean ± SEM of five animals in each group. Control group compared with rest of the treated groups. Significance at *P < 0.05; **P < 0.01; ***P < 0.001
Anti-Inflammatory Activity
There was a significant decrease in the paw thickness after 3 h HEE at the dose 250 mg/kg showed paw volume 0.182 ± 0.041 and at 500mg/kg 0.156 ± 0.024 when compared to standard (0.128 ± 0.083) [Figure 7].
Figure 7.
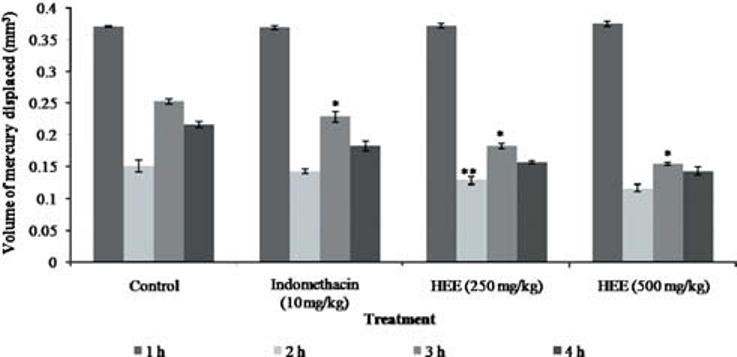
Effect of P. florida extract on carageenan induced edema in wistar rats. Results are given as mean ± SEM of five animals in each group. Control group compared with rest of the treated groups. Significance at *P < 0.05; **P< 0.01; ***P< 0.001
Evaluation of Sedative Activity
No significant result was observed which could justify the sedative potential of mushroom. In muscle strength test, latency to fall was 12.344 ± 0.125 v/s 13.175 ± 0.211 (Control v/s HEE 500 mg/kg treated).
Discussion
The present study was conducted to assess the anti-nociceptive and anti-inflammatory properties of hydroethanolic extract of P. florida. The methods selected were chemical nociception in the test model of acetic acid-induced writhing and thermal nociception hot plate and tail immersion test. These methods were selected to evaluate both centrally and peripherally mediated effects of P. florida. To study anti-inflammatory potential, carageenan induced edema method was studied.
The hot-plate test is commonly used to assess narcotic analgesia. HEE showed antinociceptive effects in this test. Hence, it is assumed that P. florida has analgesic effect at the central nervous system. This observation together with the antinociceptive effect seen with naloxone and the lack of activity with metoclopramide reinforces the notion that the antinociceptive activity of HEE is related to the activation of the opioid system.[20]
Dopamine, a catecholamine neurotransmitter, is known to demonstrate analgesic effect due to D2 receptor activation.[21] Metoclopramide is a D2 receptor blocker. In the current work, administration of metoclopramide along with mushroom extract did not altered pain perception and analgesic effect on animal (as evident by analgesic effect in presence of metoclopramide) which demonstrates noninvolvement of dopamine in analgesic effect.
It is known that the tail immersion/tail-flick response appears to be a spinal reflex, and is regarded as a specific screening method for centrally acting analgesics. On the other hand, the progressive influence of HEE on the reaction time submitted to the tail flick test is consistent with the interpretation that its analgesic property might have a central origin.[22]
The writhing test is a very sensitive method for preliminary evaluation of anti-nociceptive activity and the ED50 values obtained in animals using this test can be correlated with the analgesic doses in humans,[23] still it cannot indicate whether the effects result from central and/or peripheral actions. The analgesic potential of the extract was shown by acetic acid test to be significant but was not specific.[19] The test did not indicate if the potential resulted from central and/or peripheral actions. To clarify that, formalin test[16] was conducted which is the most predictive of the models of acute pain. Intraplantar injection of 2.5% formalin evoked a characteristic biphasic licking response, an early phase corresponding to acute neurogenic pain, sensitive to drugs that interact with the opioid system and a late phase corresponding to inflammatory pain responses inhibited by analgesic-anti-inflammatory drugs.[16] Morphine, a central nervous system analgesic drug, inhibits both phases, while aspirin, a peripherally acting drug, inhibits only the second phase. In our study, HEE produced a marked reduction of the duration of the licking in the both phase, corresponding to the inflammatory reaction suggesting its activity on CNS.
Carrageenan-induced paw oedema is a commonly used primary test for the screening of new anti-inflammatory agents and is believed to be biphasic.[24] The first phase (1-2 h) is due to the release of histamine or serotonin and the second phase of oedema is due to the release of prostaglandin.[25] The results of this study indicate that the HEE significantly reduced carrageenan induced paw oedema in rats. Therefore, the mechanism of action may be by inhibition of histamine, serotonin or prostaglandin synthesis.
Mushrooms are known to contain a large variety of myoconstituents like flavonoids, phenolics, polysaccharides and polysaccharopeptides which are documented to possess therapeutic potential.[3,7–9] In present work, these myoconstituents might be responsible to show analgesic effect. Thus in nutshell, P. florida showed excellent analgesic and anti-inflammatory activity in rats. Further studies on isolation and identification of the active compounds may provide a better source for developing new therapeutic agents.
