Abstract
Mesenchymal stem cells (MSCs) are prototypical adult stem cells with the capacity for self-renewal and differentiation with a broad tissue distribution. MSCs not only differentiate into types of cells of mesodermal lineage but also into endodermal and ectodermal lineages such as bone, fat, cartilage and cardiomyocytes, endothelial cells, lung epithelial cells, hepatocytes, neurons, and pancreatic islets. MSCs have been identified as an adherent, fibroblast-like population and can be isolated from different adult tissues, including bone marrow (BM), umbilical cord, skeletal muscle, and adipose tissue. MSCs secrete factors, including IL-6, M-CSF, IL-10, HGF, and PGE2, that promote tissue repair, stimulate proliferation and differentiation of endogenous tissue progenitors, and decrease inflammatory and immune reactions. In this paper, we focus on the role of BM-derived MSCs in organ repair.
1. Introduction
The shortage of donor organs and the need of lifelong immunosuppression for the thousands of patients suffering from end-stage diseases worldwide are problems that need to be resolved. The repair, replacement, and regeneration of organs can restore impaired functions and are regarded as a potential solution to allotransplantation [1]. The bone marrow (BM) is an invaluable source of adult pluripotent stem cells, including hematopoietic stem cells (HSCs), endothelial progenitor cells (EPCs), and mesenchymal stem cells (MSCs). MSCs are prototypical adult stem cells with the capacity for self-renewal and differentiation with a broad tissue distribution. MSCs have been identified as an adherent, fibroblast-like population, originally isolated from BM [2]. These multipotent cells can be differentiated in vitro and in vivo into various cell types of mesenchymal origin, such as osteoblasts, adipocytes, and chondrocytes [3, 4]. Recently, more reports have demonstrated that MSCs secrete a variety of factors that promote tissue repair, stimulate proliferation and differentiation of endogenous tissue progenitors, and decrease inflammatory and immune reactions [5–7]. Because MSCs do not evoke an immune response, they are useful for allogenic organ and tissue repair.
2. Source, Multilineage Potential and Definition of MSCs
MSCs were first isolated from BM and have since been isolated from different adult tissues, including skeletal muscle [8], adipose tissue [9], umbilical cord [10], synovium [11], the circulatory system [12], dental pulp [13], amniotic fluid [14], fetal blood [15], lung [16], liver, and BM [17]. Friedenstein and coworkers first reported the existence of adherent, fibroblast-like cells isolated from BM [2], and that these cells could differentiate into mesodermal lineage such as osteoblasts, adipocytes, and chondrocytes in vitro [18] and cardiomyocytes [19]. Also, MSCs have been reported to differentiate into types of cells of endodermal and ectodermal lineages, including lung [20], retinal pigment [21], skin [22], sebaceous duct cells [23], renal tubular cells [24], and neural cells [25, 26], hepatocytes [27], and pancreatic islets [28]. There has hitherto been no specific surface marker for the identification of MSCs. For the isolation of human MSCs, the International Society for Cell Therapy proposed criteria [18] that comprise (1) adherence to plastic in standard culture conditions; (2) expression of the surface molecules CD73, CD90, and CD105 in the absence of CD34, CD45, HLA-DR, CD14 or CD11b, CD79a, or CD19 surface molecules as assessed by fluorescence-activated cell sorter analysis; (3) a capacity for differentiation to osteoblasts, adipocytes, and chondroblasts in vitro. Similarly, murine MSCs have been shown to differ from human MSCs in terms of marker expression and behavior and have been identified as an adherent, fibroblast-like population, negative for CD45, CD11b, and CD 31, and positive for Scal1 and CD106 [29].
