Abstract
The metabolism of α- and γ-hexabromocyclododecane (HBCD) was investigated in adult C57BL/6 female mice. α- or γ-[14C]HBCD (3 mg/kg bw) was orally administered with subsequent urine and feces collection for 4 consecutive days; a separate group of mice were dosed and sacrificed 3 hours post-exposure to investigate tissue metabolite levels. Extractable and non-extractable HBCD metabolites were quantitated in liver, blood, fat, brain, bile, urine and feces and characterized by LC/MS (ESI-). Metabolites identified were distinct between the two stereoisomers. In mice exposed to α-HBCD, four hydroxylated metabolites were detected in fecal extracts, and one of these metabolite isomers was consistently characterized in liver, brain, and adipose tissue extracts. In contrast, mice exposed to γ-HBCD contained multiple isomers of monohydroxy-pentabromocyclododecene, dihydroxy-pentabromocyclododecene, and dihydroxy-pentabromocyclododecadiene in the feces while only a single monohydroxy-pentabromocyclododecane metabolite was measured in liver and adipose tissue. Both stereoisomers were transformed to metabolites which formed covalent bonds to proteins and/or lipids in the gut as evidenced by high fecal non-extractables. Although the potential toxicity of these free and bound metabolites remains to be determined, the presence of distinct metabolic products from the two main HBCD stereoisomers should allow biomarkers to be selected that may aid in characterizing sources of HBCD exposure.
INTRODUCTION
Hexabromocyclododecane (HBCD) is a high volume brominated flame retardant (BFR) used mainly in extruded and expanded polystyrene foams for insulation in the building industry. HBCD is a cycloaliphatic BFR, making it chemically unique among other high production volume BFRs, such as aromatic polybrominated diphenyl ethers (PBDEs) and tetrabromobisphenol-A (TBBP-A). HBCD is persistent, bioaccumulative, and known to undergo long-range transport (1- 5). HBCD is an enzyme inducer, endocrine disruptor, and developmental neurotoxicant. Specifically, HBCD exposure leads to changes in thyroid hormone systems (6-7), and neurodevelopmental effects in children (8) or mice (9), alters rat hepatic gene expression profiles for cholesterol biosynthesis and lipid metabolism in a sex specific manner (10), and induces genetic recombination (11). Because of HBCD’s environmental persistence, bioaccumulative ability, demonstrated toxicity and long-range transport, it is being considered for listing by the Stockholm Convention (12).
Commercial HBCD is a mixture of 3 major stereoisomers and is synthesized by brominating cyclododecatriene. Theoretically, this process could yield 16 possible stereoisomers, i.e. enantiomers and meso forms (13). The three major stereoisomers, present as enantiomeric pairs, differ in water solubility, polarity, and dipole moment (14), which may influence environmental stability, biological uptake, and metabolism. While γ-HBCD is the most abundant stereoisomer present in commercial mixtures (75-89%), α-HBCD levels dominate in most biota, especially animals higher on the food chain, including humans. Since 1980, an increase in α-HBCD levels in biota and food has been documented, as compared to γ-HBCD, in both Europe and North America (3, 15-16).
Current understanding of HBCD metabolism is limited. In an in vitro system with a 1:1:1 mixture of the three HBCD stereoisomers, no metabolism of α-HBCD was observed, while γ-HBCD and β-HBCD were extensively metabolized to monohydroxylated metabolites (17). Esslinger et al. (18) measured the in vitro degradation half-lives and metabolism of all six major enantiomers in the commercial mixture. At the conclusion of incubation with rat liver microsomes, (-)-α-HBCD and (+)-γ-HBCD were significantly enriched compared to their enantiomeric pairs, suggesting these two enantiomers are more resistant to metabolism. A distinct metabolite pattern of mono- and dihydroxylated products was generated from each enantiomer. These patterns enabled the researchers to propose sources of HBCDs after examining the HBCD contents of wildlife tissues. In rats exposed to a high, chronic dose of commercial HBCD oxidation and reductive debromination were common metabolic routes for HBCD, although it was not possible to determine whether individual stereoisomers underwent the same metabolism (19).
Recent work from our laboratory has shown that the stereoisomeric shift observed with the HBCD commercial product (and environmental samples) to biota may be due to both in vivo stereoisomerization of γ-HBCD (to α-HBCD), and to faster metabolism of γ-HBCD (20-21). The aim of the present study was to isolate and identify metabolites in tissues and excreta from female C57BL/6 mice after exposure to a single, oral dose of either α- or γ-[14C]HBCD. We hope to obtain a better understanding of the disposition and fate of these BFRs in biological systems on an isomer-specific basis.
MATERIALS AND METHODS
Dose, animals, and treatments
Details of the dosing materials (α- and γ-[14C]HBCD), animals (female C57BL/6 mice), and treatments (single, oral dose at 3 mg/kg) have been published previously (20-21).
Metabolite isolation from tissues
All tissue samples were taken from mice sacrificed 3-h after a [14C]HBCD exposure. Liver samples were weighed and homogenized in 0.5 mL water with a mortar and pestle. An equal volume of hexane was added, vortexed, and the hexane decanted. Extractions were repeated with ethyl acetate and then methanol. The residual tissue was combusted in a tissue oxidizer (Packard Model 307, Meridan, CT) to determine non-extractable metabolites. Each extract was evaporated to dryness with nitrogen, reconstituted with hexane, and delipidated on a sulfuric acid:silica column (0.7:1, w/w, 2.0 g). Samples were eluted with hexane, then 1:1 hexane:methylene chloride, and evaporated to dryness with nitrogen, before submission for LC/MS (ESI-) analysis (methods below).
Adipose tissue samples and brains were separately pooled and homogenized in 5 mL of water with a mortar and pestle, and transferred to centrifuge tubes; 4 mL hexane:acetone (1:3.5) was added, vortexed, and decanted. The procedure was repeated with 4 mL hexane:ether (9:1) and extracts were combined with the hexane/acetone layer. The combined extracts were evaporated to dryness, and reconstituted in methylene chloride. BioBeads SX-3 GPC packing (100-200 mesh, BioRad Laboratories, Inc.; Hercules, CA) was introduced into a 5 ¾” Pasteur pipet, equilibrated with methylene chloride, and the adipose tissue or brain extract was applied. Metabolites were eluted with 10 mL methylene chloride which was evaporated to dryness with nitrogen, and the residue was reconstituted with hexane. Lipids were removed, as above, before submission for LC/MS (ESI-) analyses.
