Abstract
Female mice exhibited higher survival rate than males after pneumonia, with a reversal of this pattern following ozone exposure. Surfactant protein A (SP-A) plays an important role in innate immunity and SP-A (−/−) mice were more susceptible to pneumonia than wild type mice. Here, we investigated underlying mechanisms of the differential susceptibility of mice to pneumonia. Wild type and SP-A (−/−) C57BL/6J male and female mice were exposed to ozone or filtered air (FA) and then infected intratracheally with Klebsiella pneumoniae. Blood, spleen, and lung were analyzed for bacterial counts, lung and spleen weights, and sex hormone and cortisol levels were measured in plasma within two days post-infection. We found: 1) in the absence of ozone-induced oxidative stress, males had higher level of bacterial dissemination compared to females; ozone exposure decreased pulmonary clearance in both sexes and ozone-exposed females were more affected than males; 2) ozone exposure increased lung weight, but decreased spleen weight in both sexes, and in both cases ozone-exposed females were affected the most; 3) plasma cortisol levels in infected mice changed: ozone-exposed > FA-exposed, females > males, and infected > non-infected; 4) no major sex hormone differences were observed in the studied conditions; 5) differences between wild type and SP-A (−/−) mice were observed in some of the studied conditions. We concluded that reduced pulmonary clearance, compromised spleen response to infection, and increased cortisol levels in ozone-exposed females, and the higher level of lung bacterial dissemination in FA-exposed males, contribute to the previously observed survival outcomes.
Keywords: Pneumonia infection, Lung inflammation, Spleen, Hormones, CFU, Cortisol
1. Introduction
The outcome of lung infection depends on several factors, including effective pulmonary clearance of pathogens and limitation of systemic or extra-pulmonary dissemination of infection. In addition to the effectiveness of lung host defense, other factors such as sex, stress hormones, and air pollution may have an impact on lung health.
Klebsiella pneumoniae, an encapsulated Gram-negative bacterium, is among the most common hospital-acquired and potential community-acquired pathogens of the respiratory tract [1,2]. Pneumonia caused by this microorganism is often difficult to treat, especially in compromised patients. Dissemination of K. pneumoniae bacteria into the bloodstream is a critical step in disease pathogenesis, as this may lead to multiple organ dysfunction [3]. K. pneumoniae bacteremia occurs more often in the warmest and more humid months of the year, indicating a seasonal variability in K. pneumoniae-induced pneumonia [4].
Several risk factors have been identified for lung disease, one of them being sex. Males, in general, are more susceptible than females to lung disease, as is the case with neonatal respiratory distress syndrome [5,6], idiopathic pulmonary fibrosis and COPD [7,8], and different types of pneumonia [8–11]. The incidence of asthma is often higher in young males than in young females [7]. In animal models, males also exhibit a higher level of susceptibility to lung disease [12–15].
Air pollution is a major problem worldwide. The United States Environmental Protection Agency (EPA) identifies six commonly found air pollutants (also known as "criteria pollutants"): particulate matter, ground-level ozone, carbon monoxide, sulfur oxides, nitrogen oxides, and lead. Ground-level ozone, a photochemical air pollutant and powerful oxidant, has the most widespread health effects. Ozone exposure can affect breathing, induce coughing, reduce lung function [16], and trigger asthma [17,18]. The effect of ozone has been associated with impairment of the entire respiratory epithelia [19]. Exposure to ozone can influence lung immunity (reviewed in [20,21]). Moreover, the effect of ozone and the overall lung immune status may be affected by sex (reviewed in [7,22–24]).
Surfactant protein A (SP-A) is an important innate immune defense protein, and it was shown to be involved in the stimulation of chemotaxis of macrophages [25], enhancement of phagocytosis of bacteria [26–30], proliferation of immune cells [31,32], linkage of innate and adaptive immunity [33], and production of proinflammatory cytokines [34– 37] in part, via NFκB activation [38,39]. Genetically modified mice lacking SP-A were shown to be more susceptible to experimental pneumonia caused by Pseudomonas aeruginosa [40], group B Streptococcus [41,42], Haemophilus influenzae [42], and K. pneumoniae [43] than wild type mice.
Increased levels of air pollution have resulted in an increased number of respiratory emergency hospital visits [44]. However, there is no consistency in the published reports about the sex-based influence of air pollution. Some epidemiological studies reported sex differences in the effect of air pollution, with females being more susceptible than males [45–51], but in other studies, differences between sexes in response to air pollution were not found [52–56]. Moreover, most studies did not consider sex as a factor. The challenge of studying differences in the susceptibility to air pollution between female and male patients has, in part, to do with the fact that these differences are the result of interactions of “sex” (genetic and biologic) and "gender" (sociocultural) factors [23]. However, animal models can in part overcome this challenge and help study the role of sex (genetic and biological) in both lung disease and in response to air pollution [57]. We have previously demonstrated that although females were initially more resistant to K. pneumoniae than males in both wild type and SP-A (−/−) mice, ozone exposure reversed this pattern, causing females to be more susceptible to K. pneumoniae infection than males [43,57]. Thus, the female advantage as in the case of pulmonary K. pneumoniae infection alone appears to become a female disadvantage when air pollution is used as a second insult.
Because the final outcome of pneumonia disease is critically dependent on the events during the first steps of infection, in this study, we investigated underlying processes in the wild type and SP-A (−/−) mice during the first two days post-infection. The goal was to determine differences in the outcome of pneumonia in both sexes in the absence or presence of oxidative stress.
2. Results
Wild type and SP-A (−/−) male and female mice were first exposed to ozone or filtered air (FA is used as a control), followed by infection with K. pneumoniae bacteria. Mouse specimens were tested with different analyses within the first two days post-infection, as described in Methods. We opted not to use later time points for the analysis because more than 50% mortality from pneumonia was detected in ozone-exposed males and females at day 3 post-infection, as we described earlier [43,57]. In general, although the results from wild type and SP-A (−/−) mice during the course of pneumonia exhibited similar trends in different assays, differences were observed in some of the studied conditions as described below.
2.1. Effect of ozone exposure, sex, and SP-A on the course of pneumonia
2.1.1. Effect of ozone exposure
2.1.1.1. Lung CFU
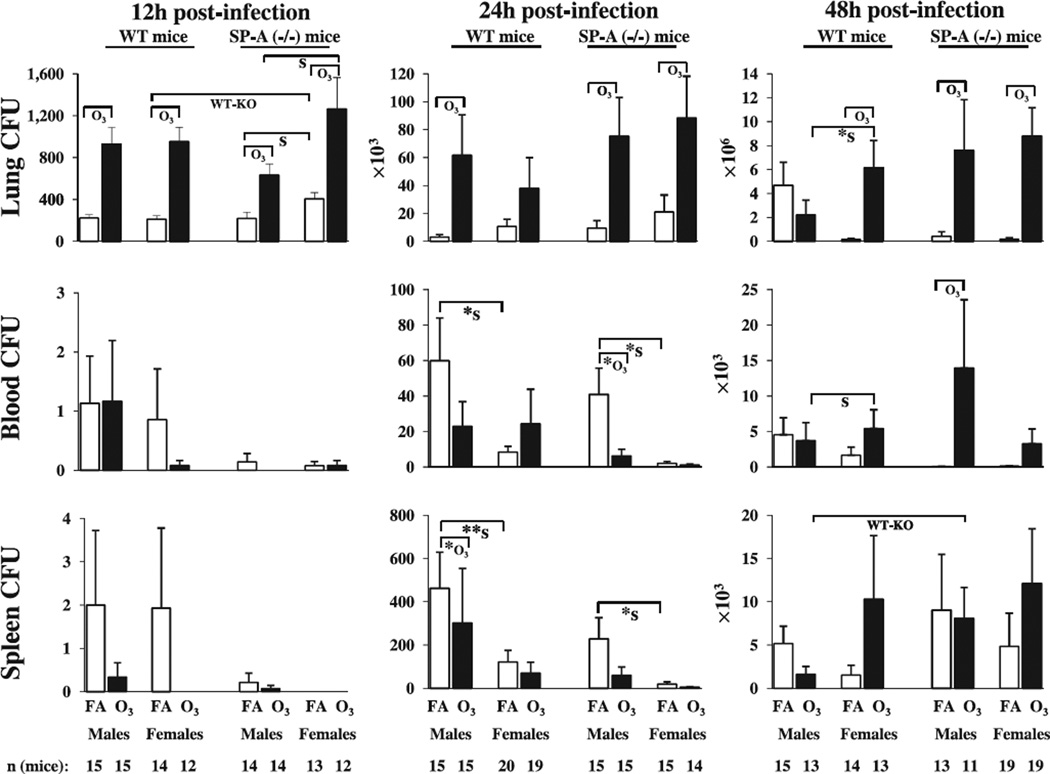
The results demonstrated that ozone exposure significantly increased lung CFU counts in both male and female mice compared to FA exposure, except for wild type females at 24 h, and wild type males at the 48 h time point (Fig. 1).
Fig. 1.
Lung, blood, and spleen CFU counts in FA-exposed and ozone-exposed wild type and SP-A (−/−) male and female mice during K. pneumoniae infection. Wild type and SP-A (−/−) male and female mice were exposed to ozone (solid bars, O3) or to filtered air (open bars, FA; control) and then intratracheally infected with K. pneumoniae bacteria. Blood, lung, and spleen were analyzed for bacterial number at 12, 24, and 48 h post-infection, as described in Methods. Note that the scale on the Y-axis differs in each experimental analysis. The CFU numbers are expressed as CFU per 20 µl of either whole blood or original organ homogenate (i.e. before serial dilutions). For statistical analysis, log10 transformed data were used. Significant differences between groups of mice are shown with brackets. If no bacteria were detected (CFU = 0) in a given sample, the “log10” of these data was assumed to be equal to zero. If significant differences of transformed data were found with the Mann–Whitney Rank Sum Test, as indicated in Methods, this was shown with “*” under the brackets. Two asterisks (**) depict a significant difference with Mann–Whitney Rank Sum Test of untransformed data only. The notations “O3”, “S”, and “WT-KO” under the brackets show significant differences between ozone-exposed and FA-exposed animals, between males and females, and between wild type and SP-A (−/−) mice, respectively. The number of independent experiments for each condition was 3, as described in Methods, except for wild type females at the 24 h time point and for SP-A (−/−) females at the 48 h time point (n = 4). The total number of mice used in each group is shown at the bottom of the figure.
