Abstract
Recent studies suggest that paclitaxel, an anticancer agent, may modulate the injury and inflammatory responses in normal tissues. However, the underlying mechanism is not fully understood. Here we have examined the effect of paclitaxel on lipopolysaccharide (LPS)–induced acute kidney injury (AKI) in mice and further studied the mechanism. At relatively low doses, paclitaxel protected against LPS-induced AKI and improved animal survival. The beneficial effects of paclitaxel were accompanied by the downregulation of tumor necrosis factor-α, interleukin-1, and interleukin-6 production. In cultured renal tubular HK-2 cells, paclitaxel decreased the DNA-binding activity of nuclear factor-κB (NF-κB) during LPS treatment, inhibited the degradation of the inhibitor of κB-α, and blocked the expression and activation of NF-κB p65. At the upstream level, paclitaxel reduced LPS-induced association of myeloid differentiation protein-2 (MD-2) with Toll-like receptor 4 (TLR4). In an in vitro assay, paclitaxel was shown to directly bind recombinant MD-2. The inhibitory effect of paclitaxel on NF-κB activation and cytokine expression disappeared in MD-2 knockdown cells, indicating that paclitaxel acts through MD-2. Collectively, these results suggest that paclitaxel may bind MD-2 to block MD-2/TLR4 association during LPS treatment, resulting in the suppression of NF-κB activation and inhibition of proinflammatory cytokine production.
Introduction
Gram-negative bacterial infection associated with septic multiple organ failure remains a significant cause of morbidity and mortality in patients (Karima et al., 1999; Gao et al., 2008; Sharma et al., 2008). In kidneys, sepsis induces acute injury, tissue damage, and decrease of renal function, diminishing the efficacy of fluid supplementation and other therapeutics (Matejovic et al., 2011; Zarjou and Agarwal, 2011; Mayeux and MacMillan-Crow, 2012). Development of new drugs to treat septic acute kidney injury (AKI) therefore has a great potential to reduce mortality and morbidity in patients (Bagshaw et al., 2009; Zarjou and Agarwal, 2011). However, up-to-date, effective therapies for septic AKI are not available.
Lipopolysaccharide (LPS) is a major pathogenic factor during infection of Gram-negative bacteria (Mayeux, 1997; Karima et al., 1999; Bosshart and Heinzelmann, 2007). In mice and other animal models, LPS induces AKI, which is accompanied by a robust inflammatory response including leukocyte infiltration (Wang et al., 2009; Zahedi et al., 2010; Eadon et al., 2012). Proximal tubular cells are thought to play a role in the interstitial leukocyte infiltration in kidneys because of their anatomic proximity and ability to produce proinflammatory mediators and chemotactic cytokines (Danoff, 1998; Wan et al., 2008). In these cells, nuclear factor-κB (NF-κB), the ubiquitous proinflammatory transcription factor, has a pivotal role in the regulation of cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α), and many early response genes during LPS treatment (Viedt et al., 2002; Zager et al., 2007; Wang et al., 2011).
Paclitaxel is an anticancer agent that stabilizes microtubules to interfere with microtubule breakdown during cell division or mitosis, resulting in cell cycle arrest and cell death in cancer (Kavallaris, 2010; Rao et al., 2012). However, recent research suggests that paclitaxel may modulate the injury and inflammatory response in nonmalignant tissues. For example, we showed that low-dose paclitaxel can efficiently reduce tubulointerstitial fibrosis in kidneys by inhibiting Smad2/3 activation and downregulating miR-192 (Zhang et al., 2010; Sun et al., 2011). In models of acute lung injury, paclitaxel may attenuate inflammation and vascular leak (Chausovsky et al., 2000; Mirzapoiazova et al., 2007). However, the molecular mechanism by which paclitaxel modulates inflammation is not fully understood. LPS is the primary ligand of Toll-like receptor 4 (TLR4), activating it through binding to its accessory protein myeloid differentiation protein-2 (MD-2) (Shimazu et al., 1999; Akashi et al., 2000). Interestingly, in in vitro binding assays, paclitaxel was shown to bind human and murine MD-2 (Zimmer et al., 2008). In view of these findings, this study was initiated to assess whether low doses of paclitaxel can block TLR4-mediated NF-κB signaling by binding to MD-2 leading to the attenuation of LPS-induced AKI.
Materials and Methods
Care and Use of Laboratory Animals
Animal experiments were performed in accordance with the regulations set by the Institutional Committee for the Care and Use of Laboratory Animals of Second Xiangya Hospital, China. C57BL/6 mice were housed on a 12-hour light/dark cycle, and were allowed free access to food and water.
Animal Model
The mice (male, aged 8–10 weeks) were divided into four groups: vehicle (saline) (n = 24), paclitaxel (n = 24), LPS (n = 24), and LPS plus paclitaxel (n = 24). LPS (from Escherichia coli O111:B4, 10 mg/kg; Santa Cruz Biotechnology, Santa Cruz, CA) was injected through the tail vein. Paclitaxel (0.15 mg/kg; Sigma-Aldrich, Shanghai, China) was injected via the jugular vein. In the LPS plus paclitaxel group, LPS and paclitaxel were injected simultaneously. The control group was administered with saline. Renal tissues were harvested for various biochemical and morphologic studies at 16 hours after LPS and/or paclitaxel treatment except for survival study.
Cell Culture and Grouping
Experiment Protocol 1.
HK-2 cells were cultured in Dulbecco’s modified Eagle's medium F-12 supplemented with 10% fetal bovine serum in an atmosphere of 5% CO2–95% air at 37°C. The cells were plated into dishes and grown 24 hours to reach 90–100% confluence before being divided into four groups for the following treatments: control, paclitaxel, LPS (10 μg/ml), or LPS plus paclitaxel. After 2 or 24 hours of treatment, the cells were processed for various analyses. All experiments were carried out in quadruplicate.