Metabolite isolation from feces
Fecal and urine analyses were conducted in samples collected from mice that were euthanized after a 96-hour collection periods. Daily fecal collections (0-24, 24-48, 48-72, and 72-96 h) were air-dried, pulverized with a mortar and pestle, and aliquots combusted for determination of total radioactivity. The remaining feces were pooled by collection day, and then sequentially extracted 3 times with 100 mL of hexane, ethyl acetate, and methanol. The radioactivity in each extract was determined by liquid scintillation counting (LSC). Extracts were evaporated to dryness, reconstituted in 1 mL hexane, and applied to silica gel columns constructed in disposable Pasteur pipets. Columns were eluted with a gradient of toluene in hexane in 2% intervals (from 0-20% toluene). Fractions containing radioactivity were analyzed by LC/MS (ESI-).
Urine
Protein binding assay
To examine whether the urinary metabolites were protein-associated, 0-24 h urine from α- and γ-[14C]HBCD treated mice were chromatographed by gel filtration on Sephadex G-75 columns (Pharmacia Biotech, Piscataway, NJ; 4.5 × 90cm; eluted with 0.05 M phosphate buffer, pH 7.2), and fractions were assayed for radioactivity and protein content, as described previously (22).
Assay for volatiles
Aliquots of 0-24 h urine (2 mL) were transferred to 20 mL glass vials, which were constructed with an inlet and outlet in each cap. The inlet was attached to a compressed air tank, while the outlet was attached to a silica gel SepPak (Waters, Milford, MA). Following a 2 h purge with air, the trapped volatiles in the SepPak were eluted with acetone, while the residual radioactivity in the vials were reconstituted with water, and both portions were assayed for radioactivity by LSS, and then submitted for LC/MS (ESI-) analyses.
Phase II metabolism
In order to discern whether urinary metabolites were composed of negatively- or positively-charged conjugates, urine (2 mL) was evaporated to dryness on a rotary evaporative centrifuge (Savant, Farmington, NY) and either (a) acidified to pH 2.0 with concentrated hydrochloric acid or (b) adjusted to pH 10.2 with 0.1 M sodium bicarbonate, then fractionated using reverse-phase solid phase extraction (SPE) cartridges (C-18 SepPak, Waters). The void volume was collected, and the cartridges were washed sequentially with distilled water (4 mL) and methanol (4 mL). Fractions were assayed by LSC. Additionally, two pooled 0-24 h urine aliquots were transferred to 4 mL vials, dried by centrifugal rotary evaporation, and hydrolyzed with either β-glucuronidase (E. coli, Sigma, St. Louis, MO) or aryl sulfatase (Type V from Patella vulgaris; Sigma, St. Louis, MO) according to manufacturer’s instructions in order to check for the presence of glucuronide or sulfate conjugates, respectively.
Serum and bile
Blood serum was diluted 1:1 with distilled water, then extracted twice with 1:1 hexane:ethyl acetate. The serum extract or the unextracted bile was chromatographed by silica gel thin layer chromatography (TLC) and/or high performance liquid chromatography (HPLC; see below).
Metabolite characterization
HPLC
Urine, bile and serum samples were injected on an HPLC system (Gilson; Middleton, WI) equipped with a C18 column (8 × 100 mm; Delta-Pak, Waters, Milford, MA), and eluted isocratically with 70:30 methanol:water for 20 min at 1 mL/min. Dual detectors were a UV (Model 117, Gilson; 254 nm) and a flow scintillation analyzer (Radiomatic 150TR, Perkin-Elmer, Shelton, CT). Radioactive peaks were collected and assayed by LSC. [14C]-α- and γ-HBCD were used as standards.
LC/MS (ESI-)
A Q-TOF Ultima API-US, Quadrupole-Time of Flight mass spectrometer (Waters, Milford, MA) equipped with an electrospray ionization source was used to characterize metabolites in the negative-ion mode. The liquid chromatography (LC) system was an Alliance 2695 Separation Model (Waters) with a Symmetry C18 column (3.5μm, 2.1 × 100 mm) and guard column (2.1 × 10 mm; Waters). The isocratic mobile phase consisted of 25% 10 mM ammonium acetate and 75% 10 mM ammonium acetate in 80:20 methanol:acetonitrile at a flow rate of 0.3 mL/min. Full scan mass spectra were obtained using the ChroTools function within MassLynx™ software (Waters) to locate putative metabolites by their [M-H]- brominated cluster. Metabolites searched for are listed in Table 1. Unlabeled α- and γ-HBCD standards were purchased from Wellington Laboratories (Guelph, ON) and used for daily instrument calibration.
Table 1.
Full scan mass spectra obtained using the ChroTools function within MassLynx™ software (Waters) to locate putative metabolites by their [M-H]- brominated cluster. The ions represent possible Phase I rat metabolites of α- and/or γ-[14C]HBCD and include all ions present in the putative brominated M-H cluster.
| Masses | Interpretation |
|---|---|
| 78.9 + 80.9 | Br |
| 554.6 + 556.6 + 558.6 + 560.6 + 562.6 + 564.6 | PBCDe |
| 556.6 + 558.6 + 560.6 + 562.6 + 564.6 + 566.6 | PBCD |
| 570.6 + 572.6 + 574.6 + 576.6 + 578.6 + 580.6 | OH-PBCDe |
| 572.6 + 574.6 + 576.6 + 578.6 + 580.6 + 582.6 | OH-PBCD |
| 586.6 + 588.6 + 590.6 + 592.6 + 594.6 + 596.6 | diOH-PBCDe |
| 588.6 + 590.6 + 592.6 + 594.6 + 596.6 + 598.6 | diOH-PBCD |
| 634.6 + 636.6 + 638.6 + 640.6 + 642.6 + 644.6 + 646.6 | HBCD |
| 650.6 + 652.6 + 654.6 + 656.6 + 658.6 + 660.6 + 662.6 | OH-HBCD |
| 668.6 + 670.6 + 672.6 + 674.6 + 676.6 + 678.6 + 680.6 | diOH-HBCD |
TLC
Untreated urine, bile, and serum, α-, β-, and γ-[14C]HBCD standards, and extracts of feces and tissues were spotted on silica gel TLC plates (5×20 cm, 250μm mesh, Analtech, Newark, DE). Plates were developed with mobile phase 1 for non-polar metabolites, i.e. 1:1 hexane:methylene chloride, or mobile phase 2 for polar metabolites, i.e. 6:2:3 n-butanol:CH3CN:H2O. Radiochemical detection was accomplished using a System 2000 Imaging Scanner (Bioscan, Washington, DC) with a typical scan time of 30 min. For qualitative LC/MS (ESI-) analyses, radioactive bands were scraped from the TLC plate and the metabolites extracted with MeOH.
