Abstract
Achromatopsia is an autosomal recessive retinal disease involving loss of cone function that afflicts approximately 1 in 30,000 individuals. Patients with achromatopsia usually have visual acuities lower than 20/200 because of the central vision loss, photophobia, complete color blindness and reduced cone-mediated electroretinographic (ERG) amplitudes. Mutations in three genes have been found to be the primary causes of achromatopsia, including CNGB3 (beta subunit of the cone cyclic nucleotide-gated cation channel), CNGA3 (alpha subunit of the cone cyclic nucleotide-gated cation channel), and GNAT2 (cone specific alpha subunit of transducin). Naturally occurring mouse models with mutations in Cnga3 (cpfl5 mice) and Gnat2 (cpfl3 mice) were discovered at The Jackson Laboratory. A natural occurring canine model with CNGB3 mutations has also been found. These animal models have many of the central phenotypic features of the corresponding human diseases. Using adeno-associated virus (AAV)-mediated gene therapy, we and others show that cone function can be restored in all three models. These data suggest that human achromatopsia may be a good candidate for corrective gene therapy.
1.1 Human Achromatopsia
The human retina has approximately 6 million cone photoreceptors and 100 million rod photoreceptors. Cones are primarily responsible for central, fine resolution and color vision while operating in low to very bright light; they are primarily concentrated in the central macula comprising nearly 100% of the fovea. In contrast, rods are responsible for peripheral, low light and night vision, and are primarily found in the peripheral retina and perimacular region. Achromatopsia, or rod monochromatism, is a recessive genetic condition characterized by cone dysfunction, thus leaving the patient with only rod mediated vision. There are two clinical forms of achromatopsia: complete and incomplete. The complete form results in serious visual deficits and affects approximately 1:30,000 Americans (Kohl et al. 2002). Those achromats exhibit total color vision loss, relatively stable central vision loss, and visual acuity of 20/200 or worse (Kohl et al. 2005), usually making them legally blind. Since these individuals see the world only with their rods, they experience photophobia or “daytime blindness” because their rods become light-saturated in normal bright light conditions.
1.2 Clinical manifestations
Clinically, the first signs of achromatopsia in infants are the presence of nystagmus, a pendular quivering of the eyes, and photophobia as evidenced by squinting in bright light (Kohl et al. 2005). Infants can be tested by the cone ERG, and, as they mature they can be given specific tests such as the Sloan Achromatopsia Test.
1.3 Current achromatopsia treatments
There are no treatments available that correct cone function in achromats to any degree. Current standard of care consists of managing symptoms by limiting retinal light exposure with tinted contact lenses (Park et al. 2004), and (or) very darkly tinted sunglasses (Young, Krefman & Fishman 1982; Young, Krefman and Anderson 1983). Tinted central contact lenses typically transmitting light at wavelengths between 400–480nm (Park et al. 2004) are an improvement over simple cutoff filters in alleviating photophobia (Schornack 2007). Additionally, these contacts have reduced the stigma of wearing dark wraparound sunglasses indoors. However, such red central contacts only reduce the amount of light entering the retina and do nothing to improve high-resolution vision or color sensitive tasks. A partial solution is to use tinted contact lenses with magnification to boost what central vision is still present from functional photoreceptors (Fonda and Thomas 1974). With respect to visual acuity, improvement can be obtained by employing microscopic eyewear, enlarged print and closed circuit TV. Even with the best external aid techniques in place, daily tasks such as driving and going to school present significant obstacles. This is especially problematic for school age children because of the usual color-based curriculum; in this context tinted lenses have been tested (Schiefer 1995). A common result of these symptoms is that achromats often gravitate towards activities normally performed in the evening or in low light.
2.1 Genetics of human achromatopsia
Autosomal recessive mutations in predominantly three genes, CNGB3, CNGA3, and GNAT2, cause achromatopsia (Kohl et al. 1998, 2000, 2002, 2005; Sundin et al. 2000; Aligianis et al. 2002). CNGB3 encodes the beta subunit of the cone cyclic nucleotide-gated cation channel and CNGA3 encodes the alpha subunit. GNAT2 encodes the cone specific alpha subunit of transducin. Of all mutations that cause complete achromatopsia, those in CNGB3 account for about 50% of cases whereas CNGA3 mutations account for about 23% and GNAT2 mutations for about 2% (Wissinger et al. 2001; Kohl et al. 2002, 2005; Aligianis et al. 2002). The majority of CNGB3 mutations result no protein or protein truncations that are functionally null (Kohl et al. 2005). In contrast, CNGA3 mutations are predominantly missense (Wissinger et al. 2001; Kohl et al. 2005). Recently, a Japanese patient with congenital achromatopsia was identified with compound heterozygous mutations in CNGA3 (Goto-Omoto et al. 2006).
