Abstract
An unusual α-(1,1)-galacturonic acid (GalA) lipid A modification has been reported in the lipopolysaccharide of a number of interesting Gram-negative bacteria, including the nitrogen-fixing bacteria Azospirillum lipoferum, Mesorhizobium huakuii and M. loti, the stalk-forming bacterium Caulobacter crescentus and the hyperthermophilic bacterium Aquifex aeolicus. However, the α-(1,1)-GalA transferase (GalAT) gene, which we have named RgtF, was not identified. Species of the Rhizobium genera produce lipid A with α-(1,4′)-GalA but not α-(1,1)-GalA. The Rhizobium GalAT, RgtD, is the lipid A α-(1–4′)-GalAT which utilizes the lipid donor dodecaprenyl-phosphate GalA (Dod-P-GalA) for GalA transfer. An additional Rhizobium GalAT, RgtE, is required for the biosynthesis of Dod-P-GalA. We predicted candidate rgtF genes in bacterial species known to produce lipid A with α-(1,1)-GalA. In order to determine the predicted rgtF gene function, we cloned the M. loti rgtF gene into an expression plasmid and introduced that plasmid into Rhizobium etli strains that do not contain the rgtF gene nor produce lipid A α-(1,1)-GalA. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis combined with NMR studies revealed that the lipid As from these rgtF-complemented strains were modified with an additional α-(1,1)-GalA attached to the proximal glucosamine.
Keywords: bacterial membrane biosynthesis, lipid A biosynthesis, novel glycosyl transferase
Introduction
Lipopolysaccharide (LPS) is a major cell surface glycoconjugate found in the outer membranes of Gram-negative bacteria and contains a carbohydrate and lipid portion. Lipid A, the lipid portion of LPS, anchors the LPS molecule to the outer leaflet of the outer membrane lipid bilayer and is the main focus of this study. The membrane stability and viability of Gram-negative bacteria, as well as the animal innate immune response to LPS, are dependent on the lipid A structure (Galanos et al. 1985; Raetz et al. 2007).
There are many examples that demonstrate the importance of LPS for the formation of complete nitrogen-fixing symbiosis between bacterial symbionts and their legume host plants (Carlson et al. 2010). To establish the function of LPS in the context of endosymbiosis and pathogenesis, it is advantageous to elucidate both the structure of the LPS and the genes required for its biosynthesis. The model organism Mesorhizobium loti MAFF303099 forms nitrogen-fixing endosymbiosis with the host legume plant Lotus japonicum (Kaneko et al. 2000). The lipid A structure of M. loti MAFF303099 has not been published. However, the lipid A structure of the closely related strain Mesorhizobium huakuii IFO15243T has been determined (Choma and Sowinski 2004); it is a unique structure that contains a nonreducing trisaccharide carbohydrate backbone. The backbone comprises a β-(1,6)-2,3-diamino-2,3-dideoxyglucosamine (DAG) disaccharide that is substituted with phosphate at the 4′ position and contains an unusual GalA residue α-(1,1)- linked to the proximal DAG in stoichiometric amounts. The presence of phosphate, DAG and GalA has also been reported in the lipid A from the closely related strain Rhizobium loti NZP2213 (Russa et al. 1995). Organisms that produce lipid A substituted with α-(1,1) GalA include Azospirillum lipoferum (Choma and Komaniecka 2008), Caulobacter crescentus (Smit et al. 2008) and Aquifex aeolicus (Plotz et al. 2000). In C. crescentus and A. aeolicus, the DAG disaccharide is bis-galacturonosylated with GalA attached to the lipid A 4′ (distal) and 1 (proximal) positions. The nitrogen-fixing endosymbionts Rhizobium etli and R. leguminosarum produce α-(1,4′) mono-galacturonosylated lipid A as well as three terminally linked GalAs on the LPS core. The genes encoding the glycosyl transferases responsible for GalA attachment to the lipid A and core have been determined in R. leguminosarum 3841 (Kanjilal-Kolar et al. 2006; Brown et al. 2012) and similar genes are present in the sequenced genomes of R. etli and R. leguminosarum strains. The Rhizobium LPS core GalA transferases (GalATs; Rgt A, B, and C), as well as the lipid A specific RgtD (1,4′)-GalAT, are 4-amino-4-deoxy-arabinosyl transferase (ArnT)-like (Trent et al. 2001) integral inner membrane proteins. They belong to the glycosyl transferase (GT) family 39 (GT-39) and require the lipid donor dodecaprenyl-phosphate GalA (Dod-P-GalA) for GalA transfer to the LPS. An additional GalAT, RgtE, is a bacterial like dolichol-phosphate mannosyl transferase (Dpm1) of the GT family 2 (GT-2) and is responsible for the biosynthesis of the Dod-P-GalA lipid donor. Therefore, we hypothesize that the GalAT responsible for the biosynthesis of α-(1,1)-GalA in the lipid A from the aforementioned organisms is an ArnT-like protein, and that a bactoprenyl phosphate GalA lipid donor will be required.
In this study, we show that the lipid A structure of the sequenced strain M. loti MAFF303099 is the same as that reported for M. huakuii lipid A (Choma and Sowinski 2004). We describe the identification of the α-(1,1)-GalAT gene, which we named rgtF. In order to identify the function of rgtF, we introduced the rgtF gene via a plasmid into the agriculturally significant nitrogen-fixing bean symbiont R. etli bv. phaseoli and determined the effects on lipid A biosynthesis.
Results
Discovery of the putative lipid A α-(1,1)-GalAT
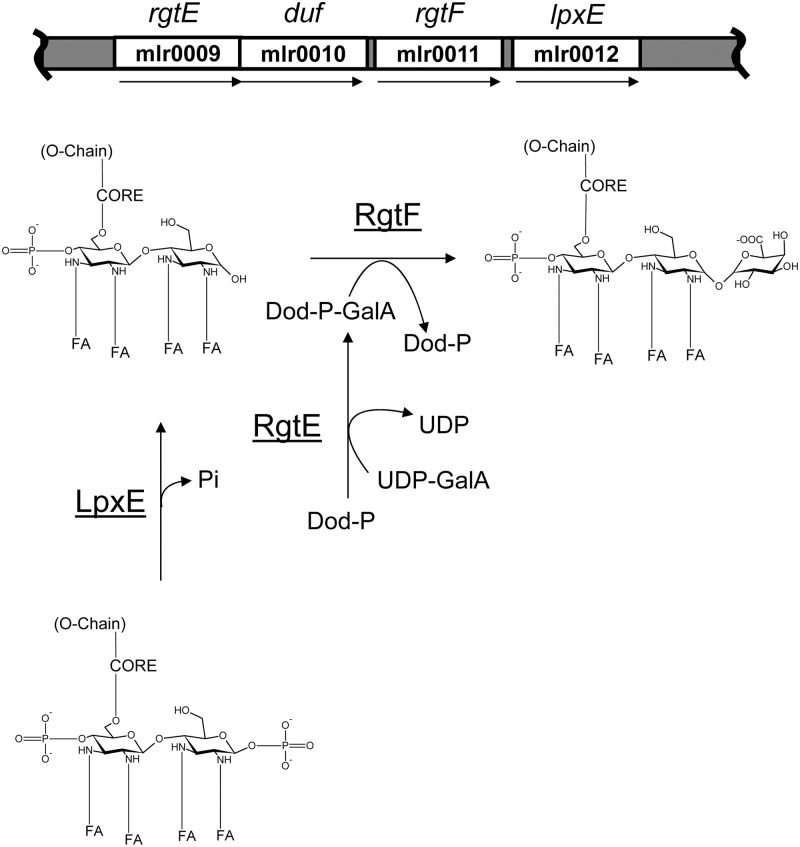
The presence of lipid A α-(1,1)-GalA has been reported in C. crescentus (Smit et al. 2008), M. huakuii (Choma and Sowinski 2004), A. lipoferum (Choma and Komaniecka 2008) and A. aeolicus (Plotz et al. 2000). So far, glycosylation of the lipid A backbone in Gram-negative bacteria has been attributed to ArnT-like glycosyl transferases that are distantly related to the eukaryotic phosphate mannosyl transferase family of glycosyl transferases. ArnT is an integral inner membrane protein that acts on the periplasmic face of the inner membrane and facilitates the transfer of 4-deoxy-4-amino-arabinose (Ara4N) from the lipid donor undecaprenyl-phosphate-Ara4N (Und-P-Ara4N) to the lipid A 4′- or 1-phosphate in Escherichia coli and other enteric pathogens (Trent et al. 2006; Raetz et al. 2007). Before deformylation, the Und-P-N-formyl arabinose (Und-P-Ara4FN) precursor lipid donor is synthesized at the inner membrane cytosolic interface by ArnC, a Dpm1-like glycosyl transferase that utilizes UDP-Ara4FN as a substrate (Trent et al. 2006; Raetz et al. 2007). ArnT- and ArnC-like enzymes have since been discovered in Francisella (Kanistanon et al. 2008; Song et al. 2009; Wang et al. 2009), Bordetella (Marr et al. 2008) and Rhizobium (Brown et al. 2012) and were shown to be involved in the synthesis of lipid donor substrates and the subsequent transfer of glycosyl residues to the lipid A disaccharide backbone. Therefore, we hypothesized that the lipid A α-(1,1)-GalAT is an ArnT-like protein. Indeed, C. crescentus, A. aeolicus, A. lipoferum and Mesorhizobium spp. each contain a predicted arnT-like gene with high peptide similarities, which we named rgtF (Marchler-Bauer et al. 2011; Table I). In addition, C. crescentus and A. aeolicus contain genes with high peptide similarity to the recently reported lipid A (1,4′)-GalAT, RgtD (Brown et al. 2012), which is consistent with the presence of both 1 and 4′ GalA in the lipid A from these organisms (Table I). These organisms also contain genes with high peptide similarity to RgtE (Table I), an ArnC-like glycosyl transferase that synthesizes the lipid donor Dod-P-GalA, which is a required substrate for GalA attachment to the LPS in R. leguminosarum (Brown et al. 2012). In M. loti MAFF303099, the rgtF gene is in a four-gene cluster that also contains the predicted lipid A biosynthetic genes lpxE (1-phosphatase), rgtE, and a gene of unknown function (Figure 1 and Table I). The predicted lipid A 1-phosphatase LpxE has high amino acid similarity to the characterized LpxE in R. leguminosarum (Karbarz et al. 2003) and R. etli (Ingram et al. 2010; Table I). As shown in Figure 1, we predict that the M. loti lpxE gene is required for lipid A dephosphorylation at the 1 position of the proximal DAG and allows the subsequent addition of the α-(1,1)-GalA by RgtF. The amino acid sequence of the predicted M. loti rgtE gene product is highly similar to the characterized R. leguminosarum (Kanjilal-Kolar and Raetz 2006; Brown et al. 2012) rgtE gene product and is likely required for the synthesis of a bactoprenyl-P-GalA lipid donor that can be utilized by RgtF (Figure 1).