3. MSCs and the Immune System
MSCs have the ability to modify and influence almost all the cells of the innate and adaptive immune systems, to interfere with and affect cellular proliferation, differentiation, maturation, and function to induce an anti-inflammatory phenotype, and to modulate the immune response mediated by MSC soluble factors, including IL-6, M-CSF, IL-10, TGFβ, HGF, and PGE2 [7, 30, 31]. The innate immune cells include neutrophils, dendritic cells (DCs), natural killer (NK) cells, eosinophils, mast cells, and macrophages. MSCs modulate DC function, indirectly regulate T and B cell activities, delay and prevent the development of acute graft versus host disease (GVHD) [32], and suppress DC function during allogeneic islet transplantation [33]. MSCs have been shown to suppress these inflammatory cells [34] and to alter NK cell phenotype and suppress proliferation, cytokine secretion, and cytotoxicity against HLA class I expressing targets [35]. MSCs mediated NK cell suppression via soluble factors such as indoleamine 2,3-dioxygenase, PGE2, and TGFβ [36]. The adaptive immune system, which is composed of T and B lymphocytes generates specific immune responses to pathogens with the production of memory cells. It has been reported that MSCs upregulate anti-inflammatory Th2 cytokines, including IL-3, -5, -10, and -13, and downregulate proinflammatory Th1 cytokines, including IL-1α and β, IFNγ, and TNFα [37]. MSCs induced an alteration of DC cytokine secretion, inducing a decreased secretion of pro-inflammatory cytokines such as TNFα, IFNγ, and IL-12, and increased IL-10, which is a suppressive cytokine and inducer of reg T cells [38]. MSCs exert an inhibitory effect on B cells, but MSCs have stimulatory effect in low doses [39]. Concerning the immunomodulatory properties of MSCs in a mouse model, one report [40] has suggested that allogeneic MSCs are not intrinsically immunoprivileged, and under appropriate conditions, allogeneic MSCs induce a memory T-cell response resulting in rejection of an allogeneic stem cell graft. Another report [41] has suggested that MSCs could potentially improve experimental autoimmune encephalomyelitis in mice.
4. Homing of MSCs
Intravenously injected MSCs can migrate to the BM [42, 43] in the steady state and home to the inflammation site by migrating across the endothelium and then entering the injured organ [20, 44–47]. The fact that MSCs confer protection cannot be entirely attributed to their ability to home and engraft to the site of damage, suggesting that they are also capable of mediating protection in an endocrine manner [1]. MSCs have many chemokine receptors that assist in their migration to inflammatory sites via the SDF1/CXCR4 pathway [48]. Moreover, studies have demonstrated that platelet-derived growth factor-AB, IGF-1, and CD44 are the most potent chemoattractants for MSCs [44, 49].
5. BM-Derived MSCs (BMMSCs) and Organ Repair
Many reports have indicated that MSCs have the capacity to differentiate into endodermal, mesodermal, and ectodermal lineage cells. Recently, a report has indicated that the ability of MSCs to alter the tissue microenvironment via the secretion of soluble factors may contribute more significantly than their capacity for differentiation in tissue repair [50]. Adipose tissue and BM are the most readily available sources of MSCs because they are easy to harvest, and because of their relative abundance of progenitors and the lack of ethical concerns. Although adipose tissue-derived MSCs and BMMSCs show the same immunoregulatory and supporting hematopoiesis [51], BMMSCs have a higher degree of commitment to differentiate into chondrogenic and osteogenic lineages than adipose tissue-derived MSCs [52]. BMMSCs have been shown to ameliorate tissue damage and to improve function after lung injury [53–55], kidney disease [56, 57], diabetes [58, 59], myocardial infarction [60, 61], liver injury [62, 63], and neurological disorders [64].
5.1. BMMSCs and Lung
The lung is an organ that is highly susceptible to edema and endothelial permeability after traumatic injury. BMMSCs inhibit endothelial cell barrier permeability and preserve pulmonary endothelial cell integrity by preserving adherent junctions, tight junctions and decreasing inflammation. BMMSCs address both components of endothelial permeability and inflammation induced by hemorrhagic shock [54]. Interstitial lung diseases are characterized by epithelial injury, fibroblast proliferation, expansion of the lung matrix, and dyspnea. Of these diseases, idiopathic pulmonary fibrosis (IPF) is the most frequent and lethal. Proinflammatory cytokines IL-1 and TNF-α induce endothelial cells to express adhesion molecules and chemokines that attract other white cells from the blood to the site of injury [65]. IL-1 and TNF-α also stimulate proliferation of endothelial cells and fibroblasts that increase the blood supply at the site of injury and repair damage by the formation of scar tissue [66]. BMMSCs protect lung tissue from bleomycin-induced injury by blocking TNF-α and IL-1, two fundamental proinflammatory cytokines in the lung [53]. BMMSCs enhance the restoration of systemic oxygenation and lung compliance and decrease lung inflammation and histological lung injury. They also secrete cytokines, enhance lung repair, and attenuate the inflammatory response following ventilator-induced lung injury [55].