2.2 GNAT2 achromatopsia
Genetic analysis points to several classes of mutations in the GNAT2 gene that give rise to the similar phenotypes. Kohl et al. (Kohl et al. 2002) analyzed 77 achromatopsia patients with GNAT2 mutations and identified six distinct disease-related sequence alterations that segregated in five apparently independent families of European descent. There was one nonsense mutation, four small deletion and/or insertion mutations and a sixth mutation containing a large intragenic deletion of 2,019 bp including exon 4 and flanking intron sequences. All mutations resulted in premature translation termination or in mutant polypeptides that lack considerable portions of the carboxy1 terminus. In rods the conserved carboxyl terminus of the corresponding rod α-transducin contains major sites of interaction with photo-excited rhodopsin (Cai, Itoh and Khorana 2001). Considering the high conservation between the rod and cone transducin α-subunits, a similar structural function is likely to exist in the cone system, and thus explain the carboxy-terminal mutations. Accordingly, Kohl et al. (Kohl et al. 2002) suggest that all mutations in these achromatopsia families represent effectively null alleles of GNAT2, which prevent either the formation of a functional heterotrimeric G-protein complex or its interaction with excited photopigments. Additionally, Aligianis et al. (Aligianis et al. 2002) identified a consanguineous Pakistani family with six members having autosomal recessive achromatopsia. The deduced frameshift mutation in exon 7 of GNAT2 is different from the one described by Kohl et al. (Kohl et al. 2002), suggesting that the complete spectrum of GNAT2 mutations causing achromatopsia is not yet known. Additionally, it is important to note that not all mutations in what would appear to be “important” GNAT2 domains cause disease. Although lysine 270 of GNAT2 has been suggested by modeling to play a key role in fixing the purine ring of GTP in the nucleotide binding cleft of GNAT2, when Pina et al. (Pina et al. 2004) examined the phenotype of a K270 deletion in homozygous carriers, segregation analysis showed that the deletion is not co-inherited with the disease phenotype and, therefore, is not the disease causing mutation. Presumably, GNAT2 function is not altered by K270 deletion possibly because of a compensatory effect of K271. Thus, GNAT2 is able to structurally and functionally tolerate a K270 deletion as shown by its ability to support normal cone function.
2.3 CNG achromatopsia
Cones like rods rely on cyclic nucleotide gated (CNG) channels for membrane hyperpolarization and signal transduction upon light absorption. Heterotetrameric cation channels in rods and cones form a central pore, with the occupancy of cGMP binding sites on each subunit regulating pore formation. Six genes control CNG expression in mammals, four alpha subunits (CNGA1-4) and two beta subunits (CNGB1 and CNGB3) (Bradley et al 2001). CNGA subunits can form functional homomeric proteins, while CNGB subunits must be associated with CNGA subunits to form functional channels (Kaupp and Seifert 2002). Two classes of CNGA subunits are found in vertebrate retinas: CNGA1 is expressed in rod outer segments and CNGA3 is expressed in cones. The latter is also expressed in sperm, kidney, cardiac and brain cells. Cone CNG channels consist of two A3 subunits and two B3 subunits (Bradley, Reisert and Frings 2005; Peng; Rich and Varnum 2004). In humans, cone photoreceptor function loss due to CNGB3 gene mutations is known as achromatopsia 1 (Khan et al, 2007). Achromatopsia 2 is caused by CNGA3 gene mutation. In European populations about 25% of patients with complete achromatopsia have CNGA3 mutations (Kaupp and Seifert 2002; Kohl et al 2005), and another 50% have CNGB3 mutations (Wissinger et al 2001)
2.4 Achromatopsia gene therapy
Thus far mutations causing achromatopsia disrupt either G-protein signaling (GNAT2) or cGMP gated cation channel function (CNGB3 and CNGA3). Since these mutations appear to result in a relatively stationary cone phenotype and therefore human achromats do not generally experience severe retinal degeneration as is seen with other diseases such as Leber’s Congenital Amaurosis. However, recent adaptive optics analysis of achromats with CNGB3 mutation suggests foveal structural abnormalities (Carroll, Choi and Williams, 2008). Taken together, achromatopsia is a potentially viable candidate for gene therapy where animal models exist for all three major genetic forms of disease in which to test this hypothesis.
