Abstract
Rapid and voltage-dependent inactivation greatly attenuates outward currents in ether-a-go-go–related gene (ERG) K+ channels. In contrast, inactivation of related ether-a-go-go (EAG) K+ channels is very slow and minimally reduces outward currents. ICA-105574 (ICA, or 3-nitro-N-[4-phenoxyphenyl]-benzamide) has opposite effects on inactivation of these two channel types. Although ICA greatly attenuates ERG inactivation by shifting its voltage dependence to more positive potentials, it enhances the rate and extent of EAG inactivation without altering its voltage dependence. Here, we investigate whether the inverse functional response to ICA in EAG and ERG channels is related to differences in ICA binding site or to intrinsic mechanisms of inactivation. Molecular modeling coupled with site-directed mutagenesis suggests that ICA binds in a channel-specific orientation to a hydrophobic pocket bounded by the S5/pore helix/S6 of one subunit and S6 of an adjacent subunit. ICA is a mixed agonist of mutant EAG and EAG/ERG chimera channels that inactivate by a combination of slow and fast mechanisms. With the exception of three residues, the specific amino acids that form the putative binding pocket for ICA in ERG are conserved in EAG. Mutations introduced into EAG to replicate the ICA binding site in ERG did not alter the functional response to ICA. Together these findings suggest that ICA binds to the same site in EAG and ERG channels to elicit opposite functional effects. The resultant agonist or antagonist activity is determined solely by channel-specific differences in the mechanisms of inactivation gating.
Introduction
Ether-a-go-go (EAG) K+ channels, first described in Drosophila (Warmke et al., 1991), are highly expressed in the mammalian central nervous system (Ludwig et al., 1994; Martin et al., 2008) and a variety of tumors (Hemmerlein et al., 2006; Mello de Queiroz et al., 2006; Pardo et al., 1999). EAG channels activate rapidly and exhibit only a very subtle and slow form of inactivation (Garg et al., 2012). The related ether-a-go-go–related gene (ERG) K+ channel was discovered by screening of a human hippocampus cDNA library (Warmke and Ganetzky, 1994), and functional analysis revealed that it activates more slowly than does EAG and undergoes a very rapid inactivation that greatly reduces channel open probability at positive potentials (Smith et al., 1996; Spector et al., 1996). Both slow (EAG) and fast (ERG) inactivation are proposed to be mediated by structural rearrangement of the selectivity filter (Stansfeld et al., 2008; Garg et al., 2012), which is commonly referred to as C- or P/C-type inactivation (Hoshi et al., 1991; Chen et al., 2000), to differentiate it from the well-characterized N-type inactivation of Kv channels (Hoshi et al., 1990).
In the human heart, ERG type 1 (hERG1, Kv11.1) channels conduct the rapid delayed rectifier K+ current (IKr) (Sanguinetti and Jurkiewicz, 1990; Sanguinetti et al., 1995; Trudeau et al., 1995). Rapid inactivation of IKr during the plateau phase of the action potentially delays repolarization and facilitates Ca2+ entry into the cardiomyocyte, which triggers excitation-contraction coupling. Pathologic reduction of IKr caused either by congenital mutations in hERG1 or by block of channels as an adverse effect of many common medications is associated with a prolonged QT interval and an increased risk of cardiac arrhythmia (Sanguinetti and Tristani-Firouzi, 2006). This potentially life-threatening adverse effect prompted the now routine screening of hERG1 channel activity of compounds during the early phase of drug development programs. An unanticipated outcome of these routine screens was the discovery of compounds that activate, rather than block, hERG1 channels. Activators of hERG1 could theoretically be used to treat or prevent arrhythmia associated with congenital long QT syndrome (Zhang et al., 2012).
3-Nitro-N-(4-phenoxyphenyl)-benzamide (ICA-105574, or ICA) is a recently discovered compound that shortens the duration of cardiac action potentials by inducing a dramatic shift (e.g., +183 mV at 2 uM) in the voltage dependence of hERG1 inactivation to more positive potentials (Gerlach et al., 2010). In striking contrast to its inhibition of hERG1 channel inactivation, we recently reported that ICA enhances inactivation of human EAG1 (hEAG1, Kv10.1) channels (Garg et al., 2012). In addition to differences in their response to ICA, inactivation of hERG1, but not hEAG1, is slowed by external tetraethylammonium and elevated [K+]e (Garg et al., 2012). Despite these differences, the selectivity filter appears to serve as the inactivation gate in both hERG1 and hEAG1 channels (Smith et al., 1996; Garg et al., 2012). The disparate effects of ICA on intrinsic inactivation of such closely related Kv channels could result from ligand binding to distinct sites on the two channels or reflect inverse modulation of distinct, channel-specific modes of inactivation. Here, we use molecular modeling and analysis of mutant hEAG1 and bovine EAG1/hERG1 chimera channels to explore whether the ICA binding site in hEAG1 is homologous to the site previously reported for hERG1 (Garg et al., 2011).
Materials and Methods
Molecular Biology. Human EAG1 (KCNH1; National Center for Biological Technology Information Reference Sequence: NM_002238.3) cDNA cloned into psGEMHE oocyte expression vector was kindly provided by the late Dr. Dennis Wray. HERG1 (KCNH2, isoform 1a, National Center for Biological Technology Information Reference Sequence: NM_000238.2), was cloned into the pSP64 oocyte expression vector. Mutations in wild-type (WT) hEAG1 cDNA were made using the QuikChange site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) and were verified by DNA sequence analyses. Plasmids were linearized using NotI (psGEMHE) or EcoR1 (pSP64). HEAG1 cRNA was in vitro transcribed with the mMessage mMachine T7 kit (Life Technologies, Grand Island, NY). HERG1 cRNA was prepared using the mMessage mMachine SP6 kit (Ambion, Austin, TX). cRNA was quantified using RiboGreen assay (Life Technologies).
Two-Electrode Voltage Clamp of Xenopus Oocytes.
Procedures for harvesting oocytes from Xenopus laevis were as described elsewhere (Garg et al., 2012) and were approved by the University of Utah Institutional Animal Care and Use Committee. The isolation, culture, and injection of oocytes with cRNA were performed as described previously (Goldin, 1991; Stühmer, 1992). Injected oocytes were incubated for 1–5 days at 18°C in Barth’s saline solution before use in voltage clamp experiments. Currents were recorded from oocytes with use of a standard two-microelectrode voltage clamp technique (Goldin, 1991; Stühmer, 1992) and agarose-cushion microelectrodes (Schreibmayer et al., 1994). A GeneClamp 500 amplifier, Digidata 1322A data acquisition system, and pCLAMP 9.0 software (Molecular Devices, Inc., Sunnyvale, CA) were used to produce command voltages and to record current and voltage signals.
Oocytes were bathed in KCM211 solution at room temperature (22–24°C). To record ionic currents, the oocyte was voltage clamped to a holding potential (Vh) of −100 mV, and 1-second pulses were applied to a test potential (Vt) of 0 mV every 10 seconds until current magnitude reached a steady-state level. During perfusion of the recording chamber with ICA solutions, the pulse interval was lengthened to 30 seconds. After currents achieved a new steady-state level in the presence of ICA, I-V relationships were determined if needed.
Solutions.