5.2. BMMSCs and Kidney
Acute and chronic kidney injuries after transplantation have a complex pathophysiology involving ischemic, inflammatory, and immunologic mechanisms, and adult stem cells have been used in the treatment of these kidney diseases. Adult BM stem cells and the kidney precursors have been demonstrated to have an ability to differentiate into the kidney's specialized structures [67]. Nephrons are of mesenchymal origin, and stromal cells are of crucial importance for signaling, leading to the differentiation of both nephrons and collecting ducts [67]. Ischemic acute renal failure (ARF), characterized by a sharp decline in the glomerular filtration rate, is a very common complication in hospitalized patients and particularly in patients with multiorgan failure. When BMMSCs are injected after ARF, they can histologically become located in the kidney and significantly enhance the recovery of renal function by transdifferentiation into renal tubular or vascular endothelial cells [24, 68]. A single intrarenal administration of BMMSCs 7 days after ischemia-reperfusion significantly improved renal function and modified renal remodeling. The improvement of renal function was associated with a reduction in extracellular matrix accumulation. In addition, MSC administration also reduced tubular dilation, which is a classical feature of progressive renal failure in a renal ischemia rat model [57].
5.3. BMMSCs and Pancreas
Diabetes is caused by absolute insulin deficiency due to autoimmune destruction of insulin-secreting pancreatic β-cells (type 1 diabetes) or by relative insulin deficiency due to decreased insulin sensitivity, usually observed in overweight individuals (type 2 diabetes). In both types of the disease, an inadequate mass of functional β-cells is the major determinant for the onset of hyperglycemia and the development of overt disease. BM and BMMSCs induce the regeneration of recipient-derived pancreatic insulin-secreting cells, and MSCs inhibit T-cell-mediated immune responses against newly formed β-cells, which are able to survive in this altered immunological milieu [69].
Acute pancreatitis (AP) is characterized by a rapid onset and disease progression, with high fatality. Pancreatic acinar cells are the functional unit for the external secretion of the pancreas, which accounts for 80% of pancreatic tissue. During the process of severe AP, inflammatory mediators, metabolic products of arachidonic acid, and oxygen-derived free radicals enhance vascular permeability and cause tissue thrombosis and hemorrhage, thereby inducing necrosis of the pancreas [70]. BMMSCs can effectively relieve injury to pancreatic acinar cells and small intestinal epithelium, promote the proliferation of enteric epithelium and repair of the mucosa, and attenuate systemic inflammation in rats with severe acute peritonitis [71].
Human BM stem cells are able to differentiate into insulin-expressing cells in vitro by a mechanism involving several transcription factors of the β-cell developmental pathway when cultured in an appropriate microenvironment [72]. Human BMMSCs can be induced to express insulin in sufficient quantities to to reduce blood glucose in a diabetic mouse model [73] and to protect human islets from proinflammatory cytokines [74]. The use of human BMMSCs could be developed as a cell therapy for pancreatitis because of the ability, as shown in a rat model of acute pancreatitis, to reduce inflammation and damage to pancreatic tissue by reducing levels of cytokines and inducing Foxp3(+) regulatory T cells [75].
5.4. BMMSCs and Heart
Cardiovascular diseases are the first cause of death worldwide, and myocardial infarction (MI) is responsible for 12.8% of all deaths [76]. BMMSCs have been shown to differentiate into myogenic phenotype [77] and show a potent antifibrotic action, as their conditioned medium decreases cardiac fibroblast proliferation and the expression of collagen types I and III [78, 79] and increases the secretion of antifibrotic molecules such as matrix metalloproteinases 2, 9, and 14 [80]. BMMSCs exhibit the ability to differentiate into cardiomyocytes, smooth muscle cells, and endothelium in a swine model of chronic ischemic cardiomyopathy [81]. They have been shown to prolong survival compared with controls when hearts of Wistar rats were transplanted to Fisher 344 rats with intravenous MSC infusion [82]. Intravenous fusion of MSCs is the easiest and most practical method for delivery, though the MSCs must travel through the pulmonary circulation, where entrapment of cells is a concern [83]. Intracoronary infusion of stem cells is delivered with a standard over-the-wire balloon angioplasty catheter placed into the target coronary artery [84]. Injected BMMSCs improve cardiac function and reduce scar size in acute MI [85, 86]. Early-phase clinical trial data demonstrate that MSC therapy for post-MI is safe and has favorable effects on cardiac structure and function [87, 88].