Experiment Protocol 2.
HK-2 cells were plated at approximately 70% density for transfection with MD-2 small interfering RNA (siRNA) (CGCAAAGAAGUUAUUUGCCGAGGAU) or control siRNA (CGCAAGAAUUGGUUUAGCCGAAGAU) (Invitrogen, Carlsbad, CA) using Lipofectamine 2000 for 24 hours. The effect of siRNA on the expression of MD-2 was verified by immunoblot analysis. After siRNA transfection, the cells were cultured for 24 hours to reach confluence. The cells were then treated with LPS and/or paclitaxel for another 24 hours in the culture medium with 20 mM of sodium lactate.
Measurement of TNF-α, IL-1, IL-6, Serum Creatinine, and Blood Urea Nitrogen
Blood samples and kidney tissues were collected from mice during the in vivo experiment and culture medium was collected during the cell culture study. The levels of TNF-α, IL-1, and IL-6 were determined by using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems Inc, Minneapolis, MN). In all cases, a standard curve was constructed from standards provided by the manufacturers. Renal cytokine levels were normalized to protein concentration in lysate. Blood urea nitrogen (BUN) and creatinine levels were measured using an AutoAnalyzer (Roche Diagnostics, Mannheim, Germany).
Antibody-Sandwich ELISA Analysis of Paclitaxel Binding to MD-2
Recombinant human MD-2 was produced in E. coli as described previously (Gruber et al., 2004). The method of antibody-sandwich ELISA for the detection of binding of paclitaxel to MD-2 was modified from a previous study (Resman et al., 2008). This method is based on the use of a mouse monoclonal antibody that binds to free MD-2, but not to LPS-bound MD-2. All antibodies in this assay were purchased from Majorbio BioTech Co. Ltd. (Shanghai, China). Briefly, the wells of a microtiter plate were precoated with a chicken polyclonal anti-MD-2 antibody. Recombinant MD-2 was added into the wells at 2 μM for 2 hours of incubation. Then 0–2 μM paclitaxel was added into the wells for another 2 hours of incubation. Finally, a mouse anti-MD-2 antibody and a goat anti-mouse IgG conjugated with horseradish peroxidase were added to the wells. After extensive washes, 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS; a horseradish peroxidase substrate) was added to detect absorbance at 420 nm.
Immunoprecipitation
Approximately 500 μg cellular protein was immunoprecipitated with 2 μg antibodies to TLR4 (gift from Dr. Ruslan Medzhitov, Yale University, New Haven, CT) for 1 hour at 4°C followed by the addition of 15 μl protein A/G-agarose beads (Santa Cruz Biotechnology) as previously described (Ha et al., 2010). The precipitates were washed 4 times with lysis buffer and subjected to immunoblotting for MD-2 and TLR4.
Histology
For histologic analysis, kidney tissues fixed with 4% buffered paraformaldehyde were embedded in paraffin, and 3-µm-thick sections were prepared. The sections were then stained with hematoxylin and eosin. Histologic changes in the cortex and the outer stripe of the outer medulla (OSOM) were scored by a blinded observer. The percentage of tubules that displayed cellular necrosis, loss of brush border, cast formation, vacuolization, and tubule dilation were scored as follows: 0 = none, 1 = <10%, 2 = 11–25%, 3 = 26–45%, 4 = 46–75%, and 5 = >76% (Miyaji et al., 2003; Brooks et al., 2009).
Immunoblot Analysis
A standard protocol was followed for immunoblot analysis. Briefly, cells were treated with a lysis buffer (Sigma-Aldrich) containing phosphatase inhibitors (Calbiochem, Beijing, China). Samples (20 μg) were resolved by SDS-PAGE and then transferred onto nitrocellulose membrane (Amersham International, Buckinghamshire, UK) for sequential incubations with primary and secondary antibodies. Antibodies used included the following: mouse monoclonal anti-NF-κB p65, rabbit polyclonal anti-p-NF-κB p65, rabbit polyclonal anti-TLR4, peroxidase-conjugated goat anti-mouse, and goat anti-rabbit IgGs were purchased from Santa Cruz Biotechnology. Mouse monoclonal anti-MD-2 and anti-inhibitor of κB-α antibodies were from Abcam (Cambridge, MA).
Statistical Analysis
Data were calculated as mean ± S.E.M. One-way analysis of variance followed by Tukey’s post hoc test was used to compare multiple treatment groups. Two-way analysis of variance was used to assess the statistical significance of the differences between multiple treatment groups at different time points. Kaplan–Meier analysis (log-rank test) was used to record animal survival. Statistical significance was set at P < 0.05.
