Abstract
Metabotropic glutamate receptor 5 (mGlu5) is a target for the treatment of central nervous system (CNS) disorders, such as schizophrenia and Alzheimer’s disease. Furthermore, mGlu5 has been shown to play an important role in hippocampal synaptic plasticity, specifically in long-term depression (LTD) and long-term potentiation (LTP), which is thought to be involved in cognition. Multiple mGlu5-positive allosteric modulators (PAMs) have been developed from a variety of different scaffolds. Previous work has extensively characterized a common allosteric site on mGlu5, termed the MPEP (2-Methyl-6-(phenylethynyl)pyridine) binding site. However, one mGlu5 PAM, CPPHA (N-(4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl)-2-hydroxybenzamide), interacts with a separate allosteric site on mGlu5. Using cell-based assays and brain slice preparations, we characterized the interaction of a potent and efficacious mGlu5 PAM from the CPPHA series termed NCFP (N-(4-chloro-2-((4-fluoro-1,3-dioxoisoindolin-2-yl)methyl)phenyl)picolinamide). NCFP binds to the CPPHA site on mGlu5 and potentiates mGlu5-mediated responses in both recombinant and native systems. However, NCFP provides greater mGlu5 subtype selectivity than does CPPHA, making it more suitable for studies of effects on mGlu5 in CNS preparations. Of interest, NCFP does not potentiate responses involved in hippocampal synaptic plasticity (LTD/LTP), setting it apart from other previously characterized MPEP site PAMs. This suggests that although mGlu5 PAMs may have similar responses in some systems, they can induce differential effects on mGlu5-mediated physiologic responses in the CNS. Such stimulus bias by mGlu5 PAMs may complicate drug discovery efforts but would also allow for specifically tailored therapies, if pharmacological biases can be attributed to different therapeutic outcomes.
Introduction
The metabotropic glutamate receptors (mGlus) include eight subtypes (mGlu1-8) of seven transmembrane-spanning G protein–coupled receptors (7TMRs) for the neurotransmitter glutamate. The mGlus play multiple roles in regulating central nervous system (CNS) function and serve as potential therapeutic targets for a variety of brain disorders (Niswender and Conn, 2010; Vinson and Conn, 2012). A growing body of evidence suggests that selective activators of the mGlu5 subtype could provide an exciting new approach for treatment of schizophrenia and other disorders that lead to impaired cognitive function (Gregory et al., 2011; Vinson and Conn, 2012). Although discovery of selective mGlu5 agonists that have drug-like properties has been challenging, there have been major advances in development of highly selective positive allosteric modulators (PAMs) for mGlu5 (Liu et al., 2008; Conn et al., 2009; Stauffer, 2011; Varnes et al., 2011; Packiarajan et al., 2012). A diverse range of selective mGlu5 PAMs have now been identified that have efficacy in animal models used to predict potential antipsychotic and cognitive enhancing activity (Gregory et al., 2011; Vinson and Conn, 2012). In addition to providing greater subtype selectivity than competitive, orthosteric ligands, the ability of mGlu5 PAMs to maintain activity dependence of receptor activation may reduce adverse effect liability that can be associated with excessive mGlu5 activation (Conn et al., 2009).
An important property of mGlu5 PAMs that is relevant for the cognition-enhancing effects of these agents is their unique profile in enhancing synaptic plasticity. Multiple mGlu5 PAMs enhance induction of both long-term depression (LTD) and long-term potentiation (LTP) of transmission at excitatory glutamatergic synapses in the hippocampus (Ayala et al., 2009; Auerbach et al., 2011; Popkirov and Manahan-Vaughan, 2011; Noetzel et al., 2012). Of importance, mGlu5 PAMs enhance both forms of synaptic plasticity without altering the specific pattern of afferent activity required for induction of LTD versus LTP. This ability of mGlu5 PAMs to potentiate both LTP and LTD while maintaining a strict dependence of both on specific patterns of afferent activity could provide an ideal profile for a cognition-enhancing agent.
Selective mGlu5 PAMs have been developed from multiple chemical scaffolds (O'Brien et al., 2004; Kinney et al., 2005; Chen et al., 2007; Liu et al., 2008; Hammond et al., 2010; Rodriguez et al., 2010; Lamb et al., 2011; Stauffer, 2011; Varnes et al., 2011; Noetzel et al., 2012; Packiarajan et al., 2012); the majority of these mGlu5 PAMs bind to the same site as the prototypical mGlu5 negative allosteric modulator (NAM) MPEP (2-Methyl-6-(phenylethynyl)pyridine), located in the top third of the transmembrane spanning domains, involving transmembrane domains 3, 6, and 7 (Gregory et al., 2011). However, at least two mGlu5 PAMs, CPPHA (N-(4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl)-2-hydroxybenzamide) (O'Brien et al., 2004; Zhao et al., 2007; Chen et al., 2008) and VU0357121 (Hammond et al., 2010), have been identified that interact noncompetitively with the MPEP site. Of interest, Zhang et al. (2005) reported that CPPHA and an MPEP site mGlu5 PAM, DFB (difluorobenzaldazine), induce similar potentiation of mGlu5-mediated calcium mobilization in cortical astrocytes. However, although DFB has similar effects on mGlu5 activation of calcium mobilization and extracellular signal-regulated kinases 1 and 2 (ERK1/2) phosphorylation in astrocytes, CPPHA inhibits ERK1/2 phosphorylation responses to maximally effective concentrations of glutamate in these cells. Similar differences in effects of allosteric modulators on different signaling pathways have also been observed in cell lines expressing other mGlu subtypes (Sheffler and Conn, 2008; Niswender et al., 2010). Although CPPHA is a relatively efficacious and potent mGlu5 PAM, this compound is not entirely selective and has weak PAM activity at mGlu1 and weak NAM activity at mGlu4 and mGlu8 (O’Brien et al., 2004; Chen et al., 2008), which complicates the use of this compound to study functional effects of this class of mGlu5 PAM in native systems. We now report a series of studies in which we characterize a novel mGlu5 PAM, termed NCFP (N-(4-chloro-2-((4-fluoro-1,3-dioxoisoindolin-2-yl)methyl)phenyl)picolinamide) (Zhao et al., 2007; patent number WO 2004087048 A2 20041014) that is structurally related to CPPHA. NCFP interacts noncompetitively with the MPEP site and likely binds instead to the same site as CPPHA. Of interest, NCFP has a fundamentally different profile from previous mGlu5 PAMs when assessing effects of hippocampal synaptic plasticity. Although NCFP potentiates multiple responses to mGlu5 activation in cell lines and potentiates mGlu5-mediated depolarization of neurons in the subthalamic nucleus, this compound does not enhance induction of either LTP or LTD at the hippocampal Schaffer collateral-CA1 (SC-CA1) synapse. These data provide strong support for the hypothesis that different mGlu5 PAMs can have fundamentally different effects on mGlu5-mediated physiologic responses in the CNS that could be important for the overall efficacy profile of these agents.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and antibiotics were purchased from Life Technologies (Carlsbad, CA). DHPG [(S)-3,5-dihydroxyphenylglycine]was obtained from Ascent Scientific (Bristol, UK). CPPHA, NCFP (Zhao et al., 2007), VU0092273 [(4-hydroxypiperidin-1-yl)(4-phenylethynyl)phenyl)methanone] (Rodriguez et al., 2010), 5-MPEP (Rodriguez et al., 2005), and MPEP (Tocris) were synthesized in house. [3H]methoxyPEPy (76.3 Ci/mmol) was custom synthesized by PerkinElmer Life and Analytical Sciences (Waltham, MA). Unless otherwise stated, all other reagents were purchased from Sigma-Aldrich (St. Louis, MO) and were of an analytical grade.
Cell Culture
Human embryonic kidney 293 (HEK293) cells stably expressing rat mGlu5 or rat mGlu1 were maintained in complete DMEM supplemented with 10% FBS, 2 mM L-glutamine, 20 mM HEPES, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, antibiotic-antimycotic (Invitrogen, Carlsbad, CA), and G418 (500 µg/ml; Mediatech, Manassas, VA) at 37°C in a humidified incubator containing 5% CO2/95%O2. HEK293 cells stably expressing G protein–coupled inwardly rectifying potassium channels (HEK293-GIRK) along with the individual group II and group III mGlu receptors were maintained in growth media containing 45% DMEM, 45% F-12, 10% FBS, 20 mM HEPES, 2 mM L-glutamine, antibiotic/antimycotic, nonessential amino acids, G418 (700 μg/ml), and puromycin (0.6 μg/ml).