5.5. BMMSCs and Liver
FGF-4 is one of the most important members of the fibroblast growth factor family; it can initiate the proliferation of mesodermal and endodermal cells and improve the development of fetal liver [89]. HGF is essential for the development of several epithelial organs and has been one of the most well-characterized cytokines for the stimulation of DNA synthesis in primary hepatocyte cultures and for liver development [90]. Oncostatin M is a member of the interleukin-6 family produced by hematopoietic cells and induces the differentiation of fetal hepatic cells, conferring various metabolic activities of adult liver [91]. These three factors participate in different developmental stages of the liver. FGF4, HGF, and oncostatin M have been shown to be key cytokines for hepatic differentiation from mouse BMMSCs [92]. Transplantation of BMMSCs alleviates GalN-induced acute liver injury in rats and stimulates the recovery systems, as evidenced by an earlier surge of cellular proliferation and differentiation into functional hepatocytes. IL-6 exerts hepatoprotective and mitogenic effects by stimulating the induction of acute-phase proteins as well as by suppressing apoptosis. Transplantation of BMMSCs could ameliorate acute liver injury. It promotes cell proliferation and organ repair, and the activation of the IL-6/gp130-mediated STAT3 signaling pathway via soluble IL-6 receptor is crucial in hepatic differentiation of BMMSCs [93].
Liver fibrosis is the excessive accumulation of extracellular matrix proteins, including collagen, that occurs in most types of chronic liver disease. Advanced liver fibrosis results in cirrhosis, liver failure, and portal hypertension, and often requires liver transplantation [94]. Although liver transplantation is by far the most effective treatment for liver cirrhosis, extensive clinical application of the technique is limited by the lack of donor organ availability [95]. Cell-based hepatocyte transplantation, a potential interventional procedure, provides an effective strategy and holds great promise for the treatment of impaired livers. BMMSCs can protect against experimental liver fibrosis through promotion of IL-10 expression in CCl4- or dimethylnitrosamine-induced rats [63, 96].
5.6. BMMSCs and Brain
The development of effective treatments for human brain and spinal cord injury remains a serious challenge. In this regard, the transplantation of stem cells may help repair injured nerve tissue through the replacement of damaged cells, neuroprotection, or the creation of an environment conductive to regeneration by endogenous cells [97]. BMMSCs have been shown to promote cell proliferation and neurotrophic function of Schwann cells in vitro and in vivo [98]. Transplantation of BMMSCs can significantly reduce the behavioral abnormalities of these animals during the six weeks after engraftment [64]. Intravenously transplanted MSCs are capable of improving functional recovery and restoring neurological deficits in experimental intracerebral hemorrhage. The mechanisms are associated with enhanced survival and differentiation of neural cells and increased expression of antiapoptotic proteins and atrophic factors [99]. Human BMMSCs can improve neurological functional recovery in mice with experimental autoimmune encephalitis, possibly via a reduction of inflammatory infiltrates and areas of demyelination, stimulation of oligodendrogenesis, and by elevating brain-derived neurotrophic factor (BDNF) expression [41, 100]. Human BMMSCs transfected with the BDNF gene also showed improved functional recovery and reduced infarct size through a reduction in apoptosis [101]. Patients with Parkinson's disease transplanted with BMMSCs in the early stages of the disease (less than 5 years) showed greater improvement than in the later stages (11–15 years) [102].
5.7. BMMSCs and Intestine
Inflammatory bowel disease comprises a spectrum of chronic and relapsing diseases, including Crohn's disease (CD) and ulcerative colitis [103]. CD is characterized by a background of mucosal T-cell dysfunction, inflammatory cell infiltration, and abnormal cytokine production leading to uncontrolled and persistent intestinal transmural inflammation. Intraperitoneally injected cryopreserved BMMSCs home to and engraft into the inflamed colon and ameliorate trinitrobenzene sulfonic acid-induced colitis in rats [104]. Similarly, the injection of adipose-derived MSCs facilitated colonic mucosal repair and reduced the infiltration of inflammatory cells in the experimental colitis model [105].
Small intestinal permeability and villi injuries were significantly reduced in an MSC-administered group compared with the control group. MSC administration accelerated the recovery of the intestinal barrier dysfunction in a rat model of ischemia/reperfusion injury [106].
5.8. BMMSCs and Bone
Bone is regarded as an organ, and small bone damage can repair spontaneously without intervention. However, bone transplantation and surgery are required when there is extensive bone damage. As adult stem cells, BMMSCs possess a number of characteristics that make them appropriate for use in promoting bone regeneration [107]. BMMSCs may differentiate into tissue cells in order to restore lost morphology as well as function and to secrete a wide spectrum of bioactive factors that help to create a repair environment through their antiapoptotic effects, immunoregulatory function, and the stimulation of endothelial progenitor cell proliferation [108]. One report shows that BMMSCs stimulate growth with osteogenesis imperfecta when children received allogeneic BMMSCs [109].
