Abstract
Because hemodialysis treatment has a limited ability to remove phosphorus, dialysis patients must restrict dietary phosphorus intake and use phosphorus binding medication. Among patients with restricted dietary phosphorus intake (1000 mg/d), phosphorus binders must bind about 250 mg of excess phosphorus per day and among patients with more typical phosphorus intake (1500 mg/d), binders must bind about 750 mg per day. To determine the phosphorus binding capacity of binder prescriptions among American hemodialysis patients, we undertook a cross-sectional study of a random sample of in-center chronic hemodialysis patients.
We obtained data for one randomly selected patient from 244 facilities nationwide. About one-third of patients had hyperphosphatemia (serum phosphorus level > 5.5 mg/dL). Among the 224 patients prescribed binders, the mean phosphorus binding capacity was 256 mg/d (SD 143). 59% of prescriptions had insufficient binding capacity for restricted dietary phosphorus intake, and 100% had insufficient binding capacity for typical dietary phosphorus intake. Patients using two binders had a higher binding capacity than patients using one binder (451 vs. 236 mg/d, p <0.001).
A majority of binder prescriptions have insufficient binding capacity to maintain phosphorus balance. Use of two binders results in higher binder capacity. Further work is needed to understand the impact of binder prescriptions on mineral balance and metabolism and to determine the value of substantially increasing binder prescriptions.
Keywords: phosphorus binders, prescriptions, adequacy, hemodialysis
Introduction
Individuals with moderate to severe renal disease have an impaired ability to excrete phosphorus. As a result, they tend to develop hyperphosphatemia, especially in settings of high phosphorus intake. Elevated serum phosphorus levels are independently associated with increased morbidity and mortality. For example, serum phosphorus levels greater than those recommended by practice guidelines (5.5 mg/dL), are independently associated with a 20%-40% increase in mortality risk among patients with end stage renal disease (ESRD). (1-9) In addition, hyperphosphatemia appears to be involved in the development of atherosclerotic heart disease, secondary hyperparathyroidism, and bone disease among patients with renal disease. (10-12)
Because hemodialysis treatment has a limited ability to remove phosphorus, dialysis patients must both restrict dietary phosphorus intake and use phosphorus binding medication. Even with these interventions, about one third to one half of ESRD patients have hyperphosphatemia. (3,7,9,13) Previous efforts to improve phosphorus management have focused on limiting phosphorus intake and improving adherence to phosphorus binders. (14-17) By contrast, little is known about phosphorus binder prescriptions. We sought to determine the adequacy of phosphorus binder prescriptions among a national sample of hemodialysis patients.
Methods
Dialysis Facilities and Randomization
The publically available database Dialysis Facility Compare was used to identify American chronic hemodialysis facilities that had at least 5 stations and 30 patients. (18) We excluded 1,057 facilities that did not have at least 5 stations or 30 patients, 588 dialysis facilities that did not report the number of patients, 38 pediatric facilities, and 9 facilities located outside the 50 United States. We used a random number generator to select 300 of the remaining 3,636 facilities for our study sample. This study was approved by the MetroHealth Medical Center Institutional Review Board.
Data Collection
To ensure that patients were randomly selected, each facility was first assigned a random letter of the alphabet. Study personnel then called each facility and asked to speak to the dietitian. The dietitian was asked to look at an alphabetical list of facility patients and provide de-identified data about the first patient on the list whose last name began with the random letter assigned to that facility. Data requested included: phosphorus binder prescription, most recent serum phosphorus level, post-dialysis weight, height, age, gender, race/ethnicity, and years on dialysis. Because binder prescriptions written in patient charts may be out of date, we asked dietitians to provide the exact prescriptions that they communicated to the identified patients. Dietitians who declined to participate or could not be contacted despite 5 attempts were excluded from the study.
