Abstract
In the present report on the preliminary safety and effectiveness of radiofrequency (RF) ablation for pheochromocytoma metastases, seven metastases were treated in six patients (mean size, 3.4 cm; range, 2.2–6 cm). α- and β-adrenergic and catecholamine synthesis inhibition and intraprocedural anesthesia monitoring were used. Safety was assessed by recording ablation-related complications. Complete ablation was defined as a lack of enhancement within the ablation zone on follow-up computed tomography. No serious adverse sequelae were observed. Complete ablation was achieved in six of seven metastases (mean follow-up, 12.3 months; range, 2.5–28 months). In conclusion, RF ablation may be safely performed for metastatic pheochromocytoma given careful attention to peri-procedural management.
Treatment of metastatic pheochromocytoma can present a challenge for surgical or nonsurgical palliative management. Minimally invasive image-guided ablation can provide a safe treatment option for patients with painful lesions, life-threatening lesions, or symptoms related to these catecholamine- producing neuroendocrine tumors (1,2). The management of meta-static pheochromocytoma is complicated by a lack of effective systemic options and clinical manifestations that are often difficult to control with suppressive pharmacologic maneuvers. Treatment goals often include stabilization of symptoms including paroxysmal hypertension, tachycardia or other arrhythmia, headache, anxiety, nausea, and pallor (1,3). The average 5-year survival rate for patients with metastatic pheochromocytoma is 50% (4–9). Metastases most commonly affect the skeleton, lymph nodes, liver, and lung, all of which are potential targets for ablative therapies like percutaneous radiofrequency (RF) thermal ablation. In fact, RF ablation has been broadly applied to liver and bone tumors during the past decade, and is approved by the United States Food and Drug Administration for such applications (10,11).
Although RF ablation is appealing as a minimally invasive targeted therapy for metastatic disease, its role in the treatment of metastatic pheochromocytoma has not been well described to date. Also, to our knowledge, recommendations concerning the periprocedural hemodynamic management of these patients are not yet described in the literature. This article reviews the preliminary safety and effectiveness of RF ablation to treat seven pheochromocytoma metastases in six patients.
MATERIALS AND METHODS
Patient Demographics and Selection
All patients participated in an institutional review board–approved protocol, and informed consent was obtained from all patients. Mean tumor size before ablation was 3.4 cm (range, 2.1–6 cm; Table 1). Patients were selected for RF ablation based on the presence of hepatic metastases (patients 2–4 and 6) painful osseous metastases (patients 1 and 4), or a solitary metastasis amenable to RF ablation based on size and location (patient 5). A total of seven lesions were treated in six patients during eight RF ablation sessions; one of the seven lesions was re-treated with RF ablation after treatment failure was detected on follow-up imaging. All tumors were associated with plasma catecholamine and metanephrine excess, although variation in plasma catecholamine levels was observed before the procedure. All patients were evaluated for surgical resection, but were not surgical candidates because of disease at other sites or rapidly growing disease and refusal to undergo surgery.
Table 1.
Clinical and Biochemical Parameters of Patients Treated with RF Ablation for Metastatic Pheochromocytoma*
| Ablation Session† | Pt. Age (y) | Pt. Sex (M/F) | Metastatic Location | Outcome | Follow-up (months) |
|---|---|---|---|---|---|
| 1 | 50 | M | Right rib | Local control | 28 |
| 2 | 45 | M | Liver | Local control | 11 |
| 3 | 36 | M | Liver | Local control | 4 |
| 4 | 68 | F | Right rib | Failure | 13 |
| 4A | Liver | Local control | 11 | ||
| 4B | Right rib (repeat) | Local control | 6 | ||
| 5 | 31 | M | Right ischium | Local control | 9 |
| 6 | 57 | F | Liver | Local control | 1.25 |
The biochemical phenotype of metastatic pheochromocytoma was noradrenergic in all cases.
An ablation session was defined as a visit to the radiology department at which the mass was treated with RF ablation.
Preprocedural Preparation
Five of the six patients were premedicated for 7–21 days before ablation with a combination of phenoxybenzamine (Dibenzyline; Wellspring Pharmaceutical Corporation, Brandenton, Florida) for α-adrenergic inhibition (10 mg two to three times a day), atenolol (Tenormin; AstraZeneca Pharmaceuticals, Wilmington, Delaware) for β-adrenergic inhibition (12.5 mg once or twice per day), and α-methyl-paratyrosine (Demser; Merck and Co., Whitehouse Station, New Jersey) for inhibition of catecholamine synthesis (250 mg two to three times per day). Target blood pressure was achieved with use of phenoxybenzamine alone (10 mg three times per day) in one of the six patients. Target blood pressure for all patients before the procedure was 110–120 mm Hg systolic and 60–70 mm Hg diastolic without severe orthostatic or underperfusion symptoms. Outpatient medication management before ablation was monitored via home blood pressure checks performed by the patients three times per day. These results were conveyed via phone or e-mail to a research nurse practitioner (K.A.), who titrated the medication dosage as needed to achieve an asymptomatic target blood pressure before RF ablation. Patients were scheduled for RF ablation only after asymptomatic target blood pressure was achieved.