Table I.
Predicted genes involved in lipid A GalA biosynthesis in a variety of bacterial species
| LpxE | LpxF | RgtD | RgtF | RgtE | |
|---|---|---|---|---|---|
| Rhizobium leguminosarum 3841 | RL4708 Reference |
RL1570 Reference |
RL0684 Reference |
ND | RL1470 Reference |
| Mesorhizobium loti MAFF303099 | mlr0012 Expect 7e−41 |
ND | ND | Mlr0011 Reference |
Mlr0009 Expect 2e−33 |
| M. ciceri WSM1271 | Mesci_4393 Expect 1e−38 |
ND | ND | Mesci_4394 Expect 0.0 |
Mesci_4733 Expect 1e−135 |
| M. opportunistum WSM2075 | Mesop_4837 Expect 8e−37 |
ND | ND | Mesop_4838 Expect 0.0 |
Mesop_4840 Expect 3e−32 |
| Caulobacter crescentus CB15 | cc_3019 Expect 7e−09 |
ND | cc_0209 Expect 7e−51 |
cc_0468 Expect 2e−81 |
Cc_0469 Expect 6e−31 |
| Aquifex aeolicus VF5 | ND | ND | aq_765 Expect 1e−45 |
aq_1695a Expect 7e−16 |
Aq_1899 Expect 2e−94 |
| Azospirillum lipoferum B510 | AZL_d01960 Expect 2e−09 |
ND | ND | AZL_e02810 Expect 2e−84 |
AZL_e02830 Expect 4e−36 |
ND, not detected; LpxE, 1-phosphatase; LpxF, 4′-phosphatase; RgtD, 4′-GalA transferase; RgtF, α-(1,1)-GalA transferase; RgtE, bactoprenyl-phosphate GalA transferase.
Fig. 1.
Chromosomal location of the rgtF gene and proposed biosynthesis of the lipid A α-(1,1)-GalA in M. loti MAFF303099. The predicted rgtE and lpxE genes are located proximal to the rgtF gene. LpxE is a predicted lipid A 1-phosphatase that putatively removes the 1-phosphate, and RgtE is a Dpm1-like glycosyl transferase that putatively synthesizes a bactoprenyl-P-GalA lipid donor for the transfer of GalA to the 1 position of the lipid A by the ArnT-like glycosyl transferase RgtF. The GenBank accession numbers for the predicted genes: mlr0009, BAB4789.1; mlr0010, BAB47688.1; mlr0011, BAB4789.1; mlr0012, BAB4790.1. FA = fatty acid, Dod-P-GalA = docecaprenyl-phospho-galacturonic acid, duf = domain of unknown function.
Characterization of lipid A from M. loti MAFF303099
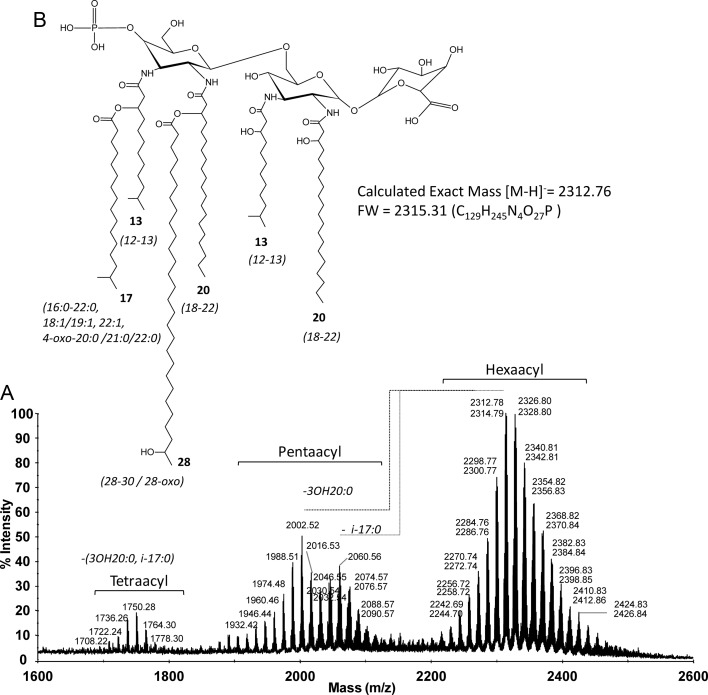
Because we cloned the rgtF gene from the genome of the sequenced strain M. loti MAFF303099, it was necessary to determine its lipid A structure to demonstrate the presence of α-(1,1)-GalA. Before this study, structural information concerning MAFF303099 lipid A was unavailable. However, the structure of the lipid A from the related strain M. huakuii IFO15243T has been determined (Choma and Sowinski 2004). We hypothesized that the general structure of M. loti MAFF303099 lipid A would be similar to that of M. huakuii due to the observed link between lipid A structural conservation and phylogeny in other bacterial systems (Carlson et al. 2010). As expected, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis (Figure 2) of isolated M. loti MAFF303099 lipid A produced molecular ions similar to those previously reported for M. huakuii lipid A (Choma and Sowinski 2004), suggesting structural similarities between the two species. We observed complex structures with two main ion clusters ranging from 2242 to 2501 and 1904 to 2117 u, which correspond to hexa- and penta-acyl lipid A species, respectively (Figure 2). Signals in each cluster differed by ±14 u due to the heterogeneous mixture of fatty acids. The observed microheterogeneity in the MALDI-TOF MS spectra was supported by the chemical identification of several fatty acids including 12:0 (3-OH), 13:0 (i-3-OH), 13:0 (3-OH), 16:0, i-17:0, 17:0, 18:1, 18:0, 19:1, 19:0, 16:0 (3-OH), 20:0, 20:0 (4-oxo), 18:0 (3-OH), 21:0, 22:1, 19:0 (3-OH), 21:0 (4-oxo), 22:0, 20:0 (3-OH), 22:0 (4-oxo), 21:0 (3-OH), 22:0 (3-OH), 28:0 (27-oxo), 28:0 (27-OH) and 30:0 (29-OH) (Supplementary data, Figure S1, panel I). The most abundant fatty acids observed were 13:0 (i-3-OH), i-17:0, 20:0, 20:0 (3-OH) and 28:0 (27-OH). In order to elucidate the amide-linked primary fatty acids, we de-O-acylated the M. loti lipid A effectively removing the O-linked secondary fatty acids. The major retained amide-linked primary fatty acids observed were 13:0 (i-3-OH) and 20:0 (3-OH) and traces of 12:0 (3-OH), 16:0 (3-OH), 19:0 (3-OH), 21:0 (3-OH) and 22 (3-OH). As expected, GalA was detected in the compositional analysis of lipid A while glucosamine (GlcN) was not detected, suggesting that M. loti MAFF303099 lipid A does not contain GlcN in the lipid A backbone. A stereochemical configuration of GalA was assigned as “d” based on the comparison of the retention times of its trimethylsilyl (TMS)-(S)-(+)-2-butyl glycoside with the authentic standard of d-GalA. These observations are consistent with other reported lipid A structures for Mesorhizobium species (Russa et al. 1995; Choma 1999; Choma and Sowinski 2004). In order to demonstrate the presence of DAG, we prepared alditol acetates from lipid A as described in the Materials and Methods section. Gas chromatography/mass spectrometry (GC/MS) analysis demonstrated the presence of 2,3-diamino-2,3-dideoxy hexitol acetate with characteristic fragment ions with mass-to-charge ratios of 144 (→103,102,85,84); 288(→228,169,168,126) and 216(→156,114) and characteristic [M+NH4]+ ions at 451 and 434 u (loss of ammonia; Supplementary data, Figure S1, panel II).
Fig. 2.
MALDI-TOF MS analysis of M. loti MAFF303099 lipid A. The recorded values are mass-to-charge ratios of the monoisotopic [M−H]− ions observed in the negative reflectron ionization mode.