Barth’s solution contained 88 mM NaCl, 2 mM KCl, 0.41 mM CaCl2, 0.33 mM Ca(NO3)2, 1 mM MgSO4, 2.4 mM NaHCO3, 10 mM HEPES, 1 mM pyruvate, and 50 mg/l gentamycin; pH was adjusted to 7.4 with NaOH. KCM211 solution contained 98 mM NaCl, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES; pH was adjusted to 7.6 with NaOH. ICA was purchased from Sigma-Aldrich (St. Louis, MO) and AKos GmBH (Steinen, Germany) and prepared as a 10 mM stock solution in dimethyl sulfoxide. Final [ICA] was obtained by dilution of the stock solution with KCM211 immediately before use for each experiment. TEA was purchased from Sigma-Aldrich.
Data Analysis.
Digitized data were analyzed off-line with pCLAMP9 (Molecular Devices), Origin 8 (OriginLab, Northhampton, MA), and Excel (Microsoft Corp., Redmond, WA) software. The concentration-effect relationship for ICA inhibition of hEAG current measured at +30 mV was fitted with a Hill equation. ICA enhanced currents at low concentrations and reduced currents at high concentrations of some mutant channels. For these mutant channels, an effective IC50 (Table 1) was determined simply by noting the concentration that reduced control current by 50%. All data are expressed as mean ± S.E.M. (n = number of oocytes) and evaluated by an unpaired Student’s t test where appropriate (P ≤ 0.05 was considered to be a statistically significant difference).
TABLE 1.
Mutant hEAG1 channels with altered response to ICA
| Mutation in hEAG1 | Mean IC50 for ICA ± S.E.M. | n | Fold increase or decrease (↓) in IC50 |
|---|---|---|---|
| µM | |||
| (WT) | 0.44 ±0.03 | 5 | – |
| V356A** | 0.08 ±0.009 | 3 | 5.5 ↓ |
| V356E** | 28.8 ±2.6 | 3 | 65 |
| F359A* | 29.6 ±9.0 | 3 | 67 |
| F359L | >30 | 4 | >68 |
| L427A** | 0.17 ±0.01 | 3 | 2.6 ↓ |
| M431A* | 1.9 ±0.34 | 5 | 4.3 |
| L434A** | 8.5 ±0.08 | 5 | 19 |
| L434C** | 4.8 ±0.2 | 3 | 11 |
| M457A | >30 | 4 | >68 |
| M458A** | 20.2 ±1.2b | 4 | 46 |
| M458Ea | 0.45 ±0.08 | 3 | no change |
| L462A** | 0.05 ±0.005 | 3 | 8.6 ↓ |
| L463A** | 41.2 ±8.2b | 3 | 94 |
| Y464A** | 0.07 ±0.014 | 3 | 6.3 ↓ |
| I467A** | 1.1 ±0.05 | 3 | 2.5 |
| I467E** | 24.8 ±4.1b | 3 | 56 |
| F468A** | 3.1 ±0.48 | 4 | 7 |
hEAG1, human ether-a-go-go type 1; ICA, -nitro-N-(4-phenoxyphenyl)-benzamide (ICA-105574); WT, wild-type.
NS, nonsignificant (P > 0.05).
Effective IC50.
P < 0.01.
P < 0.001 compared with WT.
Molecular Modeling.
Homology models of the closed and open channel conformations were generated using Modeller9v9 with the KcsA crystal structure (PDB 2HVK) as a template for the closed state model and the KvAP (1ORQ) and the high resolution Mthk (PDB 3LDC) crystal structures as templates for the open conformation. Modeling details were described previously (Stary et al., 2010).
All mutant hEAG1 channels (F359L, M431F/M458L/L463M) were generated in Pymol. Molecular Dynamic (MD) simulations of open and closed models were performed using Gromacs, version 4.5.4 (Hess et al., 2008). WT and mutant channels were embedded in an equilibrated simulation box consisting of 280 dioleolylphosphatidylcholine lipids. Lipid parameters were taken from Siu et al. (Siu et al., 2008), and the TIP3P water model was used (Jorgensen et al., 1983). The amber99sb force field (Hornak et al., 2006) was used for the protein. Electrostatic interactions were calculated explicitly at a distance <1 nm and long-range electrostatic interactions were calculated at every step by particle-mesh Ewald summation (Darden et al., 1993). Lennard-Jones interactions were calculated with a cutoff of 1 nm. All bonds were constrained by using the LINCS (Linear Constraint Solver) algorithm (Hess et al., 1997), allowing for an integration time step of 2 femtoseconds. The Nose-Hoover thermostat (Nose, 1984) was used for temperature coupling (τ = 0.1 picoseconds), and the Parrinello-Rahman barostat algorithm (Parrinello and Rahman, 1981) was used for pressure coupling. One thousand conjugate gradient energy-minimization steps were performed, followed by 2 nanoseconds of restrained MD, in which the protein atoms were restrained with a force constant of 1000kJ × mol−1nm−2 to their initial position, and ions, lipids, and solvent were allowed to move freely. Each system was then subjected to 50 nanoseconds of unrestrained MD, during which coordinates were saved every 10 picoseconds for analysis.
Coordinates of ICA were generated using Gaussview 5, and the geometry was optimized with the Hartee-Fock 3-21G basis set implemented in Gaussian09 (Frisch et al., 2009). Docking was performed using the program Gold 4.0.1 (Cambridge DataCentre, Cambridge, UK) (Jones et al., 1995). Coordinates of the geometric center calculated among residues F359, L434, M431, Y464, and F468 were taken as binding site origin. The binding site radius was set equal to 10 Å; 150,000 operations of the GOLD genetic algorithm were used to dock the selected compounds into the WT and mutant channels. Three snapshots (15, 33, and 50 nanoseconds) were taken from MD trajectories. The stability of the predicted binding modes of ICA to WT hEAG1 channels in open and closed conformation was confirmed in 50 nanosecond MD simulations as described previously (Knape et al., 2011).
Results
ICA Binds to the Pore Domain of hERG1 and hEAG1 Channels to Exert Opposite Effects on Inactivation.
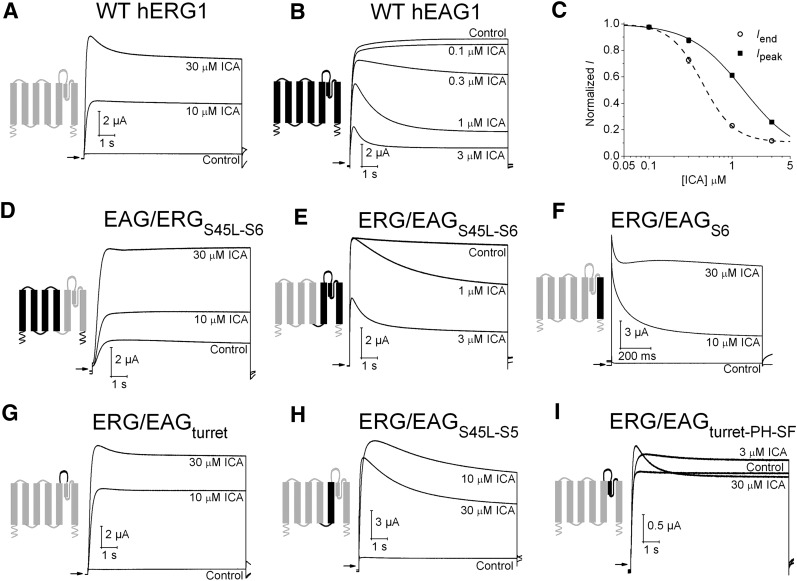
The effects of ICA on WT hERG1 and hEAG1 channels expressed in Xenopus oocytes are shown in Fig. 1. Channels were activated with a 10-second pulse to +30 mV from a holding potential of −100 mV. As reported previously (Gerlach et al., 2010; Garg et al., 2011, 2012), ICA caused a marked and concentration-dependent enhancement of hERG1 current (Fig. 1A), but inhibited hEAG1 current (Fig. 1B) by reducing both the initial peak outward current (Ipeak) and inducing a time-dependent decay of current during the pulse. Inhibition of hEAG1 current by ICA is caused by an enhancement of intrinsic inactivation and is not attributable to open channel block (Garg et al., 2012). The inhibitory effect of ICA on hEAG1 was more potent than was the activation effect on hERG1 channels. The IC50 for ICA inhibition of hEAG1 was 1.38 ± 0.04 μM for Ipeak and 0.44 ± 0.03 μM for current at the end of the pulse, Iend (n = 5) (Fig. 1C).