6. Conclusion
Figure 1 summarizes the main actions of BMMSCs. The original use of BMMSCs was to accelerate hematopoiesis, since they have the potential to differentiate into various cells, and to secrete cytokines and growth factors. BMMSCs have immunomodulatory properties through paracrine and endocrine mechanisms to repair damaged tissue. Homing and immunomodulation are important aspects of MSC functioning and their clinical effects. It has been proposed that the anti-inflammatory and antiapoptotic effects of MSCs may promote tissue regeneration. The use of allogenic nonimmunogenic BMMSCs would be a more acceptable strategy clinically. The potential role of BMMSCs to promote engraftment of organs and prevent rejection may be multifactorial and might be dependent on secretion of soluble growth factors, increasing angiogenesis, suppressing alloreactive T cells, and interacting with several arms of the immune system. However, the long-term safety of transplanted BMMSCs for organ repair needs to be proven prior to their clinical application.
Figure 1.
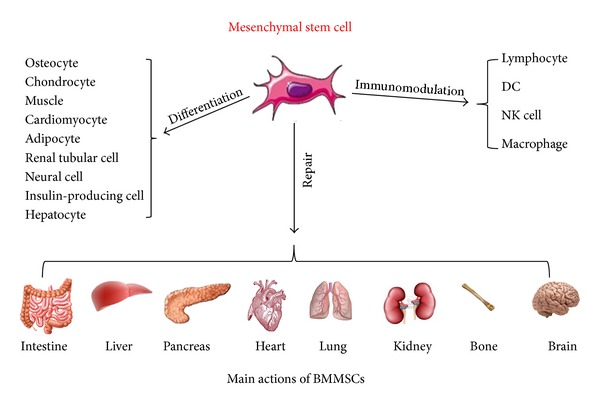
Main actions of BMMSCs.
Conflict of interests
None of the authors has conflict of interests to declare.
Acknowledgments
The authors would like to thank Mr. Hilary Eastwick-Field and Ms. Keiko Ando for their help in the preparation of the paper.
References
- 1.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. Journal of the American Society of Nephrology. 2007;18(9):2486–2496. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell and Tissue Kinetics. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein AJ, Chailakhyan RK, Latsinik NV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Caplan AI. Mesenchymal stem cells. Journal of Orthopaedic Research. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 5.Nicola MD, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 6.Raffaghello L, Bianchi G, Bertolotto M, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26(1):151–162. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 8.Williams JT, Southerland SS, Souza J, Calcutt AF, Cartledge RG. Cells isolated from adult human skeletal muscle capable of differentiating into multiple mesodermal phenotypes. American Surgeon. 1999;65(1):22–26. [PubMed] [Google Scholar]
- 9.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Engineering. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 10.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. British Journal of Haematology. 2000;109(1):235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 11.De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis & Rheumatism. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. Journal of Cell Biology. 2001;153(5):1133–1139. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.In ’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102(4):1548–1549. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 15.Noort WA, Kruisselbrink AB, In’t Anker PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34 + cells in NOD/SCID mice. Experimental Hematology. 2002;30(8):870–878. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- 16.Fan CG, Tang FW, Zhang QJ, et al. Characterization and neural differentiation of fetal lung mesenchymal stem cells. Cell Transplantation. 2005;14(5):311–321. doi: 10.3727/000000005783983070. [DOI] [PubMed] [Google Scholar]
- 17.Campagnoli C, Roberts IAG, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 18.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 19.Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. Journal of Clinical Investigation. 1999;103(5):697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(14):8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2007;245(3):414–422. doi: 10.1007/s00417-006-0382-7. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa H, Akita S, Fukui M, Fujii T, Akino K. Human mesenchymal stem cells successfully improve skin-substitute wound healing. British Journal of Dermatology. 2005;153(1):29–36. doi: 10.1111/j.1365-2133.2005.06554.x. [DOI] [PubMed] [Google Scholar]
- 23.Fu X, Fang L, Li X, Cheng B, Sheng Z. Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair and Regeneration. 2006;14(3):325–335. doi: 10.1111/j.1743-6109.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 24.Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. Journal of the American Society of Nephrology. 2004;15(7):1794–1804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 25.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(19):10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muñoz-Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. Journal of Neuroscience. 2004;24(19):4585–4595. doi: 10.1523/JNEUROSCI.5060-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz RE, Reyes M, Koodie L, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. Journal of Clinical Investigation. 2002;109(10):1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang DQ, Cao LZ, Burkhardt BR, et al. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53(7):1721–1732. doi: 10.2337/diabetes.53.7.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103(5):1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 30.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105(5):2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 31.Ramasamy R, Fazekasova H, Lam EWF, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83(1):71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 32.Zhang B, Liu R, Shi D, et al. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2 dependent regulatory dendritic cell population. Blood. 2009;113(1):46–57. doi: 10.1182/blood-2008-04-154138. [DOI] [PubMed] [Google Scholar]
