Abstract
In this work, we investigated the formation, reactivity, and antiplatelet activity of various mixed disulfide conjugates of clopidogrel. Our results showed that the production of the active metabolite (AM) from 2-oxoclopidogrel by human liver microsomes (HLMs) is greatly affected by the thiol reductants used. Among the 10 thiol compounds tested, glutathione (GSH) is most efficient in producing the AM at a rate of 167 pmoles AM/min/mg HLM. Interestingly, no AM but only the mixed disulfide conjugates were formed in the presence of 6-chloropyridazine-3-thiol (CPT), 2,5-dimethylfuran-3-thiol, and 3-nitropyridine-2-thiol (NPT). The mass spectrometry (MS) and MS2 spectra of the conjugates of these thiol compounds confirmed the presence of a mixed disulfide bond linkage between the AM and the thiol reductants. Kinetic studies revealed that the mixed disulfide conjugates were capable of exchanging thiols with GSH to release the AM with second order rate constants ranging from 1.2 to 28 M−1s−1. The mixed disulfide conjugates of CPT and NPT showed potent inhibition of platelet aggregation after pretreatment with 1 mM GSH, confirming that the AM is responsible for the antiplatelet activity of clopidogrel. Collectively, our results provide strong support for a cytochrome P450 (P450)-mediated bioactivation mechanism involving the initial formation of a glutathionyl conjugate, followed by thiol-disulfide exchange with another GSH molecule to release the AM. Furthermore, the stable mixed disulfide conjugates identified in this study provide a platform to quantitatively generate the therapeutic AM without the need for P450-mediated bioactivation. This property can be further explored to overcome the interindividual variability in clopidogrel therapy.
Introduction
Clopidogrel (Bristol-Myers Squibb, New York, NY), a thienopyridine antiplatelet agent, is widely used to prevent heart attack and stroke in patients suffering from acute cardiovascular syndromes or peripheral vascular diseases, particularly among those who undergo percutaneous coronary intervention. In spite of widespread use, clopidogrel has shown significant interindividual variability in its efficacy (Gurbel and Tantry, 2007; Freedman and Hylek, 2009; Sofi et al., 2011). Nearly one-third of patients do not respond to clopidogrel therapy (Mason et al., 2005). With the aim of overcoming this interindividual variability, several studies have been carried out in the past decade attempting to identify genetic markers that correlate with the lack of response. It has been shown that clopidogrel is less effective in patients who carry the mutant CYP2C19*2 gene (Shuldiner et al., 2009; Dick et al., 2011; Sofi et al., 2011); however, the CYP2C19*2 mutant gene accounts for only 12% of the variations in response (Shuldiner et al., 2009). Other factors are likely involved but have not been identified.
The variable response to clopidogrel therapy is closely related to the fact that clopidogrel is a prodrug that requires oxidative bioactivation by cytochromes P450 (P450s) to its pharmacologically active metabolite (AM) (Savi et al., 2000; Kazui et al., 2010). It is well documented that P450-mediated bioactivation involves two consecutive oxidative steps (Dansette et al., 2010, 2012a); clopidogrel is first monoxygenated to 2-oxoclopidogrel, which is, in turn, oxidized to the AM in the second step. Although it has been argued that esterase PON1 is responsible for converting 2-oxoclopidogrel to the AM (Bouman et al., 2011), increasing evidence supports the idea that 2-oxoclopidogrel is converted to the AM via a sulfenic acid intermediate (Dansette et al., 2009, 2010, 2012a,b), as illustrated in Scheme 1. According to Scheme 1, 2-oxoclopidogrel is first oxidized to a sulfenic acid intermediate by P450s. The highly unstable sulfenic acid is then rapidly reduced by glutathione (GSH) to form a mixed disulfide conjugate (RS-SG) that is subsequently reduced by another GSH molecule to form the AM. This is consistent with the observation that GSH is required for the formation of the AM in human liver microsomes (HLMs) (Kazui et al., 2010). It is widely accepted that the AM is responsible for inhibition of platelet aggregation through covalent modification of platelet P2Y12 receptor (Algaier et al., 2008; Ding et al., 2003). The anti-platelet activity of the mixed disulfide conjugate RS-SG remains untested.
Scheme 1.
Bioactivation of 2-oxoclopidogrel to the active metabolite of clopidogrel as proposed by Dansette et al. (2012a). 2-oxo, 2-oxoclopidogrel.
We have previously reported that metabolism of 2-oxoclopidogrel in the presence of N-acetyl-l-cysteine (NAC) and l-cysteine led to the formation of both the AM and mixed disulfide conjugates (Zhang et al., 2012). In addition, we demonstrated that the mixed disulfide conjugates of NAC and l-cysteine exchange thiols with GSH and that the equilibrium between the AM, the AM conjugate and GSH is governed by their redox potentials. The redox potential of the sulfenic acid intermediate is likely to be high because it is a reactive oxidant. In principle, if the AM is produced according to Scheme 1, the metabolism of 2-oxoclopidogrel by P450s should generate only the mixed disulfide conjugate in the presence of thiol reductants that have redox potentials between that of the sulfenic acid and the mixed disulfide conjugate. Therefore, if appropriate thiol reductants are used, we hypothesize that only the mixed disulfide conjugates should be produced as terminal products. By investigating several thiol reductants with varying redox potentials, not only can we test the validity of the mechanism shown in Scheme 1, but we can also form stable mixed disulfide conjugates to evaluate their antiplatelet activities and develop more efficacious therapeutic agents that do not require bioactivation by polymorphic P450s.
Here we investigated the formation, reactivity, and antiplatelet activity of several mixed disulfide conjugates of clopidogrel. Our results show that the production of the AM by HLMs is greatly affected by the thiol reductants present in metabolic reactions. Both the AM and the respective mixed disulfide conjugates were observed in the presence of 7 thiol reductants tested. Interestingly, only mixed disulfide conjugates were observed in the presence of 6-chloropyridazine-3-thiol (CPT), 2,5-dimethylfuran-3-thiol (DFT), and 3-nitropyridine-2-thiol (NPT). These 3 conjugates were readily reduced by GSH to release the AM. Furthermore, the GSH-treated conjugates exhibited potent inhibition of platelet aggregation induced by an ADP agonist.
Materials and Methods
Chemicals.
(S)-clopidogrel, racemic 2-oxoclopidogrel, and trans- and cis-clopidogrel-MP were purchased from Toronto Research Company (Toronto, ON, Canada). GSH, dithiothreitol (DTT), γ-l-glutamyl-l-cysteine, Cys-Gly, l-cysteine, β-mercaptoethanol (BME), N-acetyl-l-cysteine (NAC), cysteamine hydrochloride, 2,5-dimethylfuran-3-thiol, 6-chloropyridazine-3-thiol, 3-nitropyridine-2-thiol, and 2-bromo-3′-methoxyacetophenone (MPB) were purchased from Sigma-Aldrich (St. Louis, MO). Pooled HLMs and cytosol were purchased from XenoTech (Lenexa, KS).