RESULTS
Urine, serum, and bile
As previously reported, no parent α- or γ-[14C]HBCD was detected in urine, serum, or bile by normal phase TLC (20- 21). The entire radioactivity in these compartments was very polar, displaying an Rf=0.00 with the mobile phase 1 (Fig. 1B for γ-HBCD urine). However, using the more polar mobile phase 2, urine radioactivity separated into three broad bands with retention indices of 0.62, 0.73 and 0.84 (Fig. 1C). Hydrolysis of urine from both α- and γ-HBCD treated mice with β-glucuronidase or aryl sulfatase did not change the migration of radioactivity using TLC mobile phase 1 indicating the absence of glucuronide or sulfate conjugates (data not shown). No evidence of protein binding was observed with urine from either stereoisomer treatment (Figs. 2A and 2B). Acidifying the 0-24 hr urine samples and partitioning by reversed phase SPE resulted in approximately 70% of the [14C] eluting in the void volume, 10% eluting with additional water, and 20% eluting with methanol, which suggested the presence of some negatively-charged functional groups, e.g. carboxyls. In contrast, reversed phase SPE of alkaline urine (pH 10.2) resulted in more than 65% of the applied [14C] being sorbed to the column. The sorbed radiocarbon could only be eluted with methanol, suggestive of basic functional group(s), possibly amino(s).
Figure 1.
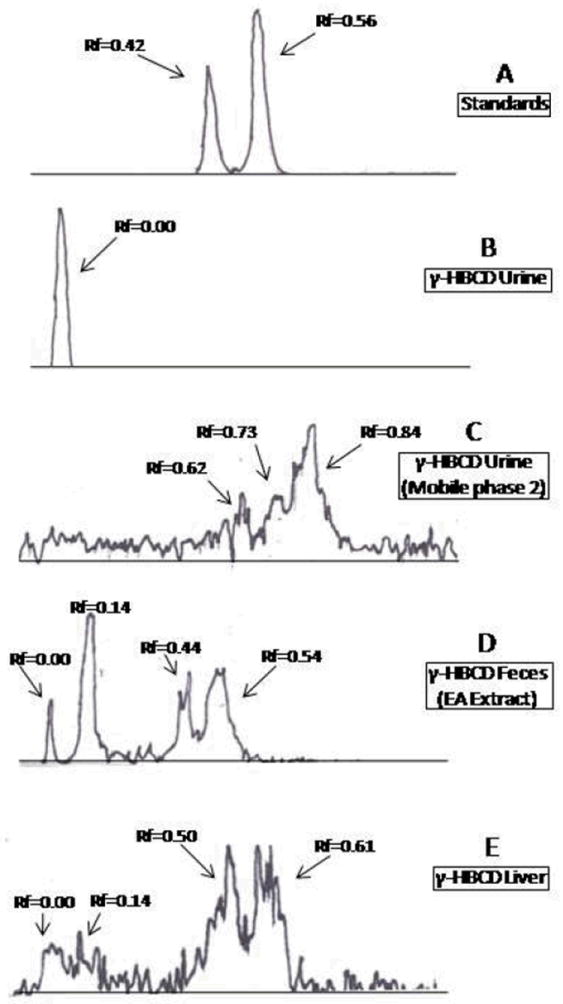
Silica gel TLC radiochromatograms of (A) β- α- and γ-[14C]HBCD standards (α- and γ-HBCD co-elute at Rf=0.56); (b) 0-24 h urine from γ-[14C]HBCD treated mice; (C) 0-24 h urine from γ-[14C]HBCD treated mice; (D) γ-[14C]HBCD treated feces (0-24 h, ethyl acetate extract), and (E) γ-[14C]HBCD treated liver extract from mice. Chromatograms were developed in mobile phase 1, except (C) which was developed in mobile phase 2 (see Material and Methods).
Figure 2.
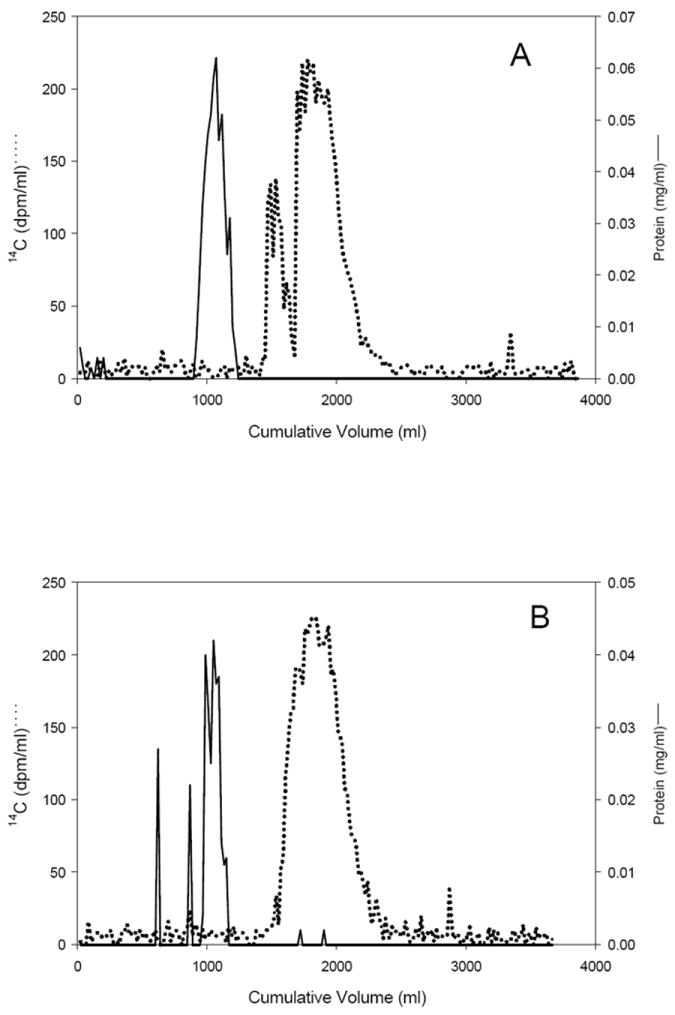
Sephadex G-75 gel filtration chromatogram of urine (0-24 h) from female mice exposed to (A) α-[14C ]HBCD or (B) γ-[14C]HBCD. Fractions off GPC column were analyzed for radioactivity by LSC and protein content by the Bradford method.