Intraprocedural Methods and RF Ablation
Ablation was performed only after extensive clinical consultation and after obtaining written informed consent under institutional review board–approved protocols; a more specific protocol was not required because the RF ablation system is approved by the Food and Drug Administration for ablation of soft tissue, unresectable liver tumors, and painful lytic bone metastases (Food and Drug Administration Act, chapter 5, section 510[k]).
General endotracheal anesthesia with radial arterial pressure monitoring was used in all cases. All procedures were performed with a Cool-Tip 200-W RF generator (Covidien, Mansfield, Massachusetts). In all cases, RF ablation procedures were performed percutaneously with a combination of direct ultrasound guidance and intermittent computed tomography (CT) guidance. Either a single 17-gauge needle electrode (n = 2) or triple parallel cluster 17-gauge needle electrodes (n = 5) were used. The type of needle electrode used was determined by lesion size and geometry and based on the location and proximity of blood vessels and nearby vulnerable regional anatomy. In general, lesions larger than 2.5 cm in diameter were treated with triple parallel cluster needle electrodes, but some lesions smaller than 2.5 cm were also treated with this electrode if their geometry (eg, ovoid rather than round) or their proximity to blood vessels would have abrogated success achievable with a single-needle electrode. One of the four hepatic metastases was treated with a single-needle electrode; the remaining were treated with triple parallel cluster needles. One of the three osseous metastases was treated with a single needle with a 3-cm long treatment tip; the remaining two were treated with triple parallel cluster needle electrodes (Figs 1, 2). Grounding pads were applied to the patient’s thighs before RF ablation. Two grounding pads were applied when a single-needle electrode was used for RF ablation, one each to the right and left anterior thighs. Four grounding pads were applied when a triple parallel cluster needle electrode was employed, one each to the anterior and posterior right and left thighs. The system’s generator software was used to monitor tissue impedance, adjusting the current output initially manually, followed eventually by an automatic standard pulsing algorithm for 12 minutes of continuous current, as described later.
Figure 1.
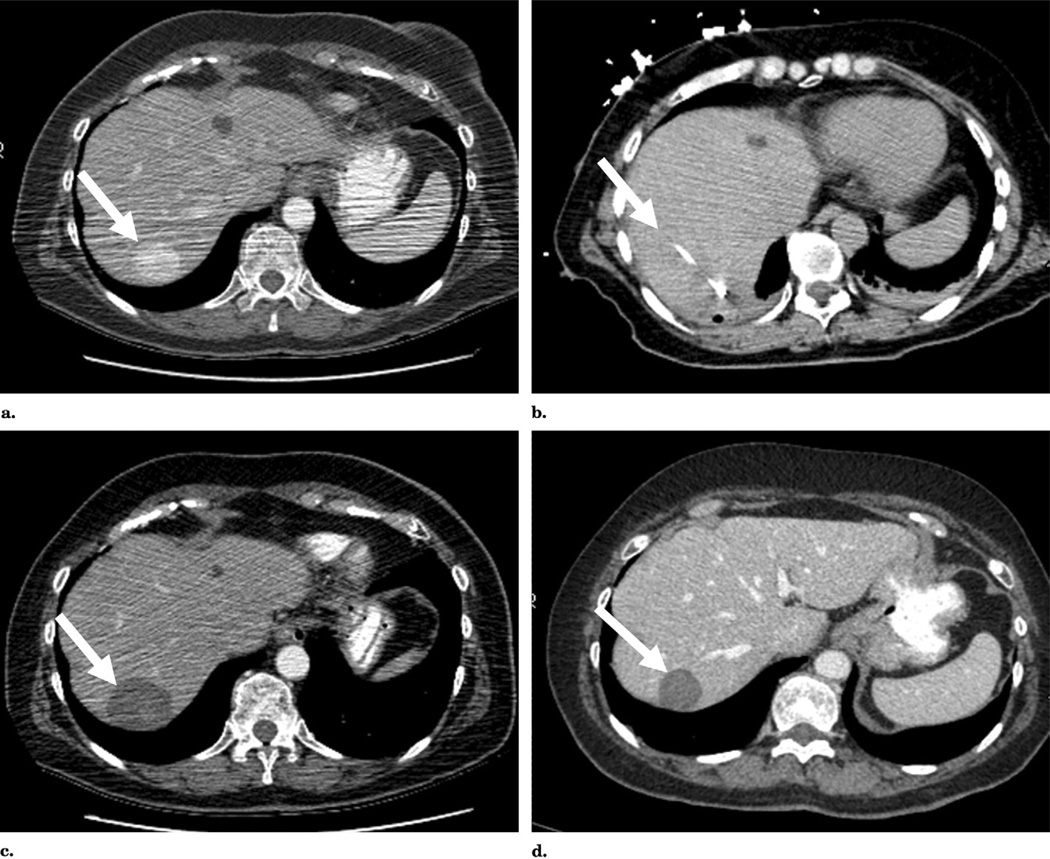
RF ablation of metastatic pheochromocytoma to liver. (a) Pre-ablation CT demonstrates an enhancing mass within segment 7 of the liver. (b) Triple parallel cluster electrode placement within the lesion with gas generated during thermal ablation. (c) Follow-up CT image obtained 4 weeks after therapy demonstrates a nonenhancing zone of ablation; internal high density within the zone of ablation is an expected finding, consistent with proteinaceous debris, necrotic tissue, and/or products of hemorrhage without residual enhancement suggestive of tumor. (d) Follow-up CT 20 months after therapy shows decreased size of the zone of ablation and no evidence of residual tumor.