Determination of the Rate for the Formation of the AM by HLMs in the Presence of Various Thiol Reductants.
To examine the effects of various thiol reductants on the formation of the AM, we determined the rates at which the AM was produced. Production of the AM was performed in 0.1 ml of 50 mM potassium phosphate (KPi) buffer (pH 7.4) containing 0.2 mg/ml HLM, 0.1 mM 2-oxoclopidogrel, the NADPH-regenerating system, and 1 mM of each of the thiol reductants except that 0.3 mM CPT, DFT, or NPT were used. The reaction was initiated by the addition of 5 units of glucose-6-phosphate dehydrogenase (G6PD) and incubated at 37°C for 20 minutes. The AM was then derivatized with 4 mM MPB at room temperature for 10 minutes, followed by acidification with acetic acid to 3% (v/v). For quantification, 50 pmoles of (S)-clopidogrel was added into each reaction mixture as internal standard (IS). The derivatized AM (AM-MP) was quantitated using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
The mass spectrometry (MS) analyses of the reaction mixture were performed on an ion-trap mass spectrometer (LCQ DecaXP; Thermo Fisher Scientific, Waltham, MA) as reported previously (Zhang et al., 2012). In brief, the analytes were separated on a reverse phase C18 column (Gemini 2×100 mm, 3 µm; Phenomenex, Torrance, CA) using a binary mobile phase at a flow rate of 0.2 ml/min. The temperature of the C18 column was maintained at 40°C using a column heater (Restek Corporation, Lancaster, PA). The mass spectrometer was operated in positive electrospray ionization mode with the following settings: heated capillary temperature, 200°C; spray voltage, +4.5 kV; sheath gas flow, 60 (arbitrary units); auxiliary gas, 20 (arbitrary units). The AM-MP and IS were fragmented in the MS through collision-induced dissociation at 35% energy level. Transitions from m/z (mass-to-charge ratio) 504 → m/z 354 for the AM-MP and from m/z 322 → m/z 212 for the IS were used to quantitate the amount of the AM-MP based on a calibration curve consisting of various concentrations of cis-clopidogrel-MP.
Analyses of the Mixed Disulfide Conjugates of Clopidogrel Using LC-MS/MS.
The mixed disulfide conjugates were produced by HLMs for both structural and semiquantitative analyses. Metabolism of 2-oxoclopidogrel was performed in 0.2 ml of 50 mM KPi buffer (pH 7.4) as previously described except that the concentration of HLMs was increased to 1 mg/ml. The reaction was incubated at 37°C for 30 minutes and then quenched by the addition of 0.1 ml of 10% of acetic acid in acetonitrile. The quenched samples were centrifuged at 13,000g for 10 minutes to remove the HLMs. Aliquots of 50 µl supernatant were loaded on a mass spectrometer to analyze both the active and conjugate metabolites.
The MS analyses were performed as previously described except that the MS detector was operated in the dependent scan mode. The precursor ions were scanned from m/z 300–700, whereas the MS2 spectra were obtained from m/z 100 to 700 for the four most abundant ions. For semiquantitative analysis, 50 pmoles of the IS was spiked into each sample that had been quenched. The relative amounts of the AM and respective conjugates were calculated as the AUC (area under the curve) ratios of the metabolites to that of the IS.
Determination of the Kinetics for the Conversion of the Mixed Disulfide Conjugates to the AM.
To examine the reactivity of the mixed disulfide conjugates, we determined the kinetics for the reduction of the mixed disulfide conjugates by GSH. The mixed disulfide conjugates were generated in 1 ml of 50 mM KPi buffer (pH 7.4) containing 1 mg/ml HLM, 0.1 mM 2-oxoclopidogrel, and 0.3 or 1 mM thiol reductants. The reaction was incubated at 37°C for 50 minutes after initiated by the addition of G6PD. The reaction mixture was then centrifuged at 13,000g to remove the HLMs, the supernatant was loaded to a preconditioned SPE cartridge (C18, 100 mg/1 ml; Agilent Technologies, Santa Clara, CA), and the mixed disulfide conjugate was eluted with 2 ml of methanol. The eluent was then dried using a Speedvac concentrator, and the dried sample was stored at −80°C until use. Prior to the kinetic measurements, the dried samples were first re-dissolved in 0.5 ml of 50 mM KPi buffer (pH 7.4) and then equilibrated at 37°C for 5 minutes. Small aliquots (1–5 µl) of stock GSH and cytosol (when present) solutions were added to the conjugate samples at 1 mM and 0.2 mg/ml, respectively, to initiate the thiol-disulfide exchange reaction. At designated times, an aliquot of 50 µl of the reaction mixture was withdrawn and mixed with 25 µl of 10% acetic acid in acetonitrile to terminate the thiol-disulfide exchange reaction. The t=0 sample was prepared immediately prior to the addition of GSH. The amounts of the mixed disulfide conjugates and the AM were analyzed using LC-MS/MS as previously described.
Formation of the Active Metabolite H4 from the Mixed Disulfide Conjugates of Clopidogrel.
Since the antiplatelet activity of the AM is closely related to its stereochemistry, we investigated the formation of the active metabolite H4 from three mixed disulfide conjugates of CPT, DFT, and NPT because of their relatively high redox potentials. Due to lack of genuine standards for the stereoisomers of the AM, we first treated the mixed disulfide conjugates with DTT to release the AM and then derivatized the AM with MPB so that we were able to compare the AM-MP derivatives with trans- and cis-clopidogrel-MP standards. The mixed disulfide conjugates of CPT, NPT, and DFT were prepared as described in the preceding section. To release the AM, the conjugate (0.2 ml) was treated with 2 mM DTT at 37°C for 20 minutes. The reaction mixture was then mixed with 0.1 ml acetonitrile and derivatized with 4 mM MPB at room temperature for 10 minutes. The derivatization reaction was then terminated by the addition of 10 µl acetic acid. The diastereomers of the derivatized AM, AM-MP, were separated on a reverse-phase C18 column (50 × 2.1 mm, 5 µm Kinetex; Phenomenex) with a binary mobile phase consisting of 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). The gradient was as follows: 35% B for 2 minutes, linearly increasing to 40% B by 7 minutes and to 90% B by 17 minutes, and then held at 90% B for 1 minute. The flow rate was 0.3 ml/min. MS analyses of the AM-MP were performed as described in the quantitation of the AM-MP.
Antiplatelet Activity of the Mixed Disulfide Conjugates of Clopidogrel.