Acknowledgments
We gratefully acknowledge Prof AK Pandey, Department of Bioscience, RD University, Jabalpur, India for providing gift sample of Pleurotus florida for research purpose. Authors are also thankful to Principal and HOD-Pharmacy department, Kelaniketan Polytechnic College, Jabalpur, for kind help in in vivo studies.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.McCurdy CR, Scully SS. Analgesic substances derived from natural products (natureceuticals) Life Sci. 2005;78:476–84. doi: 10.1016/j.lfs.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Mizuno T, Saito H, Nishitoba T, Kawagishi H. Antitumor-active substances from mushrooms. Food Rev Int. 1995;11:23–61. [Google Scholar]
- 3.Ng TB, Chan WY. Polysaccharopeptide from the mushroom Coriolus versicolor possesses analgesic activity but does not produce adverse effects on female reproductive or embryonic development in mice. Gen Pharmacol. 1997;29:269–73. doi: 10.1016/s0306-3623(96)00412-0. [DOI] [PubMed] [Google Scholar]
- 4.Stamets P. Novel antimicrobials from mushrooms. Herbal Gram. 2002;54:29–33. [Google Scholar]
- 5.Ganeshpurkar A, Rai G, Jain AP. Medicinal mushrooms: Towards a new horizon. Phcog Rev. 2010;4:127–35. doi: 10.4103/0973-7847.70904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganeshpurkar A, Bhadoriya SS, Pardhi P, Jain AP, Rai G. In vitro prevention of cataract by Oyster Mushroom Pleurotus florida extract on isolated goat eye lens. Indian J Pharmacol. 2011;43:667–70. doi: 10.4103/0253-7613.89823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy SK, Das D, Mondal S, Maiti D, Bhunia B, Maiti TK, et al. Structural studies of an immunoenhancing water-soluble glucan isolated from hot water extract of an edible mushroom, Pleurotus florida, cultivar Assam Florida. Carbohydr Res. 2009;344:2596–601. doi: 10.1016/j.carres.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Jose N, Ajith TA, Janardhanan KK. Methanol extract of the oyster mushroom, Pleurotus florida, inhibits inflammation and platelet aggregation. Phytother Res. 2004;18:43–6. doi: 10.1002/ptr.1355. [DOI] [PubMed] [Google Scholar]
- 9.Ganeshpurkar A, Bhadoriya SS, Pardhi P, Rai G. Investigation of anthelmintic potential of oyster mushroom Pleurotus florida. Indian J Pharmacol. 2012;44:539–40. doi: 10.4103/0253-7613.99349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasudewa NS, Abeytunga DT, Ratnasooriya WD. Antinociceptive activity of Pleurotus ostreatus, an Edible Mushroom in rats. Pharm Biol. 2007;45:533–40. [Google Scholar]
- 11.Harborne JB. Phytochemical Method: A guide to modern techniques of plants analysis. New York: Chapman and Hall; 1983. [Google Scholar]
- 12.Guideline 423 for testing chemicals. Paris: OECD Guidelines; 2001. Organization for Economic Cooperation and development (OECD) pp. 1–14. [Google Scholar]
- 13.Eddy NB, Leimbach DJ. Synthetic analgesics: II Dithienyl butenyl and Dithienyl butylamine. J Pharmacol Exp Ther. 1953;107:38593. [PubMed] [Google Scholar]
- 14.Kulkarni SK. Handbook of Experimental Pharmacology. 3rd ed. New Delhi: Vallabh Prakashan; 1999. [Google Scholar]
- 15.Dharmasiri MG, Jayakody JR, Galhena G, Liyanage SS, Ratnasooriya WD. Anti-inflammatory and analgesic activities of mature fresh leaves of Vitex negundo. J Ethnopharmacol. 2003;87:199–206. doi: 10.1016/s0378-8741(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 16.Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–14. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 17.Borgi W, Ghedira K, Chouchane N. Anti-inflammatory and analgesic activities of Zizyphus lotus root barks. Fitoterapia. 2007;78:16–9. doi: 10.1016/j.fitote.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Borgi W, Ghedira K, Chouchane N. Neuropharmacological activity of Solanum nigrum fruit. J Ethnopharmacol. 1998;62:43–8. doi: 10.1016/s0378-8741(98)00059-2. [DOI] [PubMed] [Google Scholar]
- 19.Godoy MC, Fighera MR, Souza FR, Flores AE, Rubin MA, Oliveira MR. α2-Adrenoceptors and 5-HT receptors mediate the antinociceptive effect of new pyrazoles, but not of dipyrone. Eur J Pharmacol. 2004;496:93–7. doi: 10.1016/j.ejphar.2004.05.045. [DOI] [PubMed] [Google Scholar]
- 20.Woolfe G, MacDonald AD. The evaluation of the analgesic action of pethidine hydrochloride (DEMEROL) J Pharmacol Exp Ther. 1944;80:300–7. [Google Scholar]
- 21.Wood PB. Role of central dopamine in pain and analgesia. Expert Rev Neurother. 2008;8:781–97. doi: 10.1586/14737175.8.5.781. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan K, Muruganandan S, Lal J, Chandra S, Tandan SK, Raviprakash V. Antinoniceptive and antipyretic activities of Pongamia pinnata leaves. Phytother Res. 2003;17:259–64. doi: 10.1002/ptr.1126. [DOI] [PubMed] [Google Scholar]
- 23.Collier HO, Dinneen LC, Johnson CA, Schneider C. The abdominal response and it suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinegar R, Schreiber W, Hugo R. Biphasic development of carrageenan edema in rats. J Pharmacol Exp Ther. 1960;166:96–103. [PubMed] [Google Scholar]
- 25.Saha A, Masud AM, Bachar SC, Kundu JK, Datta BK, Nahar L, et al. The analgesic and anti-inflammatory activities of the extracts of Phyllanthus reticulates in mice model. Pharm Biol. 2007;45:335–59. [Google Scholar]