Figure 2.
RF ablation of metastatic pheochromocytoma to bone. (a) Preablation CT demonstrates an enhancing mass within the posterior aspect of the right ilium. (b) RF ablation of osseous metastasis, with the needle electrode within the anterior margin of tumor. (c) Follow-up CT obtained 2 months after therapy demonstrates a nonenhancing zone of ablation (arrow) and no evidence of residual tumor.
Procedures were performed in sequential, step-wise fashion, with each step separated in time, and with a pause between steps to allow for resulting hemodynamic responses and pharmacologic adjustments to be made. The following steps were performed with 10–60-second pauses between steps: (i) electrode placement, (ii) brief application of low current (0.1 A, 10 seconds), and (iii) gradually increased current of longer duration until continuous current at approximately 0.1 A was tolerated without arterial pressure increases greater than 200 mm Hg systolic. Current was then increased in 0.1-A increments as tolerated. This phase of gradual increase in applied current was eventually followed by continuous current for 12 minutes after the highest current attainable with the system was achieved (2 A). At all times, heart rate and arterial blood pressure were monitored to avoid sudden increases. Of critical importance, RF ablation current was immediately turned off during the phase of gradually increasing current whenever mean arterial pressure increased 10–20 mm Hg greater than the patient’s baseline measurement. A 10–60-second pause without applied current then occurred, allowing the blood pressure to peak and then decrease while an applied nitroprusside drip also equilibrated.
Tissue temperature was also monitored after RF ablation (ie, after each standard 12-minute ablation session) to determine the need for repeated treatments in the same location. In each case, repeat ablations were performed with geographic overlap to minimize the likelihood of tumor insufficient treatment.
Before termination of the procedure, the needle tract was cauterized during needle electrode removal to decrease the theoretical risk of needle tract seeding and to prevent bleeding along the needle tract. After the procedure, patients returned to the nursing units for monitoring and pain control. Patients were kept overnight primarily because most traveled long distances to the treatment facility.
To evaluate response, contrast-enhanced CT scans were obtained 2–6 weeks after the procedure. Repeat CT scans were typically performed at regular 3–6-month intervals in the first year, then according to clinical need. Magnetic resonance imaging and positron emission tomography/CT were also performed as clinically warranted. Tumor necrosis was considered complete when tumor enhancement was eradicated, with subsequent decrease in size of the thermally treated tissue over time on follow-up CT scans. Incomplete treatment or tumor recurrence was defined as enhancement greater than 10 HU or lesion growth after RF ablation. Median follow-up was 10.3 months, with a range of 1–28 months.
Intraprocedural Monitoring
All ablations were performed under general anesthesia with a nitroprusside drip titrated during the procedure by the attending anesthesiologist. The target blood pressure during ablation was the beginning baseline pressure (ideally ≤120/80 mm Hg). Labetalol, nitroglycerine, propofol, fentanyl, and midazolam were also administered as needed for control of hypertension during ablation. Arterial pressure was monitored with the use of a radial arterial catheter in all patients. A central venous catheter was employed for intravenous medication administration in all patients. Intra-procedurally, the highest blood pressure incurred was 211/115 mm Hg. Close communication between operator and anesthesiologist was maintained throughout the procedure. Verbal queues were given to the anesthesiologist several minutes before any physical or pharmacologic manipulation expected to result in tumor catecholamine release. Announcements were made of needle insertions, needle repositioning, current adjustments, pauses in RF ablation current and expected duration of continuous current delivery, and overall treatment time. With acute pressure increases, pharmacologic therapy was tried initially. If unsuccessful, this was followed by turning off the RF ablation current completely.
Procedural complications were defined according to the Society of Interventional Radiology classification system for complications by outcome (12), with minor complications defined as those requiring no or nominal therapy and major complications requiring therapy or hospitalization (<48 hours) or resulting in permanent adverse sequelae or death. Complications were dictated into the patient’s ablation report and maintained within the hospital online medical record, thereby allowing them to be tracked in the future. Patients were monitored intra-procedurally and immediately after the procedure for complications. The specific frequency and duration of post-procedural radial arterial pressure monitoring varied based on the discretion of the attending anesthesiologist. Patients were subsequently evaluated via follow-up clinic visits and post-ablation imaging studies performed the same day. Initial postablation imaging was scheduled 2–6 weeks after the procedure, then at 3–6-month intervals for the first year, and then subsequently according to clinical need.