Fig. 1.
The pore domain of EAG1 and ERG1 channels determines functional response to ICA. (A) ICA activates WT hERG1 channel current. (B) ICA inhibits WT hEAG1 channel current. For traces shown in A and B, currents were activated with 10-second pulses to +30 mV from a holding potential of −100 mV. (C) [ICA]-response relationship for inhibition of hEAG1 channel currents (n = 5). ICA inhibited Iend, current at the end of a 10-second pulse to +30 mV (IC50 = 0.44 ± 0.03 μM; nH = 2.3) more potently than Ipeak, peak outward current (IC50 = 1.38 ± 0.04 μM; nH = 1.4). (D–I) Effect of ICA on EAG1/ERG1 chimera channels. In each panel, the diagram indicates regions of subunits contributed by bEAG1 (black) and hERG1 (gray). Currents were elicited with 10-second pulses to +30 mV from a holding potential of −100 mV. The fold-change in Iend induced by ICA for each chimera channel was as follows: (D) EAG/ERGS45L-S6 (1.9 ± 0.03 at 10 μM; 3.6 ± 0.6 at 30 μM, n = 3). (E) ERG/EAGS45L-S6 (0.58 ± 0.07 at 1 μM; 0.24 ± 0.04 at 3 μM, n = 4). (F) ERG/EAGS6 (9.6 ± 3.5 at 10 μM; 41 ± 11 at 30 μM, n = 4). (G) ERG/EAGturret (15 ± 1.9 at 10 μM; 26 ± 0.3 at 30 μM, n = 3). (H) ERG/EAGS45L-S5 (9.7 ± 1.6 at 10 μM; 6.1 ± 1.0 at 30 μM, n = 3). (I) ERG/EAGturret-pore helix-selectivity filter (% change in Iend: 9 ± 3 at 3 μM; 4 ± 2 at 10 μM; -3 ± 3 at 30 μM, n = 3).
Using scanning mutagenesis and functional analysis of mutant hERG1 channels, we recently proposed that ICA binds to a hydrophobic pocket formed by the S5/pore helix/S6 of one subunit and S6 segment of an adjacent channel subunit (Garg et al., 2011). To identify the region of the EAG1 channel responsible for the inhibitory effect of ICA, we first used an unbiased approach and examined several previously characterized chimera channels (Ficker et al., 1998) constructed from bovine EAG1 (bEAG1) and hERG1. The amino acid sequences of bovine and human EAG1 are 97% identical for the entire proteins and 100% identical in the S1–S6 regions. First, the role of the pore module was investigated. ICA was an agonist when the pore region (S45 linker-S6) of the chimera channel was contributed by hERG1 (Fig. 1D), but was an antagonist when the same pore region was supplied by bEAG1 (Fig. 1E). Next, ICA was tested on chimeras in which only a limited region of the pore domain of hERG1 was replaced by their bEAG1 counterpart. When only the S6 segment (Fig. 1F) or the turret (Fig. 1G) was supplied by bEAG1, ICA acted as an agonist. Finally, when the S45 linker-S5 (Fig. 1H) or the turret-pore helix-selectivity filter (Fig. 1I) was supplied by bEAG1, the effects of ICA were biphasic; 30 μM ICA induced or enhanced the rate of slow inactivation and caused a smaller increase in current, compared with 10 μM ICA. Together, the findings obtained with hERG1/bEAG1 chimeras indicate that the pore domain (S5–S6) region determines the channel-specific response to ICA.
ICA Is a Mixed Agonist of a Fast-Inactivating Mutant hEAG1 Channel.
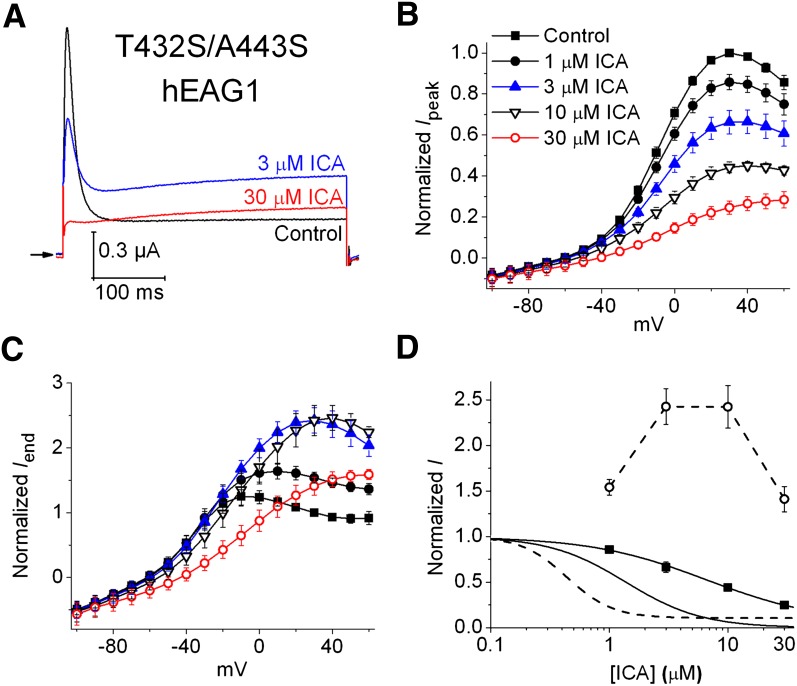
Two Ser residues, located on either side of the selectivity filter, are key determinants of fast P/C-type inactivation in hERG1 (Suessbrich et al., 1997). In bEAG1 channels, combined mutation of the residues located in the homologous position as these two Serines induces fast inactivation (Ficker et al., 1998). Introduction of the same two mutations (T432S/A443S) into hEAG1 also induces a rapid, time-dependent decay of outward current (Fig. 2A), and similar to P/C-type inactivation of hERG1 (Smith et al., 1996), its rate is slowed by extracellular tetraethylammonium (10 mM) or elevated [K+]e (Supplemental Fig. 1). In contrast, the rate of the comparatively very slow inactivation of hEAG1 is unaffected by tetraethylammonium or changes in [K+]e (Garg et al., 2012). When activated by a 0.4-second pulse to +30 mV, 3 μM ICA reduced Ipeak and the inactivating component of outward T432S/A443S hEAG1 current, but doubled the magnitude of Iend (Fig. 2A). At 30 μM, ICA eliminated fast inactivation (Fig. 2A). ICA (1–30 μM) reduced Ipeak in a concentration-dependent manner (Fig. 2B), but exhibited a biphasic (mixed agonist) effect on Iend and reduced rectification of the Iend -V relationship (Fig. 2C). At 30 μM, ICA inhibited Ipeak (all potentials) and Iend (at test potentials < +10 mV). The [ICA]-response relationship for Ipeak and Iend at a single voltage (+30 mV) for T432S/A443S hEAG1 channels is compared with WT channels in Fig. 2D. Together, these findings suggest that the multiple effects of ICA on T432S/A443S hEAG1 channels represent the sum of two opposing actions: reduced fast (ERG1-like) inactivation to increase current plus inhibition, perhaps mediated by open channel block to reduce current. We next explore whether the opposite effects of ICA on fast versus slow modes of inactivation gating are mediated by ICA binding to the same or distinct sites in hERG1 and hEAG1 channels.