2.1.1.2. Spleen and blood CFU
The CFU results in both blood and spleen (both reflect dissemination of infection) showed a similar pattern in both types (wild type, SP-A (−/−)) of mice (Fig.1). At 12 h post-infection there were no significant differences between any of the compared groups. However, differences were found at the 24 and 48 h time points. At the 24 h time point, the blood of FA-exposed SP-A (−/−) males and spleens of FA-exposed wild type males were found to have significantly higher CFU counts compared to ozone-exposed mice. However, higher (p < 0.05) blood CFU counts were found in SP-A (−/−) ozone-exposed males compared to SP-A (−/−) FA-exposed animals at 48 h post-infection. No significant differences in blood and spleen CFU counts in response to ozone were found in females in both types of mice (Fig. 1).
2.1.1.3. Lung weight
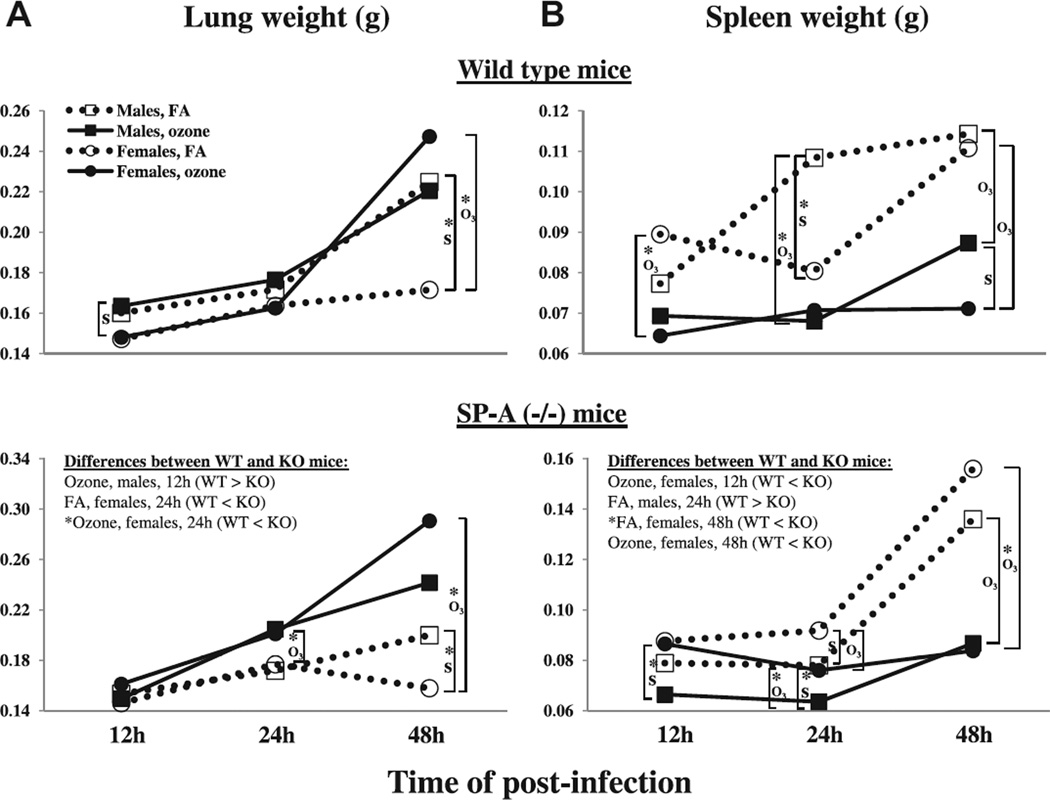
In the wild type mice, ozone exposure was found to significantly increase lung weight in females compared to FA-exposed animals at the 48 h post-infection time point (Fig. 2). No significant differences in the lung weights were found between ozone-exposed and FA-exposed male mice at any of the time points studied. Similar to the wild type mice, in the SP-A (−/−) mice, significantly higher lung weights were observed in ozone-exposed females compared to FA-exposed females at the 24 and 48 h time points, with no significant differences in response to ozone exposure in males. Fig. 2 also demonstrates that by the 48 h time point, ozone-exposed wild type and SP-A (−/−) females showed the highest increase in their lung weight, whereas FA-exposed female mice showed the least alteration in lung weight.
Fig. 2.
Lung and spleen weights in FA-exposed and ozone-exposed wild type and SP-A (−/−) male and female mice during K. pneumoniae infection. The data for this Figure were from the same mice used in Fig. 1, and the experimental design, number of experiments and number of mice were as described in the legend for Fig. 1. Panels A and B depict the results for lung and spleen weights, respectively. The results for wild type, and SP-A (−/−) mice are on the top, and on the bottom of the Figure, respectively. The organ weights for FA-exposed animals are shown with dotted lines and opened symbols, and organ weights for ozone-exposed mice are shown with solid lines and solid symbols. Squares and circles were used for males and females, respectively. Significant differences were determined within the same time point post-infection and are shown with brackets. Significant differences between wild type and SP-A (−/−) mice are shown in the SP-A (−/−) mouse panels. The notations “*”, “O3“ and “S” under the brackets are the same as those indicated in Fig. 1.
2.1.1.4. Spleen weight
In the wild type mice, ozone was found to significantly decrease spleen weight in both male (at 24 and 48 h post-infection) and female (12 and 48 h post-infection) mice compared to FA-exposed animals (Fig. 2). In the SP-A (−/−) mice, ozone exposure significantly decreased the weight of the spleen in both males and females at 24 and 48 h post-infection. Fig. 2 also demonstrates that by the 48 h time point, FA-exposed wild type and SP-A (−/−) females showed the highest increase in their spleen weight, whereas ozone-exposed female mice showed the lowest alteration in spleen weight. These trends are opposite to the trends seen in the changes of lung weight during pneumonia in response to ozone exposure in both wild type and SP-A (−/−) mice (Fig. 2).
2.1.1.5. Plasma sex hormone levels
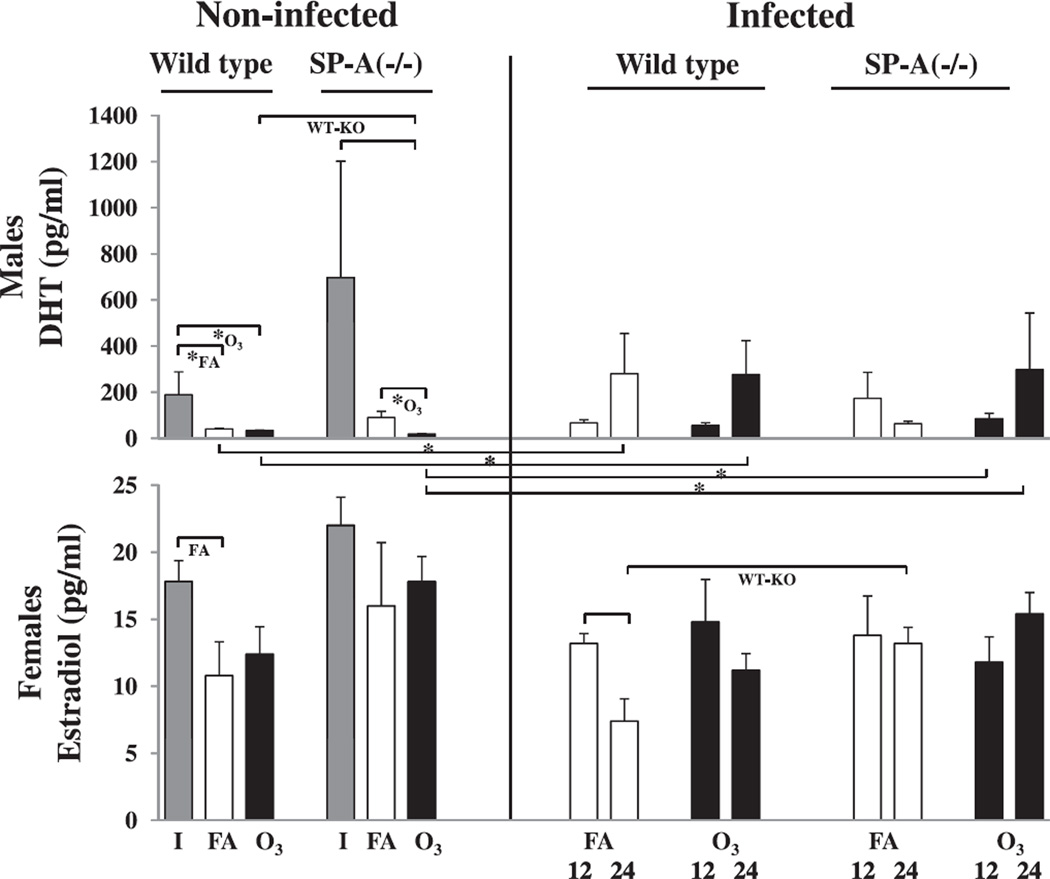
No significant sex hormone changes were observed in response to ozone either in males, or in females (Fig. 3).
Fig. 3.
Sex hormone levels in FA-exposed and ozone-exposed wild type and SP-A (−/−) male and female mice during K. pneumoniae infection. After FA or ozone exposure and subsequent infection with K. pneumoniae, as described in the legend for Fig. 1, plasma was collected and analyzed at 12 and 24 h post-infection for sex hormone concentrations in males and females, as described in Methods. Data from non-infected and infected mice are shown on the left and right, respectively. DHT and estradiol concentrations in plasma from intact (I), FA-exposed (FA), and ozone-exposed (O3) animals are shown with solid grey, open, and solid black bars, respectively. Significant differences are shown with brackets. The notations “*”, “O3“ and “WT-KO” under the brackets are the same as those indicated in Fig. 1, and the notation “FA” under the bracket show significant differences between FA-exposed and intact mice. The number of mice was 5 for each condition from three independent experiments.