Phosphorus Binding Capacity
We calculated the PBC for each patient’s binder prescription using summary statistics from a recent systematic review by Daugirdas et al. (19) PBC refers to the in vivo phosphorus binding ability of various binders and may be used to compare the potency of prescriptions involving different binders. (19) To determine the adequacy of binder prescriptions, we used standard estimates of dietary phosphorus intake, gut absorption of phosphorus, and dialytic removal of phosphorus. Practice guidelines recommend a restricted dietary phosphorus intake of 1,000 mg/d. (1) Approximately 60% of naturally occurring phosphorus is absorbed by the intestinal tract. (20-22) Conventional hemodialysis removes approximately 800 mg of phosphorus per treatment or 2400 mg/week. (19,23,24) As a result, phosphorus binders must bind about 250 mg/d of phosphorus to maintain balance. However, many processed and fast foods have phosphorus-containing additives. Such additives are completely absorbed and may increase dietary phosphorus by 500-1000 mg/d. We used 1,500 mg/d as an estimate of typical phosphorus intake. Under this scenario, binders must bind about 750 mg/d of phosphorus to maintain balance.
Sample Size Calculation
We calculated the sample size required to estimate the percent of inadequate prescriptions with a 95% confidence interval of ± 7.5%. To be conservative, we assumed that the percent of inadequate prescriptions would be as high as 50%. This leads to a sample size requirement of 171 participants. (25) To account for a possible non-participation rate as high as 40%, we approached 300 dietitians.
Statistical Analysis
Means and standard deviations are reported for continuous variables and percentages are reported for categorical variables. The t test and analysis of variance were used to determine the univariate relationship between PBC and patient and facility characteristics. Multiple linear regression was used to determine the independent relationship between PBC and patient and facility characteristics. We defined 30 mg as a clinically meaningful difference in binding capacity since this corresponds to the binding capacity of one tablet of calcium acetate. All analyses were conducted using JMP statistical software version 9.0.2 (SAS Institute, Cary, North Carolina).
Results
Patient and Facility Characteristics
We obtained data for one randomly selected patient from each of 244 facilities (participation rate 81%). As indicated in Table 1, the average age of patients was 62 years, about half were white, and one third had hyperphosphatemia (serum phosphorus level > 5.5 mg/dL). Facilities had an average of 67 patients, and most were for profit and affiliated with large dialysis organizations.
Table 1. Patient and Facility Characteristics.
| Patient Characteristics | |
| Number of patients | 244 |
| Age, mean (SD), y | 62 (15) |
| Female (%) | 45 |
| Race/Ethnicity (%) | |
| Black | 30 |
| White | 46 |
| Hispanic | 14 |
| Other | 9 |
| Weight, mean (SD), kg | 81 (23) |
| Time receiving dialysis, mean (SD), y | 3 (3) |
| Serum phosphorus, mean (SD), mg/dL | 5.2 (1.6) |
| Serum phosphorus > 5.5 mg/dL (%) | 34 |
| Facility Characteristics | |
| Number of facilities | 244 |
| Large dialysis organization (%) | 73 |
| For profit (%) | 84 |
| Number of patients, mean (SD) | 67 (32) |
| Number of stations, mean (SD) | 21 (8) |
Phosphorus Binder Prescriptions
The most commonly prescribed single binders were sevelamer carbonate and calcium acetate (Table 2). The most commonly prescribed dual binder combination was calcium acetate and sevelamer carbonate. Twenty (8%) patients were not prescribed any binders. Among the 224 patients prescribed binders, the binder pill burden averaged 8 tablets per day (SD 5) and ranged from 1-27 tablets per day.
Table 2. Phosphorus Binder Prescription and Binding Capacity.