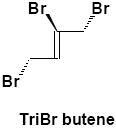
Negative-ion LC/MS of untreated urine from α-HBCD exposed mice, resulted in two unambiguous brominated ion clusters having molecular ions of [M-H]- 621.6 (3 Br) and of 697.5 (4 Br) (Table 2). The clusters differed by one bromine atom (m/z 78), and their odd-mass molecular ions, i.e. MW 623 and 699 (rounded to nearest whole-number), respectively, suggests an odd number of nitrogen atoms. Mercapturic acid pathway intermediates that could be fit to the observed molecular ions were tri- and tetrabromo hexene glutathiones, respectively (metabolites α-M2 and α-M3; Fig. 3A). Urine from γ-HBCD-treated mice yielded mass spectra that were consistent with carboxylic acid moieties, i.e. [M-H]- 696.8 (6 Br; a putative hexabromo dodecanedioic acid present as three isomers; γ-M8), and M-H 388.8 (3 Br; a possible tribromononeneoic acid; γ-M10). Another mass spectrum was consistent with tetrabromo pentanoic acid (M-H 412.8; 4 Br), but in the absence of bromine scrambling/rearrangement it was not obvious how it could have formed. Roughly 29% of the urinary radioactivity (0-24 h) was volatilized. A mass spectrum consistent with tribromobutene ([M-H]- 284.9; 3 Br; γ-M11) was the only interpretable brominated cluster detected in the volatile portion of the urine of γ-HBCD treated mice, although the presence of other compounds could not be ruled out.
Table 2.
Structures of fecal metabolites from mice exposed to α-[14C]HBCD or γ-[14C]HBCD characterizied by LC/MS (ESI-).
| Metabolites of α-HBCD | Compartment | Abbreviation | # Isomers | M-H (nominal) |
|---|---|---|---|---|
|
| ||||
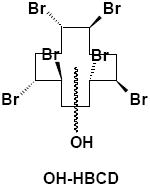
|
Liver, Brain, Adipose | α-M1 | 1 | 650.6 |
| Feces | 4 | |||
|
| ||||
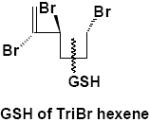
|
Urine | α-M2 | 1 | 621.6 |
|
| ||||
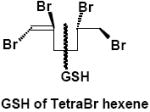
|
Urine | α-M3 | 1 | 697.5 |
|
| ||||
| Metabolites of γ-HBCD | ||||
|
| ||||
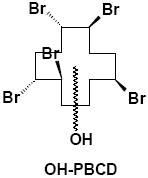
|
Liver, Adipose | γ-M4 | 1 | 572.6 |
|
| ||||
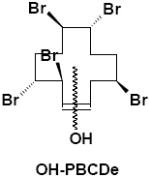
|
Feces | γ-M5 | 4 | 570.6 |
|
| ||||
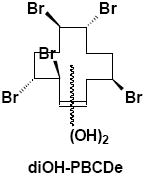
|
Feces | γ-M6 | 3 | 588.6 |
|
| ||||
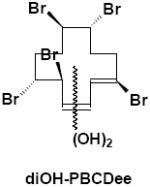
|
Feces | γ-M7 | 2 | 586.6 |
|
| ||||
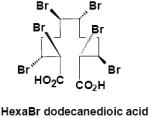
|
Urine | γ-M8 | 3 | 696.8 |
|
| ||||
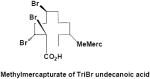
|
Feces | γ-M9 | 1 | 563.3 |
|
| ||||
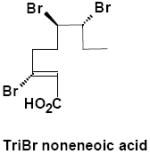
|
Urine | γ-M10 | 1 | 388.8 |
|
| ||||
|
Urine (volatile) | γ-M11 | 1 | 284.9 |
Figure 3.
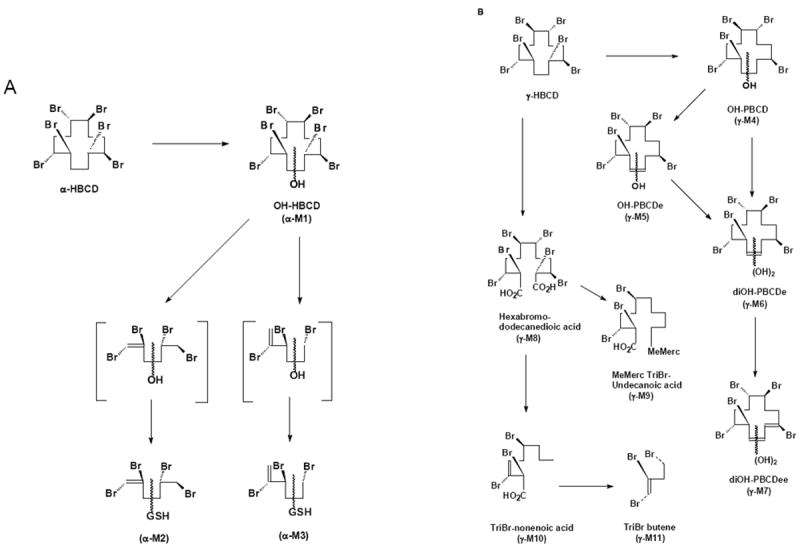
Proposed metabolic schemes for metabolites characterized in the feces, urine and selected tissues after exposure to (A) α-[14C]HBCD and (B) γ-[14C]HBCD as single, oral doses to female C57BL/6 mice.
Feces
Hexane extracts of feces generally consisted of either the parent compound or stereoisomerized parent, as reported previously (20-21). Ethyl acetate extracts of feces contained polar bands on TLC (Rfs=0.00 and 0.14; Fig. 1D). Further purification of the Rf=0.14 band from mice treated with α-HBCD was performed using gradient silica gel column chromatography and subsequent LC/MS (ESI-) analyses. This resulted in the LC-MS identification of four mono-hydroxylated HBCD isomers (OH-HBCD; [M-H]- 650.6, 6 Br; α-M1; Table 2). LC/MS (ESI-) analyses of the Rf=0.14 band from mice exposed to the γ-HBCD indicated multiple isomers of three different oxidized HBCD metabolites. Four mono-hydroxylated pentabromododecene isomers were characterized (OH-PBCDe; [M-H]- 570.6; 5 Br; γ-M5; Table 2), and also three dihydroxylated pentabromododecene isomers (diOH-PBCDe; [M-H]- 586.6, 5 Br; γ-M6), and two dihydroxylated pentabromododecadiene isomers (diOH-PBCDee; [M-H]- 584.6, 5 Br; γ-M7).
The methanolic fecal extracts were composed entirely of a very polar metabolite(s), having an Rf=0.00 with TLC mobile phase 1 (data not shown). With mobile phase 2, the radioactivity migrated as three broad bands at Rfs=0.16, 0.46 and 0.71 (data not shown). Mass spectral analyses of the methanolic fecal extracts from γ-HBCD treated feces yielded an [M-H]- 563.3 (3 Br), consistent with a methylmercapturic acid conjugate of tribromo- undecanoic acid (γ-M9). Reasonable structures could not be postulated for the mass spectra of methanolic extracts from feces of α-HBCD treated mice. Non-extractable radioactivity comprised between 29-56% of the fecal radioactivity at all time points regardless of HBCD treatment (Table 3).
Table 3.