To generate sufficient quantities of the mixed disulfide conjugates, the metabolism of 2-oxoclopidogrel was performed in 2-ml reaction mixtures containing 1 mg/ml HLM, 0.1 mM 2-oxoclopidogrel, the NADPH-regenerating system, and 0.3 mM CPT or NPT or 1 mM GSH. The reaction was initiated by the addition of 10 units G6PD and incubated at 37°C for 50 minutes. Two control samples were prepared in parallel. One control sample did not contain any G6PD (−G6PD), which was designed to examine whether 2-oxoclopidogrel and other components present in the HLMs contributed to antiplatelet activities. The other control (−SH; minus thiol) did not contain any thiol reductant, enabling us to examine whether any metabolites other than the AM and the conjugate interfered with the antiplatelet activity assay. After an incubation of 50 minutes, the reaction mixtures were centrifuged at 13,000g to remove the HLMs. The supernatants were loaded onto SPE C18 cartridges to enrich the mixed disulfide conjugates. After extensive washing with water to remove salts and other water-soluble metabolites, the conjugate samples were eluted with 2 ml of methanol. The methanolic fractions were dried using a Speedvac concentrator (Thermo Fischer Scientific), and the dried samples were then resuspended in 1 ml of platelet-poor plasma (PPP). Prior to the antiplatelet activity assays, the resuspended conjugates were divided into two equal volumes (0.5 ml each), one of which was treated with 1 mM GSH at 37°C for 30 minutes to generate the AM. Both samples were then placed on ice until use.
The procedures used to determine ex vivo antiplatelet activity were similar to those as reported by Abell and Liu (2011) and are in accordance with the guidelines of the University of Michigan University Committee on the Use and Care of Animals (National Institutes of Health publication No. 86-23). The University of Michigan Unit for Laboratory Animal Medicine provided all veterinary care.
Male New Zealand white rabbits (2.2–2.9 kg) were used as blood donors. Whole blood was drawn from a central ear artery into a plastic syringe containing 3.7% sodium citrate as the anticoagulant (1:10 volume ratio of citrate to blood). A whole blood cell count was determined with a Medonic CA620 hematology analyzer (Clinical Diagnostic Solutions, Inc., Plantation, FL). Platelet-rich plasma (PRP), the supernatant present after centrifugation of whole blood at 100g for 10 minute, was diluted with PPP to achieve a platelet count of approximately 300,000/μl. PPP was prepared by centrifuging the remaining blood at 1,500g for 10 minutes and discarding the bottom cellular layer. The diluted PRP was divided into 0.5-ml samples, centrifuged again at 170g for 10 minutes, and the resulting supernatant was discarded. The platelet pellets were resuspended in PPP containing the various chemical inhibitors (prepared as described previously) and incubated with gentle shaking at 37°C for 60 minutes to modify the P2Y12 receptor. Ex vivo platelet aggregation was assessed by established nephelometric methods with the use of a 4-channel aggregometer (BioData PAP-4; BioData Corp., Horsham, PA) by recording the increase in light transmission through a stirred suspension of PRP maintained at 37°C. Platelet aggregation was induced with ADP (10 μM). A subaggregatory concentration of epinephrine (550 nM) was used to prime the platelets before addition of the agonist.
Results
Effects of Thiol Reductants on the Formation of the Active Metabolite of Clopidogrel.
To examine the effects of thiol reductants, we determined the steady-state rates for the formation of the AM in the presence of various thiol reductants. The concentrations of the thiol reductants present in the metabolic reactions were 1 mM except for CPT, DPT and NPT. Instead, the concentrations of these three thiol reductants were 0.3 mM because of their low Km values (explained below). As shown in Fig. 1, the AM is formed in the presence of all but three thiol reductants. The highest rate for the formation of the AM was observed in the presence of GSH, an endogenous reductant in the human body. Specifically, in the presence of 1 mM GSH, the AM is produced at a rate of 167 pmol AM/min/mg HLM. Likewise, l-cysteine is ∼84% as active as GSH in producing the AM. As observed previously (Zhang et al., 2012), only a low level of the AM was formed in the presence of 1 mM NAC. The rate is only ∼7% of that observed in the presence of 1 mM GSH. No AM was observed in the presence of CPT, DFT, or NPT. Overall, the rates for the formation of the AM decrease in the order of GSH > l-cysteine > Cys-Gly > γ-l-glutamyl-l-cysteine > cysteamine > BME > NAC > CPT or DFT or NPT. This wide range of rates underscores the critical role of thiol reductants in the formation of the AM.
Fig. 1.
Effects of thiol reductants on the formation of the AM of clopidogrel. The AM was produced in 0.1 ml of 50 mM KPi buffer (pH 7.4) containing 0.2 mg/ml HLM, 0.1 mM 2-oxoclopidogrel, the NADPH-regenerating system, and the thiol reductants. The concentrations of the thiol reductants were 1 mM except for CPT, DFT, and NPT, which were 0.3 mM each. The reaction was initiated by the addition of 5 units of G6PD and incubated at 37°C for 20 minutes. The active metabolite was then quantitated as the MP derivative as described in Materials and Methods. The reported rates were averaged over three separate measurements. Abbreviations for the thiol compounds are provided in Table 1. CG, l-cysteine-l-glyceine; CYA, Cysteamine; GC, γ-l-glutamyl-l-cysteine.
Analyses of the Mixed Disulfide Conjugates of Clopidogrel.
As shown, formation of the AM was greatly affected by the thiol reductants present. In this experiment, we examined the effects of the thiol reductants on the formation of the mixed disulfide conjugates. This is particularly important to understand the cause for the lack of any AM formed in the presence of CPT, DFT, and NPT. In marked contrast to what was observed for the AM, mixed disulfide conjugates were formed in the presence of all the thiol reductants examined. The m/z for the parent ions MH+ and the retention times of these mixed disulfide conjugates are summarized in Table 1, and the extracted ion chromatograms (EICs) of four selected mixed disulfide conjugates are presented in Fig. 2. The parent ions MH+ observed are in excellent agreement with the theoretical values for these conjugates. In the presence of BME, four conjugate peaks were observed at m/z 432, eluting from 8.9 to 9.72 minutes (Fig. 2A). These four AM peaks are likely due to the formation of multiple stereoisomers of clopidogrel, as reported previously (Pereillo et al., 2002). Two major conjugate peaks were observed in the presence of CPT and NPT (Fig. 2, C and D, respectively). However, in the presence of DFT, one predominant conjugate peak with m/z 482 was observed at 15.8 minutes (Fig. 2B). The Km values for the formation of the CPT, NPT, and DFT conjugates were determined to be 23, 51, and 30 µM, respectively (unpublished data), which is significantly lower than a Km of 300 µM for GSH that we previously reported (Zhang et al., 2012).
TABLE 1.