| Binder(s) | Number of Patients |
Prescribed Dose, mean, g/d (SD) |
Pill Burden, mean, number/d (SD) |
Binding Capacity, mean, mg/d (SD) |
|---|---|---|---|---|
| Calcium Acetate Only | 74 | 5.0 (2.5) | 7.5 (3.7) | 225 (112) |
| Calcium Carbonate Only | 7 | 4.5 (2.0) | 5.0 (2.7) | 201 (88) |
| Lanthanum Only | 12 | 2.7 (0.7) | 3.0 (0.5) | 241 (59) |
| Magnesium Carbonate Only |
1 | 24(--) | 6.0 (--) | 184 (--) |
| Sevelamer Carbonate Only | 92 | 7.4 (4.2) | 8.9(5.2) | 249 (143) |
| Sevelamer Hydrochloride Only |
17 | 6.8 (3.8) | 8.5 (4.8) | 229 (129) |
| Calcium Acetate and Calcium Carbonate |
2 | 5.3 (3.8) 3.3 (3.9) |
8.0 (5.7) 3.5 (3.5) |
240 (170) 146 (175) |
| Calcium Acetate and Sevelamer Carbonate |
10 | 4.6 (1.4) 7.5 (5.2) |
6.9 (2.0) 8.8 (6.8) |
207 (61) 254 (176) |
| Calcium Acetate and Sevelamer Hydrochloride |
2 | 3.3 (3.8) 4.4 (0.6) |
5.0 (5.7) 5.5 (0.7) |
150 (170) 149 (19) |
| Calcium Acetate and Lanthanum |
2 | 6.5 (2.1) 2.8 (0.4) |
9.8 (3.2) 2.5 (0.7) |
293 (96) 248 (32) |
| Calcium Carbonate and Sevelamer Carbonate |
2 | 5.3 (1.1) 10.8 (1.7) |
10.5 (2.1) 13.5 (2.1) |
236 (48) 365 (57) |
| Calcium Carbonate and Lanthanum |
2 | 5.3 (2.1) 2.0 (1.4) |
6.0 (4.2) 2.0 (1.4) |
236 (95) 180 (127) |
| Lanthanum and Sevelamer Carbonate |
1 | 0.5 (--) 9.6 (--) |
1 (--) 12 (--) |
45 (-- ) 324 (-- ) |
| No Binders | 20 | 0 (--) | 0 (-- ) | 0 (-- ) |
Phosphorus Binding Capacity
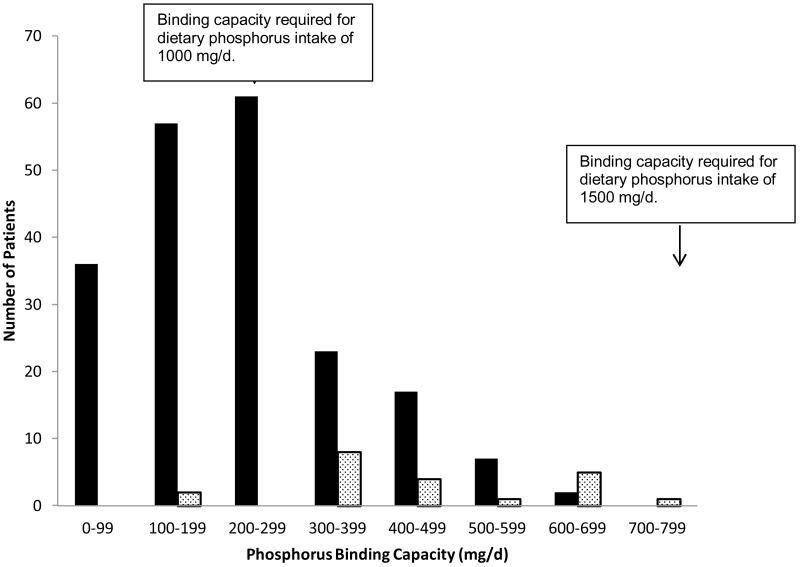
Among the 224 patients prescribed binders, the mean phosphorus binding capacity (PBC) was 256 mg/d (SD 143). As indicated in Figure 1, 59% of prescriptions had insufficient binding capacity for restricted dietary phosphorus intake, and 100% had insufficient binding capacity for typical dietary phosphorus intake. Patients using two binders had a higher binding capacity than patients using one binder (451 vs. 236 mg/d, p <0.001).
Figure 1.
Distribution of Phosphorus Binding Capacity. Dark bars represent 203 patients prescribed a single binder. Light bars represent 21 patients prescribed two binders.
On univariate analysis, increased binding capacity was associated with younger age, male gender, increased weight, longer time receiving dialysis, higher serum phosphorus level, and large dialysis organizations (Table 3). However, there were no clinically significant relationships between binding capacity and patient or facility characteristics on multivariate analyses (Table 4).