Summary of metabolite profiles (up to 96 h) in selected female mouse tissues and excreta after receiving 3 mg/kg of a single oral dose of either α- or γ-[14C]HBCD in corn oil.
| Matrix | Time (h) | %Dose | %Dose as Free Metabolites | %Dose as Non-extractables | Total Metabolites (%Dose) |
|---|---|---|---|---|---|
| α-HBCD | |||||
| Urine | 0-24 | 15 | 15 | --- | 15 |
| 24-48 | 2 | 2 | --- | 2 | |
| 48-72 | 1 | 1 | --- | 1 | |
| 72-96 | 0.5 | 0.5 | --- | 0.5 | |
| Feces | 0-24 | 42 | 14.3 | 12.2 | 26.5 |
| 24-48 | 3 | 0.9 | 1.7 | 2.2 | |
| 48-72 | 2 | 1.0 | 0.9 | 1.9 | |
| 72-96 | 1 | 0.6 | 0.3 | 0.9 | |
| Adipose | 3 | 0.006 | 0.0007 | 0 | 0.0007 |
| Brain | 3 | 1.2 | 0.68 | 0 | 0.68 |
| Liver | 3 | 2.7 | 0.5 | 0.6 | 1.1 |
| Serum | 3 | 1.3 | 1.3 | --- | 1.3 |
| γ-HBCD | |||||
| Urine | 0-24 | 25 | 25 | --- | 25 |
| 24-48 | 2 | 2 | --- | 2 | |
| 48-72 | 1 | 1 | --- | 1 | |
| 72-96 | 0.5 | 0.5 | --- | 0.5 | |
| Feces | 0-24 | 52 | 16.4 | 27.6 | 44.0 |
| 24-48 | 6 | 2.0 | 2.7 | 4.7 | |
| 48-72 | 1 | 0.01 | 0.03 | 0.04 | |
| 72-96 | 0.5 | 0.3 | 0.02 | 0.3 | |
| Adipose | 3 | 0.008 | 0.008 | 0 | 0.002 |
| Brain | 3 | 0.035 | 0.035 | 0 | 0.035 |
| Liver | 3 | 0.24 | 0.18 | 0.04 | 0.22 |
| Serum | 3 | 0.08 | 0.08 | --- | 0.08 |
Tissues
Hexane extracts of liver from α- or γ-[14C]HBCD treated mice contained parent or stereoisomerized HBCD compounds, as reported previously (20-21). Additionally, polar radioactive TLC bands were observed at Rf=0.14 and Rf=0.00 (Fig. 1E) in liver extracts of mice receiving either HBCD treatment, which suggested hydroxylation, and a highly polar metabolite(s), repectively. The Rf=0.14 band from liver extracts of α-HBCD treated mice contained a single monohydroxy-HBCD metabolite ([M-H]- 650.6; Br6; α-M1; Table 2), while the same band from γ-HBCD treated mice contained a single monohydroxy-PBCD metabolite ([M-H]- 572.8; 5 Br; γ-M4; Table 2).
Similar to liver, a single monohydroxylated-HBCD metabolite ([M-H]- 650.6; Br6; α-M1) was detected in adipose tissue extracts from α-[14C]HBCD dosed mice. A monohydroxylated-PBCD metabolite ([M-H]- 572.8; Br5; γ-M4) was characterized in adipose tissue extracts from γ-[14C]HBCD dosed mice.
The total radioactive residues in the brains of α-HBCD dosed mice were nearly 35-fold higher than for γ-HBCD dosed mice (Table 3). A single monohydroxylated HBCD metabolite ([M-H]- 650.6; Br6; α-M1) was detected in brain extracts obtained from α-[14C]HBCD dosed mice. However, silica gel TLC chromatography of brain extracts from γ-[14C]HBCD dosed mice provided no indication of oxidized products (data not shown).
A summary of metabolite compositions in tissues, sera and excreta for α-[14C]HBCD and γ-[14C]HBCD treated mice is shown in Table 3. Stereoisomerization of γ- to α- and β-HBCD, reported previously (20), is assumed to be the result of metabolic activity towards HBCD, therefore, stereoisomerized products are included in the “free metabolites” in the table.
DISCUSSION
The number of reports on HBCD metabolism is growing, but the earliest ones were not peer-reviewed, utilized mixtures rather than pure stereoisomers, and were often contradictory. For instance, both Yu and Atallah (22) and Dean and Leong (23) reported that in rats HBCD absorption was facile, half-lives were short (2 h), and elimination via feces was about 70% of the dose while elimination via urine was 16%. Four unidentified metabolites were reported by Yu and Atallah (22), however, another report did not mention metabolites (24), detected only about 30% elimination in feces per day, and no elimination via urine.
The purpose of the present study was to reconcile the wide disparity in pharmacokinetics between these earlier data, and to identify the metabolites present in mouse tissues and excreta following a single oral dose. Identifying and quantitating individual stereoisomers in sensitive tissues, like the brain, will make interpretation of future toxicity research on HBCD isomers more facile, and will permit initial toxicity assessments of metabolites to be conducted.
As will be discussed below, not only were differing metabolic routes identified by the present research for α-HBCD and γ-HBCD, but the extent of γ-HBCD metabolism was greater than α-HBCD, as determined by quantitating the metabolites detected in the urine, feces, liver and serum. Most of the quantitative difference occurred in the first 24 h, where the concentration of urinary metabolites favored γ-HBCD over α-HBCD, i.e. 25 vs. 15% of dose, and in feces where the difference was even greater, i.e. 44.0 vs. 26.5% of dose, respectively (Table 3). The fecal totals are composed of both free/extractable and non-extractable metabolites, and within these two compartments the largest contributor was the non-extractables. The source of non-extractables was assumed to be from the enzymatic activation of the HBCD molecule to reactive intermediates, followed by covalent binding to proteins and/or lipids. The present data, therefore, support that rapid metabolism and elimination of γ-HBCD, compared to α-HBCD, occurs and are in agreement with the data of several in vitro (17-18, 25) and in vivo (20-21, 26) studies.
Urine, serum, bile, and methanolic extracts of feces from α-HBCD mice all contained highly polar metabolite(s) as evidenced by TLC radioprofiles. Enzymatic hydrolysis of urine resulted in essentially identical TLC radiochromatograms, indicating that the urinary metabolites were not glucuronide or sulfate conjugates. Mass spectral data of urine metabolites suggested that α- and γ-HBCD were subject to different metabolic fates. In the case of α-HBCD, the metabolites were glutathione conjugates of a symmetrically cleaved HBCD, which depending on cleavage site, could have yielded either a tri- or a tetra-brominated C6 hydrocarbons (α-M2 and α-M3; Table 2). Glutathione conjugates may be subject to further metabolism along the mercapturic acid pathway to yield mercapturic acid, cysteine, thiol, and methyl sulfone metabolites; although these secondary metabolites were not identified in this study. No binding to urinary proteins was observed with the HBCD-derived radioactivity for either stereoisomer (Figs. 2A and 2B). We have previously reported on the binding of other POPs to urinary proteins (27-29), and it has been hypothesized that this binding facilitates elimination of lipophilic xenobiotics. The data demonstrated that urinary HBCD metabolites in urine were sufficiently polar to be eliminated without the aid of carrier proteins. To our knowledge, this is the first report to identify such highly polar metabolites after exposure to HBCD. The metabolism of α-HBCD resulted in only monohydroxylated, hexabrominated metabolites (α-M1; Table 2) being excreted in mouse feces.