Mutagenesis and Transfection
Single-point mutations of mGlu5 were generated using QuikChange II site-directed mutagenesis kits (Agilent Technologies, Santa Clara, CA) and confirmed by sequencing. Construction of the HA-tagged N-terminal truncated mGlu5 mutant was performed as described previously (Goudet et al., 2004). Transfection of HEK293A cells with wild-type mGlu5 and mutant constructs in pCI:Neo were performed using Fugene6 (Promega, Madison, WI), and passaged five times in the presence of 1 μg/ml G418 to generate stable polyclonal cell lines. Stably transfected cell lines were subsequently maintained in complete DMEM supplemented with 10% FBS, 2 mM L-glutamine, 20 mM HEPES, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, antibiotic-antimycotic, and 500 μg/ml G418 at 37°C in a humidified incubator containing 5% CO2/95% O2.
Fluorescence-Based Calcium Signaling
Measurements of compound-evoked increases in intracellular calcium were performed as described previously (Noetzel et al., 2012). In brief, HEK293 cells stably expressing rat mGlu5 and mutants thereof were plated in 96-well, poly-D-lysine–coated, black-walled, clear-bottomed plates in assay medium (DMEM supplemented with 10% dialyzed FBS, 20 mM HEPES, and 1 mM sodium pyruvate) at a density of 40–50,000 cells/well. Calcium flux was measured over time as an increase in fluorescence of the Ca2+ indicator dye, Fluo-4 AM, using a FlexStation II (Molecular Devices, Sunnyvale, CA). Either vehicle or test compound was added 60 seconds before the addition of glutamate. Compound addition 120 second before the addition of glutamate was also assessed. There was no difference observed in the potency for CPPHA or NCFP between the two time points (pEC50 CPPHA: 60 seconds, −6.14; 120 seconds, −6.14; NCFP: 60 seconds, −6.30; 120 seconds, −6.23), suggesting that the maximum effect of the PAMs has been achieved with this preincubation period. The change in fluorescence over basal was determined before normalization to the maximal response elicited by glutamate. Experiments using the N-terminal truncated mGlu5 receptor were normalized to the maximal response elicited by 1 μM ionomycin. Data were transformed and fitted using GraphPad Prism 5.01 (Graph-Pad Software, Inc., San Diego, CA). As described in Gregory et al. (2012), shifts of glutamate concentration-response curves by allosteric modulators were globally fitted to an operational model of allosterism (Leach et al., 2007) with use of Eq. 2 from Gregory et al. (2012).
Selectivity
mGlu1.
To assess the effect of test compounds at mGlu1, Ca2+ mobilization assays were performed as described previously (Hammond et al., 2010; Noetzel et al., 2012). In brief, HEK293 cells stably expressing rat mGlu1 were plated in black-walled, clear-bottomed, poly-D-lysine–coated 384-well plates (Greiner Bio-One, Monroe, NC) in assay medium at a density of 20,000 cells/well. Calcium flux was measured over time as an increase in fluorescence of the Ca2+ indicator dye, Fluo-4 AM, with use of a Functional Drug Screening System 6000 (Hamamatsu, Tokyo, Japan). Either vehicle or a fixed concentration of test compound (10 µM, final concentration) was added followed 140 seconds later by a concentration-response curve of glutamate. Data were analyzed as described above.
Group II and Group III mGlus.
The functional activity of the compound of interest was assessed at the rat group II and III mGlu receptors by measuring thallium flux through GIRK channels as previously described (Niswender et al., 2008). In brief, HEK293-GIRK cells expressing mGlu subtype 2, 3, 4, 6, 7, or 8 were plated into 384-well, black-walled, clear-bottom poly-D-lysine–coated plates at a density of 15,000 cells/well in assay medium. A single concentration of test compound (10 µM) or vehicle was added followed 140 seconds later by a concentration-response curve of glutamate (or L-(+)-2-Amino-4-phosphonobutyric acid for mGlu7) diluted in thallium buffer (125 mM NaHCO3, 1 mM MgSO4, 1.8 mM CaSO4, 5 mM glucose, 12 mM thallium sulfate, 10 mM HEPES), and fluorescence was measured using a Functional Drug Screening System 6000. Data were analyzed as described previously (Niswender et al., 2008).
Rat Cortical Astrocytes
Primary rat cortical astrocytes (from rats of mixed sex) were purchased from Lonza (Basel, Switzerland) and maintained as previously described (Noetzel et al., 2012). Astrocytes were grown in assay growth media (AGM; assay basal media supplemented with AGM Singlequots from Lonza). Two days before the assay, astrocytes were plated in 96-well, poly-D-lysine–coated, black-walled, clear-bottomed plates in AGM at a density of approximately 50,000 cells/well. The next day, astrocytes were supplemented with G5 diluted in AGM. The calcium flux assay was performed on the following day with use of assay conditions and compound preparation identical to those used for the mGlu5 HEK293A cell assay.
ERK Phosphorylation
Receptor-mediated ERK1/2 phosphorylation was determined using the AlphaScreen-based ERK SureFire kit (PerkinElmer, Waltham, MA). HEK293A cells stably expressing mGlu5 were plated at a density of 40,000 cells/well in clear 96-well poly-D-lysine–coated plates in assay medium 16–24 hours before assay. Media was aspirated and cells serum starved in serum-free media (DMEM supplemented with 16 mM HEPES) for 6 hours before assay. Serum-free media was exchanged for fresh at the start of the experiment (30 minutes before termination of the response). Cells were treated with modulators or glutamate for the indicated amount of time at room temperature. The assay was terminated by aspiration of ligand-containing media and addition of 50 μl/well of lysis buffer. Lysates were processed as described previously (Gregory et al., 2012) and AlphaScreen signal measured using an Enspire (PerkinElmer) with standard AlphaScreen settings. Data are expressed as fold increase over basal levels of phosphorylated ERK.
Radioligand Binding
Membranes were prepared from HEK293 cells expressing rat mGlu5 and mutants as previously described (Gregory et al., 2012). For inhibition binding experiments, membranes (20–50 µg/well) were incubated with 2 nM [3H]methoxyPEPy and a range of concentrations of test ligand (100 pM to 100 µM) for 1 hour at room temperature with shaking in calcium assay buffer with 1% dimethylsulfoxide final; 10 µM MPEP was used to determine nonspecific binding. Binding assays were terminated by rapid filtration through GF/B Unifilter plates (PerkinElmer Life and Analytical Sciences, Boston, MA) using a Brandel 96-well plate Harvester (Brandel Inc., Gaithersburg, MD) and three washes with ice-cold Binding Buffer (50 mM Tris-HCl, 0.9% NaCl; pH 7.4). Plates were allowed to dry overnight before addition of MicroScint20 (40 µl/well; PerkinElmer). Radioactivity was counted after at least 2 hours incubation with use of a TopCount Scintillation Counter (PerkinElmer Life and Analytical Sciences). Inhibition [3H]methoxyPEPy binding data sets were fitted to a one-site inhibition binding model, and estimates of inhibitor dissociation constants (KI) were derived using the Cheng-Prusoff equation for competitive ligands (Cheng and Prusoff, 1973) and the allosteric ternary complex model for ligands that did not fully displace radioligand (Lazareno and Birdsall, 1995).
Electrophysiology
Extracellular Field Potential Recordings.
All animals used in these studies were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals; 400 µm hippocampal slices were prepared from young adult (age, 30–40 days) male Sprague-Dawley rats (Charles River, Wilmington, MA) with use of standard techniques and buffers as previously described (Noetzel et al., 2012; Ayala et al., 2009) A bipolar-stimulating electrode was placed in the stratum radiatum near the CA3-CA1 border to stimulate the Schaffer collaterals. Recording electrodes were pulled with a Flaming/Brown micropipette puller (Sutter Instrument Company, Novato, CA), filled with ACSF (artificial cerebral spinal fluid), and placed in the stratum radiatum of area CA1. Field potential recordings were acquired using a MultiClamp 700B amplifier (Molecular Devices) and pClamp 10.2 software (Molecular Devices). A stimulus intensity that produced 50–60% [long-term depression (LTD)] and 40–50% [long-term potentiation (LTP)] of the maximum field excitatory postsynaptic potential (fEPSP) slope was set before each experiment. mGlu5 compounds were diluted to the appropriate concentrations in dimethylsulfoxide (0.1% final) in ACSF and applied to the bath for 10–20 minutes with use of a perfusion system. Chemically induced mGlu LTD was initiated by the application of DHPG in ACSF (25–75 µM) for 10 minutes. Threshold LTP was induced by one train of theta burst stimulation (TBS; nine bursts of four pluses at 100 Hz, 230-millisecond interburst interval). Saturated LTP was induced by four trains of 10 Hz TBS (nine bursts of four pulses at 100 Hz, 100-millisecond interburst interval). Data were analyzed using Clampfit 10.2 and GraphPad Prism 5.0 as described previously (Noetzel et al., 2012).
Whole-Cell Patch-Clamp Recordings.