Results
Paclitaxel Improves Animal Survival After LPS Treatment
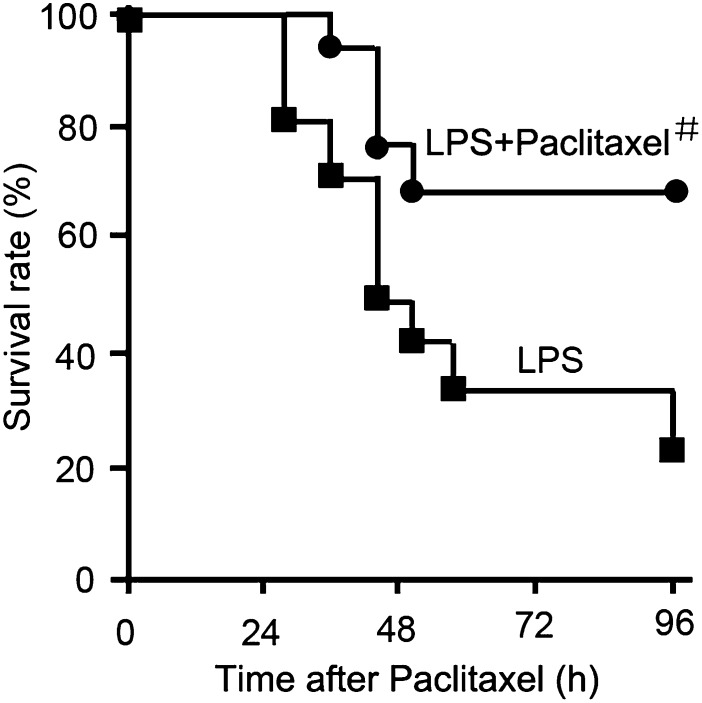
LPS treatment induced animal death within 1 day; by the end of 4 days, only 20–30% of animals survived. Cotreatment with 0.15 mg/kg paclitaxel delayed animal death by 8 hours; notably, the majority (68%) of animals survived in this group at the end of 4 days (Fig. 1).
Fig. 1.
Effect of paclitaxel on animal survival after LPS treatment. Male C57 mice were injected with 10 mg/kg LPS with or without 0.15 mg/kg paclitaxel. Animal survival was recorded at indicated time points. #P < 0.05 versus the LPS group (n = 12).
Paclitaxel Reduces Kidney Injury and Renal Dysfunction in LPS-Treated Mice
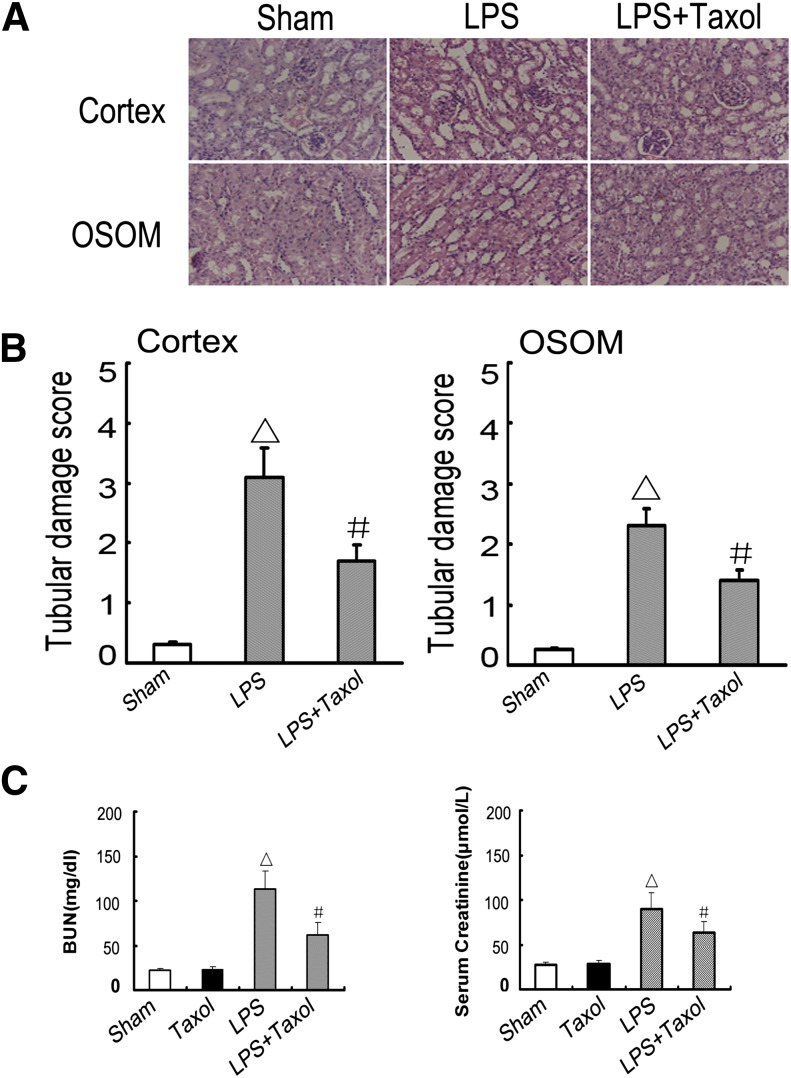
LPS-induced kidney injury was shown in the tubules in both renal cortex and OSOM. This tubular damage was significantly ameliorated by paclitaxel (Fig. 2, A and B). To note, the tubular damage observed in this study appeared more severe than that of previous studies (Wu et al., 2007; Takahashi et al., 2012). Although the cause of the severity is unclear, it may be related to the LPS dosage tested and the animal husbandry condition. To analyze kidney function, BUN and serum creatinine were determined. As shown in Fig. 2C, LPS induced significant increases in both BUN and serum creatinine levels. The increases were significantly attenuated by cotreatment with paclitaxel.
Fig. 2.
Effect of paclitaxel on LPS-induced renal tissue damage, BUN, and serum creatinine in mice. Male C57 mice were injected with 10 mg/kg LPS with or without 0.15 mg/kg paclitaxel. As a control, the sham group was injected with saline. Kidney tissues and blood samples were collected 16 h after treatment. (A) Kidney sections were stained with hematoxylin and eosin to review tubular damage. (B) Tubular damage scores of kidney cortex and OSOM. (C) BUN and serum creatinine. Data are shown as mean ± S.E.M. (n = 12). △P < 0.05 versus the sham group; #P < 0.05 versus the LPS group. Original magnification, ×200 in A.