Parent ions and retention times observed for the mixed disulfide conjugates of clopidogrel in liquid chromatography tandem mass spectrometry analysis
| Thiol Compounds | Abbreviation | MH+ (m/z) | RTa (min) | MH+ Theoreticalb (m/z) |
|---|---|---|---|---|
| Glutathione | GSH | 661.05 | 5.47 | 661.02 |
| l-cysteine | CYS | 475.05c | 5.60c | 475.06 |
| l-cysteine-l-glyceine | CG | 532.09 | 5.44 | 532.10 |
| γ-l-glutamyl-l-cysteine | GC | 604.09 | 5.71 | 604.12 |
| Cysteamine | CYA | 431.05 | 6.33 | 431.09 |
| β-mercaptoethanol | BME | 432.06 | 9.65 | 432.07 |
| N-acetyl-l-cysteine | NAC | 517.11 | 6.85 | 517.09 |
| 6-chloropyridazine-3-thiol | CPT | 499.99 | 11.97 | 500.03 |
| 2,5-dimethylfuran-3-thiol | DFT | 482.08 | 15.80 | 482.09 |
| 3-nitropyridine-2-thiol | NPT | 510.08 | 12.82 | 510.06 |
MH+, parent ions; RT, retention times.
Retention time for the most intense peak.
Exact masses calculated from molecular formula.
Data from Zhang et al. (2012).
Fig. 2.

EIC of representative mixed disulfide conjugates of clopidogrel. The mixed disulfide conjugates were produced in 0.2 ml of 50 mM KPi buffer (pH 7.4) containing 1 mg/ml HLMs, 0.1 mM 2-oxoclopidogrel, various thiol reductants, and the NADPH-regenerating system at 37°C for 30 minutes. MS analyses were performed as described in Materials and Methods. (A) EIC for the BME conjugate at m/z 432.06; (B) EIC for the DFT conjugate at m/z 482.08; (C) EIC for the CPT conjugate at m/z 499.99; (D) EIC for the NPT conjugate at m/z 510.08.
Integration of the AUC for each EIC of the conjugates gave the relative amount of the mixed disulfide conjugates produced. As shown in Fig. 3, the relative amounts of the mixed disulfide conjugates varied substantially from each other. Only a low level of the glutathionyl conjugate was formed, indicating that metabolism of 2-oxoclopidogrel in the presence of GSH greatly favors the formation of the AM. Although both the AM and the mixed disulfide conjugate were formed in the presence of BME, it is clear that formation of the conjugate is favored over the AM. In spite of lack of formation of the AM, the mixed disulfide conjugates of CPT, DFT, and NPT were formed in significant quantities. Due to lack of genuine standards of these conjugates, we were unable to quantitate the absolute amounts of these conjugates. Caution should be exercised in comparing the absolute amounts of the conjugates based on the AUC ratios because these conjugates may respond differently to the MS detector.
Fig. 3.
Relative amounts of the AM and thiol conjugates of clopidogrel produced by HLMs. The AM and conjugates were produced in 0.2 ml of 50 mM KPi (pH 7.4) as described in Fig. 2. For these analyses, 50 pmol of (S)-clopidogrel was spiked into each sample as the IS. Both the AM and the thiol conjugates were analyzed using LC-MS/MS in the dependent scan mode as described in Materials and Methods. Open bar represents AUC ratio of m/z 356 (AM) to m/z 322 (IS); solid bar represents AUC ratios of respective conjugate to IS. CG, l-cysteine-l-glyceine; CYA, Cysteamine; GC, γ-l-glutamyl-l-cysteine.
To determine the chemical structure of these conjugates, the MS and MS2 spectra were obtained. The MS spectra of all 10 conjugates showed the major MH+ peaks at expected m/z ratios as summarized in Table 1, along with a pair of 35Cl/37Cl isotope peaks that are characteristic of the presence of one Cl atom in clopidogrel. The only exception to this is the CPT conjugate that contains two chlorine atoms. Its MS and MS2 spectra are shown in Fig. 4. This conjugate was observed at m/z 499.99, which is within an experimental error of 80 ppm of the expected m/z value of 500.03. In addition, a strong isotope peak was also observed at 501.94 with ∼75% of the intensity of the base peak, indicative of the presence of two chlorine atoms in this conjugate. The MS2 of the parent ion at m/z 499.99 showed the formation of multiple daughter ions. The predominant daughter ion was observed at m/z 353.99, with other minor ones at m/z 465.90, 211.93, and 183.34. This fragmentation pattern, in addition to the presence of the 35Cl/37Cl isotope peaks, is consistent with the chemical structure of the conjugate possessing a mixed disulfide bond shown in Fig. 4D. The predominant daughter ion m/z 353.99 is assigned to the larger fragment cleaved at the mixed disulfide bond, as we reported previously for the conjugates of GSH, NAC, and l-cysteine with clopidogrel (Zhang et al., 2012). The daughter ions at m/z 212 and 183 are also characteristic of clopidogrel (Dansette et al., 2012a; Pereillo et al., 2002) . The MS2 spectrum of the isotope peak at m/z 501.94 provides further evidence for this assignment. As shown in Fig. 4C, a pair of daughter ions, instead of single ions, were observed two mass units apart, which supports the presence of two chlorine atoms. The MS2 spectra for the rest of the conjugates exhibited very similar fragmentation patterns, with the characteristic daughter ions at m/z 354 (unpublished data).
Fig. 4.
MS and MS2 spectra of the mixed disulfide conjugate of CPT. The conjugate was produced as described in Fig. 2. The MS and MS2 spectra were obtained using LC-MS/MS in the dependent-scan mode as described in Materials and Methods. (A) MS spectrum of the CPT conjugate; (B) MS2 spectrum of the parent ion m/z 499.99 for the CPT conjugate; (C) MS2 spectrum of the parent ion m/z 501.94 for the CPT conjugate; (D) Fragmentation pattern of the CPT conjugate deduced from the MS2 spectrum shown in (B).
Kinetics for the Reduction of the Mixed Disulfide Conjugates of Clopidogrel by GSH.