Table 3. Univariate Relationship Between Phosphorus Binding Capacity and Patient and Facility Characteristics.
| Patient Characteristics |
n | PBC, mg/d, mean (SD) |
p value |
Facility Characteristics |
n | PBC, mg/d, mean (SD) |
p value |
|---|---|---|---|---|---|---|---|
| Age, y | |||||||
| < 55 | 70 | 285 (161) | <0.001 | ||||
| 55-64 | 66 | 267 (165) | Large dialysis organization |
178 | 247 (157) | 0.05 | |
| 65-74 | 56 | 243 (122) | Small dialysis organization |
66 | 202 (144) | ||
| ≥ 75 | 52 | 118 (96) | |||||
| For profit | 205 | 240 (155) | 0.25 | ||||
| Sex | Non-profit | 39 | 209 (152) | ||||
| Female | 105 | 212 (140) | 0.04 | ||||
| Male | 139 | 252 (162) | Number of patients | ||||
| < 45 | 63 | 222 (160) | 0.28 | ||||
| Race/Ethnicity | 45-64 | 83 | 243 (150) | ||||
| Black | 83 | 248 (154) | 0.21 | 65-84 | 42 | 205 (127) | |
| White | 106 | 215 (146) | ≥85 | 56 | 261 (171) | ||
| Hispanic | 34 | 274 (160) | |||||
| Other | 21 | 222 (180) | Number of stations | ||||
| < 15 | 49 | 199 (135) | 0.27 | ||||
| Weight, kg | 15-19 | 58 | 231 (156) | ||||
| < 65 | 59 | 173 (132) | <0.001 | 20-24 | 83 | 250 (155) | |
| 65-74 | 53 | 225 (142) | ≥ 25 | 54 | 250 (166) | ||
| 75-84 | 47 | 223 (127) | |||||
| ≥85 | 85 | 291 (171) | |||||
| Time receiving dialysis, y | |||||||
| <1 | 41 | 137 (115) | <0.001 | ||||
| 1-2.9 | 90 | 233 (155) | |||||
| 3-4.9 | 53 | 260 (141) | |||||
| ≥5 | 60 | 283 (160) | |||||
| Serum phosphorus, mg/dL |
|||||||
| <4 | 49 | 171 (137) | <0.001 | ||||
| 4-4.74 | 57 | 186 (123) | |||||
| 4.75-5.4 | 52 | 249 (156) | |||||
| ≥5.5 | 86 | 296 (158) |
Table 4. Multivariate Relationship Between Phosphorus Binding Capacity and Patient and Facility Characteristics.
| Effect on phosphorus binding capacity, mg/d |
p value | |
|---|---|---|
| Age, per 10 years | −20 | <0.001 |
| Weight, per 5 kg | 8 | <0.001 |
| Time receiving dialysis, per 1 year | 10 | <0.001 |
| Serum phosphorus, per 0.5 mg/dL | 16 | <0.001 |
| Non-profit facility | −25 | 0.03 |
Discussion
By examining binder use in the context of a dietary and dialytic framework of phosphorus balance, we identified a previously unrecognized potential contributor to the high prevalence of hyperphosphatemia among hemodialysis patients. We found that all examined phosphorus binder prescriptions were inadequate for typical dietary phosphorus intake, and a majority were inadequate for restricted dietary intake. Although dual binder use conferred significantly more PBC, a minority of patients were prescribed two binders. In addition, there was a sizeable pill burden from binders, with an average patient prescribed 8 tablets per day. Strengths of our study include a large and nationally representative sample, a high participation rate, and the quantification of binding capacity across a variety of binder types and prescriptions.