Fig. 2.
ICA has dual effects on fast-inactivating T432S/A443S hEAG1 channels. (A) Representative traces showing the effect of 3 and 30 µM ICA on current measured at a test potential of +30 mV. Currents were elicited from a holding potential of −60 mV. (B and C) Normalized Ipeak-V relationships (B) and Iend-V (C) relationships measured before (control) and in the presence of indicated [ICA] (n = 5). (D) [ICA]-response relationships for Ipeak (solid curves) and Iend (dashed curves) measured at a single test potential (+30 mV) for T432S/A443S (data points, ▪ and ○) and WT hEAG1 (curves without data points). The IC50 for Ipeak was 7.6 ± 0.9 μM for T432S/A443S hEAG1 (n = 5).
Molecular Determinants of ICA Interaction with hEAG1 Channels.
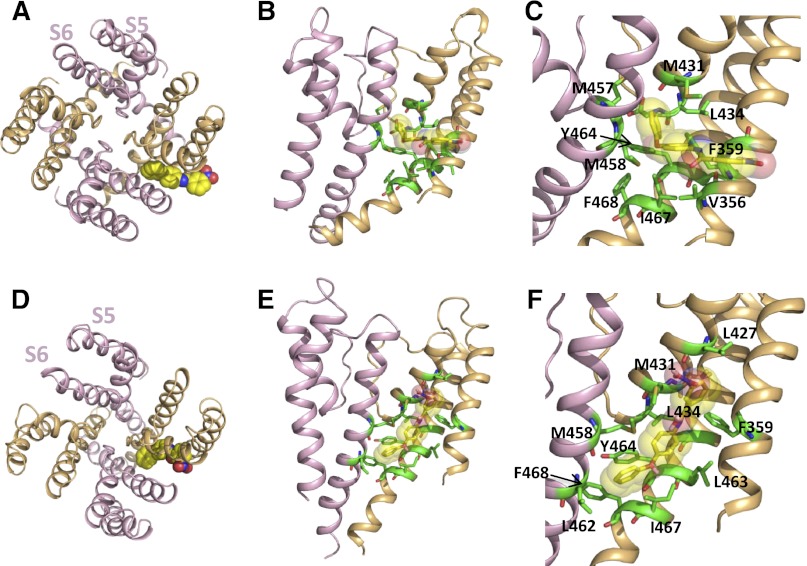
Simulated docking of ICA to molecular models of the hEAG1 pore module was performed, and the findings were compared with those from our previous model of ICA bound to the hERG1 channel. Homology models of hEAG1 were constructed using the KvAP and MthK crystal structures (Jiang et al., 2003; Ye et al., 2010) as template for the open state and the KcsA crystal structure (Doyle et al., 1998) as template for the closed state. In hERG1, ICA was predicted to be oriented perpendicular to the axis of the S5 and S6 segments in both the closed and the open state dockings, with the nitro group facing toward the pore (Garg et al., 2011). For hEAG1, ICA also favored a perpendicular orientation in the open state, but the nitro group faces away from the pore and toward the lipids that surround the pore module (Fig. 3, A–C). In the closed state of hEAG1, ICA is orientated parallel to S5 and S6 (Fig. 3, D–F). Simulated docking of ICA to the open states of hERG1 and hEAG1 are compared in Supplemental Fig. 2. In hEAG1, ICA resides in a hydrophobic pocket formed by residues Met431, Leu434, Met458, Tyr464, Ile467, and Phe359. The location of this binding pocket is quite similar to that previously described for hERG1 (Garg et al., 2011). However, sequence differences between hERG1 and hEAG1 lead to different-shaped binding pockets for ICA (Supplemental Fig. 2, C and E). ICA protrudes deeply into the cleft between two adjacent subunits in the hERG1 channel (Supplemental Fig. 2A). By contrast, in hEAG1, Tyr464 forms a barrier at the S6-S6 interface, leading to a shallower binding mode for ICA (Fig. 3; Supplemental Fig. 2D). ICA does not form π-π stacking interactions with Tyr464 in either the closed or the open state of hEAG1 and, thus, might not be able to stabilize the phenyl group in the down conformation, as previously suggested for hERG1 (Garg et al., 2011). In addition, stabilizing hydrogen bonds predicted between ICA and selectivity filter residues in hERG1 are lacking in hEAG1.
Fig. 3.
Models of ICA docked to the open and closed state of the hEAG1 pore module. (A) Single ICA molecule (shown in space-fill) docked to the open state model of the complete pore module as viewed from the extracellular space. (B) Side view of the S5-S6 regions of two adjacent hEAG1 subunits of an open state channel. ICA molecule is shown in transparent space-fill; interacting residues are shown as green sticks. (C) Close-up view of panel B with interacting residues labeled. (D–F) ICA bound to the closed state model of the hEAG1 pore module.
Considering both open and closed hEAG1 model simulations, 11 residues are predicted to be in close proximity to ICA: Leu427, Met431, and Leu434 in the pore helix; Val356 and Phe359 in S5; Leu463, Tyr464, Ile467, and Phe468 in S6 of one subunit; and Met457 and Met458 in the S6 of an adjacent subunit. To corroborate the modeling results, we mutated to Ala each of these 11 residues. Seven of the 11 Ala substitutions reduced the sensitivity (increased IC50) of the mutant channel to ICA by >4-fold, compared with WT hEAG1 (Table 1; Supplemental Fig. 3). The I467A mutation increased the IC50 by 2.5-fold, whereas V356A, L427A, and Y464A mutations reduced IC50 by 2.5–6-fold. Val356, Leu427, and Ile467 are predicted to interact with ICA in the closed but not the open state. Because of the poor expression of L427A mutant channel (requiring 100 times more cRNA and longer incubation time in comparison with WT hEAG) and its location near the selectivity filter (P/C-type inactivation gate), Leu427 residue was not analyzed further. To explore the potential significance of Ile467 and Val356 to ICA binding, each was mutated to the more perturbing Glu. Accordingly, both V356E and I467E mutations increased the IC50 for ICA by >55-fold (Supplemental Fig. 3; Table 1). Thus, mutagenesis confirmed the importance of all the residues predicted by molecular modeling to interact with ICA in either the open or the closed state of the hEAG1 channel.
Some of the molecular determinants of ICA binding to hEAG1 align with those previously described for hERG1. For example, mutation of Phe557, Phe619, Leu622, and Phe656 in hERG1 reduced the sensitivity to the activator effect of ICA (Garg et al., 2011); similarly, mutation of the corresponding residues to Ala in hEAG1 (Phe359, Met431, Leu434, and Phe468) reduced the inhibitory action of ICA. However, many homologous mutations yielded incongruous findings for the two channels. First and of most importance, the Y652A hERG1 channel is nearly insensitive to activation by ICA (Garg et al., 2011), whereas the homologous Y464A hEAG1 channel is more sensitive to inhibition (more inactivated) by ICA (Garg et al., 2012). Second, although the mutations L463A and M458A decreased the sensitivity of hEAG1 channels (IC50 increased by 94-fold and 46-fold, respectively; Table 1) and V356A enhanced sensitivity to ICA by 6-fold, the corresponding mutations in hERG1 (M651A, L646A, and M554A) were previously reported to not alter the response to ICA (Garg et al., 2011). Third, F557L hERG1 channels are insensitive to ICA, whereas the corresponding F359L hEAG1 channel is activated by ICA (Garg et al., 2012). We investigated these notable differences by further examining three of these key residues in hEAG1: Met458 and Tyr464 in S6 and Phe359 in S5.