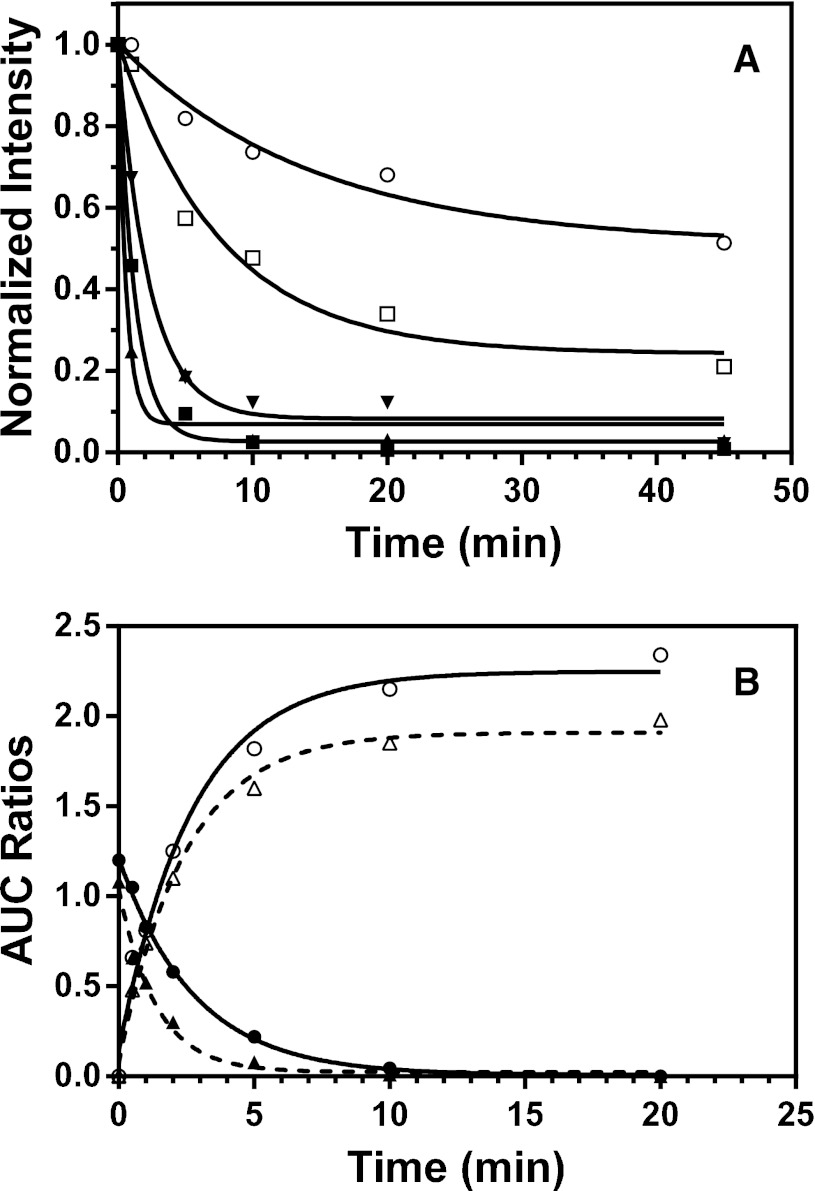
The kinetics for the thiol-disulfide exchange reaction between the various mixed disulfide conjugates and GSH were determined, and the results are shown in Fig. 5. Incubation of the conjugates with GSH and cytosol led to time-dependent decreases in the amounts of the mixed disulfide conjugates (Fig. 5A). Fitting the kinetic data to a monoexponential function gave first-order rate constants of 0.07, 0.79, 0.43, 1.65, and 0.13 minute−1 for the losses of the mixed disulfide conjugates of BME, CPT, NAC, DFT, and NPT, respectively. Since these kinetics were determined under pseudo first-order conditions with excess of GSH (1 mM), these rate constants are equivalent to the second-order rate constants of 1.2, 13, 7.2, 28, and 2.2 M−1s−1, respectively. They also demonstrated the variable reactivity of these conjugates toward GSH. Clearly, the DFT and CPT conjugates are 10- to 20-fold more reactive than the BME conjugate, respectively. It appears that ∼50% of the BME conjugate still remained even after an incubation of 40 minutes, indicating that the thiol-disulfide exchange for this conjugate had reached equilibrium.
Fig. 5.
Kinetics for the reduction of the mixed disulfide conjugates of clopidogrel by GSH. The mixed disulfide conjugates were prepared from the reaction mixtures containing 1 mg/ml HLM, 0.1 mM 2-oxoclopidogrel, the NADPH-regenerating system, and various thiol reductants. The mixture was purified using SPE C18 cartridges, as described in Materials and Methods. The purified conjugates were then mixed with 1 mM GSH and 0.2 mg/ml cytosol (when present). The conjugate remaining and the AM formed were analyzed using LC-MS/MS. (A) Reduction of the conjugates of BME (○), CPT (▪), NAC (▼), DFT (▲), and NPT (⬜) by 1 mM GSH in the presence of 0.2 mg/ml cytosol. (B) Reduction of the CPT conjugate by 1 mM GSH in the presence and absence of 0.2 mg/ml cytosol. Open circle, formation of AM in the absence of cytosol; open triangle, formation of the AM in the presence of cytosol; filled circle, reduction of the conjugate in the absence of cytosol; filled triangle, reduction of the conjugate in the presence of cytosol. The solid and dashed lines are nonlinear curve fittings to a single exponential function.
To examine the effect of cytosol and to monitor the conjugates and the AM simultaneously, we determined the kinetics for both the formation of the AM and the reduction of the CPT conjugate in the presence and absence of cytosol. As shown in Fig. 5B, the decrease in the amount of the CPT conjugate occurred with concomitant increase in the amount of the AM with almost identical rate constants. In the presence of cytosol, the rate constants for the reduction of the conjugate and for the formation of the AM are 0.73 and 0.50 minute−1, respectively. In the absence of cytosol, the reduction of the CPT conjugate is approximately one-half as fast, with a rate constant of 0.39 minute−1 for the reduction of the conjugate and 0.35 minute−1 for the formation of the AM. This indicates that cytosol accelerates the reduction of the thiol-disulfide exchange reaction, as observed previously (Hagihara et al., 2011, 2012). We confirmed this observation by use of boiled cytosol, as shown in Supplemental Fig. 1.
Formation of the Active Metabolite H4 from the Mixed Disulfide Conjugates of cCopidogrel.
As shown in Scheme 1, the active metabolite contains two chiral centers (C7 and C4) and one double bond (C3-C16). Therefore, metabolism of racemic 2-oxoclopidogrel could potentially produce up to eight stereoisomers. However, only four of the diastereomers, termed H1, H2, H3, and H4, can be separated on conventional reverse phase C18 columns, whereas the other four stereoisomers co-elute as enantiomers. It has been established that H4 is responsible for the antiplatelet activity in humans, having an S configuration at carbon 7 and a Z (or cis) configuration at C3–C16 double bond (Savi et al., 2000; Pereillo et al., 2002; Tuffal et al., 2011). To evaluate the therapeutic potential of these conjugates, we examined whether the H4 is formed in the mixed disulfide conjugates; the results are presented in Fig. 6.
Fig. 6.
Extracted ion chromatograms observed at m/z 504 showing the formation of the active metabolite H4 from the mixed disulfide conjugates of clopidogrel. The mixed disulfide conjugates were produced in the HLMs and purified with SPE cartridges as described in Fig. 5. Prior to MS analyses, the mixed disulfide conjugates were treated with DTT to release the AM that was then subsequently derivatized with MPB. The AM-MP derivatives were analyzed using LC-MS/MS as described in Materials and Methods. (A) trans- (dashed line) and cis-clopidogrel-MP (solid line) standards; (B) the AM-MP obtained in the presence of 1 mM GSH (dashed line) and 1 mM ascorbic acid (solid line); (C) the AM-MP obtained from the CPT conjugate; (D) the AM-MP obtained from the NPT conjugate; (E) the AM-MP obtained from the DFT conjugate. The amplitude was multiplied by two.
The EIC of m/z 504 for the mixed disulfide conjugate of CPT exhibited two peaks eluting at 9.4 and 10.1 minutes (Fig. 6C). Likewise, the EIC of the mixed disulfide conjugate of NPT was almost identical to that of the CPT conjugate, with two peaks observed at 9.4 and 10.2 minutes (Fig. 6D). The two peaks observed at 9.4 and 10.2 minutes in the CPT and NPT conjugates were also observed for the DFT conjugate (Fig. 6E). In addition, an extra peak was observed at 8.0 minutes, and it appears that a shoulder was also observed at 9.7 minutes.