Whole-cell patch-clamp recordings were performed using midbrain slices prepared from 15–19-day-old Sprague-Dawley rats of mixed sex (Charles River); 300 µm sagittal slices were prepared using standard techniques and buffers described previously (Noetzel et al., 2012). Whole-cell recordings were made from visually identified subthalamic nucleus (STN) neurons. Borosilicate glass patch electrodes were pulled using a Flaming/Brown micropipette puller (Sutter Instrument Company) with a resistance of 2–5 MΩ when filled with intracellular solution. All whole-cell patch-clamp recordings were performed using a MultiClamp 700B amplifier (Molecular Devices). Data were digitized with a DigiData 1331 system (Molecular Devices), filtered at 2 kHz, and acquired using pClamp10.2 (Molecular Devices). After formation of a whole-cell configuration, cells were held at −60 mV and changes in membrane potential were recorded. Data were analyzed using Clampfit 10.2 (Molecular Devices).
Results
NCFP Acts as an mGlu5 PAM.
NCFP (Zhao et al., 2007) is a close structural analog of the previously reported mGlu5 PAM CPPHA (Fig. 1A) (O'Brien et al., 2004; Zhao et al., 2007). To assess the activity of NCFP as an mGlu5 PAM, we compared the effects of NCFP on agonist-induced calcium mobilization in HEK293A cells expressing mGlu5. In common with CPPHA, NCFP had no effect on calcium mobilization when added alone but induced a concentration-dependent potentiation of the response to an EC20 concentration of glutamate (Fig. 1B; Table 1). In agreement with previous results (O'Brien et al., 2004), CPPHA also potentiated the response to an EC20 concentration of glutamate (Fig. 1B; Table 1). The ability of NCFP to potentiate the response to glutamate was also assessed in rat cortical astrocytes, a native system that expresses mGlu5 (Peavy and Conn, 1998; Zhang et al., 2005; Chen et al., 2008). In agreement with the HEK293A cell assays, NCFP and CPPHA potentiated the response to an EC20 concentration of glutamate in cortical astrocytes. However, NCFP had a slightly lower potency, compared with HEK293A cells (Fig. 1C; Table 1). Previous studies suggest that some mGlu5 PAMs can possess intrinsic mGlu5 agonist activity; we recently reported that this allosteric agonist activity is most readily observed when mGlu5 PAMs are assessed in cells expressing high levels of mGlu5 (Noetzel et al., 2012). Thus, we evaluated the effects of NCFP and CPPHA in a previously characterized cell line expressing high levels of mGlu5 (Noetzel et al., 2012). Neither NCFP nor CPPHA exhibited any agonist activity when assessed in the high mGlu5 expression cell line, and both compounds potentiated the EC20 response to glutamate in a similar manner to that observed in the lower expressing cell line (Supplemental Fig. 1A; Table 1). In the lower mGlu5-expressing cell line, both NCFP and CPPHA induced a concentration-dependent leftward shift in the concentration-response relationship of glutamate, with NCFP inducing a slightly greater shift in glutamate potency, compared with CPPHA (Fig. 1, D and E; Table 1). Allosteric modulator affinity and cooperativity estimates were determined by fitting the data to an operational model of allosterism, as described in Gregory et al. (2012) (details in Materials and Methods). Of interest, NCFP had lower affinity (pKb) and increased cooperativity (logβ), compared with CPPHA (Table 2).
Fig. 1.

NCFP potentiates the response to glutamate in a manner similar to that of CPPHA. (A) Structures of CPPHA and NCFP. (B) The potencies of CPPHA (black squares) and NCPF (open triangles) were determined by adding increasing concentrations of each PAM to HEK293 mGlu5 cells 60 seconds before the addition of a concentration of glutamate eliciting a 20% maximal response (EC20, 40–60 nM). The calcium response was normalized to the response induced by a maximally effective concentration of glutamate (10 µM). (C) Potencies of the compounds were determined as in B, except rat cortical astrocytes were used. (EC20, 600–650 nM). (D and E) Progressive fold shift values were determined by treating HEK293 mGlu5 cells with fixed concentrations (300 nM, black triangles, 1 µM black circle, 3 µM open square or 10 µM open triangle) of CPPHA (D) or NCFP (E), followed by the addition of a concentration response curve to glutamate. Calcium responses were normalized to the response induced by a maximally effective concentration of glutamate (10 µM). (F) The level of ERK1/2 phosphorylation (fold/basal) was determined by treating HEK293 mGlu5 cells with a fixed concentration of mGlu5 compounds (3 µM; control black square, NCFP open triangle, VU0092273 black circle) or glutamate (1 mM; black diamond) for the times indicated. Data represent the mean ± S.E.M. of 3–4 independent experiments performed in duplicate.
TABLE 1.
Expression level and potency values for mGlu5-expressing cell lines
Data represent the mean ± S.E.M. from a minimum of three individual experiments conducted in duplicate or triplicate.
| Cell line |
Expression levela |
pEC50b | |
|---|---|---|---|
| pmol/mg protein |
NCFP |
CPPHA |
|
| Wild-type-mGlu5 (high expression) | 2.3 ± 0.4c | 6.69 ± 0.09 (214 nM) | 6.44 ± 0.04 (370 nM) |
| Wild-type-mGlu5 (low expression) | 0.6 ± 0.1d | 6.67 ± 0.10 (225nM) | 6.16 ± 0.01 (689nM) |
| F585I-mGlu5 | 1.8 ± 0.6 | ND | ND |
| Rat cortical astrocytes | 3.4 ± 0.3ce fmol/105 cells | 6.04 ± 0.10 (969 nM) | 6.33 ± 0.28 (470 nM) |
CPPHA, N-(4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl)-2-hydroxybenzamide; mGlu, metabotropic glutamate receptor; NCFP, N-(4-chloro-2-((4-fluoro-1,3-dioxoisoindolin-2-yl)methyl)phenyl)picolinamide; ND, not determined.
Receptor expression level determined by [3H]methoxyPEPy binding.
Negative logarithm of the concentration of modulator that resulted in half-maximal potentiation of an EC20 concentration of glutamate. Values in parentheses are conversion to molar concentration.
Expression level previously reported in Noetzel et al., 2012.
Expression level previously reported in Gregory et al., 2012.
Expression level reported as fmol/105 cells because of methodological difficulties in membrane binding.ND values not determined.
TABLE 2.
Operational model parameters and fold shift values for NCFP and CPPHA modulation of intracellular Ca2+ mobilization
Data represent the mean ± S.E.M. from a minimum of three individual experiments conducted in duplicate.
| Parameter | Wild-type-mGlu5 |
F585I-mGlu5 |
||
|---|---|---|---|---|
| NCFP | CPPHA | NCFP | CPPHA | |
| pKBa | 5.56 ± 0.13 | 6.09 ± 0.09d | ND | ND |
| Logβb | 0.99 ± 0.05 | 0.59 ± 0.08d | ND | ND |
| Fold shift (max)c | 7.7 ± 0.1 | 5.9 ± 1.6 | ND | ND |
| Fold shift (3µM)c | 5.9 ± 0.1 | 4.0 ± 0.7 | 1.6 ± 0.1* | 1.3 ± 0.1* |
CPPHA, N-(4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl)-2-hydroxybenzamide; mGlu, metabotropic glutamate receptor; NCFP, N-(4-chloro-2-((4-fluoro-1,3-dioxoisoindolin-2-yl)methyl)phenyl)picolinamide; ND, not determined; PkB, protein kinase B.
Negative logarithm of the allosteric modulator dissociation constant.
Negative logarithm of the efficacy cooperativity factor.
Fold shift values were determined for the maximum fold shift determined for multiple concentrations or at a single (3 µM) concentration.
Operational model parameters were previously reported in Gregory et al., 2012.ND values not determined.
Significantly different (P < 0.05) to wild-type value.
As a second measure of mGlu5 activation, NCFP and CPPHA were evaluated for their ability to stimulate an increase in ERK1/2 phosphorylation in mGlu5-expressing HEK293 cells. Treatment of HEK293A cells with 1 mM glutamate resulted in an increase in ERK1/2 phosphorylation that peaked at 7 minutes (3.1 ± 0.8 fold/basal). When added alone, both NCFP (Fig. 1F) and CPPHA (unpublished data) also induced robust increases in ERK1/2 phosphorylation peaking at 7 minutes and were comparable to the responses to glutamate (3.0 ± 0.9 and 2.8 ± 0.5 fold/basal, respectively). In parallel, we evaluated the response to VU0092273, a known MPEP-site mGlu5 PAM (Rodriguez et al., 2010; Noetzel et al., 2012). VU0092273 alone also induced an increase in ERK1/2 phosphorylation that was similar to the responses to NCFP and CPPHA (Fig. 1F; 3.9 ± 1.1 fold/basal at 7 minutes). Similar results were observed with other MPEP-site PAMs (Gregory et al., 2012). We further tested whether the ERK1/2 phosphorylation response observed after the addition of compound alone could be blocked with an orthosteric antagonist, LY341495. Incubation with antagonist, followed by compound addition, resulted in a blockade of the ERK1/2 phosphorylation response (Supplemental Fig. 2), suggesting that observed response to the compounds alone is not the result of direct activation of the receptor by the modulator. Thus, each of these compounds induces ERK1/2 phosphorylation in HEK293A cells that is dependent on mGlu5 activation.