- 33.Aldinucci A, Rizzetto L, Pieri L, et al. Inhibition of immune synapse by altered dendritic cell actin distribution: a new pathway of mesenchymal stem cell immune regulation. Journal of Immunology. 2010;185(9):5102–5110. doi: 10.4049/jimmunol.1001332. [DOI] [PubMed] [Google Scholar]
- 34.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature Reviews Immunology. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 35.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 36.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 37.Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. Journal of Immunology. 2003;171(7):3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Ge W, Li C, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells and Development. 2004;13(3):263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 39.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 40.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EGA, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108(6):2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Li Y, Chen J, et al. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Experimental Neurology. 2005;195(1):16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Devine SM, Bartholomew AM, Mahmud N, et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Experimental Hematology. 2001;29(2):244–255. doi: 10.1016/s0301-472x(00)00635-4. [DOI] [PubMed] [Google Scholar]
- 43.Wynn RF, Hart CA, Corradi-Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104(9):2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 44.Herrera MB, Bussolati B, Bruno S, et al. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney International. 2007;72(4):430–441. doi: 10.1038/sj.ki.5002334. [DOI] [PubMed] [Google Scholar]
- 45.Chapel A, Bertho JM, Bensidhoum M, et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. Journal of Gene Medicine. 2003;5(12):1028–1038. doi: 10.1002/jgm.452. [DOI] [PubMed] [Google Scholar]
- 46.Mahmood A, Lu D, Lu M, et al. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53(3):697–703. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- 47.Orlic D, Kajstura J, Chimenti S, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(18):10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fox JM, Chamberlain G, Ashton BA, Middleton J. Recent advances into the understanding of mesenchymal stem cell trafficking. British Journal of Haematology. 2007;137(6):491–502. doi: 10.1111/j.1365-2141.2007.06610.x. [DOI] [PubMed] [Google Scholar]
- 49.Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25(7):1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 50.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25(11):2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 51.Poloni A, Maurizi G, Leoni P, et al. Human dedifferentiated adipocytes show similar properties to bone marrow-derived mesenchymal stem cells. Stem Cells. 2012;30(5):965–974. doi: 10.1002/stem.1067. [DOI] [PubMed] [Google Scholar]
- 52.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circulation Research. 2007;100(9):1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ortiz LA, DuTreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(26):11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pati S, Gerber MH, Menge TD, et al. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0025171.e25171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Curley GF, Hayes M, Ansari B, et al. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax. 2012;67(6):496–501. doi: 10.1136/thoraxjnl-2011-201059. [DOI] [PubMed] [Google Scholar]
- 56.Kunter U, Rong S, Djuric Z, et al. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. Journal of the American Society of Nephrology. 2006;17(8):2202–2212. doi: 10.1681/ASN.2005080815. [DOI] [PubMed] [Google Scholar]
- 57.Alfarano C, Roubeix C, Chaaya R, et al. Intraparenchymal injection of bone marrow mesenchymal stem cells reduces kidney fibrosis after ischemia-reperfusion in cyclosporine-immunosuppressed rats. Cell Transplant. 2012;21(9):2009–2019. doi: 10.3727/096368912X640448. [DOI] [PubMed] [Google Scholar]