To determine whether H4 was formed during the conversion of the mixed disulfide conjugates to the AM, we compared the EICs of the three mixed disulfide conjugates with those of clopidogrel-MP standards and the AM-MP obtained in the presence of ascorbic acid and GSH. As shown in Fig. 6A, the EIC of the trans-clopidogrel-MP exhibited two peaks, at 8.0 and 9.7 minutes (dashed line), whereas the EIC of the cis-clopidogrel-MP exhibited one major peak at 10.2 minutes (solid line). The two peaks observed with the trans-clopidogrel-MP are indicative of two pairs of diastereomers representing H1 and H2 (Pereillo et al., 2002; Tuffal et al., 2011). It should be pointed out that the cis-clopidogrel-MP standard available from the Toronto Research Company contained only one pair of the diastereomers (J. Bradley, personal communications);consequently, only one peak was observed at 10.2 minutes. However, it is unclear whether this peak represents H3 or H4.
To further assist peak assignment, we obtained the EICs in the presence of GSH and ascorbic acid. As shown in Fig. 6B (solid line), the AM-MP obtained in the presence of ascorbic acid exhibited only two peaks, at 9.5 and 10.2 minutes. The AM-MP obtained in the presence of GSH exhibited extra peaks in addition to the two observed at 9.5 and 10.2 minutes (dashed line, Fig. 6B). Specifically, a new peak was observed at 8.0 minutes, and a shoulder was observed at 9.7 minutes. It is known that the metabolism of clopidogrel by HLMs in the presence of GSH produces all four diastereomers (H1–H4), whereas only H3 and H4 are produced in the presence of ascorbic acid (Pereillo et al., 2002; Dansette et al., 2012b; Zhang et al., 2012). Therefore, it is clear that the peaks at 9.4 and 10.2 minutes are representative of H3 and H4, while the peaks at 8.0 and 9.7 minutes are representative of H1 and H2. On the basis of the order of elution of the MP derivatives of H1–H4 (Tuffal et al., 2011), we can conclude that the peaks observed at 8.0, 9.4, 9.7, and 10.2 minutes correspond to H1, H3, H2 and H4, respectively. It appears that H2 and H3 are not completely separated under the chromatographic conditions we used. Nonetheless, this does not affect our conclusion that the mixed disulfide conjugates of CPT, NPT, and DFT are capable of producing the active metabolite H4 in the presence of thiol reductants.
Antiplatelet Activity of the Mixed Disulfide Conjugates of CPT and NPT.
As a proof of concept, we elected to examine the antiplatelet activities of two of the mixed disulfide conjugates, the CPT and NPT conjugates, for several reasons. First, both conjugates can be generated without the formation of any AM, which eliminates any interference from the AM during the antiplatelet activity assays. Second, both conjugates exchange thiols with GSH at relatively fast rates, which prevents potential decay of the AM. Third, reduction of the two conjugates produces the H4 isomer that is known to be responsible for the antiplatelet activity in humans. The results for the ex vivo antiplatelet activity assays are shown in Fig. 7. The percentage of aggregation was normalized to that of PRP to compensate for any variations due to environmental factors, such as blood sources and PRP preparations.
Fig. 7.
Inhibition of platelet aggregation by the mixed disulfide conjugates of clopidogrel. The mixed disulfide conjugates were prepared and purified from the reaction mixtures in the presence of 0.3 mM CPT and NPT, along with three control samples containing either 1 mM GSH, no thiol reductant, or no G6PD. All samples were resuspended in 0.5 ml PPP, some of which were treated to 1 mM GSH to release the AM. Platelet aggregation was initiated by the addition of 10 µM ADP and recorded with an aggregometer. The percentage of aggregation was normalized to that of PRP and averaged over four separate measurements. For details, see Materials and Methods. CPT, metabolites produced in the presence of 0.3 mM CPT; CPT+GSH, metabolites produced in the presence of 0.3 mM CPT and then treated with 1 mM GSH; −G6PD, metabolites produced in the absence of G6PD; AM, 1 mM GSH in PRP; GSH, metabolites produced in the presence of 1 mM GSH; NPT, metabolites produced in the presence of 0.3 mM NPT; NPT+GSH, metabolites produced in the presence of 0.3 mM NPT and then treated with 1 mM GSH; PRP, untreated platelet-rich plasma; −SH, metabolites produced in the absence of any thiol reductants.
As shown, the three control samples showed no inhibition of the platelet aggregation. The first control showed that free GSH has no effect on platelet aggregation at 1 mM concentration (GSH, Fig. 7), and the second control showed that nonmetabolite components present in the reaction mixtures, such as 2-oxoclopidogrel and related impurities, did not inhibit platelet aggregation (−G6PD, Fig. 7). It is known that clopidogrel may decompose to byproducts via nonenzymatic oxidation (Mohan et al., 2008; Fayed et al., 2009). These byproducts do not seem to have any inhibitory effects on platelet aggregation. In the third control, we demonstrated that the metabolites from the reaction mixture, in the absence of any thiol reductants, have no effects on platelet aggregation either. However, we did observe ∼60% inhibition of platelet aggregation in the sample prepared from the reaction mixture containing GSH (AM, Fig. 7). This is expected, since metabolism of 2-oxoclopidogrel in the presence of 1 mM GSH generates the AM as shown in Fig. 1. Incubation of PRP with the CPT and NPT conjugates did not inhibit platelet aggregation, indicating that the conjugates themselves have no antiplatelet activity (CPT and NPT, Fig. 7). In marked contrast, incubation of PRP with the CPT and NPT conjugates that had been treated with 1 mM GSH significantly inhibited platelet aggregation by ∼50 and 70%, respectively. This inhibitory activity most likely arises from the AM released from the conjugates by GSH. These results demonstrate that the conjugates of clopidogrel have no antiplatelet activity and also confirmed that the AM is solely responsible for the inhibition of platelet aggregation. Furthermore, they demonstrate that it is possible to deliver the AM without the need for bioactivation by polymorphic P450s. Of note, the variations in the percentage of aggregation observed in the GSH, CPT+GSH, and NPT+GSH samples were most likely due to variations in the concentrations of the AM. It was estimated that the concentrations of the AM in these samples were in the range of 1–4 µM (unpublished data).
Discussion
We have demonstrated that thiol reductants greatly affect the formation of the AM of clopidogrel during the metabolism by HLMs. A wide range of rates were observed for the formation of the AM, depending on the thiol reductants present in the metabolic reactions. At one end of the spectrum, the AM was produced at a rate of 167 pmol AM/min/mg HLM in the presence of GSH, whereas no AM was produced in the presence of CPT, DFT, or NPT (see Fig. 1). Furthermore, we observed the formation of mixed disulfide conjugates in the presence of all 10 thiol compounds. Analyses of the MS and MS2 spectra of the conjugates revealed that, in all cases, the AM is bonded to the thiol reductant via a disulfide bond.