It is worth noting that the prevalence of hyperphosphatemia in our sample (34%) is less than the prevalence of inadequate prescriptions (59%). It is possible that serum phosphorus levels underestimate actual phosphorus burden, e.g. serum levels may not reflect calcium-phosphorus deposition in vasculature and soft tissue. Alternatively, malnourished patients may have a phosphorus intake much lower than 1000 mg/day. (26) It is also possible that some patients have significant residual renal function that contributes to phosphorus excretion. It is also worth noting that hyperphosphatemia increased slightly with increased PBC in our sample (Table 4). This finding is consistent with a previous study which found that more phosphorus binders do not necessarily result in lowering of serum phosphorus levels. (27) It is likely that binder prescriptions are increased in response to hyperphosphatemia among patients who have very high levels of dietary phosphorus intake and/or are non-adherent with binders. In these situations, we would expect to see a positive association between hyperphosphatemia and binding capacity. Paradoxically, reported dose-response studies for selected binders, namely lanthanum and sevelamer, show an inverse relationship between dose and phosphorus binding. However, these studies were conducted using normal volunteers or patients with non-dialysis dependent CKD. (19,28-30)
Our findings have implications for patients, providers, and policy makers. Patients and providers should work together to limit dietary phosphorus intake, maximize dialytic phosphorus removal, and optimize binder use. However, these may be difficult to accomplish. The increased use of phosphorus-containing food additives in processed meats, fast food, and beverages makes it challenging for patients to limit phosphorus intake to an amount that can be managed with dialysis and binders. (31-35) We used a conservative estimate of additive phosphorus (500 mg per day). Some reports indicate that phosphorus additives account for 1,000 mg of added phosphorus intake per day. (20) Even maximum tested dose of the commonly used binders would be insufficient to maintain phosphorus balance with these levels of additive phosphorus intake. (36-40) Therefore, our conclusion that binder prescriptions are inadequate may be tempered by the observation that the ability to formulate a reasonably adequate phosphorus binder prescription is outstripped by the high prevalence of phosphorus-containing additives in foods. Although weight-based protein requirements have been used as an estimated dietary phosphorus intake, it is recognized that this correlation may be skewed. Phosphorus intake may be underestimated if solely based on protein content of food given the increased use of phosphorus additives. (31,33,41) Optimizing binder use will also be challenging given the already high pill burden, cost, and binder non-adherence among hemodialysis patients. (27,42,43) Dialytic removal of phosphorus is roughly proportional to treatment time. As a result, increased dialytic removal would require greatly lengthened treatments, which may not be acceptable to patients or providers. (44,45) Policy makers should be aware of these challenges when utilizing serum phosphorus levels as a marker of quality of hemodialysis care. Helpful policy interventions may include restricting the widespread use of phosphorus additives and requiring labeling of phosphorus content on food packages.
Several limitations must be considered in interpreting our results. We did not directly measure phosphorus binding capacity, dietary phosphorus intake, adherence to binder prescriptions, or dialytic phosphorus removal. We obtained only a single serum phosphorus level and did not assess other influences such as vitamin D and parathyroid hormone.
We report that a majority of binder prescriptions among American hemodialysis patients appear to have insufficient binding capacity to maintain phosphorus balance. Phosphorus binders alone are insufficient to overcome a positive phosphorus balance without taking into account increased intake of phosphorus containing additives as well as increasing dialysis treatment time to accommodate phosphorus removal. Specifically, in reference to phosphorus binder therapy, further work is needed to understand the impact of binder prescriptions on mineral balance and metabolism, to determine the value of substantially increasing binder prescriptions, and to examine patient adherence to and tolerability of increased binder prescriptions.
Acknowledgements
We are grateful to the dietitians who participated in this study.
This publication was made possible by the Case Western Reserve University/Cleveland Clinic CTSA Grant Number UL1 RR024989 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Support: Anne M. Huml, MD: Supported by grant T32DK007470 from the National Institutes of Health.
Catherine M. Sullivan, MS, RD, LD: Supported by grants MD002265 and RR024989 from the National Institutes of Health.
Janeen B. Leon, MS, RD, LD: Supported by grants MD002265 and RR024989 from the National Institutes of Health.
Ashwini R. Sehgal, MD: Supported by grants MD002265 and RR024989 from the National Institutes of Health.
Footnotes
Catherine M. Sullivan, MS, RD, LD, Center for Reducing Health Disparities, MetroHealth Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109, csullivan1@metrohealth.org, fax (216) 778-8401; Janeen B. Leon, MS, RD, LD, Center for Healthcare Research and Policy, MetroHealth Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109, Janeen.Leon@case.edu, fax (216) 778-8401; Ashwini R. Sehgal, MD, Center for Reducing Health Disparities, MetroHealth Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109, sehgal@case.edu, fax (216) 778-8401
Declaration of Interest:
All authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1. [accessed July 2011]; http://www.kidney.org/professionals/kdoqi/guidelines_bone/index.htm.