2.1.1.6. Plasma cortisol levels
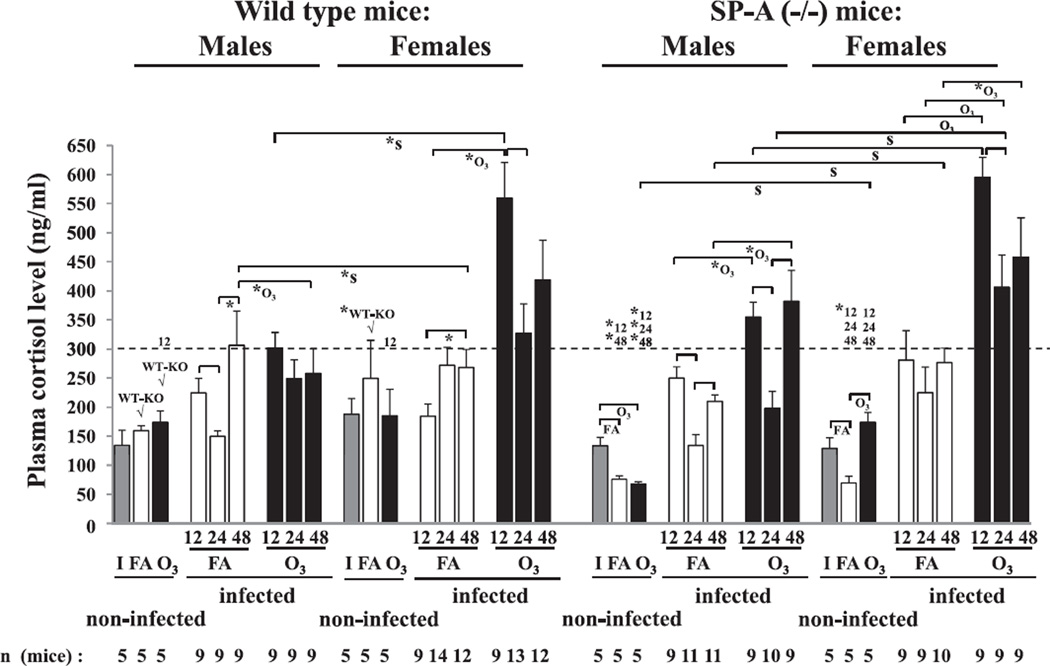
Ozone-exposed animals of both sexes in both wild type and SP-A (−/−) mice demonstrated significantly higher cortisol levels compared to FA-exposed (control) animals (Fig. 4).
Fig. 4.
Plasma cortisol levels in FA-exposed and ozone-exposed wild type and SP-A (−/−) male and female mice during K. pneumoniae infection. After FA or ozone exposure, mice were infected with K. pneumoniae as described in the legend of Fig. 1, and plasma was collected and analyzed for cortisol concentration as described in Methods. Cortisol concentration in plasma from intact (non-exposed and non-infected) animals is shown with solid grey bars (I); plasma cortisol level for FA-exposed (FA) and ozone-exposed (O3) animals is shown with open and solid black bars, respectively. Significant differences are shown with brackets. The notation “FA” is the same as that indicated in Fig. 3, and notations “*”, “O3”, “S”, and “WT-KO” under the brackets are the same as those indicated in Fig. 1. The “WT-KO” differences between FA- or ozone-exposed non-infected wild type and SP-A (−/−) males, and between FA-exposed non-infected wild type and SP-A (−/−) females were additionally indicated with “√”. The dotted reference line at the level of 300 ng/ml is shown to better visualize the differences between different groups of mice. The numbers 12, 24, and 48 above the reference line show significant differences between FA- and ozone-exposed non-infected, and infected mice of the same type (wild type or SP-A (−/−)) and sex, from the 12, 24, and 48 h time points post-infection, respectively. The number of independent experiments was 3 (for wild type females at 24 h post-infection was n = 4), and the number of mice used for analysis is shown on the bottom of the Figure.
2.1.2. Effect of sex
2.1.2.1. Lung CFU
Ozone exposure resulted in significantly higher CFU counts in females compared to their male counterparts at 12 h in SP-A (−/−) mice, and at 48 h post-infection in wild type mice (Fig.1). FA-exposed SP-A (−/−) females showed significantly higher numbers of bacteria in their lungs compared to SP-A (−/−) males at the 12 h time point (Fig. 1).
2.1.2.2. Spleen and blood CFU
FA-exposed males demonstrated higher (p < 0.05) CFU numbers in both blood and spleen compared to FA-exposed females in both wild type and SP-A (−/−) mice at the 24 h time point post-infection (Fig.1). However, significantly higher CFU counts were found in the blood of ozone-exposed wild type females compared to ozone-exposed males at the 48 h time point (Fig. 1).
2.1.2.3. Lung weight
Lungs from ozone-exposed wild type males had significantly higher weights than those from ozone-exposed wild type females at 12 h post-infection (ozone: males > females), and FA-exposed males had significantly higher lung weights than those from FA-exposed females at 48 h time point (FA: males > females) (Fig. 2). FA-exposed SP-A (−/−) male mice also demonstrated higher lung weights (p < 0.05) compared to FA-exposed SP-A (−/−) female mice at the 48 h time point (Fig. 2).
2.1.2.4. Spleen weight
Mouse spleens from FA-exposed wild type males were found to have significantly higher weight than those fromfemales at 24h post-infection (FA:males > females), and spleen weights from wild type ozone-exposed males were higher than those from females at 48 h post-infection (ozone: males > females) (Fig. 2). In contrast to wild type mice, the SP-A (−/−) ozone-exposed females demonstrated significantly higher spleen weights compared to their male counterparts at the 12 and 24h time points, and FA-exposed SPA (−/−) female mice showed higher (p < 0.05) spleen weights than males at the 24 h time point (Fig. 2).
2.1.2.5. Plasma sex hormone levels
In general, female infected mice showed a trend toward decreased estradiol levels at the 24 h time point compared to the 12 h in both FA- and ozone-exposed mice (with p < 0.05 in wild type FA-exposed females), whereas males showed a trend toward increased DHT levels at the 24 h time point compared to the 12 h in both FA- and ozone-exposed mice (Fig. 3).
2.1.2.6. Plasma cortisol levels
During the course of infection, a significant decrease in cortisol levels was observed in the FA-exposed wild type and SP-A (−/−) males (but not in females) at the 24 h time point compared to the 12 h time point (Fig. 4). Ozone-exposed mice (except for wild type males) demonstrated a similar change. In all cases in males described above there was a significant increase at the 48 h time point where the levels of cortisol reached those observed at the 12 h time point (Fig. 4).
Both FA- and ozone-exposed wild type and SP-A (−/−) female mice had higher (p < 0.05) plasma cortisol levels compared to male mice (Fig. 4). However, major sex differences were found in ozone-exposed animals. Ozone exposure in both wild type and SP-A (−/−) mice resulted in a 2–3 fold increase in the cortisol levels in females compared to males at the 12 h time point. This increase was attenuated at the 24 h time point, and in most cases became equivalent to the 12 h time point in males (Fig. 4).
2.1.3. Effect of SP-A
2.1.3.1. Lung CFU
Significantly higher lung CFU counts were found in FA-exposed SP-A (−/−) females compared to FA-exposed wild type females at the 12 h time point (Fig. 1).
Dynamic changes in bacterial CFU numbers in mouse lungs during the course of pneumonia revealed a significant increase in all groups of wild type mice, except in ozone-exposed males between 24 and 48 h, and 12 and 48 h, and FA-exposed females between 24 and 48 h (Table 1). In SP-A (−/−) mice, bacterial proliferation was not significantly increased between the 12 and 24 h and 24 and 48 h in FA-exposed males, and between 12 and 24 h in FA-exposed females (Table 1).
Table 1.
CFU changes through the early post-infection period (12–48 h) in the wild type and SP-A (−/−) mouse blood, lung, and spleen, and changes in the lung and spleen weights during the course of pneumonia.
| Sample | Sex | Treatment | CFU number | Organ weight | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period post-infection | ||||||||||||||
| 12–24 h | 24–48 h | 12–48 h | 12–24 h | 24–48 h | 12–48 h | |||||||||
| WT | KO | WT | KO | WT | KO | WT | KO | WT | KO | WT | KO | |||
| Lung | Males | FA | + | − | + | − | + | + | − | + | + | − | + | + |
| Ozone | + | + | − | ++ | − | + | ++ | + | ++ | − | + | + | ||
| Females | FA | + | − | − | + | + | + | ++ | ++ | − | *↓ | ++ | − | |
| Ozone | + | + | + | + | + | + | ++ | ++ | + | + | + | + | ||
| Blood | Males | FA | + | + | − | − | + | + | ||||||
| Ozone | + | + | − | + | + | + | ||||||||
| Females | FA | + | − | + | + | + | + | |||||||
| Ozone | + | − | + | + | + | + | ||||||||
| Spleen | Males | FA | + | + | − | + | + | + | + | − | − | + | + | + |
| Ozone | + | + | + | + | + | + | − | − | ++ | ++ | ++ | + | ||
| Females | FA | + | − | + | + | + | + | − | − | + | + | + | + | |
| Ozone | + | + | + | + | + | + | − | − | − | − | − | − | ||
The data from Figs. 1 and 2 were used for the analysis shown in this Table. Comparisons were made between groups of mice of the same type (i.e. wild type (WT) or SP-A (−/−) KO)), and the same sex that were subjected to the same treatment (i.e. to filtered air (FA) or ozone (O3) exposures) during the course of pneumonia (i.e. between the following time points post-infection: 12 and 24 h, 24 and 48 h, and 12 and 48 h). For statistical analysis, log10 transformed data for the CFU numbers were used, and untransformed data were used for the organ weights. Significant differences (p <0.05) were assessed by a t-test (shown with “++”, and by a Mann–Whitney Rank Sum Test (shown with “+”) for the cases where either normality or equal variance tests failed, as suggested by the SigmaPlot 10.0 software. If no significant differences were detected between compared groups, these are indicated with “− ”. In all cases the values for CFU numbers and organ weights were increased with increased time post-infection. However, lung weight of FA-exposed SP-A (−/−) female mice was decreased from 24 to 48 h time point (shown with asterisk).