The variable rates of the formation for the AM in the presence of various thiol reductants can be interpreted on the basis of redox potentials, as illustrated in Scheme 2. As shown, formation of the mixed disulfide conjugate (RS-SX) depends on the difference in the redox potentials between the sulfenic acid (RS-OH) and the thiol reductant used, whereas formation of the AM depends on the difference in redox potentials between the RS-SX and thiol reductant. In other words, the thiol reductants with high redox potentials disfavor the formation of the AM, whereas the thiol reductants with low redox potentials favor the formation of the AM. Of the 10 thiol compounds we examined, only the redox potentials of four are known: −0.24 V for GSH, −0.22 V for l-cysteine, −0.20 V for cysteamine, and −0.19 V for BME (Lukesh et al., 2012). It is likely that RS-OH possesses the highest redox potential because it is a highly reactive oxidant. Although the redox potentials of CPT, DFT, and NPT are unknown, it is reasonable to assume that their redox potentials are relatively high. This is because the sulfhydryl groups of CPT, DFT, and NPT are bonded to conjugated π systems containing electrophilic atoms, such as a chlorine atom in CPT, an oxygen atom in DFT, and a nitro group in NPT. The chemical structures of CPT, DFT, and NPT are shown in Fig. 8. The lone pair of electrons of these sulfhydryl groups is disproportionally delocalized away from the sulfur atom. This is expected to reduce electron density on the sulfur atoms, consequently leading to an increase in the redox potentials. The low redox potential of GSH contributes to the facile reduction of RS-OH to form the glutathionyl conjugate RS-SG and subsequent reduction of RS-SG to form the AM. This is consistent with the highest rate observed for the formation of the AM in the presence of GSH. The observation that only RS-SX is formed in the presence of CPT, DFT, and NPT indicates that reduction of RS-SX by these reductants is prohibited because the redox potential of RS-SX is less than that of CPT, DFT, or NPT. The variable rates observed for the formation of the AM provide direct evidence for the participation of the thiol reductants and are consistent with the proposed mechanism shown in Scheme 1.
Scheme 2.
Schematic illustration for the formation of the mixed disulfide conjugate and the AM based on redox potentials. The question marks indicate unknown redox potentials. CYA, Cysteamine; CYS, l-cysteine.
Fig. 8.
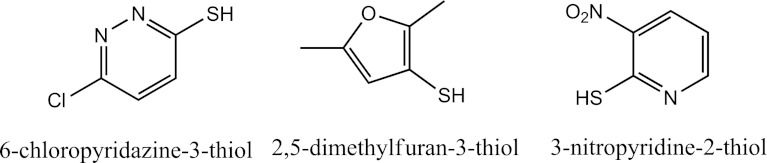
Chemical structures and generic names of three thiol compounds: 6-chloropyridazine-3-thiol (CPT), 2,5-dimethylfuran-3-thiol (DFT) and 3-nitropyridine-2-thiol (NPT).
The relatively high redox potentials of the mixed disulfide conjugates of CPT, DFT, and NPT are further illustrated in the thiol-disulfide exchange experiments (see Fig. 5). When the RS-SX of these three compounds is mixed with GSH, even in the absence of cytosol, the RS-SX undergoes thiol-disulfide exchange with GSH to release the AM. This leads to the simultaneous reduction of RS-SX and the formation of the AM. Because of its low redox potential, GSH is capable of reducing not only the conjugates of CPT, DFT, and NPT, but also the conjugates of BME and NAC. In addition, it is shown that the thiol-disulfide exchange reactions are facilitated by the cytosolic fraction of human liver. This is likely due to the involvement of glutaredoxin and/or thioredoxin in the thiol-disulfide exchange reaction as previously reported (Hagihara et al., 2011, 2012).
The availability of stable RS-SX conjugates with high redox potentials and the correct stereochemistry provides a unique platform to quantitatively generate the AM that is otherwise highly challenging because the AM of the thienopyridine antiplatelet agents are unstable. They are even more useful because GSH-treated mixed disulfide conjugates possess antiplatelet activity (see Fig. 7). This property can be further explored to improve the efficacy of thienopyridine antiplatelet drugs. The use of stable mixed disulfide conjugates as antiplatelet agents may offer several advantages over thienopyridines because they do not require bioactivation by polymorphic P450s. Eliminating this requirement removes one of the major sources of interindividual variability. Furthermore, absence of P450-mediated bioactivation would also eliminate the production of other metabolites that may be harmful and toxic. This is particularly true for ticlopidine; severe idiosyncratic events of agranocytosis have been associated with the production of reactive metabolites of ticlopidine (Ono et al., 1991; Love et al., 1998). Furthermore, elimination of no-productive metabolites would benefit dose response to clopidogrel therapy since the effective concentration of the AM is more predictable. The antiplatelet activity of the mixed disulfide conjugates in vivo merits further studies to overcome the interindividual variability in clopidogrel therapy.
In conclusion, we have demonstrated that formation of the active metabolite of clopidogrel is greatly affected by the thiol reductants used. GSH is the most efficient in producing the AM because of its low redox potential. Interestingly, no AM but only the mixed disulfide conjugates are formed in the presence of CPT, DFT, and NPT. These results provide direct evidence supporting that production of the AM proceeds via consecutive reduction of RS-OH → RS-SG → AM. We also demonstrated the usefulness of stable mixed disulfide conjugates in quantitatively producing the AM and in inhibiting platelet aggregation following treatment with GSH. These conjugates merit further studies to examine their antiplatelet activity in vivo, with the ultimate goal to improve the therapeutic efficacy of ticlopidine, clopidogrel, prasugrel, and other thienopyridine antiplatelet drugs.
Supplementary Material
Abbreviations
- AM
active metabolite of clopidogrel
- AM-MP
derivatized active metabolite of clopidogrel
- AUC
area under the curve
- BME
β-mercaptoethanol
- CPT
6-chloropyridazine-3-thiol
- DFT
2,5-dimethylfuran-3-thiol
- DTT
dithiothreitol
- EIC
extracted ion chromatogram
- G6PD
glucose-6-phosphate dehydrogenase
- GSH
glutathione
- HLM
human liver microsome
- IS
internal standard
- Km
Michaelis constant
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MPB
2-bromo-3′-methoxyacetophenone
- MS
mass spectrometry
- m/z
mass-to-charge ratio
- NAC
N-acetyl-l-cysteine
- NPT
3-nitropyridine-2-thiol
- P450
cytochrome P450
- PPP
platelet-poor plasma
- PRP
platelet-rich plasma
- RS-OH
sulfenic acid
- RS-SG
mixed glutathione disulfide conjugate
- RS-SX
mixed disulfide conjugate
Authorship Contributions
Participated in research design: Zhang, Lauver.