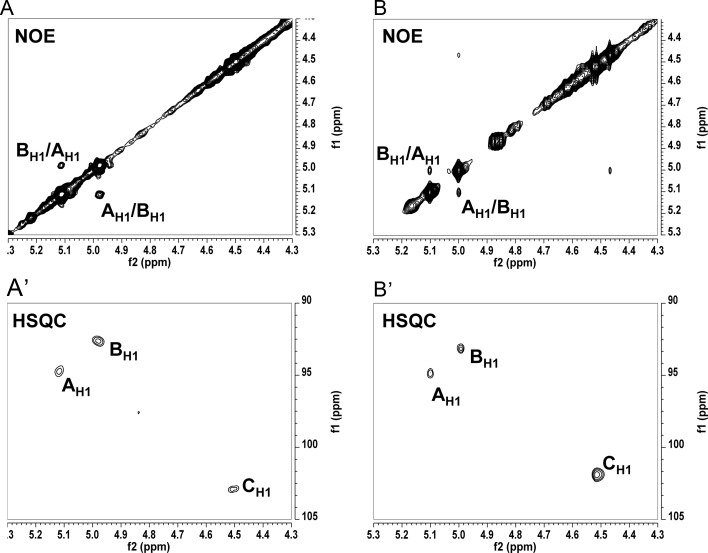
A set of 1D 1H and 2D (total correlated spectroscopy [TOCSY], nuclear overhauser effect spectroscopy [NOESY] and 1H–13C-heteronuclear single quantum correlation spectroscopy [HSQC]) NMR experiments were performed on de-O-acylated MAFF303099 lipid A to identify a sequence of glycosyl residues and glycosyl linkages in the lipid A backbone. The results of these NMR analyses were very similar to those reported in M. huakui lipid A (Choma and Sowinski 2004), showing that the lipid A structures of these two Mesorhizobium strains are very similar. Three glycosyl ring systems with anomeric proton/carbon (1H/13C) resonances at δ 5.11/94.73 for residue A, δ 4.98/92.64 for residue B and δ 4.50/102.96 for residue C (Figure 3A′) were observed and, based on the above published structure and NMR data for M. huakui lipid A and the fact that our NMR data were very similar to these published data, were assigned to the α-d-GalA, proximal α-DAG and distal β-DAG residues, respectively. Moreover, an NOESY experiment has revealed a strong inter-residue interaction between AH1 and BH1 protons at δ 5.11/4.98 ppm, respectively, supporting the α-(1,1) linkage between the GalA and proximal DAG residue. To further verify the general M. loti lipid A structure, negative and positive ionization mode MS/MS was performed on the major molecular ions observed in the MALDI-TOF analysis (Supplementary data, Figure S2). Based on the composition data taken together with the MALDI-TOF MS/MS and NMR experiments, we conclude that the general lipid A structure for the most abundant ion (2312.78 u, [M−H]−) (Figure 2) is consistent with the published structure for M. huakui (Choma and Sowinski 2004).
Fig. 3.
A partial NOE spectra of de-O-acylated lipid A from (A) M. loti MAFF303099 and (B) R. etli rgtF mutant ODB33 along with the corresponding partial HSQC spectra (A′) and (B′), respectively. A, B, C represent anomeric protons of A-α-GalA, B-proximal α-DAG in (A) and proximal α-GlcN in (B), and C- distal β-DAG in (A) and distal β-GlcN in (B).
Analysis of lipid A isolated from R. etli strains CE3, CS506, ODB32 and ODB33
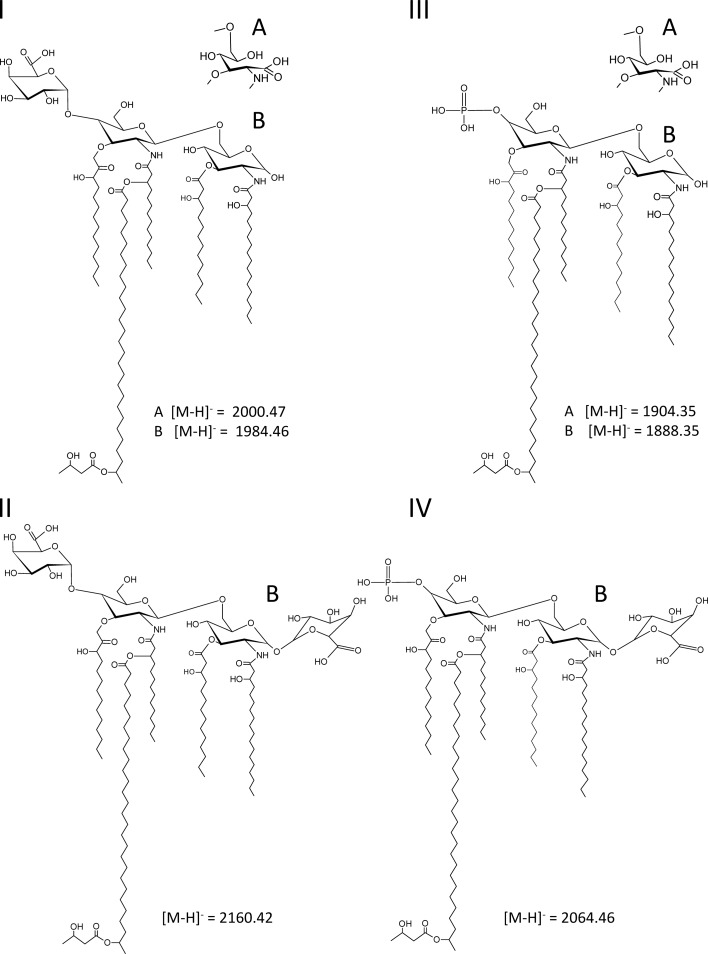
In order to determine the function of the rgtF gene from M. loti MAFF303099, we introduced the plasmid pMl_RgtF into R. etli strains CE3 and CS506 to create strains ODB32 and ODB33, respectively, which constitutively express the rgtF gene. In this discussion, we will be referring to the lipid A structures for the major ions observed for these strains, which are shown in Figure 4. Strains R. etli CE3 and CS506 were chosen because they produce the donor lipid Dod-P-GalA, their lipid A structures are known and contain a free hydroxyl group at the 1 position of the lipid A in the form of a reducing proximal GlcN (Figure 4, structures I and III; Que-Gewirth et al. 2003; Ingram et al. 2010). Therefore, these strains produce the necessary substrates for the predicted RgtF activity. RgtF activity was determined by MALDI-TOF MS and MS/MS analyses of isolated lipid A from the rgtF complemented strains ODB32 and ODB33 as well as the respective parent strains CE3 and CS506 for reference.
Fig. 4.
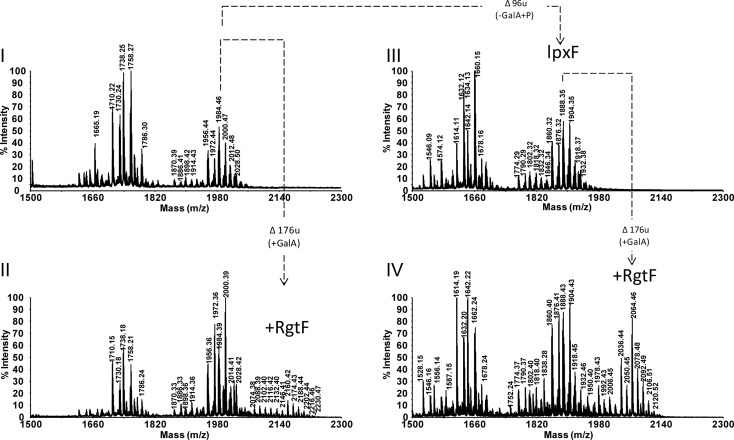
The lipid A structures for the most abundant ions determined by MALDI-TOF MS (Figures 3 and 5 and Table II). Displayed are the major lipid A structure observed for (I) strain CE3, (II) strain ODB32, (III) strain CS506 and (IV) strain ODB33. Structures “A” are due to lipid A with proximal GlcNonate and structures “B” represent lipid A with proximal GlcN. Compositions are given in Table III and mass-to-charge ratio values are highlighted in bold.
MALDI-TOF MS analysis of lipid A from the CE3 parent strain indicated the presence of two major clusters of ions due to penta- and tetra-acyl (lacking C14:0 (3-OH) acyl chain) lipid A species in which the proximal glycosyl residue can be either 2-amino-2-deoxy-gluconate (GlcNonate) or GlcN. The proximal GlcN is converted into GlcNonate in the outermembrane by the mono-oxygenase LpxQ (Que-Gewirth et al. 2003). Each cluster consists of multiple ions due to heterogeneity in fatty acyl chain length (±14 u, ±28 u) and 28:0 (27-OH) with or without a 3-hydroxybutyrate (C4:0 (3-OH), ±86 u) substitution. For details, refer to Figure 5, panel I, structures I-A and I-B in Figure 4 and Table II. These ions and proposed compositions are in agreement with the published structures (Que-Gewirth et al. 2003; Ingram et al. 2010). In the MALDI-TOF MS analysis of strain ODB32 (CE3/pMl_RgtF) lipid A, the highest molecular mass ion group was in the region between 2074.38 and 2230.47 u: +176 u higher than ions observed in the CE3 parent strain spectrum and consistent with the addition of GalA (Figure 5, panel II). These ions represented penta-acyl lipid A species with a tetrasacharide sugar backbone containing two GalA residues (structure II-B in Figure 4). The intensity of MALDI-TOF MS signals for lipid A substituted with 2 GalA residues was very low suggesting only partial substitution of proximal GlcN with GalA. The majority of signals in the ODB32 spectrum were also found in the parent strain (compare panels I and II in Figure 5, and see Table II).
Fig. 5.
MALDI-TOF MS spectra of isolated lipid A. (I) Parent strain CE3. (II) Strain ODB32 (CE3/pMl_RgtF). (III) Strain CS506, the ΔlpxF mutant of CE3. (IV) Strain ODB33 (CS506/pMl_RgtF). Proposed compositions are given in Table III.
Table II.