2.1.3.2. Spleen and blood CFU
Ozone-exposed SP-A (−/−) males had a significantly higher number of bacteria in the spleen compared to ozone-exposed wild type males at the 48 h time point (Fig. 1).
Analysis of dissemination of infection in the dynamic state revealed significant increases in the blood and spleen CFU numbers in all groups of wild type mice during the course of pneumonia, except in the blood of FA- and ozone-exposed males, and spleen of FA-exposed males during the 24–48 h post-infection interval (Table 1). In SP-A (−/−) mice, bacterial proliferation was not significantly increased in the blood of FA-exposed males and FA- and ozone-exposed females between 24 and 48 h and between 12 and 24 h, respectively, and in the spleen of FA-exposed females between 12 and 24 h post-infection (Table 1).
2.1.3.3. Lung weight
Ozone-exposed wild type male mice were found to have significantly higher lung weights compared to ozone-exposed SP-A (−/−) males at the 12 h time point, and both FA- and ozone-exposed wild type female mice had lower (p < 0.05) lung weights compared to their respective SP-A (−/−) female counterparts at the 24 h post-infection (Fig. 2)
Analysis of the wild type mouse lung weights during the course of pneumonia revealed a significant increase in the lung weight in all groups of mice, with the exception of FA-exposed males between 12 and 24 h, and FA-exposed females between 24 and 48 h post-infection (Table 1). In SP-A (−/−) mice, the lung weights did not change between the 24 and 48 h time points in FA- and ozone-exposed males, and between 12 and 48 h in FA-exposed females. Importantly, the FA-exposed SP-A (−/−) female mouse lung weights were even significantly decreased during the 24–48 h period of post-infection (Table 1).
2.1.3.4. Spleen weight
Analysis of spleen weights during the course of pneumonia revealed that only FA-exposed wild type male mice exhibited a significant increase of their spleen weights during the 12–24 h interval (Table 1). However, between the 24 and 48 h time points they did not show a significant increase in their spleen weights. Importantly, ozone-exposed wild type and SP-A (−/−) females did not show any significant changes in their spleen weights during the course of pneumonia infection (Table 1).
2.1.3.5. Plasma sex hormone levels
FA-exposed SP-A (−/−) female mice had a significantly higher plasma estradiol concentration than wild type mice at the 24 h time point post-infection (Fig. 3).
2.1.3.6. Plasma cortisol levels
No significant differences were observed between wild type and SP-A (−/−) mice in the presence or absence of ozone-induced oxidative stress (Fig. 4).
2.2. Plasma sex hormone and cortisol levels in the non-infected mice
The plasma from the non-infected mice was used for an additional control in the sex hormone and cortisol tests.
2.2.1. Sex hormone levels
Among non-infected animals, a significant reduction in the sex hormone concentrations were found between intact (not exposed to FA or ozone and not infected) and FA or ozone-exposed wild type males, and between intact or FA-exposed and ozone-exposed SP-A (−/−) male mice (Fig. 3). Ozone-exposed wild type males showed significantly higher levels of DHT compared to SP-A (−/−) mice. In females, a significant reduction in the estradiol level was only found in FA-exposed wild type females compared to intact female mice (Fig. 3).
Regarding the differences between non-infected and infected mice, FA-exposed and ozone-exposed wild type non-infected male mice had lower (p < 0.05) levels of DHT compared to FA-exposed and ozone-exposed wild type infected males at 24 h post-infection, respectively, and ozone-exposed non-infected SP-A (−/−) males demonstrated significantly higher DHT levels compared to ozone-exposed and infected animals at both 12 and 24 h time points post-infection (Fig. 3).
2.2.2. Cortisol levels
The SP-A (−/−) non-infected male mice exposed to ozone or FA, and female mice exposed to FA had significantly lower levels of plasma cortisol compared to the wild type mice, although no significant differences were observed for intact mice either between males and females or between wild type and SP-A (−/−) mice (Fig. 4). Both FA- and ozone-exposed infected SP-A (−/−) male and female mice compared to non-infected mice demonstrated an increase (p < 0.05) in cortisol levels, but in wild type mice this increase was observed only in ozone-exposed wild type mice of both sexes (Fig. 4).
2.3. Summary of results
Thus, in general, based on the CFU numbers, ozone exposure decreases the ability of mice to clear K. pneumonia bacteria from the lung, and ozone-exposed females are more affected than males. However, in the absence of ozone-induced oxidative stress, males are more predisposed to have a higher level of dissemination of infection compared to females.
For the lung and spleen weights, during the 48 h period of pneumonia, ozone exposure showed opposite trends in the mouse lung and spleen weights: increased lung weights but decreased spleen weights in both sexes, and in both cases, ozone-exposed female lungs and spleens were the most affected.
For the cortisol levels, in general, we found, both wild type and SP-A (−/−) infected mice to demonstrate the same trends in plasma cortisol level changes: ozone-exposed > FA-exposed; females > males (with the major differences observed as noted above in the ozone-exposed animals); and infected > non-infected (except for FA-exposed wild type males and females).We also found differences in cortisol levels in non-infected FA- and ozone-exposed wild type mice > non-infected FA- and ozone-exposed SP-A (−/−) mice (with no differences after infection); intact males = intact females; and intact wild type mice = intact SP-A (−/−) mice.
3. Discussion
The clinical course of lung disease is influenced by a number of factors, including sex and environment. We have shown previously [57] that although female mice had higher survival rates than males after K. pneumoniae infection, ozone exposure reversed this pattern. Our results also demonstrated that SP-A (−/−) mice are more susceptible to K. pneumoniae infection than wild type mice [43]. In this study, we investigated the hypothesis that a) with prior exposure (or lack of) to ozone-induced oxidative stress, events occurring in the first two days of K. pneumoniae infection are responsible for the previously observed survival sex differences, and b) SP-A is involved in these differences. Pulmonary clearance, dissemination of infection into the blood and spleen, plasma sex hormone and cortisol levels were examined during the first two days post-infection.
In both types of mice (wild type and SP-A (−/−)) we found that: 1) Whereas ozone exposure decreased the ability of mice to clear K. pneumoniae bacteria from the lung, with ozone-exposed females being affected more than males, in the absence of ozone-induced oxidative stress males were more prone to develop a higher level of bacterial dissemination compared to females; 2) Ozone exposure showed opposite trends in the mouse lung and spleen weights: an increase in lung weight, but a decreased in the spleen weight in both sexes, and in both cases ozone-exposed females were affected more; 3) No differences were found between FA- and ozone-exposed infected animals of both sexes for plasma DHT and estradiol levels during the first day post-infection; 4) Plasma cortisol levels were changed as follows: ozone-exposed > FA-exposed; females > males (with greater differences observed in the ozone-exposed animals); and infected > non-infected (except for FA-exposed wild type males and females that showed no differences as a function of infection). These observations together indicate that several and perhaps different factors contribute to the survival rate differences between males and females in the presence or absence of ozone-induced oxidative stress that we reported previously [43,57]. The decreased pulmonary clearance, the compromised spleen response, and the increased plasma cortisol level in females are likely contributing factors to the increased mortality of ozone-exposed females compared to ozone-exposed males; the increased bacterial dissemination of lung infection in males may contribute to the increased mortality of FA-exposed males compared to FA-exposed females.
Interaction of lung host defense mechanisms with invading bacterial pathogens results either in pulmonary clearance or in the establishment of a favorable environment for bacterial colonization and subsequent dissemination of the infection. In this study, in the absence of oxidative stress, the FA-exposed K. pneumoniae-infected female mice demonstrated an advantage over males by virtue of a lower dissemination of K. pneumoniae bacteria as assessed by the lower blood and spleen CFU counts. The lung weight was minimally changed in females compared to males during the course of infection indicating milder pathological processes in the lungs of the female mice that, in turn, may result in a better resolution of infection in females than in males. The lower bacterial dissemination from the lung into the blood in FA-exposed females could prevent or minimize multiple organ dysfunction and increased mortality rate, complications observed following Klebsiella bacteremia [3]. In fact, the expansion of bacteria into the blood and spleen and the spleen weight were all rapidly increased by 24 h post-infection in FA-exposed males compared to FA-exposed females indicating that dissemination occurs earlier and at a higher level in males. These findings are similar to those from experimental studies of trauma-hemorrhage followed by bacterial sepsis. Male mice were more susceptible to sepsis than females and it was suggested that the male sex hormones were responsible for this effect [58–60]. Our recent findings [61] indicates that sex hormones do play a role in survival after infection in the presence or absence of ozone-induced oxidative stress. Bacteremia may also result in more pronounced extra-pulmonary lesions in males compared to females. Indeed, FA-exposed male mice [62] were more predisposed to liver infarction and splenic thromboemboli. Thus, the increased bacteremia in FA-exposed males (the finding in this study) supports our previous observation. These findings together may explain the advantage of females over males in the survival from pneumonia in the absence of ozone-induced oxidative stress [43,57].
In the presence of ozone-induced oxidative stress prior to K. pneumoniae infection, the host lung immune defense mechanisms appeared to be markedly compromised. At most of the studied time points, ozone-exposed both males and females showed significantly higher lung CFU counts compared to FA-exposed mice. Moreover, ozone-exposed females exhibited significantly lower lung bacterial clearance compared to their male counterparts, with SP-A (−/−) females showing this difference earlier (12 h) than wild type mice (48 h). These observations may explain the survival advantage of males over females in the susceptibility to pneumonia [43,57].
