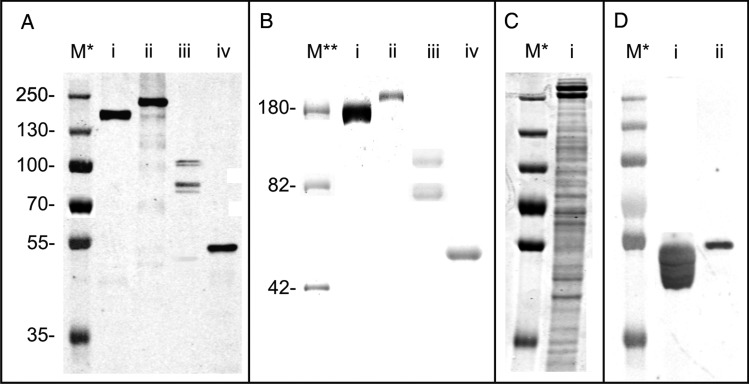
Fig. 1.
(A) Western blot analysis of (i) TfsA-His, (ii) TfsB-His, (iii) BF3567-His and (iv) BF2494-His purified from B. fragilis (i and ii) and T. forsythia (iii, iv), respectively. The observed Mw of all four proteins is larger than the one calculated, suggesting a posttranslational modification of these proteins. (B) Pro-Q Emerald-stained gel showing that both (i) TfsA-His and (ii) TfsB-His purified from B. fragilis as well as BF3567-His and BF2494-His purified from T. forsythia are glycosylated. (C) Coomassie Blue-stained SDS–PAGE gel of a T. forsythia crude extract (i), showing that native (glycosylated) TfsA (lower of the two broad bands migrating around 250 kDa) and TfsB migrate at higher apparent Mw than their heterologously expressed versions shown in A (lanes i and ii), indicating a difference in posttranslational modification. (D) Western blot probing BF2494-His expressed in (i) B. fragilis wild-type (Fletcher et al. 2009) as well as in (ii) T. forsythia with an anti-BF2494 antibody. BF2494-His from T. forsythia shows altered migration behavior compared with wild-type BF2494-His (which additionally shows a banding pattern indicating a consecutive transfer of monosaccharides), indirectly indicating altered glycosylation. M*, PageRuler Plus prestained protein ladder (Thermo Fisher Scientific, Vienna, Austria); M**, CandyCane glycoprotein molecular weight ladder (Invitrogen).