3.1 The mutant Gnat2 mouse and gene therapy
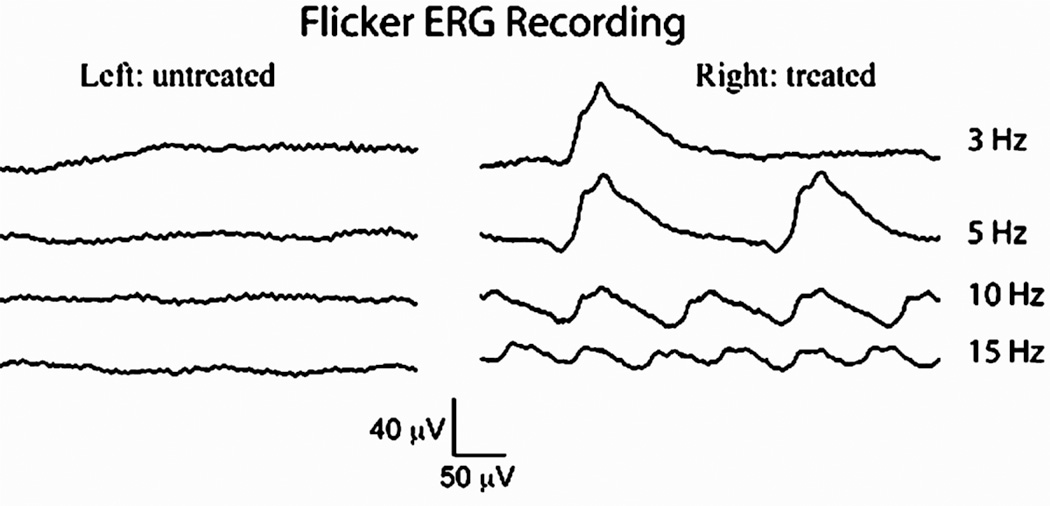
The Gnat2cpfl3 mouse carries a recessive mutation in its cone α-transducin gene that results in cone mediated vision loss, little or no cone-mediated ERG and poor visual acuity, all of which are similar to the corresponding human form of achromatopsia (Chang et al 2006; Alexander et al 2007). Homozygous Gnat2cpfl3 mice were treated with a single subretinal injection of an AAV serotype 5 vector (4 × 1010 vector genome containing particles) carrying a wild type mouse Gnat2 cDNA under control of a human red cone opsin promoter that targets vector transgene expression to cones in mice (Alexander et al 2007). In treated eyes, light-adapted (cone specific) ERG responses were stably restored to within the normal amplitude range for at least 7 months (Alexander et al 2007). Cone ERG amplitudes in untreated eyes remained undetectable. Furthermore, visual acuity was restored to normal levels as deduced through optomotor behavioral testing. These encouraging results suggest that long term, effective cone-targeted therapy is possible, providing a basis for treating a variety of related diseases. Additional testing has revealed that treated eyes also respond to cone isolating flicker ERG stimuli more robustly than untreated contralateral eyes (Figure 3.1) further confirming the efficacy to cone-target gene therapy in this model of achromatopsia.
Figure 3.1.
Light-adapted flicker ERGs recorded from a cpfl3 mouse (homozygous recessive Gnat2 mutation) at 7 months after subretinal injection of AAV vector. The right eye was vector treated and the left eye was untreated as a control. For the flicker ERG series, mice were exposed to flashes of 2.5 cd·s/m2 at 3, 5, 10, and 15 Hz in the presence of a 30 cd · s/m2 background light.
3.2 The Cnga3 mutant mouse and gene therapy
Recently a new mouse with a cone function loss, cpfl5 (Cone Photoreceptor Function Loss 5) mice, that has an ocular phenotype similar to human achromatopsia was discovered in The Jackson Laboratory. Cpfl5 is a naturally occurring mouse model of autosomal recessive achromatopsia as defined by a missense mutation in exon 5 of the Cnga3 gene (Hawes et al. 2006). Functional studies found no cone ERG response. Histological and immunohistochemical analysis revealed a migration of cone cell bodies into the outer plexiform layer of the retina from as early as postnatal week 3 suggesting early pathogenesis.