In contrast, γ-HBCD underwent ring opening and oxidation to a dicarboxylic acid (γ-M8), which presumably is decarboxylated and enters β-oxidation as an odd-numbered carboxylic acid (γ-M10; Table 2). These reactions have been demonstrated with cyclic alkanes in bacteria, which were oxidized to alcohols, then ketones, followed by cleavage via 2-oxepanone formation, and finally to dicarboxylic acids (30-32). With γ-HBCD, it would also be reasonable to invoke β-oxidation/decarboxylation as a biological pathway that could yield shorter, volatile metabolites observed in the urine, such as tribromobutene (γ-M11; Table 2; Fig. 3B). Fecal metabolite data also suggested that the metabolic pathways of α- and γ-HBCD are distinctly different. In contrast, hexabrominated metabolites of γ-HBCD were not detected in fecal extracts, but debrominated and oxidized metabolites (γ-M4 through γ-M7; Table 2) were present.
Apart from the previously mentioned, non-peer-reviewed reports (22-24), to our knowledge only one other in vitro study with HBCD isomers is available in the literature. Brandsma et al. (19) exposed rats to 30 and 100 mg/kg for 28 d with the HBCD technical mixture (containing α-, β-, and γ-HBCD isomers), and found four different groups of hydroxylated metabolites in tissues. These were (1) monohydroxy HBCD (OH-HBCD), (2) mono-debrominated, dehydrogenated metabolites (PBCDe), (3) di-debrominated, dehydrogenated metabolites (TBCDe), and (4) dihydroxy HBCD (diOH-HBCD). A fifth metabolite group, i.e. diOH-PBCDe, was detected under GC/MS analysis, but could not be confirmed by LC/MS (ESI-) methods. This latter metabolite group was identified in the present mouse study, but only in γ-HBCD dosed mice (Table 2). If one assumes that mice and rats are analogous murine species for HBCD metabolism, and the current results are applied to interpret the technical mixture dosing results, then it can be concluded that metabolite categories (2), (3) and (4) from Brandsma et al. (19) were the result of γ-HBCD metabolism, while category (1) was probably the result of α-HBCD metabolism.
Consistent with these early studies in rodents, in vitro investigations of HBCD stereoisomers have provided evidence that differential metabolism occurs among the various isomers. Zegers et al. (17) demonstrated using a 1:1:1 mixture of α-, β-, and γ-HBCD that α-HBCD was virtually resistant to degradation in an induced rat liver microsomal system, while both β- and γ-HBCD were significantly degraded (>60% after 90 min). Biotransformation was mediated by cytochrome P450s since no metabolism was observed in the absence of NADPH. Using hepatic harbor seal microsomes, the same authors identified three brominated metabolites in β-HBCD assays and two in γ-HBCD assays. Two of the three β-HBCD and both γ-HBCD metabolites were monohydroxylated HBCDs (17). Huhtala et al. (25) used induced trout liver microsomes to conclude that α-HBCD was less extensively metabolized than γ-HBCD, but that β-HBCD was the most readily metabolized isomer. With no attempt to identity the metabolites, the authors claimed that hydroxylated HBCD metabolites were produced (17). In contrast, Esslinger et al. (18) reported that there was not a large difference in degradation rates between α- and γ-HBCD in induced rat liver microsomes. They did, however, demonstrate that β-HBCD enantiomers had the highest metabolism. Again, α- and γ-HBCD showed distinct patterns of metabolites that were either monohydroxylated (three metabolites and five metabolites, respectively) or dihydroxylated (possibly 6 metabolites for each enantiomer). No metabolites of β-HBCD were detected and no debromination was detected for any of the isomers in the in vitro system.
The mechanism of debromination can only be speculated upon at this point, but whereas cytochrome P450s are known to be involved in the oxidative metabolism of BFRs like HBCD (17), they do not catalyze reductive debromination of PBDEs (33). Glutathione-S-transferases (GSTs) have been reported to catalyze the reductive dechlorination of DDT (34), but not the reductive debromination of PBDEs (35). Comparability of these reports to the present study is uncertain since the POPs previously investigated (PCDDs, PBDEs and DDT) were aromatic, while HBCD is aliphatic. However, strong evidence has recently been obtained for the involvement of deiodinases in debromination reactions (33, 36). Future research would need to be conducted to clarify which enzyme systems are responsible for these metabolic events in mice, therefore, only putative metabolic schemes can be suggested at the present time to explain the metabolites identified in mice from both α- and γ-HBCD treatments (Fig. 3A and 3B).
Since no hexabrominated forms of γ-HBCD could be detected, it is reasonable to assume that a tightly-coupled deiodinase and cytochrome P450 system exists where γ-HBCD is a preferred substrate for the debromination by deiodinase(s) as compared to α-HBCD. Initial oxidation of γ-HBCD could precede debromination to OH-γ-PBCD (γ-M4), or reductive debromination to OH-γ-PBCDe (γ-M5). Further oxidation and dehydrogenation would yield diOH-γ-PBCDe (γ-M6) or di-OH-γ-PBCDee, respectively (γ-M7; Fig. 3B).
Because of the distinct pattern of metabolites for each HBCD stereoisomer, it was a research goal that environmental exposures to particular HBCD stereoisomers could be unequivocally determined using either a unique marker metabolite or by analyzing an isomer-specific pattern of metabolites in biological samples. This approach, at the level of individual HBCD enantiomers, was used successfully to identify exposure patterns of wild pollack, and mackerel and gulls (18). Patterns of monohydroxylated metabolites in pollack, mackerel livers, and gull eggs were dominated by α-HBCD metabolites (identified from rat microsomal incubations), suggesting that fish and gulls may actually be preferentially exposed to α-HBCD rather than γ-HBCD. These data then suggest that abiotic mechanisms may be operating in nature that could lead to either isomer-selective weathering or the putative stereoisomerization of the technical mixture of HBCD, such as photolysis (37) or elevated temperatures (38), which have been shown to contribute to stereoisomerization in the laboratory. The metabolites identified in the present study may also serve as biological markers of exposure. Biomarkers could simply reside in the degree of bromination of fecal monohydroxylated metabolites, where hexabromination or pentabromination would distinguish α-HBCD exposure form γ-HBCD (α-M1 vs. γ-M4; Table 2). Urinary biomarkers for α-HBCD exposure could be the brominated hexene glutathiones (α-M2 and α-M3, Table 2), and either the brominated noneneoic acid or dodecanedioc acid for γ-HBCD exposure (γ-M8 and γ-M10, respectively; Table 2).