Paclitaxel Decreases Serum and Renal TNF-α, IL-1, and IL-6 Levels in LPS-Treated Mice
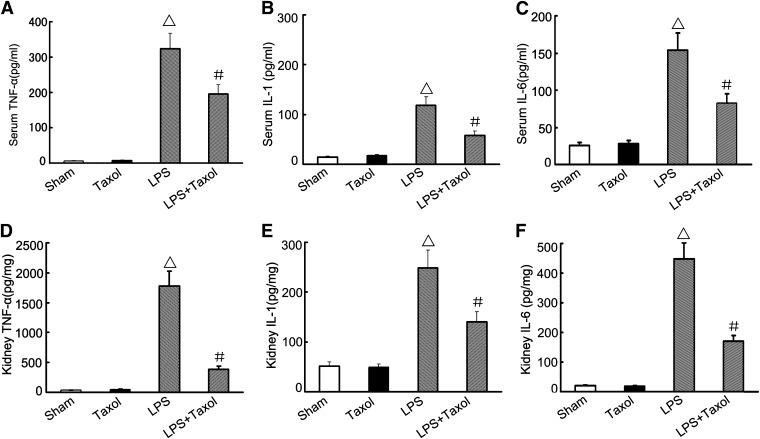
The effects of paclitaxel on serum and renal TNF-α, IL-1, and IL-6 were determined by ELISA. As shown in Fig. 3, LPS induced marked increases in the levels of TNF-α, IL-1, and IL-6, which were suppressed by paclitaxel. Paclitaxel had no effects on TNF-α, IL-1, and IL-6 expression under control conditions.
Fig. 3.
Effect of paclitaxel on LPS-induced expression of TNF-α, IL-1, and IL-6 in serum and kidney tissues. Male C57 mice were injected with 10 mg/kg LPS with or without 0.15 mg/kg paclitaxel, whereas the sham group was injected with saline. Kidney tissues and blood samples were collected 16 hours after treatment of ELISA determination of TNF-α, IL-1, and IL-6. Data are presented as mean ± S.E.M. (n = 12). △P < 0.05 versus the sham group; #P < 0.05 versus the LPS group.
Paclitaxel Attenuates LPS-Induced NF-κB Activation in Renal Tubular Epithelial Cells
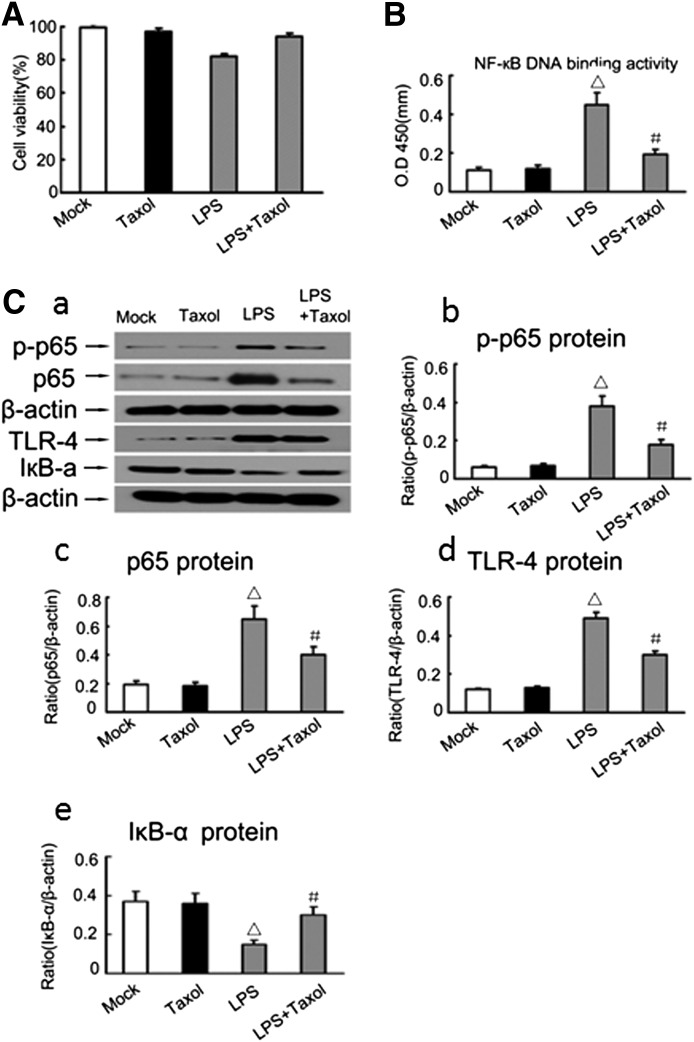
To understand the mechanism whereby paclitaxel blocks LPS-induced cytokine production (Fig. 3), we examined human renal tubular HK-2 epithelial cells after LPS/paclitaxel treatment. Within 24 hours, 2 μM paclitaxel did not have significant effects on the viability of HK-2 cells as shown by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cell viability analysis (Fig. 4A). ELISA analysis showed that LPS induced NF-κB DNA-binding activity in HK-2 cells and notably, this effect was markedly reduced by paclitaxel (Fig. 4B). Furthermore, LPS induced the expression of phospho-p65, p65, and TLR4 expression, which was also reduced by paclitaxel (Fig. 4C). Consistently, expression of the inhibitor of κB was decreased during LPS treatment and this decrease was prevented by paclitaxel (Fig. 4C). These results were verified by densitometry analysis of the immunoblots (Fig. 4, D–G).
Fig. 4.