- 58.Lee RH, Seo MJ, Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Si Y, Zhao Y, Hao H, et al. Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: identification of a novel role in improving insulin sensitivity. Diabetes. 2012;61(6):1616–1625. doi: 10.2337/db11-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iso Y, Spees JL, Serrano C, et al. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochemical and Biophysical Research Communications. 2007;354(3):700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cho J, Zhai P, Maejima Y, Sadoshima J. Myocardial injection with GSK-3β-overexpressing bone marrow-derived mesenchymal stem cells attenuates cardiac dysfunction after myocardial infarction. Circulation Research. 2011;108(4):478–489. doi: 10.1161/CIRCRESAHA.110.229658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanazawa H, Fujimoto Y, Teratani T, et al. Bone marrow-derived mesenchymal stem cells ameliorate hepatic ischemia reperfusion injury in a rat model. PLoS ONE. 2011;6(4) doi: 10.1371/journal.pone.0019195.e19195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao W, Li JJ, Cao DY, et al. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World Journal of Gastroenterology. 2012;18(10):1048–1058. doi: 10.3748/wjg.v18.i10.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edalatmanesh MA, Bahrami AR, Hosseini E, Hosseini M, Khatamsaz S. Bone marrow derived mesenchymal stem cell transplantation in cerebellar degeneration: a behavioral study. Behavioural Brain Research. 2011;225(1):63–70. doi: 10.1016/j.bbr.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 65.Kaneider NC, Leger AJ, Kuliopulos A. Therapeutic targeting of molecules involved in leukocyte-endothelial cell interactions. FEBS Journal. 2006;273(19):4416–4424. doi: 10.1111/j.1742-4658.2006.05441.x. [DOI] [PubMed] [Google Scholar]
- 66.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1β induces acute lung injury and chronic repair leading to pulmonary fibrosis. Journal of Clinical Investigation. 2001;107(12):1529–1536. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anglani F, Forino M, Del Prete D, Tosetto E, Torregrossa R, D’Angelo A. In search of adult renal stem cells. Journal of Cellular and Molecular Medicine. 2004;8(4):474–487. doi: 10.1111/j.1582-4934.2004.tb00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lange C, Tögel F, Ittrich H, et al. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney International. 2005;68(4):1613–1617. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 69.Urbán VS, Kiss J, Kovács J, et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26(1):244–253. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- 70.Liu ZH, Peng JS, Li CJ, et al. A simple taurocholate-induced model of severe acute pancreatitis in rats. World Journal of Gastroenterology. 2009;15(45):5732–5739. doi: 10.3748/wjg.15.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tu XH, Song JX, Xue XJ, et al. Role of bone marrow-derived mesenchymal stem cells in a rat model of severe acute pancreatitis. World Journal of Gastroenterology. 2012;18(18):2270–2279. doi: 10.3748/wjg.v18.i18.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moriscot C, De Fraipont F, Richard MJ, et al. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells. 2005;23(4):594–603. doi: 10.1634/stemcells.2004-0123. [DOI] [PubMed] [Google Scholar]
- 73.Zhao M, Amiel SA, Ajami S, et al. Amelioration of streptozotocin-induced diabetes in mice with cells derived from human marrow stromal cells. PLoS ONE. 2008;3(7) doi: 10.1371/journal.pone.0002666.e2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yeung TY, Seeberger KL, Kin T, et al. Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0038189.e38189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jung KH, Song SU, Yi T, et al. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011;140(3):998–1008. doi: 10.1053/j.gastro.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 76.Mazo M, Pelacho B, Prósper F. Stem cell therapy for chronic myocardial infarction. Journal of Cardiovascular Translational Research. 2010;3(2):79–88. doi: 10.1007/s12265-009-9159-9. [DOI] [PubMed] [Google Scholar]
- 77.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle and Nerve. 1995;18(12):1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 78.Li L, Zhang S, Zhang Y, Yu B, Xu Y, Guan Z. Paracrine action mediate the antifibrotic effect of transplanted mesenchymal stem cells in a rat model of global heart failure. Molecular Biology Reports. 2009;36(4):725–731. doi: 10.1007/s11033-008-9235-2. [DOI] [PubMed] [Google Scholar]
- 79.Ohnishi S, Sumiyoshi H, Kitamura S, Nagaya N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Letters. 2007;581(21):3961–3966. doi: 10.1016/j.febslet.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 80.Mias C, Lairez O, Trouche E, et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009;27(11):2734–2743. doi: 10.1002/stem.169. [DOI] [PubMed] [Google Scholar]
- 81.Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(33):14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou HP, Yi DH, Yu SQ, et al. Administration of donor-derived mesenchymal stem cells can prolong the survival of rat cardiac allograft. Transplantation Proceedings. 2006;38(9):3046–3051. doi: 10.1016/j.transproceed.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 83.Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108(7):863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 84.Sherman W, Martens TP, Viles-Gonzalez JF, Siminiak T. Catheter-based delivery of cells to the heart. Nature Clinical Practice Cardiovascular Medicine. 2006;3(supplement 1):S57–S64. doi: 10.1038/ncpcardio0446. [DOI] [PubMed] [Google Scholar]