- 2.Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 4.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12(10):2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70(4):771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Benot A, Martin-Malo A, Alvarez-Lara MA, Rodriguez M, Aljama P. Mild hyperphosphatemia and mortality in hemodialysis patients. Am J Kidney Dis. 2005;46(1):68–77. doi: 10.1053/j.ajkd.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Tentori F, Blayney M, Albert J, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52(3):519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Wald R, Sarnak M, Tighiouart H, et al. Disordered mineral metabolism in hemodialysis patients: analysis of cumulative effects in the Hemodialysis (HEMO) Study. Am J Kidney Dis. 2008;52(3):531–540. doi: 10.1053/j.ajkd.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro S, Ramos A, Brandao A, et al. Cardiac valve calcification in haemodialysis patients: role of calcium-phosphate metabolism. Nephrol Dial Transplant. 1998;13(8):2037–2040. doi: 10.1093/ndt/13.8.2037. [DOI] [PubMed] [Google Scholar]
- 11.Goodman WG. The consequences of uncontrolled secondary hyperparathyroidism and its treatment in chronic kidney disease. Semin Dial. 2004;17(3):209–216. doi: 10.1111/j.0894-0959.2004.17308.x. [DOI] [PubMed] [Google Scholar]
- 12.Malluche HH, Mawad H. Management of hyperphosphatemia of chronic kidney disease: lessons from the past and future directions. Nephrol Dial Transplant. 2002;17(7):1170–1175. doi: 10.1093/ndt/17.7.1170. [DOI] [PubMed] [Google Scholar]
- 13.Young EW, Akiba T, Albert JM, et al. Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the dialysis outcomes and practice patterns study (DOPPS) Am J Kidney Dis. 2004;44(S2):S34–S38. doi: 10.1053/j.ajkd.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan C, Sayre SS, Leon JB, et al. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: a randomized controlled trial. JAMA. 2009;301(6):629–635. doi: 10.1001/jama.2009.96. [DOI] [PubMed] [Google Scholar]
- 15.Ashurst I, Dip PG, Dobbie H. A randomized controlled trial of an educational intervention to improve phosphate levels in hemodialysis patients. J Ren Nutr. 2003;13(4):267–274. doi: 10.1016/s1051-2276(03)00116-x. [DOI] [PubMed] [Google Scholar]
- 16.Mayne TJ, Benner D, Ricketts K, et al. Results of a pilot program to improve phosphorus outcomes in hemodialysis patients. J Ren Nutr. 2011 Nov 2; doi: 10.1053/j.jrn.2011.08.006. published online ahead of print. doi:10.1053/j.jrn.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Morey B, Walker R, Davenport A. More dietetic time, better outcome? Nephron Clin Pract. 2008;109:c173–c180. doi: 10.1159/000145462. [DOI] [PubMed] [Google Scholar]
- 18. [accessed July 2011]; http://data.medicare.gov/dataset/Dialysis-Facility-Compare-Listing-by-Facility/23ew-n7w9.
- 19.Daugirdas JT, Finn WF, Emmett M, Chertow GM, the Frequent Hemodialysis Network Trial Group The phosphate binder equivalent dose. Semin Dial. 2011;24(1):41–49. doi: 10.1111/j.1525-139X.2011.00849.x. [DOI] [PubMed] [Google Scholar]
- 20.Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial. 2003;16(3):186–188. doi: 10.1046/j.1525-139x.2003.16037.x. [DOI] [PubMed] [Google Scholar]
- 21.Uribarri J. Phosphorus homeostasis in normal health and in chronic kidney disease patients with special emphasis on dietary phosphorus intake. Semin Dial. 2007;20(4):295–301. doi: 10.1111/j.1525-139X.2007.00309.x. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez JA, Emmett M, White MG, et al. The absorption of dietary phosphorus and calcium in hemodialysis patients. Kidney Int. 1986;30:753–759. doi: 10.1038/ki.1986.252. [DOI] [PubMed] [Google Scholar]
- 23.Finn WF. Phosphorus management in end-stage renal disease. Semin Dial. 2005;18(1):8–12. doi: 10.1111/j.1525-139X.2005.18116.x. [DOI] [PubMed] [Google Scholar]