Effect of paclitaxel on LPS-induced activation of p65, degradation of IκBa, and expression of TLR4 in HK-2 cells. HK-2 cells were treated with 10 μg/ml LPS in the presence or absence of 2 μM paclitaxel. (A) Cell viability determined by MTT assay at 24 hours of treatment. (B) NF-κB DNA-binding activity determined by ELISA assay at 2 hours of treatment. (C) Immunoblot analysis of phospho-p65, p65, IκBa, and TLR4 expression in HK-2 cells at 2 hours of treatment. (a) Representative immunoblot. (b,c,d,e) Results of densitometric analysis. Data are presented as mean ± S.E.M. (n = 6). △P < 0.05 versus the mock group or paclitaxel-only group; #P < 0.05 versus the LPS group. IκB-α, inhibitor of κB-α.
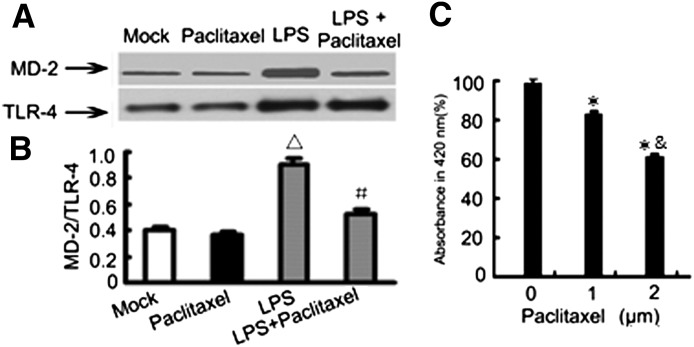
Paclitaxel Attenuates LPS-Induced Interaction between MD-2 and TLR4
A critical upstream event leading to NF-κB activation during LPS treatment is the activation and binding of MD-2 with TLR4. Therefore, we further examined the effect of paclitaxel on LPS-induced MD-2/TLR4 association by co-immunoprecipitation assay. Briefly, cell lysate was immunoprecipitated with an anti-TLR4 antibody, followed by immunoblot detection of MD-2 in the immunoprecipitates. As shown in Fig. 5A, LPS stimulation significantly increased the association between MD-2 and TLR4, which was reduced by paclitaxel. As a control, the immunoprecipitation by a control IgG did not result in precipitation of MD-2 (not shown). One possibility is that paclitaxel may block MD-2/TLR4 association by directly binding MD-2. To test this, we determined the binding of paclitaxel to MD-2. For this purpose, recombinant human MD-2 was incubated with a monoclonal antibody that binds to free MD-2, but not to paclitaxel-bound MD-2. In this assay, paclitaxel was shown to reduce the association of MD-2 with its antibody (Fig. 5C), indicating that paclitaxel indeed binds to MD-2.
Fig. 5.
(A) Paclitaxel blocks LPS-induced MD-2/TLR4 association. HK-2 cells were treated with 10 μg/ml LPS for 2 hours in the presence or absence of 2 μM paclitaxel to collect lysate for immunoprecipitation using anti-TLR4 antibodies. The immunoprecipitates were then subjected to immunoblot analysis of TLR4 and MD-2. (B) Densitometric analysis of the MD-2/TLR4 ratio in the immunoblots of the immunoprecipitates. △P < 0.05 versus the mock group or paclitaxel-only group; #P < 0.05 versus the LPS group. The results show that LPS induced MD-2/TLR4 association and this molecular association was suppressed by paclitaxel. (C) In vitro assay of paclitaxel binding of MD-2. Antibody-sandwich ELISA was conducted as described in the Materials and Methods. Decreases in absorbance at 420 nm indicate the binding of paclitaxel of recombinant MD-2. *P < 0.05 versus the 0 μM paclitaxel group; &P < 0.05 versus the 1 μM paclitaxel group.
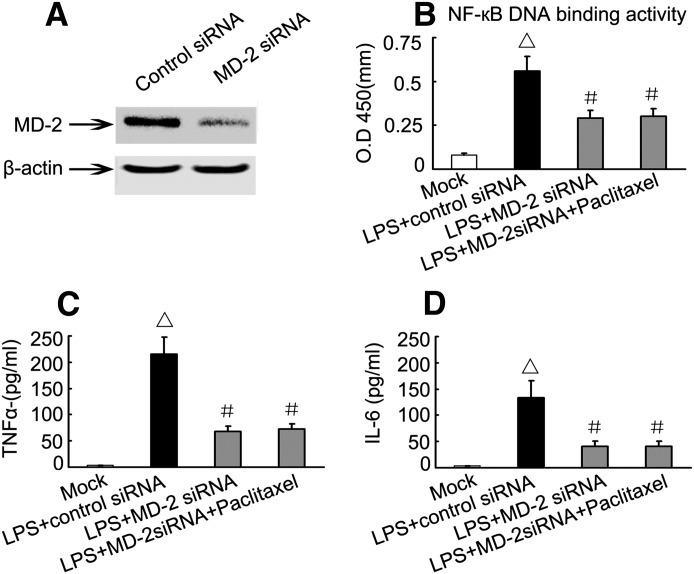
The Anti-Inflammatory Effect of Paclitaxel Depends on MD-2
To determine whether the anti-inflammatory effect of paclitaxel depends on MD-2, we downregulated MD-2 with specific siRNA. As shown in Fig. 6A, transfection of HK-2 cells with MD-2 siRNA led to a marked MD-2 downregulation. Importantly, in MD-2 siRNA transfected cells, the stimulatory effects of LPS on NF-κB DNA-binding activity and cytokine expression were diminished. In addition, paclitaxel did not show further inhibitory effects in these cells (Fig. 5C), suggesting that the anti-inflammatory effect of paclitaxel depends on MD-2.
Fig. 6.