Some interesting observations were made following analysis of the changes in lung and spleen weights at 48 h post-infection between sexes. The increase in the lung weight in ozone-exposed females correlated with respective changes in the CFU counts, indicating that pulmonary clearance is one of the major risk factors for survival of ozone-exposed females compared to ozone-exposed males. Unlike the lung weight changes, the spleen weight in both ozone-exposed males and females was significantly lower than in FA-exposed animals at 48 h post-infection. These observations indicate that ozone exposure, with subsequent lung infection and bacterial dissemination into bloodstream and spleen, may result in an inadequate spleen immune response, and thus compromise host defense. Alternatively, the lower weight of the spleen in ozone-exposed animals may reflect depletion of leukocytes through an imbalance of demand and production. However, this is unlikely because spleen red pulp myelopoesis, an indication of proliferation of leukocytes, was shown to be reduced in ozone-exposed males and females [62].Moreover, the fact that the spleen weight in the absence of infection in ozone-exposed mice was significantly (at least in wild type mice) reduced compared to FA-exposed animals (Fig. 5) indicates that the absence of an appropriate spleen immune response to bacterial infection is due primarily to ozone-induced oxidative stress and not to infection. Furthermore,we found that the spleen weight of ozone-exposed females did not change significantly in response to gradual increases of the spleen CFU counts during the course of pneumonia (12–48 h), whereas in ozone-exposed males the spleen weight was increased after 24 h. Thus, the female spleen appears to be affected more by ozone exposure than the male spleen and, this may lead to a greater functional compromise of the spleen in females in response to pneumonia infection.
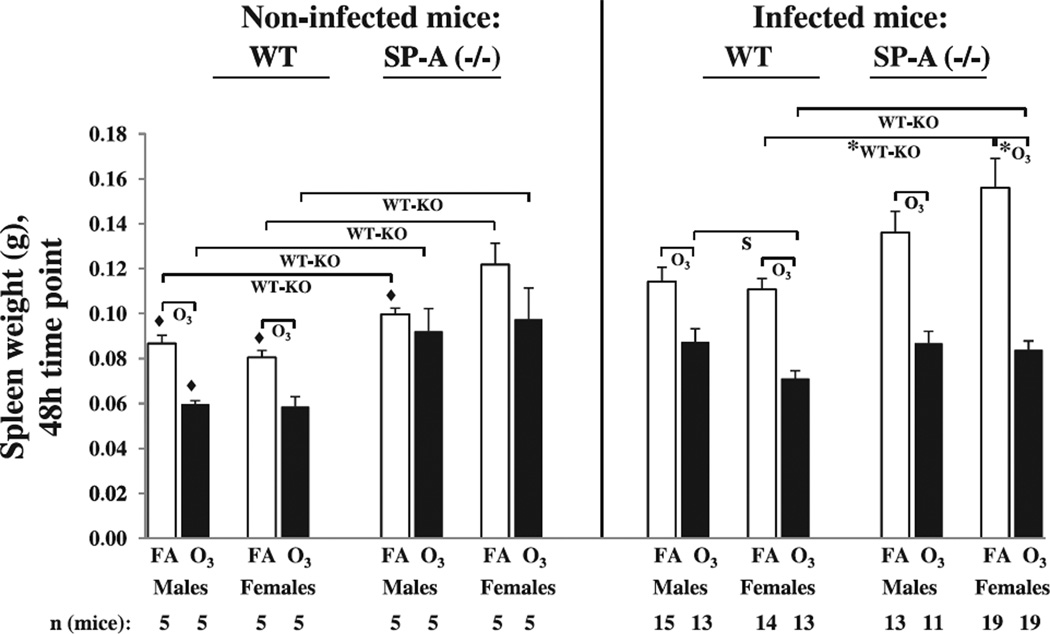
Fig. 5.
Comparison of the spleen weights in the non-infected and infected wild type and SP-A (−/−) male and female mice after filtered air and ozone exposure at 48 h post-infection. Wild type and SP-A (−/−) male and female mice were exposed to ozone (solid bars, O3) or to filtered air (open bars, FA; control) and then intratracheally infected with K. pneumoniae bacteria or inoculated with PBS only (sham control), as described in Methods. The spleen weights were analyzed at 48 h post-infection. In this Figure, the spleen weight data from infected mice were from Fig. 2. Significant differences are shown with brackets. The notations “*”, “O3“, “S”, and “WT-KO” under the brackets are the same as those indicated in Fig. 1. Symbol “◆” shows significant differences between respective groups of non-infected and infected mice. The total number of mice used in each group is shown on the bottom of the Figure.
The lack of significant changes in spleen weight indicates that ozone-exposed animals (and especially females) may not be able to respond appropriately to infection. To the contrary, a rapidly enlarging spleen in response to increased bacteremia in FA-exposed males compared to FA-exposed female mice at the 24 h post-infection reflects excessive bacterial proliferation. This produces large amounts of LPS, which, in turn, can contribute to the clogging of the blood vessels [63], cause splenic thromboemboli, and interfere with spleen function. Both, spleen hypofunction (as in ozone-exposed females) or rapid increase of dissemination and subsequent enlarging of spleen (as in FA-exposed males) represent examples of a sex-related disadvantage in the course and outcome of pneumonia in the presence or absence of oxidative stress, respectively. Thus, based on our data, we speculate that during the first two days post-infection, the major factors that affect survival from pneumonia between males and females differ in the presence or absence of oxidative stress. The increased dissemination of infection may be among the most significant risk factors for the higher mortality [43,57] observed in FA-exposed males compared to FA-exposed females, and the reduced pulmonary clearance and spleen hypofunction may be among the mechanisms contributing to the higher mortality of ozone-exposed females compared to ozone-exposed males.
Sex hormones have been shown to influence lung function, the course of disease, and the response to environmental agents in animal models [64]. However, we found that the concentration of sex hormones at 12 and 24 h post-infection time points did not differ significantly between FA- and ozone-exposed mice in either sex, indicating that early in the course of infection, sex hormones do not contribute to differences between ozone- and FA-exposed animals.
It is known that stress has an immunomodulatory effect [65], and stress hormones, glucocorticoids, inhibit production of Th1 cytokines (regulate cell-mediated immunity) and induce the production of Th2 cytokines (regulate humoral immunity) by lymphocytes [65–68]. The shift in Th1/Th2 cytokine levels toward Th2 has been demonstrated via the use of a synthetic glucocorticoid, dexamethasone [69]. Plasma cortisol levels in our infection experimental model were associated with sex and ozone-induced oxidative stress differences. In pneumonia-infected mice, the level of cortisol was increased in response to oxidative stress (ozone-exposed > FA-exposed) and in females (females > males). Because cortisol inhibits production of Th1 cytokines that are critical for cell-mediated immunity (which is especially important in the beginning of the infection process), the increased cortisol levels in ozone-exposed and infected males and females may be one of the factors responsible for reduced cell immune defense and increased susceptibility to pneumonia of these animals compared to their FA-exposed counterparts [43,57]. The higher plasma cortisol level in ozone-exposed and infected females compared to males may provide a relatively higher impairment of cell-mediated immune defense in ozone-exposed females, and this may be responsible for their higher mortality after pneumonia [43,57].
In an attempt to synthesize the findings from the present study and the relevant findings from the literature where this mouse model has been used, we provide a summary in the diagrams shown in Figs. 6 and 7, to depict the impact of ozone (Fig. 6) and sex (Fig. 7) on the previously observed survival rates [43,57]. It has been previously shown [43] that the survival and the phagocytic index of alveolar macrophages in wild type mice were higher than in SP-A (−/−) mice. The observations from the present study (of plasma estradiol levels, spleen and lung CFUs and weights), together with the previously published findings [43] indicate that the absence of SP-A may have a further negative impact on pneumonia survival.
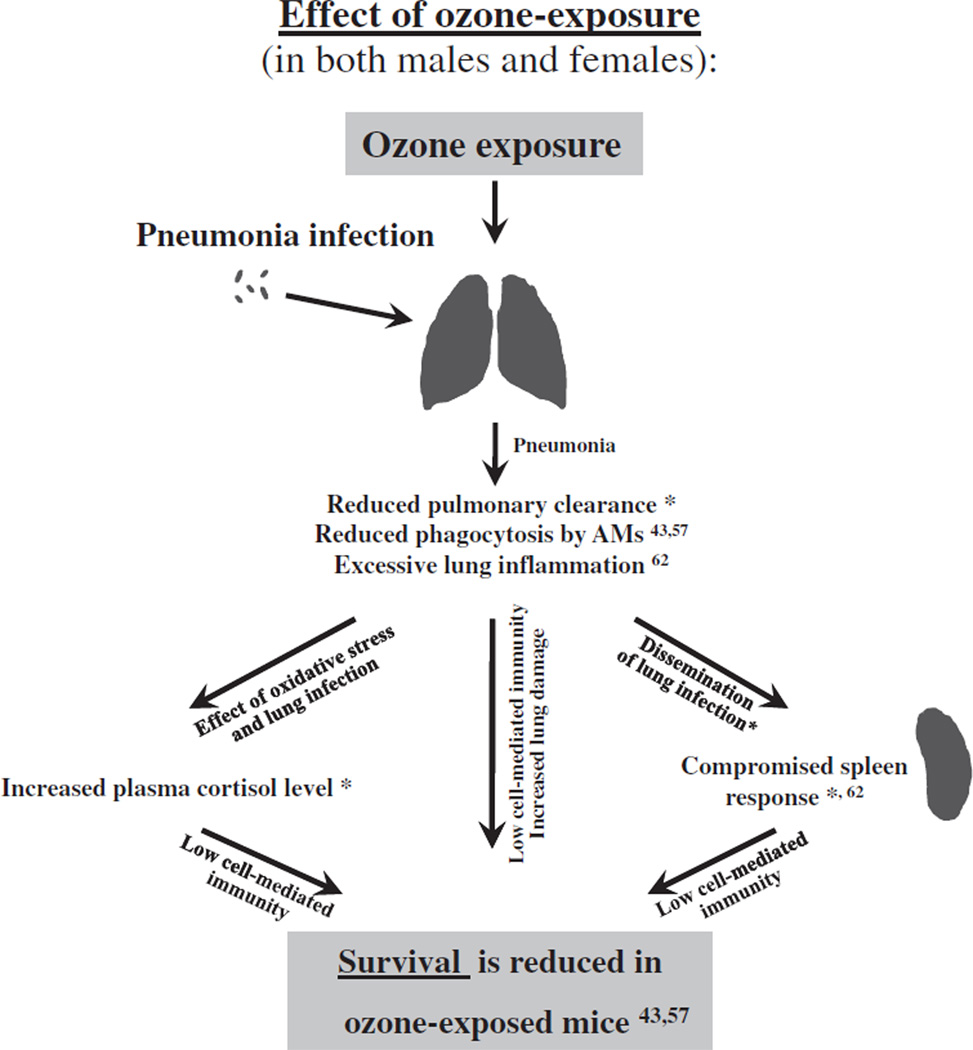
Fig. 6.