Catecholamine Sampling
Blood samples were obtained before, after, and at specific intervals during the RF ablation procedure for plasma catecholamine assays during seven of the eight ablation sessions. These included baseline measurements (ie, before RF ablation) and measurements at the time of needle insertion and initial current application, during the phase of gradually increasing current, during continuous fixed current, and after completion of RF ablation and removal of the needle electrode. Plasma norepinephrine and epinephrine values were obtained via alumina extraction of 1 mL of plasma in accordance with published methods (13).
RESULTS
RF ablation of seven pheochromocytoma metastases resulted in successful tumor necrosis in six of the seven tumors. One rib lesion exhibited growth and enhancement (>10 HU) at follow-up CT obtained 6 months after ablation (ablation session 4B). This lesion was ultimately treated again 13 months after initial ablation (ablation session 4B). Repeat treatment was delayed after detection of RF treatment failure at the preference of the patient. One procedural complication was encountered when a small pneumothorax developed in one patient during a rib lesion ablation (ablation session 1). This was treated intra-procedurally with temporary chest tube placement, resulting in no further clinical sequelae.
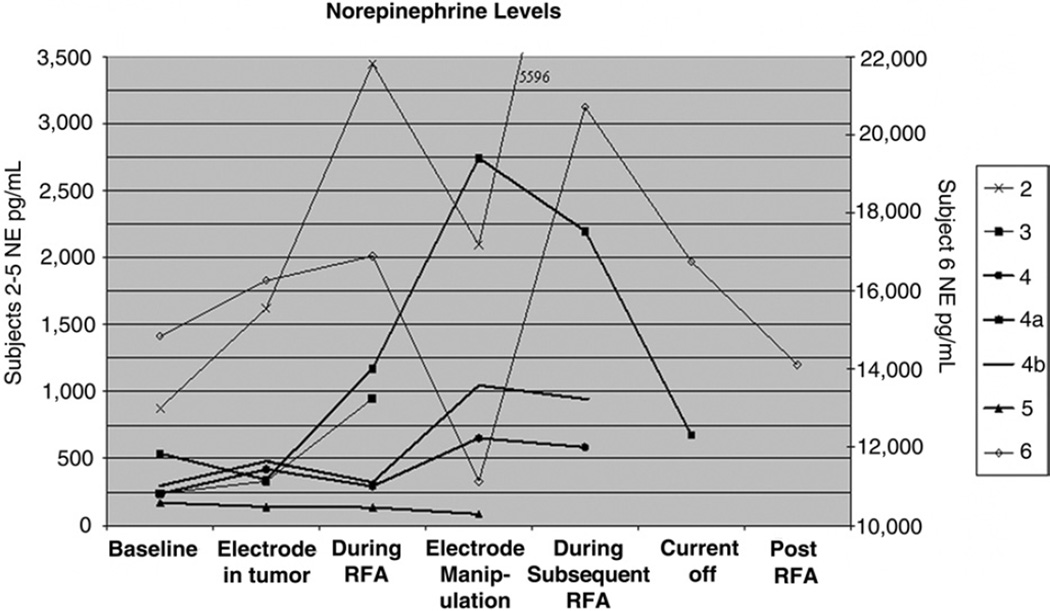
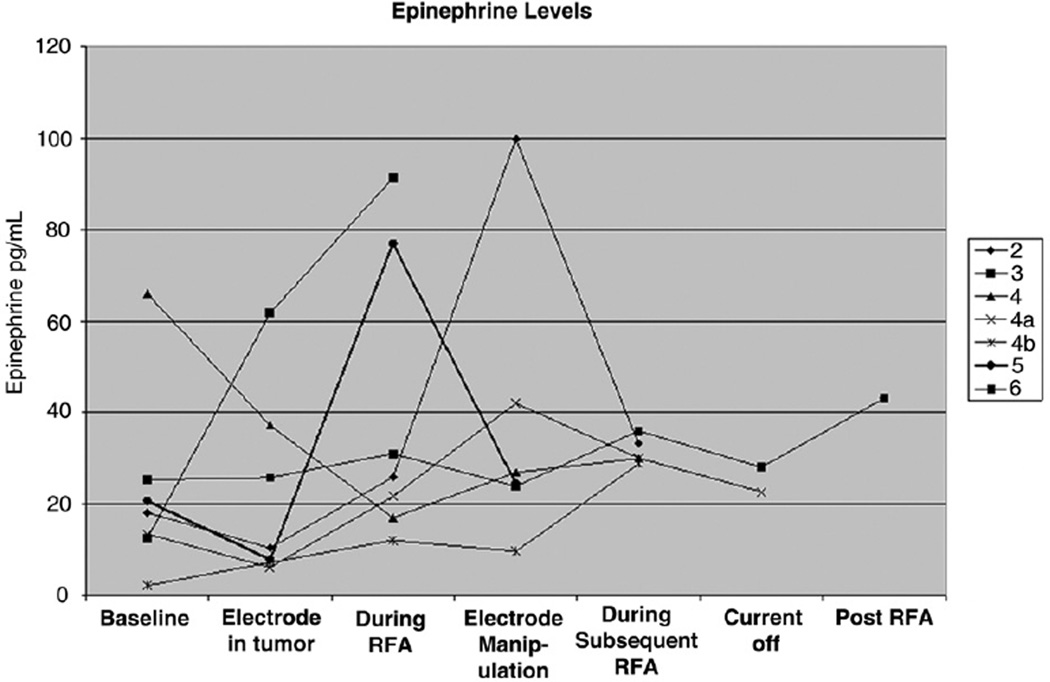
Representative dynamics of plasma epinephrine and norepinephrine as a function of procedure time (ie, before, during, and immediately after RF ablation) are presented in Figures 3 and 4. These reflect the temporal relationship of catecholamines to needle insertion, intermittent RF ablation, gradually increasing current, continuous current, and transient cessation of current for acute hypertension. Intermittent procedural hypertension was treated pharmacologically with labetalol, sodium nitroprusside, and nitroglycerin as appropriate. An increase in catecholamine levels from baseline was observed during all of the ablations for which catecholamine sampling was performed (Figs 3, 4). In six of seven cases, plasma norepinephrine levels peaked during RF ablation or electrode manipulation immediately after an RF ablation session (Fig 3). In five of the seven cases, plasma epinephrine levels peaked during RF ablation or during electrode manipulation immediately after an RF ablation session (Fig 4). Plasma catecholamine levels were noted to decrease from peak levels in all cases by the end of treatment.