The major ions observed in MALDI-TOF MS of lipid A isolated from Rhizobium CE3; rgtF complemented CE3, strain ODB32; ΔlpxF mutant (SC506) and rgtF complemented CS506, strain ODB33
| Structure (Figure 4) | Obsa [M−H]− | Calc. [M−H]− | Molecular formula | Proposed composition (penta-acyl lipid A) | Strain |
|---|---|---|---|---|---|
| I-A | 1914.43 | 1914.41 | C106H198N2O26 | GalA-GlcN-GlcNonate, (14:0(3-OH))3,18:0(3-OH), 28:0(27-OH) | CE3 and OBD32 |
| 1886.41 | 1886.39 | C104H194N2O26 | GalA-GlcN-GlcNonate, (14:0(3-OH))3, 16:0(3-OH), 28:0(27-OH) | ||
| 1942.45 | 1942.45 | C108H202N2O26 | GalA-GlcN-GlcNonate, (14:0(3-OH))2,18:0(3-OH), 16:0(3-OH), 28:0(27-OH) | ||
| 1972.44 | 1972.42 | C108H200N2O28 | GalA-GlcN-GlcNonate, (14:0(3-OH))3, 16:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 2000.47 | 2000.45 | C110H204N2O28 | GalA-GlcN-GlcNonate, (14:0(3-OH))3,18:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 2028.50 | 2028.45 | C112H208N2O28 | GalA-GlcN-GlcNonate, (14:0(3-OH))2,18:0(3-OH), 16:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| I-B | 1870.39 | 1870.39 | C104H194N2O25 | GalA-GlcN-GlcN, (14:0(3-OH))3, 16:0(3-OH), 28:0(27-OH) | CE3 and OBD32 |
| 1898.42 | 1898.42 | C106H198N2O25 | GalA-GlcN-GlcN, (14:0(3-OH))3,18:0(3-OH), 28:0(27-OH) | ||
| 1926.43 | 1926.45 | C108H202N2O25 | GalA-GlcN-GlcN, (14:0(3-OH))2,18:0(3-OH), 16:0(3-OH), 28:0(27-OH) | ||
| 1942.45 | 1942.41 | C107H198N2O25 | GalA-GlcN-GlcN, (14:0(3-OH))3, 15:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 1954.42 | 1954.48 | C110H206N2O25 | GalA-GlcN-GlcN, (14:0(3-OH))2, (18:0(3-OH))2, 28:0(27-OH) | ||
| 1956.44 | 1956.43 | C108H200N2O27 | GalA-GlcN-GlcN, (14:0(3-OH))3,16:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 1970.45 | 1970.44 | C109H202N2O27 | GalA-GlcN-GlcN, (14:0(3-OH))2, 15:0(3-OH), 16:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 1984.46 | 1984.46 | C110H204N2O27 | GalA-GlcN-GlcN, (14:0(3-OH))3,18:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 1998.47 | 1998.47 | C111H206N2O27 | GalA-GlcN-GlcN, (14:0(3-OH))2,18:0(3-OH), 15:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 2012.48 | 2012.49 | C112H208N2O27 | GalA-GlcN-GlcN, (14:0(3-OH))2,18:0(3-OH), 16:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 2040.48 | 2040.52 | C114H212N2O27 | GalA-GlcN-GlcN, (14:0(3-OH))2, (18:0(3-OH))2, 28:0(27-OH), C4:0(3-OH) | ||
| 2054.46 | 2054.53 | C115H214N2O27 | GalA-GlcN-GlcN, (14:0(3-OH))2, 15:0(3-OH), C18:0(3-OH)2, 28:0(27-OH), C4:0(3-OH) | ||
| II-B | 2074.38 | 2074.45 | C112H206N2O31 | GalA-GlcN-GlcN-GalA, (14:0(3-OH))3,18:0(3-OH), 28:0(27-OH) | OBD32 |
| 2088.39 | 2088.49 | C113H208N2O31 | GalA-GlcN-GlcN-GalA, (14:0(3-OH))2,18:0(3-OH), 15:0(3-OH), 28:0(27-OH) | ||
| 2102.40 | 2102.48 | C114H210N2O31 | GalA-GlcN-GlcN-GalA (14:0(3-OH))2,18:0(3-OH), 16:0(3-OH), 28:0(27-OH) | ||
| 2116.42 | 2116.50 | C115H212N2O31 | GalA-GlcN-GlcN-GalA, (14:0(3-OH)), 15:0(3-OH), 16:0(3-OH),18:0(3-OH), 28:0(27-OH), | ||
| 2132.40 | 2130.46 | C114H208N2O33 | GalA-GlcN-GlcN-GalA, (14:0(3-OH))3, 16:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 2146.41 | 2146.47 | C115H210N2O33 | GalA-GlcN-GlcN-GalA, (14:0(3-OH))2, 15:0(3-OH), 16:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 2160.42 | 2160.49 | C116H212N2O33 | GalA-GlcN-GlcN-GalA, (14:0(3-OH))3,18:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 2174.43 | 2174.51 | C117H214N2O33 | GalA-GlcN-GlcN-GalA, (14:0(3-OH))2,18:0(3-OH), 15:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 2188.44 | 2188.52 | C118H216N2O33 | GalA-GlcN-GlcN-GalA, (14:0(3-OH))2,18:0(3-OH), 16:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 2202.44 | 2202.53 | C119H218N2O33 | GalA-GlcN-GlcN-GalA, 14:0(3-OH), 3-OHC15:0, 16:0(3-OH),18:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 2216.46 | 2216.55 | C120H220N2O33 | GalA-GlcN-GlcN-GalA, (14:0(3-OH))2, (18:0(3-OH))2, 28:0(27-OH), C4:0(3-OH) | ||
| 2230.47 | 2230.57 | C121H222N2O33 | GalA-GlcN-GlcN-GalA, (14:0(3-OH))2, 15:0(3-OH), (18:0(3-OH))2, 28:0(27-OH), C4:0(3-OH) | ||
| III-A | 1790.27 | 1790.32 | C98H187N2O23P | P-GlcN-GlcNonate, (14:0(3-OH))3, 16:0(3-OH), 28:0(27-OH) | CS506 and OBD33 |
| 1804.31 | 1804.33 | C99H189N2O23P | P-GlcN-GlcNonate, (14:0(3-OH))2, 15:0(3-OH), 16:0(3-OH), 28:0(27-OH) | ||
| 1818.32 | 1818.35 | C100H191N2O23P | P-GlcN-GlcNonate, (14:0(3-OH))3,18:0(3-OH), 28:0(27-OH) | ||
| 1832.32 | 1832.37 | C101H193N2O23P | P-GlcN-GlcNonate, (14:0(3-OH))2, 15:0(3-OH),18:0(3-OH), 28:0(27-OH) | ||
| 1846.34 | 1846.38 | C102H195N2O23P | P-GlcN-GlcNonate, (14:0(3-OH))2,18:0(3-OH), 16:0(3-OH), 28:0(27-OH) | ||
| 1876.32 | 1876.36 | C102H193N2O25P | P-GlcN-GlcNonate, (14:0(3-OH))3, 16:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 1890.35 | 1890.37 | C103H195N2O25P | P-GlcN-GlcNonate, (14:0(3-OH))2, 15:0(3-OH), 16:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 1904.35 | 1904.39 | C104H197N2O25P | P-GlcN-GlcNonate, (14:0(3-OH))3,18:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 1918.37 | 1918.40 | C105H199N2O25P | P-GlcN-GlcNonate, (14:0(3-OH))2, 15:0(3-OH),18:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 1932.38 | 1932.42 | C106H201N2O25P | P-GlcN-GlcNonate, (14:0(3-OH))2,18:0(3-OH),16:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 1946.38 | 1946.43 | C107H203N2O25P | P-GlcN-GlcNonate, 14:0(3-OH),15:0(3-OH),18:0(3-OH), 16:0(3-OH), 28:0(27-OH), C4:0(3-OH) C4:0(3-OH) |
||
| 1960.38 | 1960.45 | C108H205N2O25P | P-GlcN-GlcNonate, 14:0(3-OH), (16:0(3-OH))2, C18:0(3-OH), C28:0(27-OH), C4:0(3-OH) | ||
| III-B | 1774.29 | 1774.32 | C98H187N2O22P | P-GlcN-GlcN, (14:0(3-OH))3, 16:0(3-OH), 28:0(27-OH) | CS506 and OBD33 |
| 1802.32 | 1802.36 | C100H191N2O22P | P-GlcN-GlcN, (14:0(3-OH))3,18:0(3-OH), 28:0(27-OH) | ||
| 1816.33 | 1816.37 | C101H193N2O22P | P-GlcN-GlcN, (14:0(3-OH))2, 15:0(3-OH),18:0(3-OH), 28:0(27-OH) | ||
| 1830.32 | 1830.39 | C102H195N2O22P | P-GlcN-GlcN, (14:0(3-OH))2,18:0(3-OH), 16:0(3-OH), 28:0(27-OH) | ||
| 1858.30 | 1858.42 | C104H199N2O22P | P-GlcN-GlcN, (14:0(3-OH))2, (18:0(3-OH))2, 28:0(27-OH) | ||
| 1860.32 | 1860.36 | C102H193N2O24P | P-GlcN-GlcN, (14:0(3-OH))3, 16:0(3-OH), C28:0(27-OH), C4:0(3-OH) | ||
| 1874.34 | 1874.38 | C103H193N2O24P | P-GlcN-GlcN, (14:0(3-OH))2, 15:0(3-OH), 16:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 1888.35 | 1888.39 | C104H197N2O24P | P-GlcN-GlcN, (14:0(3-OH))3,18:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 1902.36 | 1901.41 | C105H199N2O24P | P-GlcN-GlcN, (14:0(3-OH))2, 15:0(3-OH),18:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 1916.37 | 1916.42 | C106H201N2O24P | P-GlcN-GlcN, (14:0(3-OH))2,18:0(3-OH), 16:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 1944.37 | 1944.45 | C108H205N2O24P | P-GlcN-GlcN, (14:0(3-OH))2, (18:0(3-OH))2, 28:0(27-OH), C4:0(3-OH) | ||
| IV-B | 1950.40 | 1950.36 | C104H195N2O28P | P-GlcN-GlcN-GalA, (14:0(3-OH))3, 16:0(3-OH), 28:0(27-OH) | OBD33 |
| 1964.41 | 1964.37 | C105H197N2O28P | P-GlcN-GlcN-GalA, (14:0(3-OH))2, 15:0(3-OH), 16:0(3-OH), 28:0(27-OH) | ||
| 1978.43 | 1978.39 | C106H199N2O28P | P-GlcN-GlcN-GalA, (14:0(3-OH))3,18:0(3-OH), 28:0(27-OH), | ||
| 1992.43 | 1992.40 | C107H201N2O28P | P-GlcN-GlcN-GalA, (14:0(3-OH))2, 15:0(3-OH),18:0(3-OH), 28:0(27-OH) | ||
| 2006.45 | 2006.42 | C108H203N2O28P | P-GlcN-GlcN-GalA, (14:0(3-OH))2,18:0(3-OH), 16:0(3-OH), 28:0(27-OH) | ||
| 2034.47 | 2034.45 | C110H217N2O28P | P-GlcN-GlcN-GalA, (14:0(3-OH))2, (18:0(3-OH))2, 28:0(27-OH) | ||
| 2036.44 | 2036.39 | C108H201N2O30P | P-GlcN-GlcN-GalA, (14:0(3-OH))3, 16:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 2050.45 | 2050.41 | C109H203N2O30P | P-GlcN-GlcN-GalA, (14:0(3-OH))2, 15:0(3-OH), 16:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 2064.46 | 2064.42 | C110H205N2O30P | P-GlcN-GlcN-GalA, (14:0(3-OH))3,18:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 2078.48 | 2078.43 | C111H207N2O30P | P-GlcN-GlcN-GalA, (14:0(3-OH))2, 15:0(3-OH),18:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 2092.49 | 2092.45 | C112H209N2O30P | P-GlcN-GlcN-GalA, (14:0(3-OH))2,18:0(3-OH), 16:0(3-OH), 28:0(27-OH), C4:0(3-OH) | ||
| 2106.51 | 2106.47 | C113H211N2O30P | P-GlcN-GlcN-GalA, 14:0(3-OH), 15:0(3-OH), 16:0(3-OH),18:0(3-OH), C28:0(27-OH), C4:0(3-OH) | ||
| 2120.52 | 2120.49 | C114H213N2O30P | P-GlcN-GlcN-GalA, (14:0(3-OH))2, (18:0(3-OH))2, 28:0(27-OH), C4:0(3-OH) |
aThe recorded mass-to-charge ratio values represent the monoisotopic [M−H]− ions observed in the negative reflectron mode. The proposed structures for mass-to-charge ratio values highlighted in bold are shown in Figure 2.