Overview of the effect of ozone exposure on the outcome of pneumonia. The effect of ozone exposure on mouse survival after pneumonia is depicted diagrammatically. We have observed previously that ozone-exposed male and female mice exhibited lower survival than FA-exposed mice after pneumonia [43,57]. A summary of the findings from the present study and the literature where this mouse model has been used is provided to indicate factors or processes that may explain and/or contribute to the previously observed differential survival in response to ozone exposure. Reduced pulmonary clearance (this study*), reduced level of in vivo phagocytosis by alveolar macrophages [43,57], excessive lung inflammation [62], a compromised spleen response to infection (this study*, and [62]), and increased plasma stress hormone cortisol level (this study*) may account for the survival difference between ozone- and FA-exposed animals (males or females: ozone < FA) [43,57].
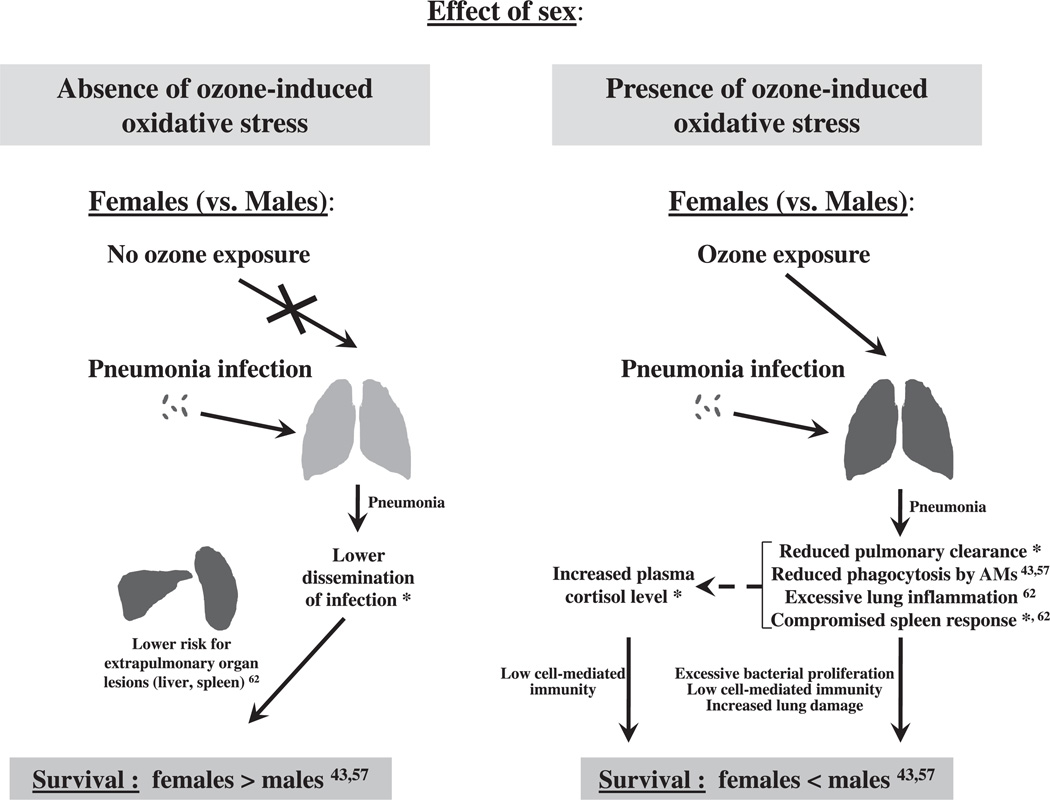
Fig. 7.
Overview of the effect of sex on the outcome of pneumonia. The effect of sex on mouse survival after pneumonia in the presence or absence of ozone-induced oxidative stress is shown diagrammatically. We have observed previously that FA-exposed females exhibited a higher survival than FA-exposed males but ozone-exposed females showed a lower survival than ozone-exposed males after pneumonia [43,57], indicating that different mechanisms may be involved. Indeed, in the absence of ozone-induced oxidative stress, decreased dissemination of lung infection in FA-exposed females (this study*) may result in a lower risk for extrapulmonary organ lesions compared to FA-exposed male mice [62]. This, in turn, may account for a higher survival in FA-exposed females after pneumonia compared to FA-exposed males (FA: females > males) [43,57]. However, in the presence of ozone-induced oxidative stress, reduced pulmonary clearance (this study*), reduced level of in vivo phagocytosis by alveolar macrophages [43,57], excessive lung inflammation [62], a compromised spleen response to the infection (this study*), and increased plasma cortisol level (this study*) in ozone-exposed females may account for their lower survival after pneumonia compared to ozone-exposed males (ozone: females < males) [43,57].
3.1. Conclusions
Our data indicate that sex differences exist in the course of pneumonia during the first two days. Pulmonary clearance, the ability to limit bacterial dissemination, spleen response to the infection, and stress hormone influences play a crucial role in the differential outcome of pneumonia in males and females in the presence or absence of oxidative stresses.
4. Materials and methods
4.1. Animals
Male and female wild type C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) and SP-A (−/−) mice on the C57BL/6J background were used at 8–12 weeks of age. Animals were maintained under approved housing conditions and fed rodent chow and autoclaved water ad libitum. SP-A (−/−) mice were propagated and raised under specific pathogen-free conditions in a barrier facility at the Penn State College of Medicine. The SP-A (−/−) mice and sentinel mice housed in the same room showed no evidence of respiratory pathogens. The Penn State Hershey Medical Center Institutional Animal Care and Use Committee (IACUC) approved all procedures involving animals.
4.2. Preparation of bacteria
K. pneumoniae bacteria (ATCC 43816) were obtained from the American Tissue Culture Collection (Rockville, MD) and prepared as described previously [57]. In brief, bacteria were grown for 18 h in TSB media at 37 °C to reach stationary phase. The overnight bacterial suspension was next diluted until the OD660 was equal to 0.4, and 200 µl were used to inoculate 50 ml of fresh TSB for sub-cultivation for 3 h to reach mid-log phase of growth. The subculture was then placed on ice to stop growth and serially diluted in PBS to obtain ~9 × 103 CFU/ml, and 50 µl of this bacterial suspension (containing ~ 450 CFU) were used immediately to infect mice. CFU per ml values were estimated based on the standard curve obtained at OD660 of the bacterial suspension, and an aliquot was also spread on TSA plates to confirm CFU estimates.
4.3. Exposure of mice to ozone
Mice were exposed to ozone (2 ppm for 3 h) or to FA (control) at the same time in separate chambers as described [70]. Mice were infected immediately after exposure.
4.4. Infection of mice with K. pneumoniae
Infection was performed as described previously [57]. Briefly, the animals were anesthetized, the trachea was surgically exposed and ~450 CFU/mouse were inoculated intratracheally in 50 µl of PBS. In cases where infected mice were moribund with no chance of recovery, they were euthanized to prevent unnecessary suffering according to Penn State University IACUC recommendations. After exposure to ozone and subsequent infection, lung, blood, and spleen were investigated for CFU numbers, lung and spleen were studied for organ weight changes, and plasma was analyzed for sex hormone and cortisol levels, as described below. In some experiments a sham control of infection was used, as described in the respective Figure legends. For this, mice were inoculated with 50 µl of PBS instead of bacterial suspension.
4.5. Blood, spleen, and lung CFU analyses
Mice were sacrificed at 12, 24, and 48 h post-infection, and their blood, spleen, and lungs were analyzed for CFU numbers. Three independent experiments were performed for each time point (except n = 4 for wild type females at the 24 h time point, and for SP-A (−/−) females at the 48 h time point). Each experiment involved 5 mice exposed to O3, and 5 exposed to FA, or a total of 180 wild type mice, and 174 SP-A (−/−) mice were analyzed. Mice were anesthetized, sacrificed, and blood was collected from the right ventricle of the heart with a 1 ml syringe and 27G 1/2 inch needle and kept on ice in 1.5 ml tubes. Immediately after sampling, 20 µl of whole blood and 1:10 serial dilutions of blood (in cold PBS) were applied in triplicate to TSA plates. The spleen was next removed, placed into a 1.5 ml tube, and kept on ice. The caudal vena cava was then cut, and the whole lungs perfused with 1 ml of 0.9% NaCl through the right ventricle of the heart. Then, the lungs were removed, placed into a 1.5 ml tube, and kept on ice. Both spleen and lung weights were recorded and the organs in their entirety were homogenized in 1 ml of cold PBS on ice, using glass homogenizers (Pyrex 7727-15). Homogenates were serially diluted 1:10 in cold PBS on ice, and aliquots (20 µl) of each undiluted homogenate and the corresponding 1:10 serial dilutions were applied to TSA plates in triplicate. CFU counts were done after the overnight incubation of plates at 37 °C with blood, spleen, or lung samples.
4.6. Plasma cortisol level measurement
The experimental design was the same as that described for blood, spleen, and lung CFU analyses. Blood from mice was collected at 12, 24, and 48 h post-infection and kept on ice. After sampling the blood specimens were spun at 15,000 g at +4 °C for 15 min. Plasma was kept at −80 °C until use. Cortisol levels in plasma were analyzed with a Corticosterone 125 RIA Kit (MP Biomedicals, LLC, Orangeburg, NY) according to the manufacturer’s protocol.