Figure 3.
Norepinephrine levels as a function of time before, during, and immediately after RF ablation of pheochromocytoma metastases (norepinephrine levels relevant to ablation sessions 2–5 are depicted on the left vertical axis; levels for ablation session 6 are depicted on the right vertical axis). In six of the seven cases, plasma norepinephrine levels peaked during RF ablation or during electrode manipulation immediately after an RF ablation session. (Plasma norepinephrine normal range, 80–498 pg/mL; plasma epinephrine normal range, 4–83 pg/mL.)
Figure 4.
Epinephrine levels as a function of time before, during, and immediately after RF ablation of pheochromocytoma metastases. In five of the seven cases, plasma epinephrine levels peaked during RF ablation or during electrode manipulation immediately after an RF ablation session. Plasma catecholamine levels were noted to decrease from peak level in all cases by the end of the treatment. (Plasma norepinephrine normal range, 80–498 pg/mL; plasma epinephrine normal range, 4–83 pg/mL.)
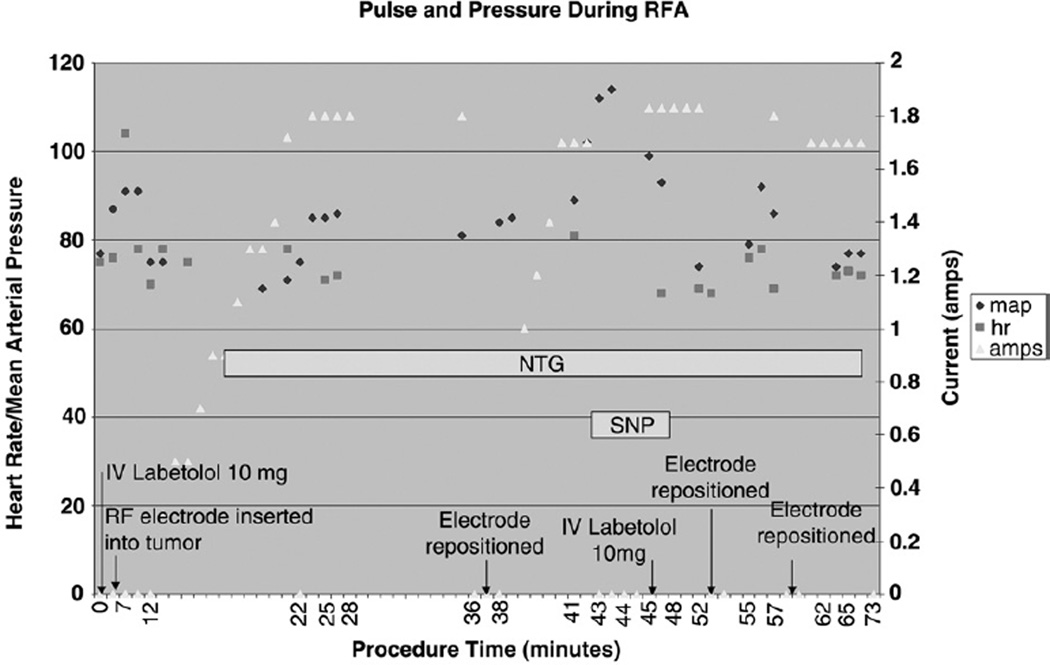
Figure 5 depicts changes in pulse and mean arterial pressure during treatment of patient 2 as a function of procedural maneuvers, including needle electrode positioning, current manipulation, and medication administration, which are representative of what was observed in all cases performed. A temporal relationship was suggested between the application and amount of RF ablation current and the intra-procedural mean arterial pressure in our patients. A delay of 1–4 minutes was noted between current application or current increase and changes in mean arterial pressure. A delay was also noted between cessation of RF ablation current and the changes in the mean arterial pressure, with a latency period of less than 1 minute observed between RF current arrest and blood pressure peak in all cases.