- 85.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(32):11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schuleri KH, Amado LC, Boyle AJ, et al. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. American Journal of Physiology. 2008;294(5):H2002–H2011. doi: 10.1152/ajpheart.00762.2007. [DOI] [PubMed] [Google Scholar]
- 87.Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. American Journal of Cardiology. 2004;94(1):92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 88.Williams AR, Trachtenberg B, Velazquez DL, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circulation Research. 2011;108(7):792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rappolee DA, Basilico C, Patel Y, Werb Z. Expression and function of FGF-4 in peri-implantation development in mouse embryos. Development. 1994;120(8):2259–2269. doi: 10.1242/dev.120.8.2259. [DOI] [PubMed] [Google Scholar]
- 90.Schmidt C, Bladt F, Goedecke S, et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373(6516):699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 91.Miyajima A, Kinoshita T, Tanaka M, Kamiya A, Mukouyama Y, Hara T. Role of oncostatin M in hematopoiesis and liver development. Cytokine and Growth Factor Reviews. 2000;11(3):177–183. doi: 10.1016/s1359-6101(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 92.Dong XJ, Zhang H, Pan RL, Xiang LX, Shao JZ. Identification of cytokines involved in hepatic differentiation of mBM-MSCs under liver-injury conditions. World Journal of Gastroenterology. 2010;16(26):3267–3278. doi: 10.3748/wjg.v16.i26.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lam SP, Luk JM, Man K, et al. Activation of interleukin-6-induced glycoprotein 130/signal transducer and activator of transcription 3 pathway in mesenchymal stem cells enhances hepatic differentiation, proliferation, and liver regeneration. Liver Transplantation. 2010;16(10):1195–1206. doi: 10.1002/lt.22136. [DOI] [PubMed] [Google Scholar]
- 94.Bataller R, Brenner DA. Liver fibrosis. Journal of Clinical Investigation. 2005;115(2):209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee DS, Gil WH, Lee HH, et al. Factors affecting graft survival after living donor liver transplantation. Transplantation Proceedings. 2004;36(8):2255–2256. doi: 10.1016/j.transproceed.2004.08.073. [DOI] [PubMed] [Google Scholar]
- 96.Zhao DC, Lei JX, Chen R, et al. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World Journal of Gastroenterology. 2005;11(22):3431–3440. doi: 10.3748/wjg.v11.i22.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplantation. 2007;40(7):609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 98.Wang J, Ding F, Gu Y, Liu J, Gu X. Bone marrow mesenchymal stem cells promote cell proliferation and neurotrophic function of Schwann cells in vitro and in vivo. Brain Research. 2009;1262:7–15. doi: 10.1016/j.brainres.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 99.Wang SP, Wang ZH, Peng DY, Li SM, Wang H, Wang XH. Therapeutic effect of mesenchymal stem cells in rats with intracerebral hemorrhage: reduced apoptosis and enhanced neuroprotection. Molecular Medicine Reports. 2012;6(4):848–854. doi: 10.3892/mmr.2012.997. [DOI] [PubMed] [Google Scholar]
- 100.Uccelli A, Zappia E, Benvenuto F, Frassoni F, Mancardi G. Stem cells in inflammatory demyelinating disorders: a dual role for immunosuppression and neuroprotection. Expert Opinion on Biological Therapy. 2006;6(1):17–22. doi: 10.1517/14712598.6.1.17. [DOI] [PubMed] [Google Scholar]
- 101.Kurozumi K, Nakamura K, Tamiya T, et al. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Molecular Therapy. 2004;9(2):189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 102.Venkataramana NK, Pal R, Rao SA, et al. Bilateral transplantation of allogenic adult human bone marrow-derived mesenchymal stem cells into the subventricular zone of Parkinson's disease: a pilot clinical study. Stem Cells International. 2012;2012:12 pages. doi: 10.1155/2012/931902.931902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Podolsky DK. Inflammatory bowel disease. The New England Journal of Medicine. 2002;347(6):417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 104.Castelo-Branco MT, Soares ID, Lopes DV, et al. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033360.e33360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ando Y, Inaba M, Sakaguchi Y, et al. Subcutaneous adipose tissue-derived stem cells facilitate colonic mucosal recovery from 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats. Inflammatory Bowel Diseases. 2008;14(6):826–838. doi: 10.1002/ibd.20382. [DOI] [PubMed] [Google Scholar]
- 106.Jiang H, Qu L, Li Y, et al. Bone marrow mesenchymal stem cells reduce intestinal ischemia/reperfusion injuries in rats. Journal of Surgical Research. 2011;168(1):127–134. doi: 10.1016/j.jss.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 107.Zomorodian E, Eslaminejad MB. Mesenchymal stem cells as a potent cell source for bone regeneration. Stem Cells International. 2012;2012:9 pages. doi: 10.1155/2012/980353.980353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Granero-Moltó F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27(8):1887–1898. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Horwitz EM, Gordon PL, Koo WKK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]