4.7. Plasma sex hormones level measurement
The experimental design was as described above for blood, spleen, and lung CFU analyses. Blood was collected at 24, and 48 h post-infection, kept on ice, and plasma was collected and kept as described for plasma cortisol level measurement. The quantification of dihydrotestosterone (DHT) in the plasma of males was performed with the Dihydrotestosterone ELISA kit (APLCO Diagnostics, Salem, NH), and the estradiol was measured in the plasma of females using the Estradiol radioimmunoassay kit (Siemens Healthcare Diagnostics Inc., Deerfield, IL) according to the manufacturer’s protocols.
4.8. Statistics
All data were analyzed with a t-test using SigmaPlot 10.0 software. In cases where log10 transformed data were used for the statistical analysis, it is indicated in the Figure or Table legends. If either normality or equal variance tests failed, the Mann–Whitney Rank Sum Test was used instead of t-test, as suggested by the SigmaPlot 10.0 software. In Fig. 1, one FA-exposed, and two ozone-exposed SP-A (−/−) male mice at the 48 h time point were eliminated from analysis because their CFU values were ~10 times higher compared to the average in the group (differences were more than 2 standard deviations). Results were considered statistically significant at p < 0.05.
Acknowledgments
We gratefully thank Dr. Robert Bonneau from Pennsylvania State University College of Medicine for his critical review of this manuscript and helpful suggestions. This work was supported by National Institute of Environmental Health Sciences Grant 1RO1-ES09882.
References
- 1.Kofteridis DP, Papadakis JA, Bouros D, Nikolaides P, Kioumis G, Levidiotou S, et al. Nosocomial lower respiratory tract infections: prevalence and risk factors in 14 Greek hospitals. Eur J Clin Microbiol Infect Dis. 2004;23(12):888–891. doi: 10.1007/s10096-004-1245-y. [DOI] [PubMed] [Google Scholar]
- 2.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11(4):589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yinnon AM, Butnaru A, Raveh D, Jerassy Z, Rudensky B. Klebsiella bacteraemia: community versus nosocomial infection. Qjm. 1996;89(12):933–941. doi: 10.1093/qjmed/89.12.933. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DJ, Richet H, Chen LF, Spelman DW, Hung YJ, Huang AT, et al. Seasonal variation in Klebsiella pneumoniae bloodstream infection on 4 continents. J Infect Dis. 2008;197(5):752–756. doi: 10.1086/527486. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen HC. Testosterone regulation of sex differences in fetal lung development. Proc Soc Exp Biol Med. 1992;199(4):446–452. doi: 10.3181/00379727-199-43379. [DOI] [PubMed] [Google Scholar]
- 6.Perelman RH, Palta M, Kirby R, Farrell PM. Discordance between male and female deaths due to the respiratory distress syndrome. Pediatrics. 1986;78(2):238–244. [PubMed] [Google Scholar]
- 7.Caracta CF. Gender differences in pulmonary disease. Mt Sinai J Med. 2003;70(4):215–224. [PubMed] [Google Scholar]
- 8.Gordon HS, Rosenthal GE. The relationship of gender and in-hospital death: increased risk of death in men. Med Care. 1999;37(3):318–324. doi: 10.1097/00005650-199903000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez F, Masia M, Mirete C, Soldan B, Rodriguez JC, Padilla S, et al. The influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogens. J Infect. 2006;53(3):166–174. doi: 10.1016/j.jinf.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Gannon CJ, Pasquale M, Tracy JK, McCarter RJ, Napolitano LM. Male gender is associated with increased risk for postinjury pneumonia. Shock. 2004;21(5):410–414. doi: 10.1097/00024382-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Loeb M, McGeer A, McArthur M, Walter S, Simor AE. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch Intern Med. 1999;159(17):2058–2064. doi: 10.1001/archinte.159.17.2058. [DOI] [PubMed] [Google Scholar]
- 12.Yancey AL, Watson HL, Cartner SC, Simecka JW. Gender is a major factor in determining the severity of mycoplasma respiratory disease in mice. Infect Immun. 2001;69(5):2865–2871. doi: 10.1128/IAI.69.5.2865-2871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Saito H, Setogawa T, Tomioka H. Sex differences in host resistance to Mycobacterium marinum infection in mice. Infect Immun. 1991;59(11):4089–4096. doi: 10.1128/iai.59.11.4089-4096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashman RB, Kay PH, Lynch DM, Papadimitriou JM. Murine candidiasis: sex differences in the severity of tissue lesions are not associated with levels of serum C3 and C5. Immunol Cell Biol. 1991;69(Pt 1):7–10. doi: 10.1038/icb.1991.2. [DOI] [PubMed] [Google Scholar]
- 15.Huber SA, Job LP, Auld KR. Influence of sex hormones on Coxsackie B–3 virus infection in Balb/c mice. Cell Immunol. 1982;67(1):173–179. doi: 10.1016/0008-8749(82)90210-6. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein JA, Alexis N, Barnes C, Bernstein IL, Bernstein JA, Nel A, et al. Health effects of air pollution. J Allergy Clin Immunol. 2004;114(5):1116–1123. doi: 10.1016/j.jaci.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 17.McBride DE, Koenig JQ, Luchtel DL, Williams PV, Henderson WR., Jr Inflammatory effects of ozone in the upper airways of subjects with asthma. Am J Respir Crit Care Med. 1994;149(5):1192–1197. doi: 10.1164/ajrccm.149.5.8173759. [DOI] [PubMed] [Google Scholar]
- 18.Currie WD, van Schaik S, Vargas I, Enhorning G. Breathing and pulmonary surfactant function in mice 24 h after ozone exposure. Eur Respir J. 1998;12(2):288–293. doi: 10.1183/09031936.98.12020288. [DOI] [PubMed] [Google Scholar]
- 19.Kehrl HR, Vincent LM, Kowalsky RJ, Horstman DH, O'Neil JJ, McCartney WH, et al. Ozone exposure increases respiratory epithelial permeability in humans. Am Rev Respir Dis. 1987;135(5):1124–1128. doi: 10.1164/arrd.1987.135.5.1124. [DOI] [PubMed] [Google Scholar]
- 20.Hollingsworth JW, Kleeberger SR, Foster WM. Ozone and pulmonary innate immunity. Proc Am Thorac Soc. 2007;4(3):240–246. doi: 10.1513/pats.200701-023AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang W, Omaye ST. Air pollutants, oxidative stress and human health. Mutat Res. 2009;674(1–2):45–54. doi: 10.1016/j.mrgentox.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol. 2007 doi: 10.1152/ajplung.00174.2007. [DOI] [PubMed] [Google Scholar]
- 23.Annesi-Maesano I, Agabiti N, Pistelli R, Couilliot MF, Forastiere F. Subpopulations at increased risk of adverse health outcomes from air pollution. Eur Respir J Suppl. 2003;40:57s–63s. doi: 10.1183/09031936.03.00402103. [DOI] [PubMed] [Google Scholar]
- 24.Carey MA, Card JW, Voltz JW, Arbes SJ, Jr, Germolec DR, Korach KS, et al. It's all about sex: gender, lung development and lung disease. Trends Endocrinol Metab. 2007;18(8):308–313. doi: 10.1016/j.tem.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright JR, Youmans DC. Pulmonary surfactant protein A stimulates chemotaxis of alveolar macrophage. Am J Physiol Lung Cell Mol Physiol. 1993;264(4 Pt 1):L338–L344. doi: 10.1152/ajplung.1993.264.4.L338. [DOI] [PubMed] [Google Scholar]
- 26.Mikerov AN, Umstead TM, Huang W, Liu W, Phelps DS, Floros J. SP-A1 and SP-A2 variants differentially enhance association of Pseudomonas aeruginosa with rat alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2005;288(1):L150–L158. doi: 10.1152/ajplung.00135.2004. [DOI] [PubMed] [Google Scholar]
- 27.Ding J, Umstead TM, Floros J, Phelps DS. Factors affecting SP-A-mediated phagocytosis in human monocytic cell lines. Respir Med. 2004;98(7):637–650. doi: 10.1016/j.rmed.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Mikerov AN, Wang G, Umstead TM, Zacharatos M, Thomas NJ, Phelps DS, et al. Surfactant protein A2 (SP-A2) variants expressed in CHO cells stimulate phagocytosis of Pseudomonas aeruginosa more than Do SP-A1 variants. Infect Immun. 2007;75(3):1403–1412. doi: 10.1128/IAI.01341-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mariencheck WI, Savov J, Dong Q, Tino MJ, Wright JR. Surfactant protein A enhances alveolar macrophage phagocytosis of a live, mucoid strain of P. aeruginosa. Am J Physiol Lung Cell Mol Physiol. 1999;277(4 Pt 1):L777–L786. doi: 10.1152/ajplung.1999.277.4.L777. [DOI] [PubMed] [Google Scholar]
- 30.Khubchandani KR, Oberley RE, Snyder JM. Effects of surfactant protein A and NaCl concentration on the uptake of Pseudomonas aeruginosa by THP-1 cells. Am J Respir Cell Mol Biol. 2001;25(6):699–706. doi: 10.1165/ajrcmb.25.6.4366. [DOI] [PubMed] [Google Scholar]
- 31.Borron P, McCormack FX, Elhalwagi BM, Chroneos ZC, Lewis JF, Zhu S, et al. Surfactant protein A inhibits T cell proliferation via its collagen-like tail and a 210-kDa receptor. Am J Physiol Lung Cell Mol Physiol. 1998;275(4 Pt 1):L679–L686. doi: 10.1152/ajplung.1998.275.4.L679. [DOI] [PubMed] [Google Scholar]
- 32.Kremlev SG, Umstead TM, Phelps DS. Effects of surfactant protein A and surfactant lipids on lymphocyte proliferation in vitro. Am J Physiol Lung Cell Mol Physiol. 1994;267(4 Pt 1):L357–L364. doi: 10.1152/ajplung.1994.267.4.L357. [DOI] [PubMed] [Google Scholar]