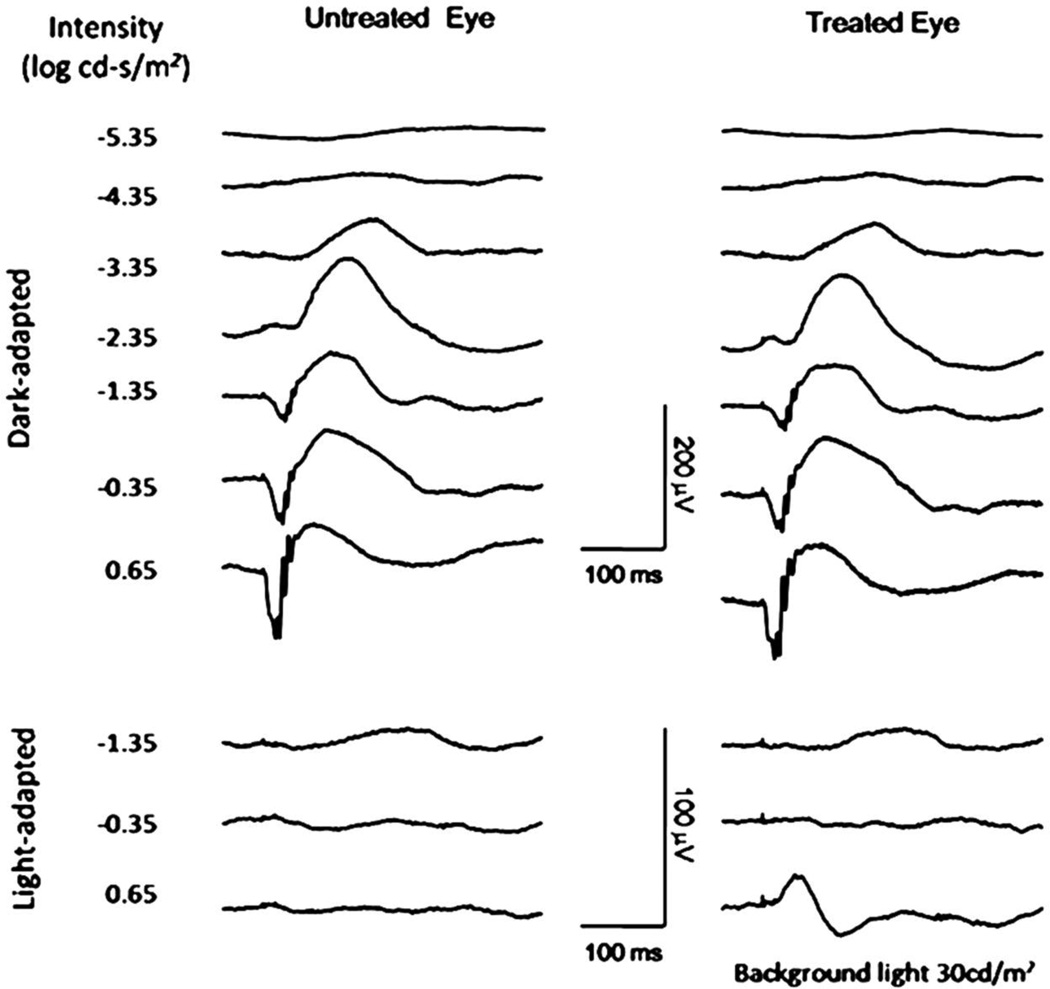
To test whether AAV-mediated Cnga3 gene therapy could restore cone function to cpfl5 mice, we delivered an AAV vector encoding the wild type mouse Cnga3 gene driven by a human blue cone promoter (HB570) that preferentially targeted transgene expression to cones to cpfl5 mice. One µl of AAV5-HB570-Cnga3 vector (1×1010 genome containing viral particles) was injected subretinally into one eye of 10 cpfl5 mice and the untreated contralateral eyes were used as controls. Treatment was at postnatal day 14 (P14) before the cone degeneration initiated. Dark- and light-adapted ERGs were recorded periodically from 3 to 10 weeks after injections. In the treated eyes, measurable light-adapted ERG signals were observed at 3 weeks after vector administration. This restored light-adapted ERGs remained stable for at least 2 months after treatment (Figure 3.2) with b-wave amplitudes about half of that recorded in normal C57BL/6J mice. In the contralateral untreated eyes, cone-driven ERGs remained unrecordable. Dark-adapted ERG analysis confirmed that the rod function is normal and unaffected by vector treatment in cpfl5 mice at this age (Figure 3.2).
Figure 3.2.