As expected and consistent with the previously reported data (Ingram et al. 2010), the lipid A MALDI-TOF MS spectra of strain CS506 (ΔlpxF) lipid A contained two major groups of ions representing penta-acyl- (e.g. 1774–1932 u) and tetra-acyl-lipidA (e.g. 1614–1678 u) species (Figure 5, panel III). Because strain CS506 lacks an active lipid A 4′ phosphatase (LpxF), the distal GlcN retains the 4′ phosphate and lacks the 4′ GalA (Figure 5, panel III; structures III-A and III-B in Figure 4 and Table II). The observed clusters of ions are shifted down by 96 u (-GalA, +P) with respect to the parent strain CE3 lipid A.
Glycosyl composition analysis of isolated lipid A revealed that GalA is not present in the lipid A of strain CS506. However, GalA is present in the lipid A of the rgtF-complemented strain ODB33, demonstrating that the rgtF gene was responsible for adding GalA to the lipid A. As with the MAFF303099 lipid A, the Gal A in ODB33 lipid A was in the “d” configuration. Furthermore, in the MALDI-TOF MS spectrum of the lipid A from ODB33 (CS506/pMl_RgtF), the higher mass ion cluster was due to phosphorylated penta-acyl-lipid A with an additional GalA residue (see, panel IV in Figure 5, structure IV-B in Figure 4 and Table II). Ions due to structures containing both the GalA and proximal GlcNonate residue were not observed in the ODB33 lipid A, which is consistent with the conclusion that RgtF transfers GalA to the proximal GlcN and not to other locations, i.e. conversion of the proximal GlcN into GlcNonate by LpxQ destroys the site for GalA addition.
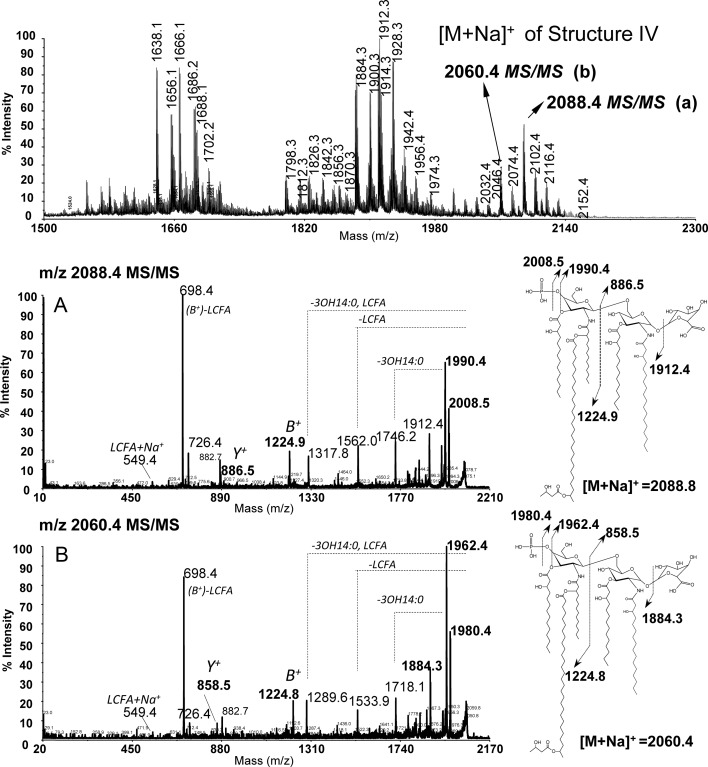
To further support the hypothesis that the RgtF protein is transferring a GalA residue to the proximal GlcN residue, we performed MALDI-TOF MS/MS analysis on lipid A in the positive ionization mode. The major [M + Na]+ ions of the penta-acyl phosphorylated and galacturonosylated ODB33 lipid A were 2088.4 u [P-GlcN-GlcN-GalA, (14:0 [3-OH])3, 18:0 (3-OH), 28:0 (27-OH), C4:0 (3-OH)] and 2060.4 u [P-GlcN-GlcN-GalA, (14:0 [3-OH])3, 16:0 (3-OH), 28:0 (27-OH), C4:0 (3-OH)]. Each of these was selected for MS/MS cleavage (Figure 6). The parent ion 2088.4 u generated a fragment ion 1912.4 u due to the cleavage of the GalA residue from the main structure. Moreover, the observed Y fragment ion at 886.5 u is consistent with the proximal GlcN being substituted with GalA and acylated with 18:0 (3-OH) and 14:0 (3-OH). The observed B+ fragment ion 1224.8 u is consistent with the distal side of the molecule containing 4′ phosphorylated GlcN that is acylated with two C14:0 (3-OH) and 28:0 (27-O-[C4:0(3-OH)]) substituents, and the ion at 698.4 u is consistent with a fragment containing the distal phosphorylated GlcN substituted with two C14:0 (3-OH) acyl groups. The fragment ions at 2008.5 and 1990.4 u are due to the loss of the distally located 4′ phosphate group as −PO3−2 or −PO4−2, respectively.
Fig. 6.
MALDI-TOF MS/MS spectra of strain ODB33 (CS506/pMl_RgtF) lipid A. Spectra were obtained in the positive reflectron mode and ions were selected for MS/MS analysis. (A) MS/MS spectrum obtained from the parent ion 2088.4 (structure IV, Figure 4). (B) MS/MS spectrum obtained from the parent ion 2060.4.
MS/MS analysis of the 2060.4 u precursor ion generated fragments identical to those observed in the MS/MS spectrum of the parent ion with 2088.4 u as well as new fragments 28 u less due to the substitution of the proximal GlcN by 16:0 (3-OH) instead of 18:0 (3-OH) (compare Figure 6A and B). We observed a fragment ion at 1884.3 u due to the cleavage of the GalA residue (−176 u) from the main structure. The Y ion with 858.5 u is consistent with the proximal GlcN being substituted with GalA and acylated with 14:0 (3-OH) and 16:0 (3-OH). The B+ fragment ion with 1224.8 u was similar to the B+ ions from the fragmentation of parent 2088.4 ion obtained from the strain ODB33 as well as B+ ions obtained from the parent strain CS506, further suggesting that the GalA is added to the proximal GlcN.
In order to further characterize the RgtF GalAT activity, the lipid A from ODB 33 (R. etli CS506/pMlRgtF) was de-O-acylated and characterized by the series of 1D 1H and 2D (correlation spectroscopy [COSY], TOCSY, NOESY and 1H–13C-HSQC) NMR spectroscopy. The anomeric regions of the HSQC and NOESY spectra are shown in Figure 3B′ and B and can be compared with those spectra of the M. loti de-O-acylated lipid A in Figure 3A′ and A. We identified three anomeric ring systems consistent with the addition of a glycosyl residue to this lipid A, which in the parent strain contains a GlcN-disaccharide backbone (see structure III in Figure 4). Based on the similarity of the anomeric region to that of the M. loti lipid A (Figure 3A′), the proton/carbon (1H/13C) resonances at δ 5.10/94.88 ppm (residue A), δ 4.99/93.33 ppm (residue B) and δ 4.51/101.83 (residue C) were assigned to the added α-GalA residue, the proximal GlcN and the distal GlcN, respectively. An NOESY experiment demonstrated that the anomeric proton of α-GalA interacts with the anomeric proton of the proximal α-GlcN as indicated by the strong inter-residue cross-peak of AH1 and BH1 at δ 5.10/4.99 ppm, respectively (Figure 3B). This comparison of the NMR spectra of ODB33 lipid A with those of the M. loti lipid A supports the conclusion that the rgtF gene in M. loti MAFF303099 encodes for a specific α-(1,1)-GalAT.