NCFP Interacts with the CPPHA Site on mGlu5.
Previous studies demonstrated that CPPHA has little or no effect on [3H]methoxyPEPy binding (O'Brien et al., 2004) in membranes from cells expressing a high level of mGlu5, suggesting that CPPHA does not bind at the MPEP site. We performed similar binding studies using the mGlu5 cell line expressing high levels of mGlu5 (Table 1; Noetzel et al., 2012). Consistent with O’Brien et al. (2004), MPEP induced a concentration-dependent inhibition of [3H]methoxyPEPy binding, whereas CPPHA was without effect at concentrations up to 30 µM (Fig. 2). In addition, NCFP had no effect on [3H]methoxyPEPy binding under these conditions (Fig. 2), suggesting that neither NCFP nor CPPHA act by competitive binding to the MPEP binding site.
Fig. 2.
NCFP does not inhibit [3H]methoxyPEPy binding in cells expressing high levels of mGlu5. Cells expressing high levels of mGlu5 were treated with increasing concentrations of NCFP (open triangles), CPPHA (black squares), or MPEP (black circles) and 2 nM [3H]methoxyPEPy. Reactions were allowed to incubate for 1 hour before termination. Nonspecific binding was determined using 10 µM MPEP. Data represent the mean ± S.E.M. of three individual experiments conducted in duplicate.
A single point mutation in the first transmembrane domain of mGlu5, mGlu5-F585I, has been shown to inhibit the ability of CPPHA to potentiate the response to glutamate but has no effect on responses to PAMs that act at the MPEP site (Chen et al., 2008). In agreement with these previous results, we found that CPPHA was unable to potentiate the response to glutamate in HEK293A cells expressing mGlu5-F585I (Fig. 3A; Table 2). In contrast, the MPEP site mGlu5 PAM, VU0092273 induced a robust potentiation of the response to glutamate in cells expressing the mGlu5-F585I mutant receptor (maximum fold-shift 6.8 ± 0.6; unpublished data). As for CPPHA, introduction of the F585I mutation resulted in a loss of the ability of NCFP to potentiate the response to glutamate (Table 2), suggesting that NCFP interacts with mGlu5 in a similar manner to CPPHA (Fig. 3A). To further support the hypothesis that NCFP potentiates mGlu5 responses via interaction with a second allosteric site, we performed Schild regression analysis of NCFP potentiator concentration–response curves in the absence and presence of 5MPEP (5-methyl-2-phenylethynyl-pyridine), a neutral MPEP site ligand. As previously shown, 5MPEP will induce parallel rightward shifts in the concentration-response curves of PAMs that act competitively at the MPEP site, with no change in the maximum response (Rodriguez et al., 2005; Chen et al., 2007). Previous studies with CPPHA suggest that the actions of this PAM are not mediated by interactions at the MPEP site; 5MPEP has minimal effects on CPPHA potency and depresses the maximal response to CPPHA (Chen et al., 2007). Consistent with these studies, treatment with increasing concentrations of 5MPEP (3–30 µM) caused a small reduction in potency (∼2-fold) and reduced the maximal response (∼20%) when measuring potentiation of the response to an EC20 concentration of glutamate (Fig. 3B). These data provide strong evidence that the functional response to NCFP is not mediated via competitive interaction with the MPEP binding site; instead, an allosteric interaction between the two sites is potentially occurring.
Fig. 3.

NCFP activity is blocked by a mutation in the CPPHA site, but not affected by an MPEP site ligand. (A) Cells expressing the F585I mutant mGlu5 receptor were treated with a fixed concentration (3 µM) of NCFP (open triangles) or CPPHA (black triangles), followed by the addition of increasing concentrations of glutamate. (B) mGlu5 cells were first treated with fixed concentrations of 5MPEP (3 µM black triangles, 10 µM black circles or 30 µM black diamonds); then, increasing concentrations of NCFP were added, and finally an EC20 concentration of glutamate was applied. In all cases, the calcium response was normalized to the response induced by a maximally effective concentration of glutamate (10µM). Data represent the mean ± S.E.M. of four independent experiments performed in duplicate.
CPPHA and NCFP Can Allosterically Inhibit Binding to the MPEP Site under Conditions of Low Receptor Expression.
Although these studies provide clear evidence that NCFP and CPPHA do not bind directly to the MPEP site, it is somewhat surprising that these compounds have no effect on [3H]methoxyPEPy binding. The functional studies outlined suggest that neutral ligands that act at the MPEP site can induce weak noncompetitive inhibition of actions of CPPHA and NCFP, suggesting that ligands that act at one of these distinct sites can regulate interactions of ligands at the other allosteric site. Of note, the radioligand binding studies were performed using membranes from cell lines that intentionally express high levels of mGlu5 to provide high specific binding and reduce the signal to noise ratio of the assay. It is possible that this high expression and other differences in the cell background used for binding versus functional studies could introduce an unexpected change in results of the radioligand binding studies. Thus, we evaluated ability of CPPHA and NCFP to inhibit [3H]methoxyPEPy binding to the MPEP site with use of the same physiologic buffer system used in the calcium mobilization assays and of membranes from the lower expressing mGlu5 cell line. Of interest, under these conditions, CPPHA induces weak partial (64.2% ± 1.7%) inhibition of [3H]methoxyPEPy binding (Gregory et al., 2012) (Fig. 4A). Similarly, NCFP induced a weak partial inhibition of [3H]methoxyPEPy binding with a maximal inhibition of 31.4% ± 8.5%. This partial inhibition of [3H]methoxyPEPy binding is consistent with an allosteric interaction between the modulators at CPPHA/NCFP site and [3H]methoxyPEPy (Fig. 4A) and in agreement with the results from Fig. 3 B and Bradley et al. (2011). These studies suggest that receptor expression level or other differences in the cellular background may influence the ability to detect an interaction between these two sites. With the ability to detect interactions between the CPPHA and MPEP site under conditions where receptor expression is low, we next sought to quantify the influence of the F585I mutation on modulator affinity. Receptor expression levels were lower in mGlu5-F585I–expressing cells than in the high mGlu5-expressing cell line (Table 1). Of interest, introduction of the F585I mutation into the mGlu5 receptor had no effect on affinity of [3H]methoxyPEPy or VU0092273 (unpublished data) but completely abolished the ability of NCFP to inhibit [3H]methoxyPEPy binding (Fig. 4B), providing further support for the hypothesis that this effect is attributable to interactions with the CPPHA/NCFP binding site.
Fig. 4.

NCFP inhibits [3H]methoxyPEPy binding in a manner suggestive of a noncompetitive interaction. (A) Cell membranes from HEK293A cells expressing a low level of mGlu5 were treated with increasing concentrations of CPPHA (black squares), NCFP (open triangles), or MPEP (black circles) and 2 nM [3H]methoxyPEPy. Reactions were allowed to incubate for 1 hour before termination. Nonspecific binding was determined using 10 µM MPEP. (B) F585I cell membranes were treated the same as in (A). Data represent the mean ± S.E.M. of 3–7 independent experiments performed in duplicate or triplicate.
NCFP Has Minimal Efficacy at the N-Terminal Truncated Receptor.
Glutamate binds to and activates mGlus via the orthosteric site located in the N-terminal domain of the receptor (Goudet et al., 2004). Truncation of the N-terminal domain prevents mGlus from being activated by orthosteric ligands (Goudet et al., 2004). Although N-terminal truncation eliminates responses to glutamate and other orthosteric ligands, previous studies have demonstrated that mGlu5 PAMs that act at the MPEP site retain their activity and act as agonists of the N-terminal truncated receptor (Goudet et al., 2004; Chen et al., 2007, 2008). On the basis of these observations, we tested the hypothesis that NCFP would also stimulate a response in the N-terminal–truncated mGlu5 construct by measuring NCFP-induced calcium mobilization. Ionomycin was used in place of glutamate to determine the maximum response, because it is able to stimulate calcium release independent of mGlu5 activation. Of surprise, in contrast to the response induced by the MPEP site PAM VU0092273, NCFP has low efficacy for calcium mobilization in cells expressing the N-terminal–truncated mGlu5, compared with the MPEP-site PAM VU0092273 (Fig. 5A; NCFP, 1.3% ± 0.5% of ionomycin; VU0092273, 19.9% ± 2.7% of ionomycin).
Fig. 5.