Subretinal administration of AAV5-HB570-cnga3 vector restores the cone-driven function in cpfl5 mouse. Representative ERGs recorded at 2 months after treatment at P14. The left column shows ERGs of an untreated control eye and the right column shows ERGs of the contralateral vector treated eye. Comparison between eyes demonstrates that gene transfer restored cone-driven function. Dark-adapted ERGs of the two eyes are comparable, suggesting the treatment had minimal effects on normal rod-driven ERGs in these mice.
3.3 The Cngb3 mutant dog and gene therapy
One autosomal recessive canine disease that occurs naturally in the Alaskan Malamute has been found to be related to CNGB3 mutations. Its phenotype is similar to human achromatopsia, and is characterized by day-blindness and absence of retinal cone function (Sidjanin et al. 2002). Since CNGB3 mutations account for 50% of human achromatopsia, this dog provides a valuable large animal model for exploring disease mechanisms and evaluating potential genetic therapeutic intervention (Sidjanin et al. 2002).
AAV-mediated gene therapy in this canine model of achromatopsia has achieved major therapeutic effect (Komaromy, 2008). ERG restoration has been observed and maintained for over 14 months in the CNGB3 Malamute following a subretinal injection of an AAV5 vector containing human CNGB3 cDNA controlled by a truncated human red cone opsin promoter (Komaromy, 2008).
4.1 Prospects for achromatopsia gene therapy
AAV mediated gene transfer of the corresponding wild type gene corrects cone functional deficiencies in two mouse models and one dog model representing all three (Gnat3,Cnga3 and CNGB3) genetic forms of human achromatopsia. Such intervention effectively restores cone system function as demonstrated by ERGs and/ or by visually elicited behavior. These data suggests achromatopsia may be a viable candidate for gene-based therapy.
Acknowledgement
This research is supported by NIH grants, EY018331, EY13729, EY11123, NS36302, EY08571, EY007758 and FFB, MVRF, RPB, Lions of Central NY.
References
- 1.Alexander JJ, Yumiko Umino Y, Everhart D, et al. Restoration of cone vision in a mouse model of achromatopsia. Nat Med. 2007;13:685–687. doi: 10.1038/nm1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aligianis IA, Forshew T, Johnson S, et al. Mapping of a novel locus for achromatopsia (ACHM4) to 1p and identification of a germline mutation in the alpha subunit of cone transducin (GNAT2) J Med Genet. 2002;39:656–660. doi: 10.1136/jmg.39.9.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley J, Frings S, Yau KW, et al. Nomenclature for ion channel subunits. Science. 2001;294:209–2096. doi: 10.1126/science.294.5549.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley J, Reisert J, Frings S. Regulation of cyclic nucleotide gated channels. Curr Opin Neurobiol. 2005;15:343–349. doi: 10.1016/j.conb.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Cai K, Itoh Y, Khorana HG. Mapping of contact sites in complex formation between transducin and light-activated rhodopsin by covalent crosslinking: use of a photoactivatable reagent. PNAS. 2001;98:4877–4882. doi: 10.1073/pnas.051632898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll J, Choi SS, Williams DR. In vivo imaging of the photoreceptor mosaic of a rod monochromat. Vision Res. 2008;48(26):2564–2568. doi: 10.1016/j.visres.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang B, Dacey MS, Hawes NL, et al. Cone photoreceptor function loss-3, a novel mouse model of achromatopsia due to a mutation in Gnat2. Invest Ophthalmol Vis Sci. 2006;47:5017–5021. doi: 10.1167/iovs.05-1468. [DOI] [PubMed] [Google Scholar]
- 8.Fonda G, Thomas H. Correction of low visual acuity in achromatopsia. Use of corrective lenses as an aid to educational and vocational placement. Arch Ophthalmol. 1974;91:20–23. doi: 10.1001/archopht.1974.03900060024006. [DOI] [PubMed] [Google Scholar]
- 9.Goto-Omoto S, Hayashi T, Gekka T, et al. Compound heterozygous CNGA3 mutations (R436W, L633P) in a Japanese patient with congenital achromatopsia. Vis Neurosci. 2006;23:395–402. doi: 10.1017/S095252380623308X. [DOI] [PubMed] [Google Scholar]