Discussion
In this study, we describe for the first time the identification and function of the lipid A α-d-(1,1)-GalAT gene rgtF from M. loti MAFF303099. The MAFF303099 putative rgtF gene was polymerase chain reaction (PCR) cloned into a plasmid vector and introduced into R. etli strains that do not produce α-(1,1)-GalA. Mass spectrometric, carbohydrate composition and NMR analysis of the lipid A from the rgtF-complemented strains unequivocally demonstrated that the expression of rgtF leads to the addition of GalA to the proximal GlcN. This result is consistent with the observation that other bacterial species that produce lipid A α-(1,1)-GalA contain genes with high peptide similarity to rgtF in their genomes along with the 1-phosphatase lpxE gene and the bactoprenyl-phosphate GalAT rgtE gene (Table I). It was previously shown that the lipid donor Dod-P-GalA is required for GalA attachment to the LPS in R. leguminosarum bv. viciae (Kanjilal-Kolar and Raetz 2006; Brown et al. 2012), and that LpxE removes the lipid A 1-phosphate (Karbarz et al. 2003; Ingram et al. 2010). Interestingly, the M. loti MAFF303099 genes rgtF and putative genes rgtE and lpxE are found together in a gene cluster on the chromosome (Figure 1). These rgtF and lpxE genes are also localized together on the chromosomes of C. crecsentus, A. lipoferum and Mesorhizobium spp. (Table I), suggesting a functional relationship between the 1-phosphatase and the RgtF GalAT. Therefore, we suggest a model for the biosynthesis of the lipid A α-(1,1)-GalA (Figure 1), whereby the 1-phosphate is removed by LpxE followed by the transfer of GalA from bactoprenyl-P-GalA to the 1 position of the lipid A by RgtF.
It is not known why some organisms produce lipid A with GalA. Escherichia coli and many other well-studied enteric pathogens contain lipid A with 1 and 4′ phosphates, and it has been suggested that the phosphates facilitate cross-bridging of LPS molecules in the outer membrane through divalent cationic interactions with metal ions, e.g. Ca2+ and Mg2+ (Snyder et al. 1999; Raetz and Whitfield 2002). Because the biosynthesis of the precursor molecule lipid IVA is a well-conserved pathway (Raetz et al. 2007) that follows a sequential order and the lipid IVA biosynthetic genes are widespread throughout the sequenced genomes of Gram-negative bacteria, it is thought that most Gram-negative organisms initially produce 1 and 4′ phosphates (Raetz et al. 2007). The modification of this commonly produced bis-phosphorylated lipid A structure in certain bacterial pathogens results in altering the ability of the host innate defense system to respond to the pathogen and in the ability of the pathogen to avoid host defense molecules such as antimicrobial cationic peptides. Organisms having lipid A that lack one or both phosphates produce inner membrane phosphatases that remove them on the periplasmic side of the inner membrane. The 1- and/or 4′- lipid A phosphatases have been described in R. leguminosarum, R. etli, Francisella spp., Porphyromonas gingivalis and Helicobacter pylori (Wang et al. 2006; Coats, Jones, et al. 2009; Coats, To, et al. 2009, Karbarz et al. 2009; Ingram et al. 2010; Coats et al. 2011; Cullen et al. 2011). The periodontal pathogen P. gingivalis may produce 4′ monophosphorylated lipid A and nonphosphorylated lipid A (Coats, Jones, et al. 2009). Interestingly, the lipid A 1-dephosphorylation activity is regulated by host-derived hemin, a common nutrient in the oral cavity. The removal of the lipid A phosphates in P. gingivalis is crucial for resistance to cationic antimicrobial peptides and the evasion and modulation of the Toll-like receptor 4 immune response (Coats, Jones, et al. 2009; Coats et al. 2011). Lipid A phosphatases are also necessary for establishing normal host infection by the animal pathogens H. pylori and Francisella novicida (Wang et al. 2007; Cullen et al. 2011) as demonstrated in mouse models. In addition to phosphate removal, some mammalian pathogens demonstrate the ability to dynamically modify their lipid A backbone with glycosyl residues in response to the host environment. For example, several pathogens such as E. coli, Salmonella spp., Yersinia pestis, Pseudomonas aeruginosa and Bordetella spp. can mask their phosphates with positively charged residues (Ara4N, GalN, GlcN or phosphoethanolamine; Trent et al. 2006; Marr et al. 2008). These modifications promote survival within the host and result in the organisms being less immunogenic and more resistant to cationic antimicrobial peptides, presumably due to charge repulsion. Francisella novicida is able to glycosylate its nonphosphorylated distal GlcN with a neutral sugar and to mask the 1-phosphate with GalN (Raetz et al. 2009). These glycosylations are important for establishing a normal infection, and mutant strains that lack proper lipid A glycosylation are promising candidates for whole cellular vaccine development (Kanistanon et al. 2008).
Unlike the dynamic lipid A glycosylation in mammalian pathogens, the lipid A GalA glycosyl residues are constitutively produced in the nitrogen-fixing endosymbionts Rhizobium spp. and Mesorhizobium spp. Our laboratory has demonstrated that R. leguminosarum mutants that are disrupted in the production of GalA in their lipid A backbone or LPS core are compromised in membrane stability (Brown et al. 2012). An rgtE-minus mutant of R. leguminosarum produces LPS completely devoid of GalA and is severely compromised in membrane stability. Therefore, the presence of GalA on the LPS functions to stabilize the outer membrane likely through divalent cationic bridging similar to mammalian pathogens that produce phosphate residues in the lipid A and LPS core (Raetz and Whitfield 2002). Perhaps the lipid A GalA residue of M. loti also functions to stabilize the bacterial envelope. Mutational analysis of the rgtF and the putative lpxE (lipid A 1-phosphatase) genes in M. loti and related organisms will provide deeper insights into the function of the lipid A α-(1,1)-GalA as it relates to lipopolysaccharide biosynthesis, membrane stability and endosymbiotic infection.
Materials and methods
Bacterial strains, plasmids and growth conditions
For a list of strains and plasmids used in this work see Table III. Escherichia coli strains were grown at 37°C on Luria-Bertani media, and R. etli and M. loti strains were grown at 30°C on tryptone-yeast (TY) extract media (Beringer 1974) containing 10 mM CaCl2. Antibiotics were used at the following concentrations where indicated: streptomycin (50 μg/mL), spectinomycin (50 μg/mL) and tetracycline (15 μg/mL).
Table III.
Bacterial strains and plasmids
| Strains and plasmids | Characterization | Source |
|---|---|---|
| E. coli | ||
| Top10 | F- mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(araleu) 7697 galU galK rpsL (Strr) endA1 nupG |
Invitrogen |
| Rhizobium etli | ||
| CE3 | Parent strain, Nod+, Fix+, Strr | Noel et al. (1984) |
| CS506 | ΔlpxF mutant of strain CE3, Nod+, Fix+, Strr, Specr | Ingram et al. (2010) |
| ODB32 | Strain CE3 containing plasmid pMl_RgtF, Strr, Tetr | This study |
| ODB33 | Strain CS506 containing plasmid pMl_RgtF, Strr, Specr, Tetr | This study |
| Mesorhizobium loti | ||
| MAFF303099 | Nod+, Fix+ | MAFF GenBank |
| Plasmids | ||
| pRK404E1 | Broad host range shuttle vector for Tcr | Lehman et al. (1990) |
| pRK2013 | Mobilizing plasmid for pMl_RgtF, Col E1 replicon, Kanr | Phadnis and Das (1987) |
| pMl_RgtF | Plasmid pRK404E1 containing the rgtF gene from strain MAFF303099 |
This study |
Creation and introduction of the M. loti rgtF expression vector in Rhizobium strains
The putative M. loti MAFF303099 rgtF gene (accession number, BAB4789.1; gene i.d., mlr0011) was PCR cloned from the MAFF303099 genomic DNA into plasmid pRK404E1 (Lehman et al. 1990) using the following primers engineered with BamHI (underlined) and EcoRI (underlined), respectively (ATCGATGGATCCGTCACTTCCGAGAAACTG and ATCGATGAATTCCCAAGGAGCCGACTGG). The PCR insert product was 1.774 kb in size containing 35 bp upstream and 89 bp downstream of the rgtF gene and no other open-reading frame. The gene was oriented parallel to the lac operon promoter, and the resulting plasmid was named pMl_RgtF. Plasmid pMl_RgtF was transformed into E. coli Top10 (Invitrogen, Inc.) cloning strain. In order to introduce plasmid pMl_RgtF into Rhizobium strains, a triparental mating was performed as previously described (Brown et al. 2011) using E. coli/pMl_RgtF as a donor, E. coli/pRK2013 (Phadnis and Das 1987) as a helper and Rhizobium strains as acceptors. The following diagnostic primers were created to recognize regions outside of the pRK404E1 multiple cloning site (GTGGCGAAACCCGACAG and GACTGGAAAGCGGGCAG).
Isolation of LPS and lipid A
Bacteria were grown under vigorous shaking at 30°C in TY media for 48 h. Finally, cells were harvested by centrifugation at 3500 × g and then washed with sterile PBS followed by washing with distilled water. LPSs were isolated from bacteria using the hot phenol water extraction procedure (Westphal and Jann 1965). Crude phenol and water phases were dialyzed against distilled water using 12–14,000 MWCO dialysis bag, freeze-dried and were further purified by ultracentrifugation at 100,000 × g at 4°C for 18 h. The LPS was mainly recovered from the water phase, and the resulting LPS pellets were suspended in a small volume of deionized water, and lyophilized. LPS was washed with 95% ethanol at 4°C to remove possible contamination of phospholipids, resuspended in water and freeze-dried. Lipid A was released by mild hydrolysis as previously described (Ryan and Conrad 1974). Briefly, samples (5 mg/mL in 1% acetic acid) were hydrolyzed at ∼100°C for 70 min with constant stirring, freeze-dried and extracted three times with a biphasic mixture of chloroform, methanol and deionized water in a ratio of 2/2/1.8 (v/v/v), respectively. The organic phase (chloroform) was pooled and extracted back with H2O and dried under a stream of N2.