- 24.Kooienga L. Phosphorus balance with daily dialysis. Semin Dial. 2007;20(4):342–345. doi: 10.1111/j.1525-139X.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 25.Browner WS, Newman TB, Hulley SB. Estimating sample size and power: applications and examples. In: Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB, editors. Designing Clinical Research. 3rd ed. Lippincott Williams and Wilkins; Philadelphia, PA: 2007. pp. 65–94. [Google Scholar]
- 26.Kalantar-Zadeh K, Ikizler A, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42(5):864–881. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089–1096. doi: 10.2215/CJN.00290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sprague SM, Abboud H, Qiu P, Dauphin M, Zhang P, Finn W. Lanthanum carbonate reduces phosphorus burden in patients with CKD stages 3 and 4: a randomized trial. Clin J Am Soc Nephrol. 2009;4(1):178–185. doi: 10.2215/CJN.02830608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finn W, Kingma-Johnson I. Urinary phosphorus excretion is a valuable measure of total body phosphorus burden in patients with CKD stages 3 and 4. J Am Soc Nephrol. 2008;19:320A–321A. [Google Scholar]
- 30.Burke SK, Slatopolsky EA, Goldberg DI. RenaGel, a novel calcium- and aluminium-free phosphate binder, inhibits phosphate absorption in normal volunteers. Nephrol Dial Transplant. 1997;12:1640–1644. doi: 10.1093/ndt/12.8.1640. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan CM, Leon JB, Sehgal AS. Phosphorus-containing food additives and the accuracy of nutrient databases: implications for renal patients. J Ren Nutr. 2007;17(5):350–354. doi: 10.1053/j.jrn.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarathy S, Sullivan C, Leon JB, Sehgal AR. Fast food, phosphorus containing food additives, and the renal diet. J Ren Nutr. 2008;18(5):466–470. doi: 10.1053/j.jrn.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Sherman RA, Mehta O. Phosphorus and potassium content of enhanced meat and poultry products: implications for patients who receive dialysis. Clin J Am Soc Nephrol. 2009;4(8):1370–1373. doi: 10.2215/CJN.02830409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calvo MS. Dietary considerations to prevent loss of bone and renal function. Nutrition. 2000;16(7-8):564–566. doi: 10.1016/s0899-9007(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 35.Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:519–530. doi: 10.2215/CJN.06080809. [DOI] [PubMed] [Google Scholar]
- 36.PhosLo [package insert] Fresenius Medical Care; Waltham, MA: 2007. [Google Scholar]
- 37.Renagel [package insert] Genzyme Corporation; Cambridge, MA: 2007. [Google Scholar]
- 38.Renvela [package insert] Genzyme Corporation; Cambridge, MA: 2010. [Google Scholar]
- 39.Fosrenol [package insert] Shire US Inc.; Wayne, PA: 2011. [Google Scholar]
- 40.Murray L. PDR for Nonprescription Drugs, Dietary Supplements, and Herbs. Thomson PDR; Montvale, NJ: 2005. Dosing information for dyspepsia products; p. 160. [Google Scholar]
- 41.Benini O, D’Alessandro C, Gianfaldoni D, Cupisti A. Extra-phosphate load from food additives in commonly eaten foods: a real and insidious danger for renal patients. J Ren Nutr. 2011;21(4):303–308. doi: 10.1053/j.jrn.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 42.Manley HJ, Garvin CG, Drayer DK, et al. Medication prescribing patterns in ambulatory haemodialysis patients: comparisons of USRDS to a large not-for-profit dialysis provider. Nephrol Dial Transplant. 2004;19:1842–1848. doi: 10.1093/ndt/gfh280. [DOI] [PubMed] [Google Scholar]
- 43.Karamanidou C, Clatworthy J, Weinman J, Horne R. A systematic review of the prevalence and determinants of nonadherence to phosphate binding medication in patients with end-stage renal disease. BMC Nephrol. 2008;9:2. doi: 10.1186/1471-2369-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schorr M, Manns BJ, Culleton B, et al. The effect of nocturnal and conventional hemodialysis on markers of nutritional status: results from a randomized trial. J Ren Nutr. 2011;21(3):271–276. doi: 10.1053/j.jrn.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Kjellstrand CM, Ing TS, Kjellstrand PT, Odar-Cederlof I, Lagg CR. Phosphorus dynamics during hemodialysis. Hemodial Int. 2011;15(2):226–233. doi: 10.1111/j.1542-4758.2011.00538.x. [DOI] [PubMed] [Google Scholar]