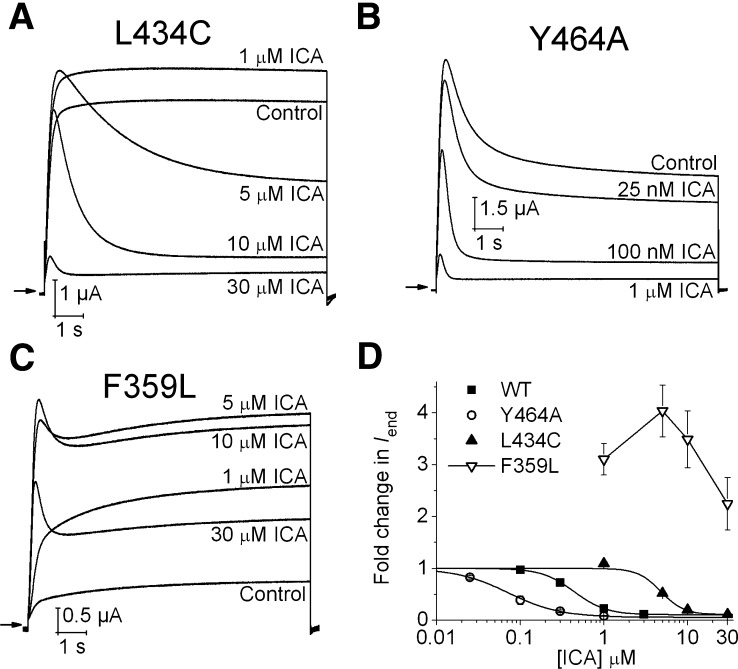
Simulated dockings predicted that both Met458 in hEAG1 and the homologous residue Leu646 in hERG1 are in close proximity to ICA. Nonetheless, substitution of Leu646 to either Ala (Garg et al., 2011) or Glu (Supplemental Fig. 4) did not alter hERG1 channel sensitivity to ICA. As noted above, M458A mutation in hEAG1 reduced ICA sensitivity; however, similar to L646E in hERG1, M458E did not alter hEAG1 sensitivity to ICA (Supplemental Fig. 4; Table 1), presumably because the acidic side chain of Glu is repelled from the hydrophobic pocket and, thus, does not affect ICA binding. In hERG1, three specific point mutations, Y652A (S6), F557L (S5), and L434C (pore helix), rendered the channel insensitive to 30 µM ICA, consistent with molecular modeling predictions (Garg et al., 2011). The effect of multiple ICA concentrations on mutant channels harboring the homologous substitutions in hEAG1 (Y464A, F359L, L434C) were examined (Fig. 4, A–D). Consistent with Leu434 contributing to ICA binding, L434C hEAG1 was less inhibited by ICA (IC50 = 4.8 ± 0.2 µM), compared with WT channels (Fig. 4, A and D). As we reported previously, Y464A promotes and ICA accentuates prominent inactivation from an open state (Fig. 4, B and D), whereas F359L (Fig. 4C) appears to promote and ICA reverses inactivation from closed states (Garg et al., 2012). Molecular modeling predicts that the F557L mutation in hERG1 excludes ICA from interaction with its hydrophobic binding pocket (Garg et al., 2011), consistent with its insensitivity to the drug. In contrast, molecular modeling predicts that the hydrophobic pocket in F359L hEAG1 channels can accommodate ICA (Supplemental Fig. 5), albeit in a different orientation, compared with the WT hEAG1 channel. Together, molecular modeling predictions and functional analysis of many mutant channels indicate that ICA modulates inactivation gating of both hEAG1 and hERG1 channels by interacting with the same hydrophobic pocket defined by the S5-pore helix-S6 region of one subunit and S6 of an adjacent subunit. We next sought to determine whether the functional effect of ICA could be reversed (i.e., switched from inhibitor to agonist) if the putative hydrophobic pocket of hEAG1 was modified by mutagenesis to mimic the pocket present in hERG1 channels.
Fig. 4.
Concentration-dependent effects of ICA on L434C, Y464A, and F359L hEAG1 channels. (A) Effect of ICA (1–30 µM) on L434C hEAG1 channel currents measured in response to 10-second depolarization to +30 mV. (B) Effect of ICA (25 nM to 1 µM) on Y464A hEAG1 channel currents during 10-second pulse to +30 mV. (C) Biphasic response of F359L hEAG1 channels to ICA (1–30 µM) during 10-second pulse to +30 mV. (D) [ICA]-response (normalized Iend) relationships for indicated WT and mutant hEAG1 channels. The IC50 for Iend was 0.07 ± 0.01 μM for Y464A (n = 3), 4.8 ± 0.2 μM for L434C (n = 3) and >30 μM for F359L (n = 4) hEAG1 channels. WT data are same as that plotted in Fig. 1C.
Introducing Putative hERG1 Binding Pockets into hEAG1 Does Not Alter Response to ICA.