- 10.Hawes NL, Wang X, Hurd RE, et al. A Point Mutation in the Cnga3 Gene Causes Cone Photoreceptor Function Loss (cpfl5) in Mice. Paper presented at ARVO, E Abstract--4579; April 30 to May 4, 2006; Fort Lauderdale. 2006. [Google Scholar]
- 11.Kaupp UB, Seifert R. Cyclic nucleotide gated ion channels. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 12.Khan NW, Wissinger B, Kohl S, et al. CNGB3 Achromatopsia with Progressive Loss of Residual Cone Function and Impaired Rod-Mediated Function. Invest Ophthalmol Vis Sci. 2007;48:3864–3871. doi: 10.1167/iovs.06-1521. [DOI] [PubMed] [Google Scholar]
- 13.Kohl S, Marx T, Giddings I, Jägle H, et al. Total color blindness is caused by mutations in the gene encoding the alpha-subunit of the cone photoreceptor cGMP-gated cation channel. Nat Genet. 1998;19:257–259. doi: 10.1038/935. [DOI] [PubMed] [Google Scholar]
- 14.Kohl S, Baumann B, Broghammer M, et al. Mutations in the CNGB3 gene encoding the beta-subunit of the cone photoreceptor cGMP-gated channel are responsible for achromatopsia (ACHM3) linked to chromosome 8q21. Hum Mo. Genet. 2000;9:2107–2116. doi: 10.1093/hmg/9.14.2107. [DOI] [PubMed] [Google Scholar]
- 15.Kohl S, Baumann B, Rosenberg T, et al. Mutations in the cone photoreceptor G-protein alpha-subunit gene GNAT2 in patients with achromatopsia. Am J Hum Gene. 2002;71:422–425. doi: 10.1086/341835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohl S, Varsanyi B, Antunes GA, Baumann B, et al. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet. 2005;13:302–308. doi: 10.1038/sj.ejhg.5201269. [DOI] [PubMed] [Google Scholar]
- 17.Komaromy AM, Alexander JJ, Chiodo AV, et al. Long-Term Rescue of Cone Function in a Canine Model of Achromatopsia by rAAV-mediated Gene Therapy. Paper presented at ARVO, E Abstract--1131; April 27 to May 1, 2008; Fort Lauderdale. 2008. [Google Scholar]
- 18.Park WL, Sunness JS. Red contact lenses for alleviation of photophobia in patients with cone disorders. Am J Ophthalmol. 2004;137:774–775. doi: 10.1016/j.ajo.2003.09.061. [DOI] [PubMed] [Google Scholar]
- 19.Peng C, Rich ED, Varnum MD. Subunit configuration of heteromeric cone cyclic nucleotide gated channels. Neuron. 2004;42:401–410. doi: 10.1016/s0896-6273(04)00225-9. [DOI] [PubMed] [Google Scholar]
- 20.Pina AL, Baumert U, Loyer M, et al. A three base pair deletion encoding the amino acid (lysine-270) in the alpha-cone transducin gene. Mol Vis. 2004;10:265–271. [PubMed] [Google Scholar]
- 21.Schiefer U, Kurtenbach A, Braun E, et al. Centrally tinted contact lenses. A useful visual aid for patients with achromatopsia. Ger J Ophthalmol. 1995;4:52–56. [PubMed] [Google Scholar]
- 22.Schornack MM, Brown WL, Siemsen DW. The use of tinted contact lenses in the management of achromatopsia. Optometry. 2007;78:17–22. doi: 10.1016/j.optm.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Sidjanin DJ, Lowe JK, McElwee JL, et al. CNGB3 mutations establish cone degeneration as orthologous to the human achromatopsia locus ACHM3. Hum Mol Genet. 2002;11:1823–1833. doi: 10.1093/hmg/11.16.1823. [DOI] [PubMed] [Google Scholar]
- 24.Sundin OH, Yang JM, Li Y, et al. Genetic basis of total colorblindness among the Pingelapese islanders. Nat Genet. 2000;25:289–293. doi: 10.1038/77162. [DOI] [PubMed] [Google Scholar]
- 25.Wissinger B, Gamer D, Jagle H, et al. CNGA3 mutations in hereditary cone photoreceptor disorders. Am J Hum Genet. 2001;69:722–737. doi: 10.1086/323613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young RS, Krefmanm RA, Fishman GA. Visual improvements with red-tinted glasses in a patient with cone dystrophy. Arch Ophthalmol. 1982;100:268–271. doi: 10.1001/archopht.1982.01030030270007. [DOI] [PubMed] [Google Scholar]
- 27.Young RS, Krefman RA, Anderson RJ, et al. Two additional benefits of dark glasses on rod vision in patients with congenital achromatopsia. Am J Optom Physiol Opt. 1983;60:56–60. doi: 10.1097/00006324-198301000-00010. [DOI] [PubMed] [Google Scholar]