Figure 5.
Changes in pulse and mean arterial pressure during RF ablation for patient 2 as a function of procedural maneuvers (needle electrode positioning, current manipulation, and medication administration). Pulse (hr, squares) and mean arterial pressure (map, diamonds) are represented as function of needle insertion (arrow), intermittent RF ablation current and gradually increasing current (amps, triangles), needle repositioning (arrow), cessation of current, and administration of antihypertensive medications, including nitroglycerin (NTG) and sodium nitroprusside (SNP).
DISCUSSION
Since its introduction, percutaneous RF ablation has been applied to tumors in multiple locations in the body, including the liver, kidney, lung, musculoskeletal system, and adrenal glands. Compared with the more established use of RF ablation in the treatment of hepatic and renal tumors, the use of this therapy in the treatment of adrenal tumors remains less common (14). An emerging body of literature describes the role of RF ablation in the treatment of primary and metastatic adrenal neoplasms, including adrenocortical carcinoma (15,16). In a study of RF ablation used to treat 15 adrenocortical carcinoma primary or metastatic tumors in eight patients over a period of 27 months, a complete response was achieved in 67% of tumors no larger than 5 cm, as defined by decreasing size and complete loss of tumor contrast enhancement on cross-sectional imaging (15).
Severe hypertensive crisis resulting from catecholamine release has been reported as a result of inadvertent injury to the adrenal during RF ablation of proximal hepatocellular carcinomas and renal cell carcinomas (17,18). Even minimal direct physical manipulation of pheochromocytomas can precipitate the uncontrolled release of catecholamines (9). Therefore, extreme care must be taken before and during the procedure when ablating pheochromocytoma metastases. Interestingly, as all the patients in the present study had metastatic pheochromocytoma, they would be expected to manifest an excess of norepinephrine (ie, not epinephrine). Plasma catecholamine sampling at baseline and during RF ablation appears to corroborate this assumption (Figs 3,4). Higher absolute values of baseline plasma norepinephrine were observed compared with baseline plasma epinephrine levels, and greater increases above baseline measurements were observed for norepinephrine compared with epinephrine during the majority of ablation sessions (Table 2). As norepinephrine excess primarily affects blood pressure whereas epinephrine excess primarily affects heart rate, it might be anticipated that the patients in the present study would be more prone to changes in blood pressure than in heart rate during tumor ablation. Indeed, patients in this study sustained greater intra-procedural blood pressure fluctuation compared with changes in heart rate, despite both α- and β-adrenergic blockade before ablation (Fig 5). In patients with metastatic pheochromocytoma undergoing RF ablation, it is crucial to be aware of the potential for significant increases in plasma norepinephrine levels and their consequences. Blood pressure elevation and/or lability must be treated accordingly during the procedure. Of interest, we observed increases in norepinephrine and epinephrine levels during repeat treatment of a pheochromocytoma rib metastasis (ablation session 4B). These preliminary data suggest equal propensity for catecholamine release during repeat treatment of previously ablated pheochromocytoma metastases. Identical precautions should therefore be taken during repeat RF ablation as with primary ablations, and this should include pre-ablation adrenergic blockade, careful intra-procedural monitoring and hemodynamic management, and post-procedural follow-up. Additionally, RF ablation–induced cell necrosis and cell death have been observed to occur even hours after a procedure is completed (14,15,18). Therefore, an overnight stay after RF ablation of pheochromocytoma may be prudent to monitor postprocedural catecholamine- induced changes of blood pressure and heart rate.
Table 2.