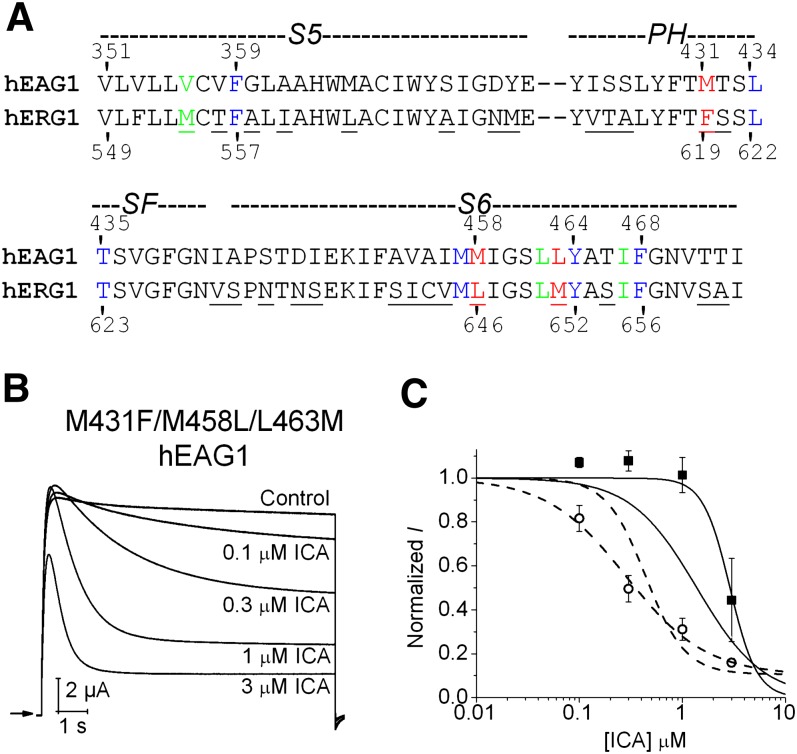
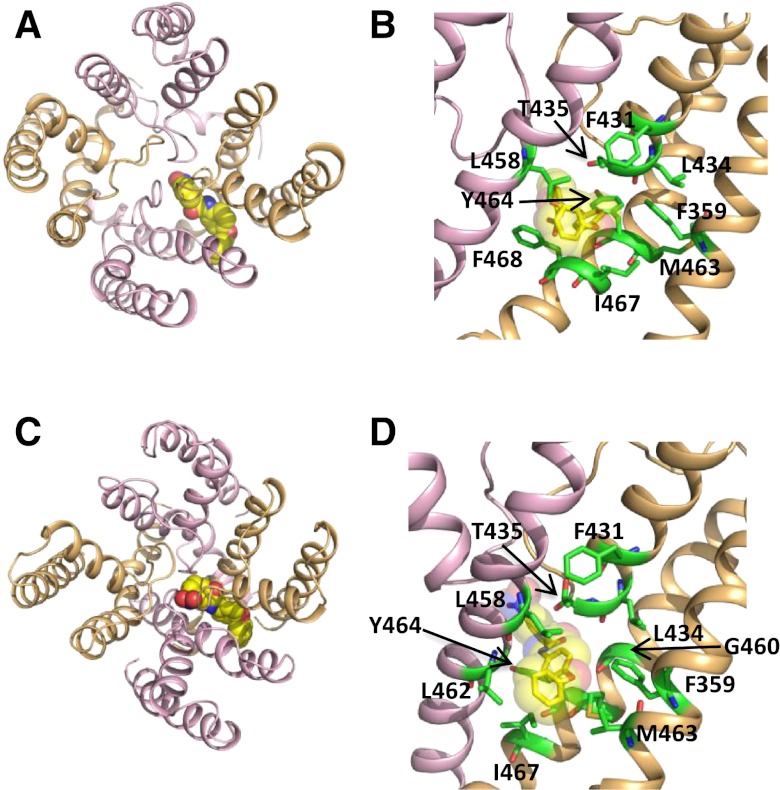
The S5-pore helix-S6 regions of hERG1 and hEAG1 are composed of 79 residues, and protein sequence alignment (Fig. 5A) indicates several differences between the two channels, including 8 residues in S5, 5 residues in the pore helix, and 14 residues in S6. However, in the putative ICA binding pocket defined by docking simulations using the open state models of hERG1 (Garg et al., 2011) and hEAG1 (Fig. 3), only one residue in the pore helix and two residues in S6 differ between the two channels (Fig. 5A). Three amino acid substitutions were introduced into hEAG1 (M431F in the pore helix; M458L and L463M in S6) to match the corresponding residues in hERG1. The resulting triple-mutant (M431F/M458L/L463M) channel retained WT hEAG1 biophysical properties and response to ICA (Fig. 5B), including a similar IC50 value for inhibition of Iend (0.49 ± 0.15 μM, n = 3; Fig. 5C). Can the putative ICA binding site in hERG1 be adequately recapitulated in hEAG1 by just three amino acid substitutions? Molecular modeling suggests remarkable similar binding modes of the triple hEAG1 channel, compared with the WT hERG1 channel. Simulated docking predicts that ICA binds perpendicular to the axis of the S5 and S6 segments in both the closed and the open state, and the nitro group faces toward the pore (Fig. 6), similar to the orientation of ICA in hERG1 (Garg et al., 2011). Furthermore, mutations M431F/M458L/L463M render the shape of the binding site to be more hERG1-like, allowing the drug to protrude deeply into the cleft formed by the interface of two adjacent subunits. Together, these modeling and experimental findings support the notion that intrinsic differences in the mechanisms of slow versus fast inactivation gating, and not differences in the binding site, determines whether ICA is a channel antagonist (hEAG1) or agonist (hERG1).
Fig. 5.
ICA-induces inactivation of mutant hEAG1 channel with putative drug-binding pocket engineered to mimic hERG1 channel. (A) Amino acid sequence alignment of S5 and pore helix (PH)/selectivity filter (SF)/S6 region of hEAG1 and hERG1. Nonconserved amino acids are underlined. Conserved residues (blue) and nonconserved residues (red) predicted to line the hydrophobic ICA binding pocket in hERG1 are highlighted. Residues colored green were predicted to contribute to ICA binding site in closed state of hEAG1 (but not predicted to interact with hERG1 in closed or open state). (B) Concentration-dependent inhibition of M431F/M458L/L463M hEAG1 channel currents by ICA. Currents were elicited with 10-second pulses to +30 mV. (C) [ICA]-response relationships for Ipeak (solid curve) and Iend (dashed curve) quantified as fold change in current measured at a test potential of +30 mV for M431F/M458L/L463M (data points, ▪ and ○) and WT hEAG1 (curves only, replotted from Fig. 1C). The IC50 was 0.49 ± 0.15 μM for Iend and 2.96 ± 0.82 μM for Ipeak (n = 4).
Fig. 6.
Molecular models of ICA docked to M431F/M458L/L463M hEAG1 channel pore module. (A and B) Open state model. (C and D) Closed state model.
Discussion
ICA Binds to a Common Site of EAG1 and ERG1 Channels to Exert Opposite Effects on Inactivation.
ICA inhibits outward K+ hEAG1 channel currents by enhancing slow inactivation (i.e., it is an agonist of intrinsic slow inactivation gating). In contrast, ICA enhances outward hERG1 K+ channel currents by inhibiting inactivation (i.e., it is an antagonist of intrinsic fast inactivation gating). Despite the opposite functional response to ICA, analysis of chimera ERG/EAG channels and multiple mutant channels clearly establish that the compound binds to a similar region, in a hydrophobic cleft between two adjacent subunits of the pore module in both hERG1 and hEAG1. A recent MD simulation study of hERG1 proposed that the binding pocket for ICA is located between the pore helices of two adjacent subunits and that the selectivity filter adopts a collapsed conformation in the inactivated state, precluding entry of the compound into the pocket (Kopfer et al., 2012). However, this binding mode does not include interaction with residues in S5, including F557, a residue that we find to be of particular importance in modification of channel gating by ICA. Because both channels are homotetramers, there could be four identical ICA binding sites on each channel. Consistent with multiple binding sites, activation of hERG1 channels by ICA exhibits strong cooperativity, with a Hill coefficient of 3.3 estimated for the concentration-response relationship (Gerlach et al., 2010).
Slow inactivation of hEAG1 channels is modulated by a proposed interaction among three residues in close proximity and located in the S5 (Phe359), pore helix (Leu434), and S6 (Tyr464) of each subunit (Garg et al., 2012). In WT channels, inactivation is very slow and barely detectable but is greatly enhanced by ICA or mutations of Tyr464. Y464A hEAG1 channels exhibit far greater intrinsic slow inactivation than do WT channels, and ICA accentuates this altered mode of gating. Inactivation of Y464A channels can be prevented (WT gating restored) by introducing a second mutation of either Leu434 or Phe359 (Garg et al., 2012). In contrast to Tyr464, multiple mutations of Leu434 or Phe359 do not alter the biophysical properties of hEAG1 (Garg et al., 2012), but do affect the response to ICA. F359A and L434A/C reduce the efficacy of ICA to induce inactivation, whereas highly inactivated F359L channels are activated by ICA. Together, these findings suggest that ICA directly affects the molecular machinery of slow inactivation in hEAG1 channels.