NCFP acts differently at the N-terminal–truncated receptor, compared with MPEP- site ligands. (A) Cells expressing the N-terminal–truncated mGlu5 receptor were treated with increasing concentrations of NCFP (open triangles) or VU0092273 (black diamonds). The calcium response was normalized to the response induced by a maximally effective concentration of ionomycin (1 µM). (B) The level of ERK1/2 phosphorylation (fold/basal) was determined by treating cells with a fixed concentration of mGlu5 compound (3 µM; NCFP open triangles, VU0092273 black diamonds), FBS (10%, black triangles), or control (black squares) for the times indicated. (C) Cell membranes were treated with increasing concentrations of NCFP (open triangles), VU0092273 (black diamonds), or MPEP (black circles) and 2 nM [3H]methoxyPEPy. Reactions were allowed to incubate for 1 hour before termination. Nonspecific binding was determined using 10 µM MPEP. Data represent the mean ± S.E.M. of 3–6 independent experiments conducted in duplicate.
As discussed above, the MPEP site PAMs that have been tested for agonist activity at the N-terminal–truncated receptor can have also weak agonist activity at wild-type mGlu5 when measuring calcium mobilization, whereas CPPHA and NCFP have no agonist activity at the wild-type receptor when measuring the calcium mobilization response. Thus, it is possible that this difference in agonist activity for calcium mobilization is simply reflected in the weak partial agonism of NCFP at the N-terminal–truncated receptor. Because calcium mobilization and ERK1/2 phosphorylation can occur through different pathways, we tested the ability of NCFP to induce ERK1/2 phosphorylation with use of the N-terminal–truncated mutant. In this case, FBS was used as a positive control, because it will stimulate ERK1/2 phosphorylation in the absence of receptor activation. Similar to the results observed in the calcium mobilization assay, NCFP had no effect on the level of ERK1/2 phosphorylation, compared with control (0.9 ± 0.04 fold/basal; Fig. 5B), whereas VU0092273 induced a robust increase in ERK1/2 phosphorylation (2.1 ± 0.4 fold/basal; Fig. 5B) that was similar to the level of phosphorylation observed with the wild-type receptor (Fig. 1F). In addition to greatly reduced or no effects in functional assays, NCFP was no longer able to inhibit the binding of [3H]methoxyPEPy to the N-terminal–truncated receptor (2.2% ± 4.8% inhibition; Fig. 5C). In contrast, MPEP retained the ability to completely inhibit [3H]methoxyPEPy binding in a concentration-dependent manner (Fig. 5C). The level of receptor expression was similar for the wild-type mGlu5 cells (Table 1) and the N-terminal–truncated receptor construct (0.69 ± 0.16 pol/mg protein), suggesting that receptor expression level was not likely to be responsible for the lack of [3H]methoxyPEPy inhibition by NCFP. In addition, VU0092273 also maintained the ability to completely inhibit [3H]methoxyPEPy binding (Fig. 5C). Although the precise molecular mechanisms underlying the differences between NCFP and MPEP site ligands, such as VU0092273, are not yet understood, these results further support the hypothesis that NCFP interacts with mGlu5 in a manner that is distinct from MPEP site ligands.
NCFP Is Selective for mGlu5 and Does Not Exhibit Probe Dependence for Glutamate Versus the Orthosteric mGlu5 Agonist DHPG.
To use mGlu5 allosteric modulators in brain slice assays, it is important to determine whether they are selective for mGlu5, compared with other mGlu subtypes. We previously reported that CPPHA has weak PAM activity at mGlu1 and weak NAM activity at mGlu4 and mGlu8 (O'Brien et al., 2004; Chen et al., 2008). On the basis of the lack of selectivity of CPPHA, we initiated chemistry efforts to develop a CPPHA analog with a better selectivity profile that could be used to assess mGlu5 function in a more complex system (Zhao et al., 2007). Of the CPPHA analogs that came from this effort, NCFP had properties that prompted us to characterize this compound more extensively. To assess the selectivity of NCFP, cells expressing each mGlu subtype were treated with 10 µM NCFP to determine whether this compound could modulate the response to glutamate (or L-(+)-2-Amino-4-phosphonobutyric acid for mGlu7). NCFP did not shift the concentration-response curves for glutamate in cells expressing any of the mGlu receptor subtypes (Supplemental Fig. 3). Thus, although NCFP and CPPHA behave in a very similar manner in potentiating responses to mGlu5 activation, NCFP is more selective for mGlu5 than is CPPHA. Thus, NCFP provides an improved tool for studies in native preparations and was used for all subsequent experiments.
In addition to determining compound selectivity for use in brain slice-based assays, it is also important to consider the choice of agonist used for these experiments. It is not practical to use glutamate in brain slice–based electrophysiology experiments when studying mGlu5 activation, because glutamate will modulate multiple targets, including other mGlu subtypes, ionotropic glutamate receptors, and glutamate transporters. In addition, glutamate is a substrate for glutamate transporters that are abundant in brain slice preparations, therefore making it difficult to achieve reliable and stable glutamate concentrations within the slice. For these reasons, the group I orthosteric agonist DHPG is routinely used for studies of the physiologic effects of activation of group I mGlus. However, previous work has shown that some allosteric modulators can potentiate responses of other 7TMRs to some, but not all orthosteric agonists, a phenomenon termed probe-dependence (Keov et al., 2011). Therefore, before using DHPG to study effects of NCFP in rat brain slices, it was critical to assess the ability of NCFP to potentiate the response to DHPG. Similar to the results observed with glutamate, NCFP potentiated the response to an EC20 concentration of DHPG with a potency of pEC50 6.94 ± 0.16 (133 nM; Supplemental Fig. 4). Thus, NCFP does not exhibit probe dependence for glutamate relative to DHPG, allowing this combination of allosteric and orthosteric ligands to be used in brain slice preparations.
NCFP Potentiates DHPG-Induced Depolarization of Subthalamic Nucleus Neurons.
One of the most well-established responses to mGlu5 activation in the CNS is depolarization of projection neurons in the STN (Awad et al., 2000). DHPG-induced depolarization of these neurons is completely blocked by MPEP and other mGlu5 NAMs and is potentiated by previously characterized mGlu5 PAMs (Awad et al., 2000; O'Brien et al., 2004; Rodriguez et al., 2005; Chen et al., 2007; Rodriguez et al., 2010; Noetzel et al., 2012). In agreement with previous results, treatment of STN neurons with 100 µM DHPG resulted in a robust membrane depolarization (Fig. 6; 10.7 ± 1.3 mV), whereas 1 µM DHPG induced a small depolarization that was just above threshold for detection (Fig. 6; 2.4 ± 0.8 mV). Treatment of midbrain slices with 10 µM NCFP alone had no effect on STN membrane voltage (Fig. 6; 0.5 ± 0.7 mV). However, 10 µM NCFP, followed by co-addition of 10 µM NCFP and 1 µM DHPG, resulted in a robust potentiation of the depolarization relative to that observed with 1 µM DHPG alone (Fig. 6; 5.7 ± 0.6 mV). We observed similar results with CPPHA, in agreement with data published by Chen et al. (2007) demonstrating the potentiation of the DHPG response in STN neurons by CPPHA. These results suggest that NCFP potentiates mGlu5-mediated depolarization of STN neurons in a manner similar to that observed in HEK293 cells and in cortical astrocytes.
Fig. 6.
NCFP potentiates the DHPG-induced change in membrane voltage in STN neurons. Bath application of 100 µM DHPG induced a robust change in membrane voltage, whereas application of 1 µM DHPG induced a small change in membrane voltage. Application of 10 µM NCFP for 10 minutes had no effect on membrane voltage alone. Co-application of 10 µM NCFP and 1 µM DHPG resulted in a significant enhancement in the change in membrane voltage, compared with 1µM DHPG alone. Data represent the mean ± S.E.M. for five individual experiments for each treatment. * P < 0.5, when compared with 1µM DHPG. Sample traces from individual experiments are presented on the right.
Unlike Other mGlu5 PAMs, NCFP Does Not Potentiate Induction of LTD and LTP in the Hippocampus.
Activation of mGlu5 plays an important role in regulating hippocampal synaptic plasticity, and previous studies have shown that mGlu5 PAMs induce a robust potentiation of DHPG-induced LTD at the hippocampal SC-CA1 synapse (Ayala et al., 2009; Auerbach et al., 2011; Popkirov and Manahan-Vaughan, 2011; Noetzel et al., 2012). In addition, selective mGlu5 PAMs potentiate induction of LTP at this synapse in response to a weak threshold TBS protocol (Ayala et al., 2009). To assess the effects of mGlu5 PAMs on DHPG-induced LTD, fEPSPs were recorded from the dendritic layer of CA1 after stimulation of the Schaffer collaterals. In agreement with our previous studies (Noetzel et al., 2012), 75 µM DHPG induced robust LTD at the hippocampal SC-CA1 synapse measured 55 minutes after washout of the compound (Fig. 7, A and B; 52.2% ± 3.9% of baseline fEPSP slope). In contrast, a lower concentration of DHPG (25 µM) induced only slight depression of fEPSPs at this synapse (Fig. 7, A and B; 92.3% ± 3.7% of baseline fEPSP slope). Previous work has shown that mGlu5 PAMs can potentiate mGlu-LTD induced by low concentrations of DHPG (Ayala et al., 2009; Auerbach et al., 2011; Popkirov and Manahan-Vaughan, 2011; Noetzel et al., 2012). All of the compounds that have been tested thus far are thought to bind at or near the MPEP site. Consistent with our previous report (Noetzel et al., 2012), 10 µM VU0092273 significantly potentiated the response to 25 µM DHPG, inducing LTD that lasted more than 55 minutes after washout of the compound (Fig. 7B; VU0092273 51.6% ± 5.8% baseline, P < 0.05). In contrast, NCFP (10 µM) did not induce a significant potentiation of the LTD response to 25 µM DHPG (Fig. 7, A and B; NCFP 79.2% ± 6.0% baseline). Of interest, there was a small effect observed with CPPHA (70.9% ± 4.7% of baseline). However, mGlu1 has been established to play a potential role in inducing LTD at this synapse; thus, this small effect may be a result of activation of mGlu1 rather than mGlu5.