- 33.Brinker KG, Garner H, Wright JR. Surfactant protein A modulates the differentiation of murine bone marrow-derived dendritic cells. American journal of physiology. 2003;284(1):L232–L241. doi: 10.1152/ajplung.00187.2002. [DOI] [PubMed] [Google Scholar]
- 34.Kremlev SG, Phelps DS. Surfactant protein A stimulation of inflammatory cytokine and immunoglobulin production. Am J Physiol Lung Cell Mol Physiol. 1994;267(6 Pt 1):L712–L719. doi: 10.1152/ajplung.1994.267.6.L712. [DOI] [PubMed] [Google Scholar]
- 35.Wang G, Phelps DS, Umstead TM, Floros J. Human SP-A protein variants derived from one or both genes stimulate TNF-alpha production in the THP-1 cell line. Am J Physiol Lung Cell Mol Physiol. 2000;278(5):L946–L954. doi: 10.1152/ajplung.2000.278.5.L946. [DOI] [PubMed] [Google Scholar]
- 36.Wang G, Umstead TM, Phelps DS, Al-Mondhiry H, Floros J. The effect of ozone exposure on the ability of human surfactant protein A variants to stimulate cytokine production. Environ Health Perspect. 2002;110(1):79–84. doi: 10.1289/ehp.0211079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borron P, McIntosh JC, Korfhagen TR, Whitsett JA, Taylor J, Wright JR. Surfactant-associated protein A inhibits LPS-induced cytokine and nitric oxide production in vivo. Am J Physiol Lung Cell Mol Physiol. 2000;278(4):L840–L847. doi: 10.1152/ajplung.2000.278.4.L840. [DOI] [PubMed] [Google Scholar]
- 38.Koptides M, Umstead TM, Floros J, Phelps DS. Surfactant protein A activates NF-kappa B in the THP-1 monocytic cell line. Am J Physiol. 1997;273(2 Pt 1):L382–L388. doi: 10.1152/ajplung.1997.273.2.L382. [DOI] [PubMed] [Google Scholar]
- 39.Song M, Phelps DS. Interaction of surfactant protein A with lipopolysaccharide and regulation of inflammatory cytokines in the THP-1 monocytic cell line. Infection and immunity. 2000;68(12):6611–6617. doi: 10.1128/iai.68.12.6611-6617.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LeVine AM, Kurak KE, Bruno MD, Stark JM, Whitsett JA, Korfhagen TR. Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 1998;19(4):700–708. doi: 10.1165/ajrcmb.19.4.3254. [DOI] [PubMed] [Google Scholar]
- 41.LeVine AM, Bruno MD, Huelsman KM, Ross GF, Whitsett JA, Korfhagen TR. Surfactant protein A-deficient mice are susceptible to group B streptococcal infection. J Immunol. 1997;158(9):4336–4340. [PubMed] [Google Scholar]
- 42.LeVine AM, Whitsett JA, Gwozdz JA, Richardson TR, Fisher JH, Burhans MS, et al. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J Immunol. 2000;165(7):3934–3940. doi: 10.4049/jimmunol.165.7.3934. [DOI] [PubMed] [Google Scholar]
- 43.Mikerov AN, Haque R, Gan X, Guo X, Phelps DS, Floros J. Ablation of SP-A has a negative impact on the susceptibility of mice to Klebsiella pneumoniae infection after ozone exposure: sex differences. Respir Res. 2008;9:77. doi: 10.1186/1465-9921-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peel JL, Tolbert PE, Klein M, Metzger KB, Flanders WD, Todd K, et al. Epidemiology. 2. Vol. 16. Cambridge: Mass; 2005. Ambient air pollution and respiratory emergency department visits; pp. 164–174. [DOI] [PubMed] [Google Scholar]
- 45.Kennedy SM, Chambers R, Du W, Dimich-Ward H. Environmental and occupational exposures: do they affect chronic obstructive pulmonary disease differently in women and men? Proc Am Thorac Soc. 2007;4(8):692–694. doi: 10.1513/pats.200707-094SD. [DOI] [PubMed] [Google Scholar]
- 46.Jarvis D, Chinn S, Sterne J, Luczynska C, Burney P. The association of respiratory symptoms and lung function with the use of gas for cooking. Eur Respir J. 1998;11(3):651–658. European Community Respiratory Health Survey. [PubMed] [Google Scholar]
- 47.Schwela D. Air pollution and health in urban areas. Rev Environ Health. 2000;15(1–2):13–42. doi: 10.1515/reveh.2000.15.1-2.13. [DOI] [PubMed] [Google Scholar]
- 48.Dow L, Fowler L, Phelps L, Waters K, Coggon D, Kinmonth AL, et al. Prevalence of untreated asthma in a population sample of 6000 older adults in Bristol. UK. Thorax. 2001;56(6):472–476. doi: 10.1136/thorax.56.6.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters JM, Avol E, Gauderman WJ, Linn WS, Navidi W, London SJ, et al. A study of twelve Southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. Am J Respir Crit Care Med. 1999;159(3):768–775. doi: 10.1164/ajrccm.159.3.9804144. [DOI] [PubMed] [Google Scholar]
- 50.Li YF, Gilliland FD, Berhane K, McConnell R, Gauderman WJ, Rappaport EB, et al. Effects of in utero and environmental tobacco smoke exposure on lung function in boys and girls with and without asthma. Am J Respir Crit Care Med. 2000;162(6):2097–2104. doi: 10.1164/ajrccm.162.6.2004178. [DOI] [PubMed] [Google Scholar]
- 51.Medina-Ramon M, Schwartz J. Epidemiology. 5. Vol. 19. Cambridge: Mass; 2008. Who is more vulnerable to die from ozone air pollution? pp. 672–679. [DOI] [PubMed] [Google Scholar]
- 52.Bates DV. The effects of air pollution on children. Environ Health Perspect. 1995;103(Suppl 6):49–53. doi: 10.1289/ehp.95103s649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L, Lubin JH, Zhang SR, Metayer C, Xia Y, Brenner A, et al. Lung cancer and environmental tobacco smoke in a non-industrial area of China. Int J Cancer. 2000;88(1):139–145. doi: 10.1002/1097-0215(20001001)88:1<139::aid-ijc22>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 54.Oosterlee A, Drijver M, Lebret E, Brunekreef B. Chronic respiratory symptoms in children and adults living along streets with high traffic density. Occup Environ Med. 1996;53(4):241–247. doi: 10.1136/oem.53.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abbey DE, Nishino N, McDonnell WF, Burchette RJ, Knutsen SF, Lawrence Beeson W, et al. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers. Am J Respir Crit Care Med. 1999;159(2):373–382. doi: 10.1164/ajrccm.159.2.9806020. [DOI] [PubMed] [Google Scholar]
- 56.Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329(24):1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 57.Mikerov AN, Gan X, Umstead TM, Miller L, Chinchilli VM, Phelps DS, et al. Sex differences in the impact of ozone on survival and alveolar macrophage function of mice after Klebsiella pneumoniae infection. Respir Res. 2008;9:24. doi: 10.1186/1465-9921-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, Chaudry IH. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med. 1997;25(1):106–110. doi: 10.1097/00003246-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 59.Wichmann MW, Ayala A, Chaudry IH. Male sex steroids are responsible for depressing macrophage immune function after trauma-hemorrhage. Am J Physiol. 1997;273(4 Pt 1):C1335–C1340. doi: 10.1152/ajpcell.1997.273.4.C1335. [DOI] [PubMed] [Google Scholar]
- 60.Wichmann MW, Zellweger R, DeMaso CM, Ayala A, Chaudry IH. Enhanced immune responses in females, as opposed to decreased responses in males following haemorrhagic shock and resuscitation. Cytokine. 1996;8(11):853–863. doi: 10.1006/cyto.1996.0114. [DOI] [PubMed] [Google Scholar]
- 61.Durrani F, Phelps DS, Weisz J, Silveyra P, Hu S, Mikerov AN, et al. Gonadal hormones and oxidative stress interaction differentially affects survival of male and female mice after lung Klebsiella pneumoniae infection. Exp Lung Res. 2012 doi: 10.3109/01902148.2011.654045. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mikerov AN, Cooper TK, Wang G, Hu S, Umstead TM, Phelps DS, et al. Histopathologic evaluation of lung and extrapulmonary tissues show sex differences in Klebsiella pneumoniae - infected mice under different exposure conditions. Int J Physiol Pathophysiol Pharmacol. 2011;3(3):176–190. [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X. Lipopolysaccharide augments venous and arterial thrombosis in the mouse. Thromb Res. 2008;123(2):355–360. doi: 10.1016/j.thromres.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 64.Card JW, Zeldin DC. Hormonal influences on lung function and response to environmental agents: lessons from animal models of respiratory disease. Proc Am Thorac Soc. 2009;6(7):588–595. doi: 10.1513/pats.200904-020RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 66.Blotta MH, DeKruyff RH, Umetsu DT. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J Immunol. 1997;158(12):5589–5595. [PubMed] [Google Scholar]
- 67.Wilckens T, De Rijk R. Glucocorticoids and immune function: unknown dimensions and new frontiers. Immunol today. 1997;18(9):418–424. doi: 10.1016/s0167-5699(97)01111-0. [DOI] [PubMed] [Google Scholar]
- 68.Spellberg B, Edwards JE., Jr Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32(1):76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 69.Agarwal SK, Marshall GD., Jr Glucocorticoid-induced type 1/type 2 cytokine alterations in humans: a model for stress-related immune dysfunction. J Interferon Cytokine Res. 1998;18(12):1059–1068. doi: 10.1089/jir.1998.18.1059. [DOI] [PubMed] [Google Scholar]
- 70.Haque R, Umstead TM, Ponnuru P, Guo X, Hawgood S, Phelps DS, et al. Role of surfactant protein-A (SP-A) in lung injury in response to acute ozone exposure of SP-A deficient mice. Toxicol Appl Pharmacol. 2007;220(1):72–82. doi: 10.1016/j.taap.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]