F359A and F359L hEAG1 channels exhibited altered responses to ICA. F359A channels were less sensitive to inhibition by ICA (67-fold increase in IC50), indicating a reduced binding affinity. In addition, ICA reduced F359A channel currents without inducing the prominent time-dependent decay of current during a depolarizing pulse seen with WT channels. We interpret this later effect to indicate that ICA enhances closed (but not open) state inactivation of F359A channels. Inhibition of F359A channels could also result from open channel block; however, we have previously presented extensive evidence that ICA induces both closed and open state inactivation of WT hEAG1 channels with no evidence of open channel block (Garg et al., 2012). On the basis of our molecular modeling results, ICA binds similarly to the open state of WT and F359L hEAG1 channels (Supplemental Fig. 2, D and E, WT; Supplemental Fig. 5, A and B, F359L). However, unlike WT channels, the activation of F359L channels was biphasic: currents were activated at all concentrations examined (1–30 µM) and peaked at 3 µM. ICA concentrations >3 µM led to progressively less activation that was accompanied by progressively more extensive time-dependent decay of outward currents (indicative of enhanced open channel inactivation). On the basis of injection of oocytes with equivalent amounts of cRNA, F359L hEAG1 channel currents are much smaller than WT channel currents, suggesting that these mutant channels are either highly inactivated or have a lower than normal single channel open probability. As discussed previously (Garg et al., 2012), we propose that ICA-mediated increase in F359L channel currents may be caused by a reduced rate of closed to inactivated state transitions.
Modification of channel gating has also been proposed as the mechanism responsible for activation of KCNQ2-5 (Kv7.2-Kv7.5) channels by retigabine, an anticonvulsant drug that shifts the voltage dependence of activation to more negative potentials. The putative binding site for retigabine is a hydrophobic binding pocket (Schenzer et al., 2005; Wuttke et al., 2005; Lange et al., 2009) located in the same region described here for ICA binding to ERG and EAG channels. The primary molecular determinants of retigabine binding in KCNQ3 are Trp265 (S5), Leu314 (pore helix), and Leu338 (S6), homologous to key components of the ICA binding site in hEAG1 (Phe359, Leu434, Met458) and hERG1 (Phe557, Leu622, Leu646). Moreover, mutation of the aromatic residue in S5 to Leu renders hERG (F557L) channels as insensitive to ICA and KCNQ3 (W265L) channels as insensitive to retigabine. The homologous mutation in hEAG1 (F359L) reversed the effect of ICA from antagonist to agonist activity, and the reverse mutation at the corresponding residue in KCNQ1 (L266W) leads to inhibition in a channel that is normally insensitive to retigabine (Schenzer et al., 2005). Another interesting analogy between EAG and KCNQ (specifically, KCNQ1) channels is that, in both, a tripartite mode of inactivation gating has been proposed, involving specific residues in the S5, pore helix, and S6 (Seebohm et al., 2005; Garg et al., 2012). Thus, gating of the selectivity filter in multiple, unrelated Kv channels is modulated by binding of lipophilic compounds to the hydrophobic cleft situated between two adjacent subunits in the pore module.
Clinical Relevance.
Treatments for congenital and acquired long QT syndrome are limited. The recent discovery of several compounds that activate hERG1 channels initiates a promising pathway toward development of genotype-specific therapy for this life-threatening disorder. As a consequence of its profound inhibition of inactivation, ICA increases the magnitude of outward hERG1 currents more than has been observed for other activators, such as RPR260243 (Kang et al., 2005), PD-118057 (Perry et al., 2007), or NS1643 (Grunnet et al., 2011; Hansen et al., 2006). A hERG1 activator that has more modest effect on current magnitude than ICA would be less prone to induce excessive shortening of action potentials and avoid the potential conversion of long to short QT syndrome. In addition, our findings warn that hERG1 agonists may also affect the gating of highly related hEAG1 channels with potential functional consequences in the central nervous system.
Supplementary Material
Acknowledgments
The authors thank Alison Gardner and Jennifer Abbruzzese for technical assistance. The computational results were achieved using the Vienna Scientific Cluster.
Abbreviations
- bEAG1
bovine ether-a-go-go type 1
- hEAG1
human ether-a-go-go type 1
- hERG1
human ether-a-go-go-related gene type 1
- ICA
3-nitro-N-(4-phenoxyphenyl)-benzamide (ICA-105574)
- Iend
current at the end of the pulse
- IKr
rapid delayed rectifier K+ current
- Ipeak
peak outward current
- MD
molecular dynamics
- WT
wild type
Authorship Contributions
Participated in research design: Garg, Stary-Weinzinger, Sanguinetti.
Conducted experiments: Garg.
Performed data analysis: Garg, Stary-Weinzinger, Sanguinetti.
Wrote or contributed to the writing of the manuscript: Garg, Stary-Weinzinger, Sanguinetti.
Footnotes
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL055236] (to M.S.); the American Heart Association (Western States Affiliate; postdoctoral fellowship to V.G.); and The Austrian Science Fund [Grant P22395] (to A.S.-W.).
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Chen J, Avdonin V, Ciorba MA, Heinemann SH, Hoshi T. (2000) Acceleration of P/C-type inactivation in voltage-gated K(+) channels by methionine oxidation. Biophys J 78:174–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darden T, York D, Pedersen L. (1993) Particle mesh Ewald: An N-log(N) method for Ewald sums in large systems. J Chem Phys 98:10089–10092 [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. (1998) The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280:69–77 [DOI] [PubMed] [Google Scholar]
- Ficker E, Jarolimek W, Kiehn J, Baumann A, Brown AM. (1998) Molecular determinants of dofetilide block of HERG K+ channels. Circ Res 82:386–395 [DOI] [PubMed] [Google Scholar]
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G. (2009) Gaussian 09, Revision A.1, Gaussian, Inc., Wallingford [Google Scholar]
- Garg V, Sachse FB, Sanguinetti MC. (2012) Tuning of EAG K(+) channel inactivation: molecular determinants of amplification by mutations and a small molecule. J Gen Physiol 140:307–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg V, Stary-Weinzinger A, Sachse F, Sanguinetti MC. (2011) Molecular determinants for activation of human ether-à-go-go-related gene 1 potassium channels by 3-nitro-n-(4-phenoxyphenyl) benzamide. Mol Pharmacol 80:630–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach AC, Stoehr SJ, Castle NA. (2010) Pharmacological removal of human ether-à-go-go-related gene potassium channel inactivation by 3-nitro-N-(4-phenoxyphenyl) benzamide (ICA-105574). Mol Pharmacol 77:58–68 [DOI] [PubMed] [Google Scholar]
- Goldin AL. (1991) Expression of ion channels by injection of mRNA into Xenopus oocytes. Methods Cell Biol 36:487–509 [DOI] [PubMed] [Google Scholar]
- Grunnet M, Abbruzzese J, Sachse FB, Sanguinetti MC. (2011) Molecular determinants of human ether-à-go-go-related gene 1 (hERG1) K+ channel activation by NS1643. Mol Pharmacol 79:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen RS, Diness TG, Christ T, Demnitz J, Ravens U, Olesen SP, Grunnet M. (2006) Activation of human ether-a-go-go-related gene potassium channels by the diphenylurea 1,3-bis-(2-hydroxy-5-trifluoromethyl-phenyl)-urea (NS1643). Mol Pharmacol 69:266–277 [DOI] [PubMed] [Google Scholar]