Fig. 7.

NCFP does not significantly enhance DHPG-induced LTD at the Schaffer collateral-CA1 synapse in hippocampus. A stimulus intensity that produced 50–60% of the maximal fEPSP response was used as the baseline response and was determined for each individual experiment. Insets are sample fEPSP traces measured pre-drug (black) and 55 minutes after drug washout (gray). (A) Bath application of 75 µM DHPG for 10 minutes (open circle, solid line) resulted in LTD of the fEPSP slope (n = 8). In contrast, bath application of 25 µM DHPG for 10 minutes (black circles, solid line) resulted in a slight decrease in fEPSP slope 55 minutes after compound washout (n = 9). Application of 10 µM NCFP for 10 minutes (dashed line), first alone and then in combination with 25 µM DHPG (solid line) for 10 minutes (gray circles), resulted in no significant change in fEPSP slope (n = 7). (B) Quantification of the change in fEPSP slope measured 55 minutes after compound washout. Error bars represent S.E.M. * P < 0.5, when compared with control (25 µM DHPG).
To determine the effects of mGlu5 PAMs on induction of LTP at the SC-CA1 synapse, fEPSPs were recorded from the dendritic layer of CA1 after stimulation of the Schaffer collaterals. In agreement with previous results from our laboratory (Ayala et al., 2009), maximal LTP was initiated using a standard TBS protocol (saturation TBS; four trains of 10Hz TBS), which resulted in a 153.4% ± 4.9% increase in fEPSP slope, compared with baseline, when measured 35 minutes after stimulation (Fig. 8B). To study the ability of mGlu5 PAMs to potentiate LTP, a threshold TBS protocol was used to induce submaximal potentiation (Ayala et al., 2009). With use of this protocol, a single train of lower frequency bursts resulted in a slight potentiation of the fEPSP slope (Fig. 8, A and B; 118.5% ± 4.1% of baseline). Treatment of hippocampal slices with 10 µM NCFP for 20 minutes, followed by threshold TBS, had no effect on fEPSP slope, compared with threshold TBS alone (Fig. 8, A and B; NCFP 112.8% ± 2.5% of baseline). Similar results were observed with CPPHA (112.8% ± 1.8% of baseline). In contrast, 1 µM VU0092273 for 20 minutes, followed by threshold TBS, resulted in a significant increase in the fEPSP slope (Fig. 8, A and B; 134.9% ± 6.8% of baseline, P < 0.05). Of note, in a subset of slices, there was an increase in fEPSP slope with VU0092273 alone, although this did not appear to affect the level of potentiation. In addition, in previous studies, another MPEP site PAM (VU29) potentiated threshold TBS LTP while having no effect on fEPSPs when added alone (Ayala et al., 2009). Collectively, these results suggest that NCFP shows a clearly distinct profile in its effects on hippocampal synaptic plasticity and does not share the ability of other mGlu5 PAMs that have been characterized to potentiate either hippocampal LTD or LTP.
Fig. 8.
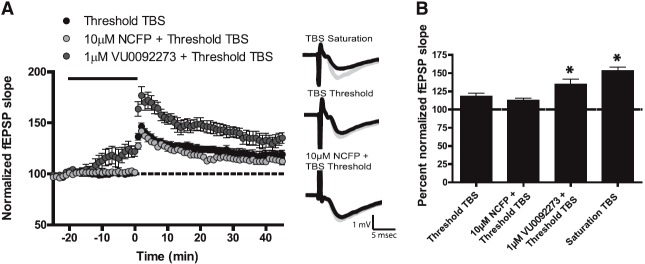
NCFP has no effect on potentiation of threshold TBS LTP at the Schaffer collateral-CA1 synapse in hippocampus. A stimulus intensity that produced 40–50% of the maximal fEPSP response was used as the baseline response and was determined for each individual experiment. Insets are sample fEPSP traces measured pre-drug (black) and 35 minutes after TBS stimulation for LTP (gray). (A) Threshold TBS (black circles) resulted in a small increase in fEPSP slope measured 35 minutes after stimulation (n = 11). Bath application of 10 µM NCFP for 20 minutes (solid line), followed by threshold TBS (light gray circles), resulted in no change in fEPSP slope from threshold TBS alone (n = 8). In contrast, bath application of 1 µM VU0092273 for 20 minutes (solid line), followed by threshold TBS (dark gray circles), resulted in a significant enhancement of fEPSP slope measured 35 minutes after stimulation (n = 9). (B) Quantification of the change in fEPSP slope measured 35 minutes after TBS stimulation. Error bars represent S.E.M. * P < 0.5, when compared with control (TBS threshold).
Discussion
Preclinical studies suggest that mGlus are viable candidates as drug targets for treatment of a variety of CNS disorders. Much of the current research around mGlus is focused on the development of allosteric modulators because of their potential for subtype selectivity and ability to maintain activity dependence of receptor activation. At present, the most advanced efforts have been in discovery and development of mGlu5 allosteric modulators; both mGlu5 PAMs and NAMs are now progressing through preclinical and clinical development. A broad range of mGlu5 modulators from a variety of different scaffolds have been discovered and characterized. Many of these compounds have been shown to have efficacy in animal models used to predict efficacy in treatment of CNS disorders. However, the mechanisms through which they mediate their different in vivo effects are not fully understood. Furthermore, it is now becoming increasingly clear that distinct allosteric modulators can differentially modulate coupling of a 7TMR to different signaling pathways of functional responses (Sheffler and Conn, 2008; Niswender et al., 2010; Gregory et al., 2012), a phenomenon referred to as functional selectivity or stimulus bias. We found that a novel mGlu5 PAM, NCFP, potentiates multiple responses to mGlu5 activation but fails to enhance hippocampal synaptic plasticity. These data provide a striking example of the potential impact of biased signaling on physiologic responses that have been postulated to be critical for the cognition-enhancing effects of mGlu5 PAMs.
Allosteric modulators have now been identified for each of the eight mGlu subtypes, and selective PAMs for mGlu2 and mGlu4 are now progressing rapidly for clinical testing in patients with schizophrenia and Parkinson’s disease, respectively (Gregory et al., 2011). The potential impact of modulators with stimulus bias with respect to in vivo and/or clinical efficacy has yet to be realized; however, understanding this could ultimately prove to be critical in optimizing allosteric modulators as therapeutic agents. Conceivably, neglect to recognize biased effects on specific responses to 7TMR activation could lead to an unexpected failure to observe the predicted in vivo or clinical effects. Consider the lack of efficacy exhibited by NCFP to enhance hippocampal synaptic plasticity. If the ability of mGlu5 PAMs to potentiate induction of LTP and LTD is important for the effects of previous agents on cognitive function, compounds that have a profile similar to that of NCFP may not have the desired therapeutic effects. Of importance, the failure of NCFP to enhance synaptic plasticity does not reflect a difference in the activity of this compound in cell lines versus native systems. For instance, we found that NCFP potentiates responses to mGlu5 activation in cortical astrocytes and in recordings from STN neurons in midbrain slices. The effects of NCFP in both astrocytes and STN neurons are similar to effects of other mGlu5 PAMs that have been reported previously (O'Brien et al., 2004; Rodriguez et al., 2005; Chen et al., 2007; Noetzel et al., 2012). Thus, the ability of NCFP to stimulate some but not all responses to mGlu5 activation more likely reflects an ability of this PAM to selectively potentiate coupling of mGlu5 to some but not all downstream responses. In addition, mGlu5 has been shown to mediate effects in other brain regions, such the striatum, where it has been shown to modulate NMDA receptor responses in medium spiny neurons (Pisani et al., 2001), and activation of mGlu1/5 by DHPG has been shown to induce LTD in the ventral striatum (Jung et al., 2012). Thus, studying the ability of CPPHA or MPEP site ligands at additional synapses in the brain will be critical to aid in the understanding of modulation of signaling through these two sites, because the effects cannot be generalized to all forms of synaptic plasticity or to all regions in the CNS. These data highlight a potential pitfall in many drug discovery programs, that is, the use of in vitro assays that may not be predictive of desired physiologic responses.