Acknowledgments
We would like to thank Colleen Pfaff, Jason Holthusen, Sara Lupton, and Barbara Magelky for technical assistance. This work is funded in part by a cooperative agreement between the University of North Carolina in Chapel Hill and the Environmental Protection Agency CR 833237 predoctoral training grants.
Footnotes
Publisher's Disclaimer: This manuscript does not reflect USEPA, NIH or USDA policy. The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the United States Department of Agriculture, the Agricultural Research Service, or the Food Safety and Inspection Service of any product or service to the exclusion of others that may be suitable. USDA is an equal opportunity provider and employer.
Contributor Information
Heldur Hakk, Email: heldur.hakk@ars.usda.gov.
Janice Huwe, Email: janice.huwe@ars.usda.gov.
Janet Diliberto, Email: jjdiliberto@yahoo.com.
Linda S. Birnbaum, Email: birnbaumls@niehs.nih.gov.
References
- 1.Covaci A, Gerecke AC, Law RJ, Voorspoels S, Kohler M, Heeb NV, Leslie H, Alchin CR, de Boer J. Hexabromocyclododecanes (HBCDs) in the environment and humans: A review. Environ Sci Technol. 2006;40:3679–3688. doi: 10.1021/es0602492. [DOI] [PubMed] [Google Scholar]
- 2.Janák K, Covaci A, Voorspoels S, Becher G. Hexabromocyclododecane in marine species from the western Scheldt estuary: diastereomer- and enantiomer-specific accumulation. Environ Sci Technol. 2005;39:1987–1994. doi: 10.1021/es0484909. [DOI] [PubMed] [Google Scholar]
- 3.Tomy GT, Pleskach K, Oswald T, Halldorson T, Helm PA, MacInnes G, Marvin CH. Enantioselective bioaccumulation of hexabromocyclododecane and congener-specific accumulation of brominated diphenyl ethers in an eastern Canadian Arctic marine food web. Environ Sci Technol. 2008;42:3634–3639. doi: 10.1021/es703083z. [DOI] [PubMed] [Google Scholar]
- 4.Hiebl J, Vetter W. Detection of hexabromocyclododecane and its metabolite pentabromocyclododecane in chicken egg and fish from the official food control. J Agr Food Chem. 2007;55:3319–3324. doi: 10.1021/jf063428b. [DOI] [PubMed] [Google Scholar]
- 5.Arnot J, McCarty L, Armitage J, Toose-Reid L, Wania F, Cousins I. An evaluation of hexabromocyclododecane (HBCD) for Persistent Organic Pollutant (POP) properties and the potential for adverse effects in the environment. Submitted to European Brominated Flame Retardant Industry Panel (EBFRIP) 2009 May 26 [Google Scholar]
- 6.Palace V, Park B, Pleskach K, Gemmill B, Tomy G. Altered thyroxine metabolism in rainbow trout (Oncorhynchus mykiss) exposed to hexabromocyclododecane (HBCD) Chemosphere. 2010;80:165–9. doi: 10.1016/j.chemosphere.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Saegusa Y, Fujimoto H, Woo GH, Inoue K, Takahashi M, Mitsumori K, Hirose M, Nishikawa A, Shibutani M. Developmental toxicity of brominated flame retardants, tetrabromobisphenol A and 1,2,5,6,9,10 hexabromocyclododecane, in rat offspring after maternal exposure from mid-gestation through lactation. Reprod Toxicol. 2009;28:456–67. doi: 10.1016/j.reprotox.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Roze E, Meijer L, Bakker A, Van Braeckel K, Sauer PJJ, Bos AF. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect. 2009;117:1953–1958. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriksson P, Fischer C, Wallin M, Jakobsson E, Fredriksson A. Impaired behaviour, learning and memory, in adult mice neonatally exposed to hexabromocyclododecane (HBCDD) Environ. Toxicol Pharmacol. 2006;21:317–322. doi: 10.1016/j.etap.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Cantón RF, Peijnenburg AACM, Hoogenboom RLAP, Piersma AH, van den Ven LTM, van den Berg M, Heneweer M. Subacute effects of hexabromocyclododecan (HBCD) on hepatic gene expression profiles in rats. Technol Appl Pharmacol. 2008;231:267–272. doi: 10.1016/j.taap.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Helleday T, Tuominen KL, Bergman Å, Jenssen D. Brominated flame retardants induce intragenic recombination in mammalian cells. Mutat Res -Genet Toxicol Environ Mutagen. 1999;439:137–147. doi: 10.1016/s1383-5718(98)00186-7. [DOI] [PubMed] [Google Scholar]
- 12.Stockholm Convention on Persistent Organic Pollutants website. [11/29/11]; http://chm.pops.int/Home/tabid/2121/mctl/ViewDetails/EventModID/1007/EventID/225/xmid/6921/Default.aspx#.