Norepinephrine and Epinephrine Levels (pg/mL) before, during, and Immediately after Thermal Ablation of Pheochromocytoma Metastases*
| Baseline | Needle Placed in Lesion | Needle Manipulation | During RF Ablation | Current off after Maxed | During Subsequent RF Ablation | After RF Ablation (Needle out) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ablation Session |
NE | EPI | NE | EPI | NE | EPI | NE | EPI | NE | EPI | NE | EPI | NE | EPI | ||||
| 2 | 876 | 18 | 1,622 (185) | 10 (56) | 3,446 (393) | 26 (144) | 2,094 (239) | 100 (556) | — | 5,596 (639) | 33 (183) | — | ||||||
| 3 | 238 | 13 | 332 (139) | 62 (477) | — | 947 (398) | 91 (700) | — | — | — | ||||||||
| 4 | 237 | 66 | 420 (177) | 37 (56) | — | 294 (124) | 17 (26) | 653 (276) | 27 (41) | — | 585 (247) | 30 (45) | ||||||
| 4A | 539 | 14 | 1,172 (217) | 22 (157) | — | 2,743 (509) | 42 (300) | 2,199 (408) | 30 (214) | — | 676 (125) | 23 (164) | ||||||
| 4B | 297 | 2 | 479 (161) | 7 (350) | — | 1,047 (353) | 10 (500) | — | 995 (335) | 44 (2,200) | 945 (318) | 29 (1,450) | ||||||
| 5 | 170 | 21 | 140 (82) | 8 (38) | — | 135 (79) | 77 (367) | — | — | 86 (51) | 25 (119) | |||||||
| 6 | 14,845 | 25 | 16,273 (110) | 26 (104) | — | 16,896 (114) | 31 (124) | 11,130 (75) | 24 (96) | 20,706 (139) | 36 (144) | 14,111 (95) | 43 (172) | |||||
Note.—Values in parentheses are percentages of baseline measurements.
Normal plasma ranges: norepinephrine, 80–498 pg/mL; epinephrine, 4–83 pg/mL.
To our knowledge, there exists one recent case report concerning safe biopsies and RF ablations in a patient with metastatic pheochromocytoma (19). The case report of Mamlouk et al (19) underscores the importance of careful periprocedural management for RF ablation of pheochromocytoma metastases, as the patient in their report experienced cardiac arrest during RF ablation before being referred to their institution. The peri-procedural medical management employed for the patients within the present study is similar to that advocated by Mamlouk et al (19), mirroring the peri-operative adrenergic blockade employed for surgical resection of these tumors (20–22). In addition to the use of phenoxybenzamine advocated by these authors (10), our experience suggests concomitant β-adrenergic blockade in view of refractory hypertension and reflex tachycardia that may result from isolated α-adrenergic blockade. Additionally, safe ablation may also rely on blocking catecholamine synthesis with administration of α-methyl-paratyrosine. In the surgical literature, use of this agent has been associated with improved control of blood pressure, reduced blood loss, and less need for intraoperative fluid replacement compared with single-agent α-adrenergic blockade (20–22). Five of our six patients exhibited an asymptomatic target blood pressure before RF ablation with the use of these three medications, and none experienced hemodynamic clinical sequelae during ablation.
During RF ablation, barring rapid fluctuations, lower baseline pressures may allow better tolerance of the variable hemodynamics sustained from plasma catecholamine release caused by in situ tumor destruction. In this study, dynamic mean arterial pressure elevations occurred as rapidly as 1 mm Hg/sec with the application of RF ablation current. We observed a less pronounced rate of change of pulse as a function of procedural maneuvers, likely attributable to patients’ peri-procedural β-adrenergic blockade (Fig 5). With the RF ablation system we employed, patients’ systemic response to subsequent cessation of current occurred promptly, typically within 1 minute, and often within 15 seconds. Based on our preliminary experience, acute blood pressure elevation caused by catecholamine release would be expected to result in achievement of peak blood pressure within less than 1 minute after cessation of applied current, with subsequent pressure equilibration well below the peak.
Intra-procedural attention to anesthesia details, including arterial pressure monitoring, central venous access for medication administration, and timing and titration of a nitroprusside drip, can facilitate safe management of the potentially labile hemodynamics encountered during pheochromocytoma RF ablation. Advance knowledge of the potential temporal relationship among RF ablation current, plasma catecholamines, and blood pressure may also facilitate procedure success and safety. Close coordination among members of a multidisciplinary team, including interventional radiologists, medical and surgical oncologists, endocrinologists, and anesthesiologists is crucial to effective peri-procedural management. Baseline blood pressure, comorbidities, and age should be taken into account during pre- and intra-procedural dose titration to stratify and mitigate the risk for rapid changes in blood pressure that may be sustained during thermal ablation.
Although we report largely successful local control of target lesions, there are limitations to this study, including the small number of patients treated and the limited follow-up period. At this time, the routine application of RF ablation for pheochromocytomas should be considered experimental, except in the hands of experienced operators and multidisciplinary teams. Surgical excision remains the conventional means of tumor removal and should be considered during evaluation.
In our preliminary experience, meticulous attention to detail, preprocedural preparation, and a multidisciplinary approach can minimize the risk for catastrophic complications related to catecholamine surge during pheochromocytoma RF ablation. Advance awareness of the need for multiple-agent pharmacologic blockade, continuous intraprocedural arterial hemodynamic monitoring, and an understanding of the potential temporal relationship between RF ablation current and arterial blood pressure may facilitate successful outcomes in experienced hands for this emerging treatment for pheochromocytoma.
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institutes of Health and the NIH Center for Interventional Oncology.
Abbreviation
- RF
radiofrequency
Footnotes
None of the authors have identified a conflict of interest.