Effect of MD-2 knockdown on LPS-induced NF-κB DNA-binding activity and expression of TNF-α and IL-6 levels. HK-2 cells were transfected with MD-2 siRNA or control siRNA, followed by treatment with 10 μg/ml LPS in the presence or absence of 2μM paclitaxel. (A) Immunoblot analysis to verify MD-2 knockdown by a specific siRNA. (B) NF-κB DNA-binding activity determined using ELISA assay. (C and D) TNF-α and IL-1 expression analyzed by ELISA assay. Data are presented as mean ± S.E.M. (n = 6). △P < 0.05 versus the mock group; #P < 0.05, versus the LPS plus control siRNA group.
Discussion
Septic AKI is a major clinical problem that leads to significant mortality. Currently, there are no effective therapies. In this study, we have demonstrated that the cancer therapy drug, paclitaxel, has protective effects on LPS-induced AKI and improves survival. LPS is the main endotoxin produced by Gram-negative bacteria, but it does not represent the entire spectrum of pathogenic factors in sepsis. In designing renoprotective therapeutics for the treatment of AKI, it is also important to consider both injury and subsequent kidney repair. Ideally, the therapeutics can limit AKI at the early phase and promote kidney repair and/or prevent fibrosis at the progression phase. In this regard, paclitaxel may adversely affect kidney repair, but this will certainly depend on the dosage used and the pharmacokinetics of paclitaxel. Further preclinical and clinical studies may need to examine the usefulness of paclitaxel in the treatment of septic AKI.
In LPS-treated mice, our ELISA assay demonstrated the inhibitory effect of paclitaxel on the production of proinflammatory cytokines, including TNF-α, IL-1, and IL-6. TNF-α and related cytokines are known to contribute to the toxic effect of LPS (Beutler and Kruys, 1995). However, it is controversial regarding the effects of paclitaxel on the expression of proinflammatory factors. In isolated murine macrophages, paclitaxel can increase TNF-α and IL-1 release (Ding et al., 1990; Bogdan and Ding, 1992). In contrast, taxanes (paclitaxel and docetaxel) decrease the levels of IL-1 and TNF-α and increase levels of interferon-γ, IL-2, and IL-6 in the serum of breast cancer patients (Tsavaris et al., 2002). In LPS-induced lung injury, Mirzapoiazova et al. (2007) demonstrated that paclitaxel may reduce the serum levels of cytokines including TNF-α and IL-6. Our data are consistent with the latter studies. Although the exact reason underlying the result discrepancy between these studies is unclear, it may be related to the different models and doses of paclitaxel used. For example, the chemotherapy dose of paclitaxel (30 μM) used in breast cancer patients is significantly higher than those used in our study (2 μM) and in the study by Mirzapoiazova et al. (10 μM). In addition to the proinflammatory cytokines, it would be interesting to test whether paclitaxel affects the expression of anti-inflammatory cytokines such as IL-10.
Mechanistically, our results suggest that paclitaxel may suppress TLR4-mediated NF-κB signaling by binding MD-2 to inhibit cytokine production in renal tubular cells. Recent studies indicate that microtubule stabilization by paclitaxel may interfere with the effects of LPS on cell–cell junctions, since microtubules and adherence junctions interact dynamically (Bogdan and Ding, 1992; Beutler and Kruys, 1995; Chausovsky et al., 2000). Furthermore, paclitaxel may suppress LPS-induced inflammation by attenuating vascular leak in vivo (Mirzapoiazova et al., 2007). Interestingly, Lee and colleagues reported that paclitaxel, like LPS, is able to stimulate the translocation of the 50- and 65-kDa heterodimers of NF-κB to the nucleus in 70Z/3 pre-B cells; however, this effect is dose dependent and requires at least 5 μM of paclitaxel (Lee and Jeon, 2001), which is significantly higher than the effective dosage in our study. Furthermore, the stimulatory effect on NF-κB nuclear translocation was shown after pre-exposing the cells to paclitaxel (Lee and Jeon, 2001). On the other hand, several recent studies have suggested that LPS and paclitaxel share a common receptor/signaling complex (Byrd et al., 1999; Kawasaki et al., 2000; Lee et al., 2000). In line with these studies, our study provides evidence that at low doses, paclitaxel suppresses NF-κB signaling in LPS-induced injury in mouse kidneys and in renal tubular HK-2 cells. We showed that paclitaxel blocks the DNA-binding activity of NF-κB, which is accompanied by the inhibition of cytokine production during LPS treatment. To investigate the underlying mechanisms, we showed that paclitaxel inhibits the association between MD-2 and TLR4 during LPS treatment in HK-2 cells. MD-2 is an extracellular molecule, which, after LPS binding, associates with TLR4 via a specific region to form a complex to trigger downstream signaling (Shimazu et al., 1999; Kawasaki et al., 2001). In our study, MD-2 knockdown suppressed LPS-induced signaling and cytokine production in HK-2 cells. Notably, the anti-inflammatory effect of paclitaxel was attenuated in MD-2 knockdown cells, indicating that paclitaxel functions through MD-2. Using an in vitro binding assay, we further verified that paclitaxel can directly bind to recombinant MD-2, suggesting that paclitaxel may bind to MD-2 to interrupt the MD-3/TLR4 association during LPS treatment. The blockade of MD-2/TLR4 association by paclitaxel explains the observed effects of paclitaxel on downstream proinflammatory responses, including the inhibition of NF-κB activation and suppression of cytokine production. Our observations are consistent with a previous work suggesting that paclitaxel acts through TLR4/MD2 (Kawasaki et al., 2000). According to these results, paclitaxel acts extracellularly by binding to MD-2 to modulate TLR4 signaling. However, paclitaxel is also known to stabilize microtubules to exert related cell biologic effects intracellularly.