Mild hydrazinolysis of MAFF303099 and ODB33 lipid A
Lipid A was de-O-acylated in the presence of anhydrous hydrazine at 35°C for 1 h. The reaction was slowed down by immediate cooling to 0°C and chilled (−70°C) acetone was added dropwise to the sample until precipitate of de-O-acyl lipid A was formed and was kept overnight at −20°C. The de-O-acyl lipid A precipitate was washed several times with chilled acetone followed by centrifugation at 3500 × g for 30 min at 0°C and finally used for MS, compositional and NMR structural studies.
MALDI-TOF MS analysis of lipid A
Lipid A samples were dissolved in a chloroform/methanol mixture (3:1 for R. etli and 2:1 for M. loti; v/v) and then mixed 1:1 (v/v) with 0.5 M 2,4,6-trihydroxyacetophenone monohydrate matrix in methanol and 0.5 µL (1 µg) was spotted onto a stainless steel target plate. Finally, spectra were recorded in the negative or positive reflectron ion mode using an ABSCIEX TOF/TOF 5800 System, Applied Biosystems, USA. The MS/MS spectra were acquired in the positive and negative ionization modes. All acquisitions were calibrated with a standard of CE3 lipid A and verified with peptide standards.
Glycosyl and fatty acid composition analysis of lipid A
The composition of lipid A was determined by GC/MS of TMS methyl glycosides and fatty acid methyl esters (FAMEs) as described previously (York et al. 1986; Bhat et al. 1994). Optionally, for FAME analysis only, lipid A was hydrolyzed with 4 M HCl for 4 h at 100°C, and fatty acids were 3-fold extracted from the reaction using chloroform. An organic phase that contained acyl groups and inorganic phase that contained sugar residues were evaporated to dryness and methanolyzed for 1 h with 0.5 M methanolic HCl at 80°C. Sugar residues were re-N-acetylated and converted into TMS methyl glycosides and hydroxyl-FAMES into TMS-FAMES and analyzed by GC/MS. The absolute configuration of GalA residues in lipid A of MAFF303099 and ODB 33 lipid A was performed according to the method of Gerwig et al. (1978).
In order to verify the presence of 2,3-diamino-2,3-dideoxy-d-glucose (GlcN3N, DAG) in MAFF303099, lipid A was subjected to strong acid hydrolysis with 4 M HCl 4 h at 121°C. Acyl chains were extracted from reaction with 10% (v/v) ether in hexane and the remaining glycosyl residues were peracetylated, reduced with NaBD4 and converted into alditol acetates as reported elsewhere (Roppel et al. 1975). The presence of GlcN3N was assigned based on characteristic mass fragmentation observed in GC/Electron Impact (EI) MS and GC/chemical ionization (CI) MS. All analyses were performed on a Hewlett-Packard HP5890 gas chromatograph equipped with mass selective detector 5970 MSD using Alltech AT-1 fused silica capillary column (30 m × 0.25 mm I.D) where helium served as the carrier gas.
NMR analysis of lipid A
The purified and dry de-O-acylated lipid A samples were exchanged in twice with CDCl3-d 99.9 at.% D and finally dissolved in 0.5 mL solution of 2/1 (v/v), CDCl3-d 99.9 at.% D (with 0.03% TMS)/DMSO-d6 99.9 at.% D, respectively. All NMR solvents manufactured by Sigma. Proton NMR spectra were acquired at 40°C on a Varian 600-MHz spectrometer equipped with an 5-mm cold probe (Varian, Inova Palo Alto, CA) and processed using the standard Varian software. COSY experiments were recorded using sets of eight time increments with 1024 scans per increment with a saturation delay of 1 s and an acquisition time of 0.54 s. TOCSY experiments were recorded using an acquisition time of 0.4 s and sets of 256 time increments at 32 scans per increment and with a 1-s saturation delay. Proton–carbon multiplicity enabled HSQC experiments were recorded with an acquisition time of 0.2 s, sets of 128 time increments with 96 scans per increment and with a 1-s relaxation delay. NOESY experiments were recorded with a mixing time of 0.2 s, sets of 256 increments with 32 scans per increment, an acquisition time of 0.54 s and a 1-s relaxation delay. All the spectra were calibrated to the internal standard of TMS. The data sets were processed using MestReNova ver.8 (Mestrelab Research, Santiago de Compostela, Spain).
Supplementary data
Supplementary data for this article are available online at http://glycob.oxfordjournals.org/.
Funding
This work was supported, in whole or in part, by National Institutes of Health (grant GM39583 to R.W.C.) and by the Department of Energy (grant DE-FG-02-93ER20097 to the Complex Carbohydrate Research Center). This work is also in partial fulfillment for the PhD degree of D.B.B.
Conflict of interest
None.
Abbreviations
Ara4N, 4-deoxy-4-amino-arabinose; ArnC, undecaprenyl phosphate-L-Ara4FN transferase; ArnT, 4-amino-4-deoxy-arabinosyl transferase; COSY, correlation spectroscopy; DAG, β-(1,6)-2,3-diamino-2,3-dideoxyglucosamine; Dod-P-GalA, dodecaprenyl-phosphate galacturonic acid; EI, electron impact; FAMEs, fatty acid methyl esters; GalA, galacturonic acid; GalAT, GalA transferase; GC/MS, gas chromatography/mass spectrometry; GlcN, glucosamine; GlcNonate, 2-amino-2-deoxy-gluconate; HSQC, heteronuclear single-quantum correlation spectroscopy; LPS, lipopolysaccharide; MALDI-TOF-MS/MS, matrix-assisted desorption/ionization time-of-flight mass spectrometry; MWCO, molecular weight cut off; NMR, nuclear magnetic resonance; NOESY, nuclear overhauser effect spectroscopy; PBS, physiological buffered saline; TMS, trimethylsilyl; TOCSY, total correlated spectroscopy; TY, tryptone-yeast; UDP, uridine diphosphate; Und-P-Ara4FN, Und-P-N-formyl arabinose; Und-P-Ara4N, undecaprenyl-phosphate-Ara4N.
Supplementary Material
Acknowledgements
The authors thank Alicja Ignatowicz for her help in determining the 2,3-diamino-2,3-dideoxy glucose in MAFF303099 lipid A. We also thank Otto Geiger for providing R. etli strain CS506.
References
- Beringer JE. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974;84:188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Bhat UR, Forsberg LS, Carlson RW. Structure of lipid A component of Rhizobium leguminosarum bv. phaseoli lipopolysaccharide. Unique nonphosphorylated lipid A containing 2-amino-2-deoxygluconate, galacturonate, and glucosamine. J Biol Chem. 1994;269:14402–14410. [PubMed] [Google Scholar]
- Brown DB, Forsberg LS, Kannenberg EL, Carlson RW. Characterization of galacturonosyl transferase genes rgtA, rgtB, rgtC, rgtD, and rgtE responsible for lipopolysaccharide synthesis in nitrogen-fixing endosymbiont Rhizobium leguminosarum. J Biol Chem. 2012;287:935–949. doi: 10.1074/jbc.M111.311571. doi:10.1074/jbc.M111.311571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DB, Huang Y-C, Kannenberg EL, Sherrier DJ, Carlson RW. An acpXL mutant in Rhizobium leguminosarum bv. phaseolus lacks 27-hydroxyoctacosanoic acid in its lipid A and is developmentally delayed during symbiotic infection of the determinate nodulating host plant Phaseolus vulgaris. J Bacteriol. 2011;193(18):4766–4778. doi: 10.1128/JB.00392-11. doi:10.1128/JB.00392-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson RW, Forsberg LS, Kannenberg EL. Lipopolysaccharides in Rhizobium-Legume symbioses. In: Wang X, Quinn PJ, editors. Endotoxins: structure, function and recognition. The Netherlands: Springer; 2010. pp. 339–386. [DOI] [PubMed] [Google Scholar]
- Choma A. Fatty acid composition of Mesorhizobium huakuii lipopolysaccharides. Identification of 27-oxooctacosanoic acid. FEMS Microbiol Lett. 1999;177:257–262. doi:10.1111/j.1574-6968.1999.tb13741.x. [Google Scholar]
- Choma A, Komaniecka I. Characterization of a novel lipid A structure isolated from Azospirillum lipoferum lipopolysaccharide. Carbohydr Res. 2008;343:799–804. doi: 10.1016/j.carres.2008.01.006. doi:10.1016/j.carres.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Choma A, Sowinski P. Characterization of Mesorhizobium huakuii lipid A containing both D-galacturonic acid and phosphate residues. Eur J Biochem. 2004;271:1310–1322. doi: 10.1111/j.1432-1033.2004.04038.x. doi:10.1111/j.1432-1033.2004.04038.x. [DOI] [PubMed] [Google Scholar]