References
- 1.Lenders JM, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 2.Reisch N, Peczkowska M, Januszewicz A, Neumann HH. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens. 2006;24:2331–2339. doi: 10.1097/01.hjh.0000251887.01885.54. [DOI] [PubMed] [Google Scholar]
- 3.Mantero F, Terzolo M, Arnaldi G, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85:637–644. doi: 10.1210/jcem.85.2.6372. [DOI] [PubMed] [Google Scholar]
- 4.Scholz T, Eisenhofer G, Pacak K, Drall H, Lehnert H. Current treatment of malignant pheochromocytoma. J Clin Endocrinol Metab. 2007;92:1217–1225. doi: 10.1210/jc.2006-1544. [DOI] [PubMed] [Google Scholar]
- 5.Schlumberger M, Gicquel C, Lumbroso J, et al. Malignant pheochromocytoma: clinical, biological, histologic and therapeutic data in a series of 20 patients with distant metastases. J Endocrinol Invest. 1992;15:631–642. doi: 10.1007/BF03345807. [DOI] [PubMed] [Google Scholar]
- 6.O’Riordain DS, Young WF, Jr, Grant CS, Carney JA, van Heerden JA. Clinical spectrum and outcome of functional extra- adrenal paraganglioma. World J Surg. 1996;20:916–921. doi: 10.1007/s002689900139. [DOI] [PubMed] [Google Scholar]
- 7.Eisenhofer G, Bornstein SR, Brouwers FM, et al. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer. 2004;11:423–436. doi: 10.1677/erc.1.00829. [DOI] [PubMed] [Google Scholar]
- 8.John H, Ziegler WH, Hauri D, Jaeger P. Pheochromocytomas: can malignant potential be predicted? Urology. 1999;53:679–683. doi: 10.1016/s0090-4295(98)00612-8. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein RE, O’Neill JA, Holcomb GW, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229:755–764. doi: 10.1097/00000658-199906000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhim H, Goldberg SN, Dodd GD, et al. Essential techniques for successful radiofrequency thermal ablation of malignant hepatic tumors. Radiographics. 2001;21(Suppl):S17–S39. doi: 10.1148/radiographics.21.suppl_1.g01oc11s17. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg. 1998;80:815–821. doi: 10.2106/00004623-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Sacks D, McClenny J, Cardella J, Lewis C. Society of Interventional Radiology Clinical Practice Guidelines. J Vasc Interv Radiol. 2003;14(Suppl):S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhofer G, Goldstein DS, Stull R, et al. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem. 1986;32:2030–2033. [PubMed] [Google Scholar]
- 14.Lo WK, VanSonnenberg E, Shankar S. Percutaneous CT-guided radiofrequency ablation of symptomatic bilateral adrenal metastases in a single session. J Vasc Interv Radiol. 2006;17:175–179. doi: 10.1097/01.rvi.0000188748.51764.ce. [DOI] [PubMed] [Google Scholar]
- 15.Wood BJ, Abraham J, Hvizda JL, Alexander HR, Fojo T. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer. 2003;97:554–560. doi: 10.1002/cncr.11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayo-Smith WW, Dupuy DE. Adrenal neoplasms: CT-guided radiofrequency ablation-preliminary results. Radiology. 2004;231:225–230. doi: 10.1148/radiol.2311031007. [DOI] [PubMed] [Google Scholar]
- 17.Onik G, Onik C, Medary I, et al. Life-threatening hypertensive crises in two patients undergoing hepatic radiofrequency ablation. AJR Am J Roentgenol. 2003;181:495–497. doi: 10.2214/ajr.181.2.1810495. [DOI] [PubMed] [Google Scholar]
- 18.Chini EN, Brown MJ, Farrell MA, Charboneau W. Hypertensive crisis in a patient undergoing percutaneous radiofrequency ablation of an adrenal mass under general anesthesia. Anesth Analg. 2004;99:1867–1869. doi: 10.1213/01.ANE.0000136803.54212.E1. [DOI] [PubMed] [Google Scholar]
- 19.Mamlouk MD, vanSonnenberg E, Stringfellow G, Smith D, Wendt A. Radiofrequency ablation and biopsy of metastatic pheochromocytoma: emphasizing safety issues and dangers. J Vasc Interv Radiol. 2009;20:670–673. doi: 10.1016/j.jvir.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhofer G, Rivers G, Rosas AL, Quezado Z, Manger WM, Pacak K. Adverse drug reactions in patients with phaeochromocytoma: incidence, prevention and management. Drug Saf. 2007;30:1031–1062. doi: 10.2165/00002018-200730110-00004. [DOI] [PubMed] [Google Scholar]
- 21.Perry RR, Keiser HR, Norton JA, Wall RT, Robertson CN, Travis W. Surgical management of pheochromocytoma with the use of metyrosine. Ann Surg. 1990;212:621–628. doi: 10.1097/00000658-199011000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinsapir J, Carr AA, Prisant M, Bransome ED. Metyrosine and pheochromocytoma. Arch Intern Med. 1997;157:901–906. [PubMed] [Google Scholar]