In conclusion, our study demonstrates that low doses of paclitaxel can protect against LPS-induced proinflammatory response and kidney injury, suggesting a potential use of the chemotherapy drug in the treatment of septic AKI. Mechanistically, paclitaxel may directly bind MD-2 to block MD-2/TLR4 association, resulting in the inhibition of NF-κB signaling and consequent production of cytokines.
Abbreviations
- AKI
acute kidney injury
- BUN
blood urea nitrogen
- ELISA
enzyme-linked immunosorbent assay
- IL-1
interleukin-1
- IL-6
interleukin-6
- LPS
lipopolysaccharide
- MD-2
myeloid differentiation protein-2
- NF-κB
nuclear factor-κB
- OSOM
outer stripe of the outer medulla
- TLR4
Toll-like receptor 4
- TNF-α
tumor necrosis factor-α
Authorship Contributions
Participated in research design: Zhang.
Conducted experiments: Zhang, Li, Liu.
Performed data analysis: Zhang, Xiang, Li, Dong.
Wrote or contributed to the writing of the manuscript: Zhang, Dong.
Footnotes
This research was supported in part by the National Natural Science Foundation of China [Grant 81100507/H0509]; the National Basic Research Program of China 973 Program No. 2012CB517600 [Grant 2012CB517601]; the Central South University Free Exploration Research Program [Grant 2011QNZT172]; the National Institutes of Health [Grants 2R01-DK58831 and 1R01-DK87843]; and the US Department of Veterans Affairs [Merit Review].
References
- Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. (2000) Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol 164:3471–3475 [DOI] [PubMed] [Google Scholar]
- Bagshaw SM, Lapinsky S, Dial S, Arabi Y, Dodek P, Wood G, Ellis P, Guzman J, Marshall J, Parrillo JE, et al. Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group (2009) Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med 35:871–881 [DOI] [PubMed] [Google Scholar]
- Beutler B, Kruys V. (1995) Lipopolysaccharide signal transduction, regulation of tumor necrosis factor biosynthesis, and signaling by tumor necrosis factor itself. J Cardiovasc Pharmacol 25 (Suppl 2):S1–S8 [DOI] [PubMed] [Google Scholar]
- Bogdan C, Ding A. (1992) Taxol, a microtubule-stabilizing antineoplastic agent, induces expression of tumor necrosis factor alpha and interleukin-1 in macrophages. J Leukoc Biol 52:119–121 [DOI] [PubMed] [Google Scholar]
- Bosshart H, Heinzelmann M. (2007) Targeting bacterial endotoxin: two sides of a coin. Ann N Y Acad Sci 1096:1–17 [DOI] [PubMed] [Google Scholar]
- Brooks C, Wei Q, Cho SG, Dong Z. (2009) Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119:1275–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd CA, Bornmann W, Erdjument-Bromage H, Tempst P, Pavletich N, Rosen N, Nathan CF, Ding A. (1999) Heat shock protein 90 mediates macrophage activation by Taxol and bacterial lipopolysaccharide. Proc Natl Acad Sci USA 96:5645–5650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chausovsky A, Bershadsky AD, Borisy GG. (2000) Cadherin-mediated regulation of microtubule dynamics. Nat Cell Biol 2:797–804 [DOI] [PubMed] [Google Scholar]
- Danoff TM. (1998) Chemokines in interstitial injury. Kidney Int 53:1807–1808 [DOI] [PubMed] [Google Scholar]
- Ding AH, Porteu F, Sanchez E, Nathan CF. (1990) Shared actions of endotoxin and taxol on TNF receptors and TNF release. Science 248:370–372 [DOI] [PubMed] [Google Scholar]
- Eadon MT, Hack BK, Xu C, Ko B, Toback FG, Cunningham PN. (2012) Endotoxemia alters tight junction gene and protein expression in the kidney. Am J Physiol Renal Physiol 303:F821–F830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Evans TW, Finney SJ. (2008) Bench-to-bedside review: sepsis, severe sepsis and septic shock - does the nature of the infecting organism matter? Crit Care 12:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber A, Mancek M, Wagner H, Kirschning CJ, Jerala R. (2004) Structural model of MD-2 and functional role of its basic amino acid clusters involved in cellular lipopolysaccharide recognition. J Biol Chem 279:28475–28482 [DOI] [PubMed] [Google Scholar]
- Ha T, Hu Y, Liu L, Lu C, McMullen JR, Kelley J, Kao RL, Williams DL, Gao X, Li C. (2010) TLR2 ligands induce cardioprotection against ischaemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Cardiovasc Res 87:694–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karima R, Matsumoto S, Higashi H, Matsushima K. (1999) The molecular pathogenesis of endotoxic shock and organ failure. Mol Med Today 5:123–132 [DOI] [PubMed] [Google Scholar]
- Kavallaris M. (2010) Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer 10:194–204 [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, Nishijima M. (2000) Mouse toll-like receptor 4.MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by Taxol. J Biol Chem 275:2251–2254 [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, Nishijima M. (2001) Involvement of TLR4/MD-2 complex in species-specific lipopolysaccharide-mimetic signal transduction by Taxol. J Endotoxin Res 7:232–236 [PubMed] [Google Scholar]