Unfortunately, despite significant effort, it has been impossible to achieve sufficient free brain concentrations of either NCFP or CPPHA to allow use of these compounds in behavioral studies (unpublished data). In the future, it will be important to optimize mGlu5 PAMs that have different pharmacological profiles for in vivo use to allow direct studies of the behavioral effects of mGlu5 PAMs that bind to different allosteric sites or differentially modulate physiologic responses to mGlu5 activation. Provision of such tools will be essential in elucidating whether biased modulation is a desirable trait for a particular therapeutic outcome or, alternatively, whether a particular pharmacological profile can be predictive of efficacy. The issue of functional selectivity and the potential impact of in vivo efficacy is a critical consideration for allosteric modulators of not just other mGlu but all 7TMRs.
NCFP is derived from the same scaffold as CPPHA, and the current data suggest that these mGlu5 PAMs interact with mGlu5 receptor at a unique site that is distinct from the common binding site used by the prototypical mGlu5 NAM, MPEP, and many other mGlu5 modulators. It is tempting to speculate that the unique profile of NCFP on mGlu5 signaling will be shared by all mGlu5 PAMs that interact with this site and that mGlu5 PAMs that interact with the MPEP site will all share the effects on mGlu5 signaling, including effects on synaptic plasticity, that have been described for previous MPEP site PAMs. Although possible, it would be premature to assume that the differences observed between NCFP and PAMs that are competitive with the MPEP site are strictly related to the different binding sites of these agents. Previous studies have shown that small changes within a chemical scaffold can lead to fundamental changes in responses to allosteric modulators. For example, there are multiple examples of single atom changes in allosteric modulators converting a partial NAM, a full NAM, or a PAM to a robust allosteric agonist (Sharma et al., 2008, 2009; Wood et al., 2011; Sheffler et al., 2012). If subtle changes within a scaffold can have such dramatic effects on the mode of modulation of mGlu5, it is also possible that such changes could alter the signaling pathways that are most efficiently engaged by the receptor. It is possible that other biased allosteric modulators of mGlu5 have been discovered, but remain unappreciated, because of a lack of in-depth assessment of their physiologic effects. Further studies are required to fully understand the molecular basis for this pharmacological bias by NCFP.
In addition to the differential effects of NCFP on different functional responses to mGlu5 activation, there were other interesting differences between NCFP and previously characterized mGlu5 PAMs. For instance, previous work by Goudet et al. (2004) demonstrated that N-terminal truncation of mGlu5 eliminates the orthosteric glutamate binding site and prevents glutamate from activating the receptor but that the mGlu5 PAM DFB behaves as an agonist at the N-terminal–truncated receptor (Goudet et al., 2004). This phenomenon has now been observed with multiple mGlu5 PAMs (Chen et al., 2007, 2008) and is replicated here with the previously characterized MPEP site mGlu5 PAM VU0092273. Furthermore, PAMs acting at other mGlu subtypes have now been shown to behave as agonists when assessed using the N-terminal–truncated receptor (Fazio et al., 2012), suggesting that this is a common feature of mGlu PAMs. In the present studies, it was interesting to find that NCFP has minimal or no agonist activity at the N-terminal–truncated receptor when assessed by measuring mGlu5 coupling to calcium mobilization or ERK1/2 phosphorylation, respectively. Of interest, CPPHA has been shown to act as an agonist in activating inositol phosphate production via N-terminal–truncated mGlu5 (Chen et al., 2008), suggesting that this does not entirely reflect differences between ligands interacting with the MPEP site versus the CPPHA site. In the absence of a radioligand to assess binding of NCFP to mGlu5, it is impossible to determine whether this reflects a loss of affinity of the N-terminal–truncated receptor for NCFP or a difference in the mechanism by which NCFP regulates receptor function.
In summary, we have demonstrated that NCFP is a potent and selective mGlu5 PAM that interacts with the mGlu5 receptor in a manner similar to the non-MPEP site ligand CPPHA. Although NCFP potentiates multiple responses to mGlu5 activation in both recombinant and native systems, this novel mGlu5 PAM does not have similar effects to previously described mGlu5 PAMs in enhancing hippocampal LTD and LTP. These results suggest that different mGlu5 allosteric modulators may behave similarly in multiple assays used to assess their effects on mGlu5 function but could have fundamentally different effects in specific circuits in the CNS. This functional selectivity could complicate drug discovery efforts but could also be exploited to develop functionally selective ligands that can be used to tailor drug therapies.
Supplementary Material
Acknowledgments
The authors thank Dr. J. Scott Daniels for the unpublished pharmacokinetic findings, and Kiran Gogi for technical expertise.
Abbreviations
- AGM
assay growth media
- ACSF
artificial cerebral spinal fluid
- CNS
central nervous system
- CPPHA
N-(4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl)-2-hydroxybenzamide
- DFB
difluorobenzaldazine
- DHPG
(S)-3,5-dihydroxyphenylglycine
- DMEM
Dulbecco’s modified Eagle’s medium
- ERK1/2
extracellular signal-regulated kinases 1 and 2
- FBS
fetal bovine serum
- fEPSP
field excitatory postsynaptic potential
- GIRK
G protein–coupled inwardly rectifying potassium channels
- HEK293 cells
human embryonic kidney 293 cells
- L-AP4
L-(+)-2-Amino-4-phosphonobutyric acid
- LTD
long-term depression
- LTP
long-term potentiation
- methoxyPEPy
3-methoxy-5-(2-pyridinylethynyl)pyridine
- mGlus
metabotropic glutamate receptors
- 5MPEP
5-methyl-2-phenylethynyl-pyridine
- MPEP
2-Methyl-6-(phenylethynyl)pyridine
- NAM
negative allosteric modulator
- NCFP
N-(4-chloro-2-((4-fluoro-1,3-dioxoisoindolin-2-yl)methyl)phenyl)picolinamide
- PAMs
positive allosteric modulators
- SC-CA1 synapse
Schaffer collateral–CA1 synapse
- STN
subthalamic nucleus
- TBS
theta burst stimulation
- 7TMRs
7 transmembrane-spanning G protein-coupled receptors
- VU0092273
(4-hydroxypiperidin-1-yl)(4-phenylethynyl)phenyl)methanone
Authorship Contributions
Participated in research design: Noetzel, Gregory, Vinson, Niswender, Xiang, Conn.
Conducted experiments: Noetzel, Gregory, Vinson.
Contributed new reagents or analytic tools: Manka, Stauffer, Lindsley.
Performed data analysis: Noetzel, Gregory, Vinson.
Wrote or contributed to the writing of the manuscript: Noetzel, Niswender, Conn.
Footnotes
This work is supported by the National Institutes of Health National Institute of Mental Health [Grant 2R01 MH062646-13]; the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant 2R01NS031373-16A2]; the National Institutes of Health National Institute on Drug Abuse [Grant 1R01DA023947]; Molecular Libraries Probe Production Centers Network [Grants 5 u54 MH84659-03 and 5 u54 MH84659-03S1]; and National Research Service Award from the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant F32 NS071746] (to M.J.N.); NARSAD Young Investigator Award 2011 (to K.J.G.); American Australian Association Merck Foundation Fellowship 2010 (to K.J.G.); and National Health and Medical Research Council (Australia) Overseas Biomedical Postdoctoral Training Fellowship (KJG). The content is solely the responsibility of the authors and does not necessarily represent the official view of the organizations listed above.