Conducted experiments: Zhang, Lauver.
Contributed new reagents or analytic tools: Zhang, Lauver.
Performed data analysis: Zhang, Lauver.
Wrote or contributed to the writing of the manuscript: Zhang, Lauver, Lucchesi, Hollenberg.
Footnotes
This work is in part supported by the National Institutes of Health [Grants AA020090 and CA016954].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Abell LM, Liu EC. (2011) Dissecting the activation of thienopyridines by cytochromes P450 using a pharmacodynamic assay in vitro. J Pharmacol Exp Ther 339:589–596 [DOI] [PubMed] [Google Scholar]
- Algaier I, Jakubowski JA, Asai F, von Kügelgen I. (2008) Interaction of the active metabolite of prasugrel, R-138727, with cysteine 97 and cysteine 175 of the human P2Y12 receptor. J Thromb Haemost 6:1908–1914 [DOI] [PubMed] [Google Scholar]
- Bouman HJ, Schömig E, van Werkum JW, Velder J, Hackeng CM, Hirschhäuser C, Waldmann C, Schmalz HG, ten Berg JM, Taubert D. (2011) Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med 17:110–116 [DOI] [PubMed] [Google Scholar]
- Dansette PM, Libraire J, Bertho G, Mansuy D. (2009) Metabolic oxidative cleavage of thioesters: evidence for the formation of sulfenic acid intermediates in the bioactivation of the antithrombotic prodrugs ticlopidine and clopidogrel. Chem Res Toxicol 22:369–373 [DOI] [PubMed] [Google Scholar]
- Dansette PM, Rosi J, Bertho G, Mansuy D. (2012a) Cytochromes P450 catalyze both steps of the major pathway of clopidogrel bioactivation, whereas paraoxonase catalyzes the formation of a minor thiol metabolite isomer. Chem Res Toxicol 25:348–356 [DOI] [PubMed] [Google Scholar]
- Dansette PM, Rosi J, Debernardi J, Bertho G, Mansuy D. (2012b) Metabolic activation of prasugrel: nature of the two competitive pathways resulting in the opening of its thiophene ring. Chem Res Toxicol 25:1058–1065 [DOI] [PubMed] [Google Scholar]
- Dansette PM, Thébault S, Bertho G, Mansuy D. (2010) Formation and fate of a sulfenic acid intermediate in the metabolic activation of the antithrombotic prodrug prasugrel. Chem Res Toxicol 23:1268–1274 [DOI] [PubMed] [Google Scholar]
- Dick RJ, Dear AE, Byron KA. (2011) Clopidogrel Resistance: case reports of CYP2C19 gene variants in suspected coronary stent thrombosis. Heart Lung Circ 20:657–658 [DOI] [PubMed] [Google Scholar]
- Ding Z, Kim S, Dorsam RT, Jin J, Kunapuli SP. (2003) Inactivation of the human P2Y12 receptor by thiol reagents requires interaction with both extracellular cysteine residues, Cys17 and Cys270. Blood 101:3908–3914 [DOI] [PubMed] [Google Scholar]
- Fayed AS, Weshahy SA, Shehata MA, Hassan NY, Pauwels J, Hoogmartens J, Van Schepdael A. (2009) Separation and determination of clopidogrel and its impurities by capillary electrophoresis. J Pharm Biomed Anal 49:193–200 [DOI] [PubMed] [Google Scholar]
- Freedman JE, Hylek EM. (2009) Clopidogrel, genetics, and drug responsiveness. N Engl J Med 360:411–413 [DOI] [PubMed] [Google Scholar]
- Gurbel PA, Tantry US. (2007) Clopidogrel resistance? Thromb Res 120:311–321 [DOI] [PubMed] [Google Scholar]
- Hagihara K, Kazui M, Kurihara A, Ikeda T, Izumi T. (2012) Glutaredoxin is involved in the formation of the pharmacologically active metabolite of clopidogrel from its GSH conjugate. Drug Metab Dispos 40:1854–1859 [DOI] [PubMed] [Google Scholar]
- Hagihara K, Kazui M, Kurihara A, Kubota K, Ikeda T. (2011) Glutaredoxin and thioredoxin can be involved in producing the pharmacologically active metabolite of a thienopyridine antiplatelet agent, prasugrel. Drug Metab Dispos 39:208–214 [DOI] [PubMed] [Google Scholar]
- Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, Ikeda T, Kurihara A. (2010) Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos 38:92–99 [DOI] [PubMed] [Google Scholar]
- Love BB, Biller J, Gent M. (1998) Adverse haematological effects of ticlopidine. Prevention, recognition and management. Drug Saf 19:89–98 [DOI] [PubMed] [Google Scholar]
- Lukesh JC, 3rd, Palte MJ, Raines RT. (2012) A potent, versatile disulfide-reducing agent from aspartic acid. J Am Chem Soc 134:4057–4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason PJ, Jacobs AK, Freedman JE. (2005) Aspirin resistance and atherothrombotic disease. J Am Coll Cardiol 46:986–993 [DOI] [PubMed] [Google Scholar]
- Mohan A, Hariharan M, Vikraman E, Subbaiah G, Venkataraman BR, Saravanan D. (2008) Identification and characterization of a principal oxidation impurity in clopidogrel drug substance and drug product. J Pharm Biomed Anal 47:183–189 [DOI] [PubMed] [Google Scholar]
- Ono K, Kurohara K, Yoshihara M, Shimamoto Y, Yamaguchi M. (1991) Agranulocytosis caused by ticlopidine and its mechanism. Am J Hematol 37:239–242 [DOI] [PubMed] [Google Scholar]
- Pereillo JM, Maftouh M, Andrieu A, Uzabiaga MF, Fedeli O, Savi P, Pascal M, Herbert JM, Maffrand JP, Picard C. (2002) Structure and stereochemistry of the active metabolite of clopidogrel. Drug Metab Dispos 30:1288–1295 [DOI] [PubMed] [Google Scholar]
- Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP, Pascal M, Herbert JM. (2000) Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost 84:891–896 [PubMed] [Google Scholar]
- Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, et al. (2009) Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 302:849–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi F, Giusti B, Marcucci R, Gori AM, Abbate R, Gensini GF. (2011) Cytochrome P450 2C19*2 polymorphism and cardiovascular recurrences in patients taking clopidogrel: a meta-analysis. Pharmacogenomics J 11:199–206 [DOI] [PubMed] [Google Scholar]
- Tuffal G, Roy S, Lavisse M, Brasseur D, Schofield J, Delesque Touchard N, Savi P, Bremond N, Rouchon MC, Hurbin F, et al. (2011) An improved method for specific and quantitative determination of the clopidogrel active metabolite isomers in human plasma. Thromb Haemost 105:696–705 [DOI] [PubMed] [Google Scholar]
- Zhang H, Lau WC, Hollenberg PF. (2012) Formation of the thiol conjugates and active metabolite of clopidogrel by human liver microsomes. Mol Pharmacol 82:302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.