- Lee M, Jeon YJ. (2001) Paclitaxel-induced immune suppression is associated with NF-kappaB activation via conventional PKC isotypes in lipopolysaccharide-stimulated 70Z/3 pre-B lymphocyte tumor cells. Mol Pharmacol 59:248–253 [DOI] [PubMed] [Google Scholar]
- Lee M, Yea SS, Jeon YJ. (2000) Paclitaxel causes mouse splenic lymphocytes to a state hyporesponsive to lipopolysaccharide stimulation. Int J Immunopharmacol 22:615–621 [DOI] [PubMed] [Google Scholar]
- Matejovic M, Chvojka J, Radej J, Ledvinova L, Karvunidis T, Krouzecky A, Novak I. (2011) Sepsis and acute kidney injury are bidirectional. Contrib Nephrol 174:78–88 [DOI] [PubMed] [Google Scholar]
- Mayeux PR. (1997) Pathobiology of lipopolysaccharide. J Toxicol Environ Health 51:415–435 [DOI] [PubMed] [Google Scholar]
- Mayeux PR, MacMillan-Crow LA. (2012) Pharmacological targets in the renal peritubular microenvironment: implications for therapy for sepsis-induced acute kidney injury. Pharmacol Ther 134:139–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzapoiazova T, Kolosova IA, Moreno L, Sammani S, Garcia JG, Verin AD. (2007) Suppression of endotoxin-induced inflammation by taxol. Eur Respir J 30:429–435 [DOI] [PubMed] [Google Scholar]
- Miyaji T, Hu X, Yuen PS, Muramatsu Y, Iyer S, Hewitt SM, Star RA. (2003) Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int 64:1620–1631 [DOI] [PubMed] [Google Scholar]
- Rao CV, Kurkjian CD, and Yamada HY (2012) Mitosis-targeting natural products for cancer prevention and therapy. Curr Drug Targets 13:1820–1830. [DOI] [PubMed]
- Resman N, Gradisar H, Vasl J, Keber MM, Pristovsek P, Jerala R. (2008) Taxanes inhibit human TLR4 signaling by binding to MD-2. FEBS Lett 582:3929–3934 [DOI] [PubMed] [Google Scholar]
- Sharma U, Rhaleb NE, Pokharel S, Harding P, Rasoul S, Peng H, Carretero OA. (2008) Novel anti-inflammatory mechanisms of N-Acetyl-Ser-Asp-Lys-Pro in hypertension-induced target organ damage. Am J Physiol Heart Circ Physiol 294:H1226–H1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. (1999) MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med 189:1777–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Zhang D, Liu F, Xiang X, Ling G, Xiao L, Liu Y, Zhu X, Zhan M, Yang Y, et al. (2011) Low-dose paclitaxel ameliorates fibrosis in the remnant kidney model by down-regulating miR-192. J Pathol 225:364–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Mizukami H, Kamata K, Inaba W, Kato N, Hibi C, Yagihashi S. (2012) Amelioration of acute kidney injury in lipopolysaccharide-induced systemic inflammatory response syndrome by an aldose reductase inhibitor, fidarestat. PLoS ONE 7:e30134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsavaris N, Kosmas C, Vadiaka M, Kanelopoulos P, Boulamatsis D. (2002) Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer 87:21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viedt C, Dechend R, Fei J, Hänsch GM, Kreuzer J, Orth SR. (2002) MCP-1 induces inflammatory activation of human tubular epithelial cells: involvement of the transcription factors, nuclear factor-kappaB and activating protein-1. J Am Soc Nephrol 13:1534–1547 [DOI] [PubMed] [Google Scholar]
- Wan L, Bagshaw SM, Langenberg C, Saotome T, May C, Bellomo R. (2008) Pathophysiology of septic acute kidney injury: what do we really know? Crit Care Med 36(4, Suppl)S198–S203 [DOI] [PubMed] [Google Scholar]
- Wang W, Bansal S, Falk S, Ljubanovic D, Schrier R. (2009) Ghrelin protects mice against endotoxemia-induced acute kidney injury. Am J Physiol Renal Physiol 297:F1032–F1037 [DOI] [PubMed] [Google Scholar]
- Wang WM, Chen H, Zhong F, Lu Y, Han L, Chen N. (2011) Inhibitory effects of rosiglitazone on lipopolysaccharide-induced inflammation in a murine model and HK-2 cells. Am J Nephrol 34:152–162 [DOI] [PubMed] [Google Scholar]
- Wu L, Tiwari MM, Messer KJ, Holthoff JH, Gokden N, Brock RW, Mayeux PR. (2007) Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiol Renal Physiol 292:F261–F268 [DOI] [PubMed] [Google Scholar]
- Zager RA, Johnson AC, Geballe A. (2007) Gentamicin suppresses endotoxin-driven TNF-alpha production in human and mouse proximal tubule cells. Am J Physiol Renal Physiol 293:F1373–F1380 [DOI] [PubMed] [Google Scholar]
- Zahedi K, Barone S, Kramer DL, Amlal H, Alhonen L, Jänne J, Porter CW, Soleimani M. (2010) The role of spermidine/spermine N1-acetyltransferase in endotoxin-induced acute kidney injury. Am J Physiol Cell Physiol 299:C164–C174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarjou A, Agarwal A. (2011) Sepsis and acute kidney injury. J Am Soc Nephrol 22:999–1006 [DOI] [PubMed] [Google Scholar]
- Zhang D, Sun L, Xian W, Liu F, Ling G, Xiao L, Liu Y, Peng Y, Haruna Y, Kanwar YS. (2010) Low-dose paclitaxel ameliorates renal fibrosis in rat UUO model by inhibition of TGF-beta/Smad activity. Lab Invest 90:436–447 [DOI] [PubMed] [Google Scholar]
- Zimmer SM, Liu J, Clayton JL, Stephens DS, Snyder JP. (2008) Paclitaxel binding to human and murine MD-2. J Biol Chem 283:27916–27926 [DOI] [PMC free article] [PubMed] [Google Scholar]