- Coats SR, Berezow AB, To TT, Jain S, Bainbridge BW, Banani KP, Darveau RP. The lipid A phosphate position determines differential host Toll-like receptor 4 responses to phylogenetically related symbiotic and pathogenic bacteria. Infect Immun. 2011;79:203–210. doi: 10.1128/IAI.00937-10. doi:10.1128/IAI.00937-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats SR, Jones JW, Do CT, Braham PH, Bainbridge BW, To TT, Goodlett DR, Ernst RK, Darveau RP. Human Toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1- and 4′-phosphatase activities. Cell Microbiol. 2009;11:1587–1599. doi: 10.1111/j.1462-5822.2009.01349.x. doi:10.1111/j.1462-5822.2009.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats SR, To TT, Jain S, Braham PH, Darveau RP. Porphyromonas gingivalis resistance to polymyxin B is determined by the lipid A 4′-phosphatase, PGN_0524. Int J Oral Sci. 2009;1:126–135. doi: 10.4248/IJOS.09062. doi:10.4248/IJOS.09062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen TW, Giles DK, Wolf LN, Ecobichon C, Boneca IG, Trent MS. Helicobacter pylori versus the host: Remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog. 2011;7(12):e1002454. doi: 10.1371/journal.ppat.1002454. doi:10.1371/journal.ppat.1002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C, Luderitz O, Rietschel ETh, Westphal O, Brade H, Brade L, Freudenberg MA, Shade FU, Imoto M, Yoshimura S, et al. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur J Biochem. 1985;148:1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. doi:10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- Gerwig GJ, Kamerling JP, Vliegenthart JF. Determination of the D and L configuration of neutral monosaccharides by high-resolution capillary G.L.C. Carbohydr Res. 1978;62:349–357. doi:10.1016/S0008-6215(00)80881-2. [Google Scholar]
- Ingram BO, Sohlenkamp C, Geiger O, Raetz CR. Altered lipid A structures and polymyxin hypersensitivity of Rhizobium etli mutants lacking the LpxE and LpxF phosphatases. Biochim Biophys Acta. 2010;1801:593–604. doi: 10.1016/j.bbalip.2010.02.001. doi:10.1016/j.bbalip.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Nakamura Y, Sato S, Asamizu E, Kato T, Sasamoto S, Watanabe A, Idesawa K, Ishikawa A, Kawashima K, et al. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 2000;7:331–338. doi: 10.1093/dnares/7.6.331. doi:10.1093/dnares/7.6.331. [DOI] [PubMed] [Google Scholar]
- Kanistanon D, Hajjar AM, Pelletier MR, Gallagher LA, Kalhorn T, Shaffer SA, Goodlett DR, Rohmer L, Brittnacher MJ, Skerrett SJ, et al. A Francisella mutant in lipid A carbohydrate modification elicits protective immunity. PLoS Pathog. 2008;4:e24. doi: 10.1371/journal.ppat.0040024. doi:10.1371/journal.ppat.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjilal-Kolar S, Basu SS, Kanipes MI, Guan Z, Garrett TA, Raetz CR. Expression cloning of three Rhizobium leguminosarum lipopolysaccharide core galacturonosyl transferases. J Biol Chem. 2006;281:12865–12878. doi: 10.1074/jbc.M513864200. doi:10.1074/jbc.M513864200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjilal-Kolar S, Raetz CR. Dodecaprenyl phosphate-galacturonic acid as a donor substrate for lipopolysaccharide core glycosylation in Rhizobium leguminosarum. J Biol Chem. 2006;281:12879–12887. doi: 10.1074/jbc.M513865200. doi:10.1074/jbc.M513865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbarz MJ, Kalb SR, Cotter RJ, Raetz CR. Expression cloning and biochemical characterization of a Rhizobium leguminosarum lipid A 1-phosphatase. J Biol Chem. 2003;278:39269–39279. doi: 10.1074/jbc.M305830200. doi:10.1074/jbc.M305830200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbarz MJ, Six DA, Raetz CRH. Purification and characterization of the lipid A 1-phosphatase LpxE of Rhizobium leguminosarum. J Biol Chem. 2009;284:414–425. doi: 10.1074/jbc.M808390200. doi:10.1074/jbc.M808390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman LJ, Fitzmaurice WP, Roberts GP. The cloning and functional characterization of the nifH gene of Rhodospirillum rubrum. Gene. 1990;95:143–147. doi: 10.1016/0378-1119(90)90426-r. doi:10.1016/0378-1119(90)90426-R. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, et al. CDD: A conserved domain database for the functional annotation of proteins. Nucleic Acids Res. 2011;39:D225–D229. doi: 10.1093/nar/gkq1189. (data base issue) doi:10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr N, Tirsoaga A, Blanot D, Fernandez R, Caroff M. Glucosamine found as a substituent of both phosphate groups in Bordetella lipid A backbones: Role of a BvgAS-activated ArnT ortholog. J Bacteriol. 2008;190:4281–4290. doi: 10.1128/JB.01875-07. doi:10.1128/JB.01875-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel KD, Sanchez A, Fernandez L, Leemans J, Cevallos MA. Rhizobium phaseoli symbiotic mutants with transposon Tn5 insertions. J Bacteriol. 1984;158:148–155. doi: 10.1128/jb.158.1.148-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadnis SH, Das HK. Use of the plasmid pRK 2013 as a vehicle for transposition in Azotobacter vinelandii. J Biosci. 1987;12:131–135. doi:10.1007/BF02702964. [Google Scholar]
- Plotz BM, Lindner B, Stetter KO, Holst O. Characterization of a novel lipid A containing d-galacturonic acid that replaces phosphate residues. The structure of the lipid A of the lipopolysaccharide from the hyperthermophilic bacterium Aquifex pyrophilus. J Biol Chem. 2000;275:11222–11228. doi: 10.1074/jbc.275.15.11222. doi:10.1074/jbc.275.15.11222. [DOI] [PubMed] [Google Scholar]
- Que-Gewirth NL, Karbarz MJ, Kalb SR, Cotter RJ, Raetz CR. Origin of the 2-amino-2-deoxy-gluconate unit in Rhizobium leguminosarum lipid A. Expression cloning of the outer membrane oxidase LpxQ. J Biol Chem. 2003;278:12120–12129. doi: 10.1074/jbc.M300379200. doi:10.1074/jbc.M300379200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz CR, Guan Z, Ingram BO, Six DA, Song F, Wang X, Zhao J. Discovery of new biosynthetic pathways: The lipid A story. J Lipid Res. 2009;50(Suppl):S103–S108. doi: 10.1194/jlr.R800060-JLR200. doi:10.1194/jlr.R800060-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz CRH, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. doi:10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. doi:10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roppel J, Mayer H, Weckesser J. Identification of a 2,3-diamino-2,3-dideoxyhexose in the lipid A component of lipopolysaccharides of Rhodopseudomonas viridis and Rhodopseudomonas palustris. Carbohydr Res. 1975;40:31–40. doi: 10.1016/s0008-6215(00)82666-x. doi:10.1016/S0008-6215(00)82666-X. [DOI] [PubMed] [Google Scholar]
- Russa R, Urbanik-Sypniewska T, Lindström K, Mayer H. Chemical characterization of two lipopolysaccharide species isolated from Rhizobium loti NZP2213. Arch Microbiol. 1995;163:345–351. doi: 10.1007/BF00404207. doi:10.1007/BF00404207. [DOI] [PubMed] [Google Scholar]
- Ryan JM, Conrad HE. Structural heterogeneity in the lipopolysaccharide of Salmonella newington. Arch Biochem Biophys. 1974;162(2):530–536. doi: 10.1016/0003-9861(74)90213-6. doi:10.1016/0003-9861(74)90213-6. [DOI] [PubMed] [Google Scholar]
- Smit J, Kaltashov IA, Cotter RJ, Vinogradov E, Perry MB, Haider H, Qureshi N. Structure of a novel lipid A obtained from the lipopolysaccharide of Caulobacter crescentus. Innate Immun. 2008;14:25–36. doi: 10.1177/1753425907087588. doi:10.1177/1753425907087588. [DOI] [PubMed] [Google Scholar]
- Snyder S, Kim D, McIntosh TJ. Lipopolysaccharide bilayer structure: Effect of chemotype, core mutations, divalent cations, and temperature. Biochem. 1999;38:10758–10767. doi: 10.1021/bi990867d. doi:10.1021/bi990867d. [DOI] [PubMed] [Google Scholar]
- Song F, Guan Z, Raetz CR. Biosynthesis of undecaprenyl phosphate-galactosamine and undecaprenyl phosphate-glucose in Francisella novicida. Biochemistry. 2009;48:1173–1182. doi: 10.1021/bi802212t. doi:10.1021/bi802212t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent MS, Ribeiro AA, Lin S, Cotter RJ, Raetz CR. An inner membrane enzyme in Salmonella and Escherichia coli that transfers 4-amino-4-deoxy-L-arabinose to lipid A: Induction on polymyxin-resistant mutants and role of a novel lipid-linked donor. J Biol Chem. 2001;276:43122–43131. doi: 10.1074/jbc.M106961200. doi:10.1074/jbc.M106961200. [DOI] [PubMed] [Google Scholar]
- Trent MS, Stead CM, Tran AX, Hankins JV. Diversity of endotoxin and its impact on pathogenesis. J Endotoxin Res. 2006;12:205–223. doi: 10.1179/096805106X118825. doi:10.1179/096805106X118825. [DOI] [PubMed] [Google Scholar]
- Wang X, McGrath SC, Cotter RJ, Raetz CRH. Expression cloning and periplasmic orientation of the Francisella novicida lipid A 4′-phosphatase LpxF. J Biol Chem. 2006;281:9321–9330. doi: 10.1074/jbc.M600435200. doi:10.1074/jbc.M600435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ribeiro AA, Guan Z, Abraham SN, Raetz CRH. Attenuated virulence of a Francisella mutant lacking the lipid A 4′-phosphatase. Proc Natl Acad Sci. 2007;104:4136–4141. doi: 10.1073/pnas.0611606104. doi:10.1073/pnas.0611606104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ribeiro AA, Guan Z, Raetz CRH. Identification of undecaprenyl phosphate-β-d-galactosamine in Francisella novicida and its function in lipid A modification. Biochemistry. 2009;48:1162–1172. doi: 10.1021/bi802211k. doi:10.1021/bi802211k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal O, Jann K. Bacterial lipopolysaccharides. Meth Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- York WS, Darvill AG, McNeil M, Stevenson TT, Albersheim P. Isolation and characterization of plant-cell walls and cell-wall components. Meth Enzymol. 1986;118:3–40. doi:10.1016/0076-6879(86)18062-1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.