- 13.Becher G. The stereochemistry of 1,2,5,6,9,10-hexabromocyclododecane and its graphic representation. Chemosphere. 2005;58:989–999. doi: 10.1016/j.chemosphere.2004.09.071. [DOI] [PubMed] [Google Scholar]
- 14.Kuromochi H, Suzuki S, Kawamoto K, Osako M, Sakai S. Measurements of water solubility and 1-octanol/water partition coefficient of three hexabromocyclododecane diastereoisomers. BFR 2010 Proceedings; April 7-9; Kyoto, Japan. 2010. pp. 102–105. [Google Scholar]
- 15.Morris S, Allchin CR, Zegers BN, Haftka JJH, Boon JP, Belpaire C, Leonards PEG, Van Leeuwen SPJ, de Boer J. Distribution and fate of HBCD and TBBPA brominated flame retardants in North Sea estuaries and aquatic food webs. Environ Sci Technol. 38:5497–5504. doi: 10.1021/es049640i. [DOI] [PubMed] [Google Scholar]
- 16.Tomy GT, Budakowski W, Halldorson T, Whittle DM, Keir MJ, Marvin C, MacInnes G, Alaee M. Biomagnification of the α- and γ-isomers of hexabromocyclododecane (HBCD) in a Lake Ontario food web. Environ Sci Technol. 2004;38:2298–2303. doi: 10.1021/es034968h. [DOI] [PubMed] [Google Scholar]
- 17.Zegers BN, Mets A, Van Bommel R, Minkenberg C, Hamers T, Kamstra JH, Pierce GJ, Boon JP. Levels of hexabromocyclododecane in harbor porpoises and common dolphins from western European seas, with evidence for stereoisomer-specific biotransformation by cytochrome P450. Environ Sci Technol. 2005;39:2095–2100. doi: 10.1021/es049209t. [DOI] [PubMed] [Google Scholar]
- 18.Esslinger S, Becker R, Maul R, Nehls I. Hexabromocyclododecane enantiomers: microsomal degradation and patterns of hydroxylated metabolites. Environ Sci Technol. 2011;45:3938–3944. doi: 10.1021/es1039584. [DOI] [PubMed] [Google Scholar]
- 19.Brandsma SH, Van der Ven LT, de Boer J, Leonards PE. Identification of hydroxylated metabolites of hexabromocyclododecane in wildlife and 28-days exposed Wistar rats. Environ Sci Technol. 2009;43:6058–63. doi: 10.1021/es900879k. [DOI] [PubMed] [Google Scholar]
- 20.Szabo DT, Diliberto JJ, Hakk H, Huwe JK, Birnbaum LS. Toxicokinetics of the flame retardant hexabromocyclododecane gamma: Effect of dose, timing, route, repeated exposure and metabolism. Toxicol Sci. 2010;117:282–293. doi: 10.1093/toxsci/kfq183. [DOI] [PubMed] [Google Scholar]
- 21.Szabo DT, Diliberto JJ, Hakk H, Huwe JK, Birnbaum LS. Toxicokinetics of the flame retardant hexabromocyclododecane alpha: Effect of dose, timing, route, repeated exposure and metabolism. Toxicol Sci. 2011;121:234–244. doi: 10.1093/toxsci/kfr059. [DOI] [PubMed] [Google Scholar]
- 22.Yu CC, Atallah YH. In: Pharmacokinetics of HBCD in rats Velsicol Chemicals, unpublished paper translated into English. Hakk H, Letcher RL, editors. 2003. [Google Scholar]; Metabolism in the toxicokinetics and fate of brominated flame retardants-A review. Environ Int. 29:801–828. doi: 10.1016/S0160-4120(03)00109-0. [DOI] [PubMed] [Google Scholar]
- 23.Dean WP, Leong BKJ. Acute toxicity studies in rabbits and rats. International Research and Development Corp. Sponsor: Velsicol Chemical Corporation; 1977. pp. 163–499. Study No. EPA/OTS Doc #86 900000266. [Google Scholar]
- 24.Ryuich A, Katsumi M, Shutoko M. Studies on pharmacodynamics of hexabromocyclododecane. Department of Pharmacy, Hokkaido University Hospital; 1983. Test on chemical substances used in household items. unpublished paper translated into English. [Google Scholar]
- 25.Huhtala S, Schultz E, Nakari T, MacInnis G, Marvin C, Alaee M. Analysis of hexabromocyclododecanes and their hydroxyl metabolites from in vitro and environmental samples by LCMSMS. Organohalogen Cmpd. 2006;68:1987–1990. [Google Scholar]
- 26.Szabo DT, Diliberto JJ, Huwe JK, Birnbaum LS. Differences in tissue distribution of HBCD alpha and gamma between adult and developing mice. Toxicol Sci. 2011;123:256–63. doi: 10.1093/toxsci/kfr161. [DOI] [PubMed] [Google Scholar]
- 27.Larsen GL, Bergman Å, Klasson-Wehler E. A methylsulphonyl metabolite of a polychlorinated biphenyl can serve as a ligand for α2u-globulin in rat and major-urinary-protein in mice. Xenobiotica. 1990;20:1343–1352. doi: 10.3109/00498259009046632. [DOI] [PubMed] [Google Scholar]
- 28.Hakk H, Diliberto JJ, Birnbaum LS. The effect of dose on 2,3,7,8-TCDD tissue distribution, metabolism and elimination in CYP1A2 (-/-) knockout and C57BL/6N parental strains of mice. Toxicol Appl Pharmacol. 2009;241:119–126. doi: 10.1016/j.taap.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Hakk H, Larsen G, Bergman Å, Örn U. Binding of brominated diphenyl ethers to male rat carrier proteins. Xenobiotica. 2002;32:1079–1092. doi: 10.1080/0049825021000016345. [DOI] [PubMed] [Google Scholar]
- 30.Perry JJ. Microbial metabolism of cyclic alkanes. In: Atlas RM, editor. Petroleum microbiology. Macmillan; New York: 1984. pp. 61–98. [Google Scholar]
- 31.Kostichka K, Thomas SM, Gibson KJ, Nagarajan V, Cheng Q. Cloning and characterization of a gene cluster for cyclododecanone oxidation in Rhodococcus ruber SC1. J Bacteriol. 2001;183:6478–6486. doi: 10.1128/JB.183.21.6478-6486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraas S, Steinbach AK, Tabbert A, Harder J, Ermler U, Tittmann K, Meyer A, Kroneck PMH. Cyclodexane-1,2-dione hydrloase: A new tool to degrade alicyclic compounds. J Molec Catal B Enzym. 2009;61:47–49. [Google Scholar]
- 33.Benedict RT, Stapleton HM, Letcher RJ, Mitchelmore CL. Debromination of polybrominated diphenyl ether-99 (BDE-99) in carp (Cyprinus carpio) microflora and microsomes. Chemosphere. 2007;69(6):987–993. doi: 10.1016/j.chemosphere.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Tang AH, Tu CP. Biochemical characterization of Drosophila glutathione S-transferases D1 and D21. J Biol Chem. 1994;269(45):27876–27884. [PubMed] [Google Scholar]
- 35.Stapleton HM, Brazil B, Holbrook RD, Mitchelmore CL, Benedict R, Konstantinov A, Potter D. In vivo and in vitro debromination of decabromodiphenyl ether (BDE 209) by juvenile rainbow trout and common carp. Environ Sci Technol. 2006;40(15):4653–4658. doi: 10.1021/es060573x. [DOI] [PubMed] [Google Scholar]
- 36.Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PRS, Birnbaum LS. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci. 2009;107:27–39. doi: 10.1093/toxsci/kfn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrad S, Abdallah MA-E, Covaci A. Causes of variability in concentrations and diastereomer patterns of hexabromocyclododecanes in indoor dust. Environ Intl. 2009;35:573–579. doi: 10.1016/j.envint.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Köppen R, Becker R, Jung C, Nehls I. On the thermally induced isomerisation of hexabromocyclododecane stereoisomers. Chemosphere. 2008;71:656–662. doi: 10.1016/j.chemosphere.2007.11.009. [DOI] [PubMed] [Google Scholar]