- Hemmerlein B, Weseloh RM, Mello de Queiroz F, Knötgen H, Sánchez A, Rubio ME, Martin S, Schliephacke T, Jenke M, Heinz-Joachim-Radzun, et al. (2006) Overexpression of Eag1 potassium channels in clinical tumours. Mol Cancer 5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess B, Bekker H, Berendsen HJC, Fraaije JGEM. (1997) LINCS: A linear constraint solver for molecular simulations. J Comput Chem 18:1463–1472 [Google Scholar]
- Hess B, Kutzner C, van der Spoel D, Lindahl E. (2008) GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4:435–447 [DOI] [PubMed] [Google Scholar]
- Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C. (2006) Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 65:712–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. (1990) Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 250:533–538 [DOI] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. (1991) Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron 7:547–556 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. (2003) X-ray structure of a voltage-dependent K+ channel. Nature 423:33–41 [DOI] [PubMed] [Google Scholar]
- Jones G, Willett P, Glen RC. (1995) Molecular recognition of receptor sites using a genetic algorithm with a description of desolvation. J Mol Biol 245:43–53 [DOI] [PubMed] [Google Scholar]
- Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW. and Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935 [Google Scholar]
- Kang J, Chen XL, Wang H, Ji J, Cheng H, Incardona J, Reynolds W, Viviani F, Tabart M, Rampe D. (2005) Discovery of a small molecule activator of the human ether-a-go-go-related gene (HERG) cardiac K+ channel. Mol Pharmacol 67:827–836 [DOI] [PubMed] [Google Scholar]
- Knape K, Linder T, Wolschann P, Beyer A, Stary-Weinzinger A. (2011) In silico analysis of conformational changes induced by mutation of aromatic binding residues: consequences for drug binding in the hERG K+ channel. PLoS ONE 6:e28778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köpfer DA, Hahn U, Ohmert I, Vriend G, Pongs O, de Groot BL, Zachariae U. (2012) A molecular switch driving inactivation in the cardiac K+ channel HERG. PLoS ONE 7:e41023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange W, Geissendörfer J, Schenzer A, Grötzinger J, Seebohm G, Friedrich T, Schwake M. (2009) Refinement of the binding site and mode of action of the anticonvulsant Retigabine on KCNQ K+ channels. Mol Pharmacol 75:272–280 [DOI] [PubMed] [Google Scholar]
- Ludwig J, Terlau H, Wunder F, Brüggemann A, Pardo LA, Marquardt A, Stühmer W, Pongs O. (1994) Functional expression of a rat homologue of the voltage gated either á go-go potassium channel reveals differences in selectivity and activation kinetics between the Drosophila channel and its mammalian counterpart. EMBO J 13:4451–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Lino de Oliveira C, Mello de Queiroz F, Pardo LA, Stühmer W, Del Bel E. (2008) Eag1 potassium channel immunohistochemistry in the CNS of adult rat and selected regions of human brain. Neuroscience 155:833–844 [DOI] [PubMed] [Google Scholar]
- Mello de Queiroz F, Suarez-Kurtz G, Stühmer W, Pardo LA. (2006) Ether à go-go potassium channel expression in soft tissue sarcoma patients. Mol Cancer 5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose S. (1984) A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys 81:511–519 [Google Scholar]
- Pardo LA, del Camino D, Sánchez A, Alves F, Brüggemann A, Beckh S, Stühmer W. (1999) Oncogenic potential of EAG K(+) channels. EMBO J 18:5540–5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello M, Rahman A. (1981) Polymorphic transitions in single crystals: A new molecular dynamics method. J Appl Phys 52:7182–7190 [Google Scholar]
- Perry M, Sachse FB, Sanguinetti MC. (2007) Structural basis of action for a human ether-a-go-go-related gene 1 potassium channel activator. Proc Natl Acad Sci USA 104:13827–13832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Jiang C, Curran ME, Keating MT. (1995) A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81:299–307 [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK. (1990) Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol 96:195–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Tristani-Firouzi M. (2006) hERG potassium channels and cardiac arrhythmia. Nature 440:463–469 [DOI] [PubMed] [Google Scholar]
- Schenzer A, Friedrich T, Pusch M, Saftig P, Jentsch TJ, Grötzinger J, Schwake M. (2005) Molecular determinants of KCNQ (Kv7) K+ channel sensitivity to the anticonvulsant retigabine. J Neurosci 25:5051–5060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibmayer W, Lester HA, Dascal N. (1994) Voltage clamping of Xenopus laevis oocytes utilizing agarose-cushion electrodes. Pflugers Arch 426:453–458 [DOI] [PubMed] [Google Scholar]
- Seebohm G, Westenskow P, Lang F, Sanguinetti MC. (2005) Mutation of colocalized residues of the pore helix and transmembrane segments S5 and S6 disrupt deactivation and modify inactivation of KCNQ1 K+ channels. J Physiol 563:359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu SW, Vácha R, Jungwirth P, Böckmann RA. (2008) Biomolecular simulations of membranes: physical properties from different force fields. J Chem Phys 128:125103. [DOI] [PubMed] [Google Scholar]
- Smith PL, Baukrowitz T, Yellen G. (1996) The inward rectification mechanism of the HERG cardiac potassium channel. Nature 379:833–836 [DOI] [PubMed] [Google Scholar]
- Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC. (1996) Fast inactivation causes rectification of the IKr channel. J Gen Physiol 107:611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfeld PJ, Grottesi A, Sands ZA, Sansom MS, Gedeck P, Gosling M, Cox B, Stanfield PR, Mitcheson JS, Sutcliffe MJ. (2008) Insight into the mechanism of inactivation and pH sensitivity in potassium channels from molecular dynamics simulations. Biochemistry 47:7414–7422 [DOI] [PubMed] [Google Scholar]
- Stary A, Wacker SJ, Boukharta L, Zachariae U, Karimi-Nejad Y, Aqvist J, Vriend G, de Groot BL. (2010) Toward a consensus model of the HERG potassium channel. ChemMedChem 5:455–467 [DOI] [PubMed] [Google Scholar]
- Stühmer W. (1992) Electrophysiological recording from Xenopus oocytes. Methods Enzymol 207:319–339 [DOI] [PubMed] [Google Scholar]
- Suessbrich H, Schönherr R, Heinemann SH, Lang F, Busch AE. (1997) Specific block of cloned Herg channels by clofilium and its tertiary analog LY97241. FEBS Lett 414:435–438 [DOI] [PubMed] [Google Scholar]
- Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. (1995) HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269:92–95 [DOI] [PubMed] [Google Scholar]
- Warmke J, Drysdale R, Ganetzky B. (1991) A distinct potassium channel polypeptide encoded by the Drosophila eag locus. Science 252:1560–1562 [DOI] [PubMed] [Google Scholar]
- Warmke JW, Ganetzky B. (1994) A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci USA 91:3438–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuttke TV, Seebohm G, Bail S, Maljevic S, Lerche H. (2005) The new anticonvulsant retigabine favors voltage-dependent opening of the Kv7.2 (KCNQ2) channel by binding to its activation gate. Mol Pharmacol 67:1009–1017 [DOI] [PubMed] [Google Scholar]
- Ye S, Li Y, Jiang Y. (2010) Novel insights into K+ selectivity from high-resolution structures of an open K+ channel pore. Nat Struct Mol Biol 17:1019–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zou B, Yu H, Moretti A, Wang X, Yan W, Babcock JJ, Bellin M, McManus OB, Tomaselli G, et al. (2012) Modulation of hERG potassium channel gating normalizes action potential duration prolonged by dysfunctional KCNQ1 potassium channel. Proc Natl Acad Sci USA 109:11866–11871 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.