This work was previously presented in abstract form: Noetzel MJ, Gregory KJ, Vinson PN, Manka J, Stauffer SR, Xiang Z, Daniels JS, Niswender CM, Lindsley CW, and Conn PJ (2012) Characterization of a potent and efficacious metabotropic glutamate receptor 5 positive allosteric modulator from the CPPHA series, in 2012 Neuroscience Meeting Planner; 2012 Oct 13–17; New Orleans, LA. Society for Neuroscience, Washington, D.C.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Auerbach BD, Osterweil EK, Bear MF. (2011) Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature 480:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. (2000) Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci 20:7871–7879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, et al. (2009) mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology 34:2057–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SJ, Langmead CJ, Watson JM, Challiss RA. (2011) Quantitative analysis reveals multiple mechanisms of allosteric modulation of the mGlu5 receptor in rat astroglia. Mol Pharmacol 79:874–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Goudet C, Pin JP, Conn PJ. (2008) N-4-Chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl-2-hydroxybenzamide (CPPHA) acts through a novel site as a positive allosteric modulator of group 1 metabotropic glutamate receptors. Mol Pharmacol 73:909–918 [DOI] [PubMed] [Google Scholar]
- Chen Y, Nong Y, Goudet C, Hemstapat K, de Paulis T, Pin JP, Conn PJ. (2007) Interaction of novel positive allosteric modulators of metabotropic glutamate receptor 5 with the negative allosteric antagonist site is required for potentiation of receptor responses. Mol Pharmacol 71:1389–1398 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 [DOI] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. (2009) Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov 8:41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio F, Lionetto L, Molinaro G, Bertrand HO, Acher F, Ngomba RT, Notartomaso S, Curini M, Rosati O, Scarselli P, et al. (2012) Cinnabarinic acid, an endogenous metabolite of the kynurenine pathway, activates type 4 metabotropic glutamate receptors. Mol Pharmacol 81:643–656 [DOI] [PubMed] [Google Scholar]
- Goudet C, Gaven F, Kniazeff J, Vol C, Liu J, Cohen-Gonsaud M, Acher F, Prézeau L, Pin JP. (2004) Heptahelical domain of metabotropic glutamate receptor 5 behaves like rhodopsin-like receptors. Proc Natl Acad Sci USA 101:378–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KJ, Dong EN, Meiler J, Conn PJ. (2011) Allosteric modulation of metabotropic glutamate receptors: structural insights and therapeutic potential. Neuropharmacology 60:66–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KJ, Noetzel MJ, Rook JM, Vinson PN, Stauffer SR, Rodriguez AL, Emmitte KA, Zhou Y, Chun AC, Felts AS, Chauder BA, Lindsley CW, Niswender CM and Conn PJ (2012) Investigating mGlu5 allosteric modulator cooperativity, affinity and agonism: enriching structure-function studies and structure-activity relationships. Mol Pharmacol 82:860–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond AS, Rodriguez AL, Townsend SD, Niswender CM, Gregory KJ, Lindsley CW, Conn PJ. (2010) Discovery of a novel chemical class of mGlu(5) allosteric ligands with distinct modes of pharmacology. ACS Chem Neurosci 1:702–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, Ginger M, Frick A, DiPatrizio NV, Mackie K, Katona I, Piomelli D and Manzoni OJ (2012) Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun 3:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keov P, Sexton PM, Christopoulos A. (2011) Allosteric modulation of G protein-coupled receptors: a pharmacological perspective. Neuropharmacology 60:24–35 [DOI] [PubMed] [Google Scholar]
- Kinney GG, O’Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, et al. (2005) A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J Pharmacol Exp Ther 313:199–206 [DOI] [PubMed] [Google Scholar]
- Lamb JP, Engers DW, Niswender CM, Rodriguez AL, Venable DF, Conn PJ, Lindsley CW. (2011) Discovery of molecular switches within the ADX-47273 mGlu5 PAM scaffold that modulate modes of pharmacology to afford potent mGlu5 NAMs, PAMs and partial antagonists. Bioorg Med Chem Lett 21:2711–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazareno S, Birdsall NJ. (1995) Detection, quantitation, and verification of allosteric interactions of agents with labeled and unlabeled ligands at G protein-coupled receptors: interactions of strychnine and acetylcholine at muscarinic receptors. Mol Pharmacol 48:362–378 [PubMed] [Google Scholar]
- Leach K, Sexton PM, Christopoulos A. (2007) Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol Sci 28:382–389 [DOI] [PubMed] [Google Scholar]
- Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Popiolek M, Wantuch C, Khawaja X, et al. (2008) ADX47273 [S-(4-fluoro-phenyl)-3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piperidin-1-yl-methanone]: a novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. J Pharmacol Exp Ther 327:827–839 [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50:295–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Johnson KA, Luo Q, Ayala JE, Kim C, Conn PJ, Weaver CD. (2008) A novel assay of Gi/o-linked G protein-coupled receptor coupling to potassium channels provides new insights into the pharmacology of the group III metabotropic glutamate receptors. Mol Pharmacol 73:1213–1224 [DOI] [PubMed] [Google Scholar]
- Niswender CM, Johnson KA, Miller NR, Ayala JE, Luo Q, Williams R, Saleh S, Orton D, Weaver CD, Conn PJ. (2010) Context-dependent pharmacology exhibited by negative allosteric modulators of metabotropic glutamate receptor 7. Mol Pharmacol 77:459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noetzel MJ, Rook JM, Vinson PN, Cho HP, Days E, Zhou Y, Rodriguez AL, Lavreysen H, Stauffer SR, Niswender CM, et al. (2012) Functional impact of allosteric agonist activity of selective positive allosteric modulators of metabotropic glutamate receptor subtype 5 in regulating central nervous system function. Mol Pharmacol 81:120–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JA, Lemaire W, Wittmann M, Jacobson MA, Ha SN, Wisnoski DD, Lindsley CW, Schaffhauser HJ, Rowe B, Sur C, et al. (2004) A novel selective allosteric modulator potentiates the activity of native metabotropic glutamate receptor subtype 5 in rat forebrain. J Pharmacol Exp Ther 309:568–577 [DOI] [PubMed] [Google Scholar]
- Packiarajan M, Mazza Ferreira CG, Hong SP, White AD, Chandrasena G, Pu X, Brodbeck RM, Robichaud AJ. (2012) N-Aryl pyrrolidinonyl oxadiazoles as potent mGluR5 positive allosteric modulators. Bioorg Med Chem Lett 22:5658–5662 [DOI] [PubMed] [Google Scholar]
- Peavy RD, Conn PJ. (1998) Phosphorylation of mitogen-activated protein kinase in cultured rat cortical glia by stimulation of metabotropic glutamate receptors. J Neurochem 71:603–612 [DOI] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. (2001) Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience 106:579–587 [DOI] [PubMed] [Google Scholar]
- Popkirov SG, Manahan-Vaughan D. (2011) Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses. Cereb Cortex 21:501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AL, Grier MD, Jones CK, Herman EJ, Kane AS, Smith RL, Williams R, Zhou Y, Marlo JE, Days EL, et al. (2010) Discovery of novel allosteric modulators of metabotropic glutamate receptor subtype 5 reveals chemical and functional diversity and in vivo activity in rat behavioral models of anxiolytic and antipsychotic activity. Mol Pharmacol 78:1105–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AL, Nong Y, Sekaran NK, Alagille D, Tamagnan GD, Conn PJ. (2005) A close structural analog of 2-methyl-6-(phenylethynyl)-pyridine acts as a neutral allosteric site ligand on metabotropic glutamate receptor subtype 5 and blocks the effects of multiple allosteric modulators. Mol Pharmacol 68:1793–1802 [DOI] [PubMed] [Google Scholar]
- Sharma S, Kedrowski J, Rook JM, Smith RL, Jones CK, Rodriguez AL, Conn PJ, Lindsley CW. (2009) Discovery of molecular switches that modulate modes of metabotropic glutamate receptor subtype 5 (mGlu5) pharmacology in vitro and in vivo within a series of functionalized, regioisomeric 2- and 5-(phenylethynyl)pyrimidines. J Med Chem 52:4103–4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Rodriguez AL, Conn PJ, Lindsley CW. (2008) Synthesis and SAR of a mGluR5 allosteric partial antagonist lead: unexpected modulation of pharmacology with slight structural modifications to a 5-(phenylethynyl)pyrimidine scaffold. Bioorg Med Chem Lett 18:4098–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler DJ, Conn PJ. (2008) Allosteric potentiators of metabotropic glutamate receptor subtype 1a differentially modulate independent signaling pathways in baby hamster kidney cells. Neuropharmacology 55:419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler DJ, Wenthur CJ, Bruner JA, Carrington SJ, Vinson PN, Gogi KK, Blobaum AL, Morrison RD, Vamos M, Cosford ND, et al. (2012) Development of a novel, CNS-penetrant, metabotropic glutamate receptor 3 (mGlu3) NAM probe (ML289) derived from a closely related mGlu5 PAM. Bioorg Med Chem Lett 22:3921–3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer SR. (2011) Progress toward positive allosteric modulators of the metabotropic glutamate receptor subtype 5 (mGlu(5)). ACS Chem Neurosci 2:450–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnes JG, Marcus AP, Mauger RC, Throner SR, Hoesch V, King MM, Wang X, Sygowski LA, Spear N, Gadient R, et al. (2011) Discovery of novel positive allosteric modulators of the metabotropic glutamate receptor 5 (mGlu5). Bioorg Med Chem Lett 21:1402–1406 [DOI] [PubMed] [Google Scholar]
- Vinson PN, Conn PJ. (2012) Metabotropic glutamate receptors as therapeutic targets for schizophrenia. Neuropharmacology 62:1461–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MR, Hopkins CR, Brogan JT, Conn PJ, Lindsley CW. (2011) “Molecular switches” on mGluR allosteric ligands that modulate modes of pharmacology. Biochemistry 50:2403–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rodriguez AL, Conn PJ. (2005) Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J Pharmacol Exp Ther 315:1212–1219 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Wisnoski DD, O’Brien JA, Lemaire W, Williams DL, Jr, Jacobson MA, Wittman M, Ha SN, Schaffhauser H, Sur C, et al. (2007) Challenges in the development of mGluR5 positive allosteric modulators: the discovery of CPPHA. Bioorg Med Chem Lett 17:1386–1391 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




