Abstract
Our knowledge of how androgens influence the cardiovascular system is far from complete, and this lack of understanding is especially true of how androgens affect resistance vessels. Our aim was to identify the signaling mechanisms stimulated by testosterone (TES) in microvascular arteries and to understand how these mechanisms mediate TES-induced vasodilation. Mesenteric microvessels were isolated from male Sprague-Dawley rats. Tension studies demonstrated a rapid, concentration-dependent, vasodilatory response to TES that did not involve protein synthesis or aromatization to 17β-estradiol. Dichlorofluorescein fluorescence and nitrotyrosine immunoblot experiments indicated that TES stimulated peroxynitrite formation in microvessels, and functional studies demonstrated that TES-induced vasodilation was inhibited by scavenging peroxynitrite. As predicted, TES enhanced the production of both peroxynitrite precursors (i.e., superoxide and nitic oxide), and xanthine oxidase was identified as the likely source of TES-stimulated superoxide production. Functional and biochemical studies indicated that TES signaling involved activity of the phosphoinositide 3 (PI3) kinase-protein kinase B (Akt) cascade initiated by activation of the androgen receptor and culminated in enhanced production of cGMP and microvascular vasodilation. These findings, derived from a variety of analytical and functional approaches, provide evidence for a novel nongenomic signaling mechanism for androgen action in the microvasculature: TES-stimulated vasodilation mediated primarily by peroxynitrite formed from xanthine oxidase-generated superoxide and NO. This response was associated with activation of the PI3 kinase-Akt signaling cascade initiated by activation of the androgen receptor. We propose this mechanism could account for TES-stimulated cGMP production in microvessels and, ultimately, vasodilation.
Introduction
Our knowledge of how gonadal steroids impact cardiovascular function is far from complete, and this incomplete understanding is especially true of androgens. Nonetheless, U.S. prescription sales for androgens have increased by more than 500% since the 1990s (Bhasin and Buckwalter, 2001). In light of their increased use, it is troublesome that our understanding of the cellular and molecular basis of androgen action on blood vessels is lacking. Moreover, what we do know of androgen action on cardiovascular physiology is controversial. For example, androgens increase the incidence of hypertension and cardiac disease in men (Sullivan et al., 1998); however, there is an inverse correlation between testosterone (TES) levels and blood pressure in middle-aged men (Khaw and Barrett-Connor, 1988). Further, endogenous TES lowers cardiovascular mortality, and declining TES levels are a strong independent predictive marker for cardiovascular disease in men (Keating et al., 2006; Khaw et al., 2007; Maggio et al., 2007). Interestingly, acute administration of TES improves exercise-induced myocardial ischemia (Rosano et al., 2007), but the mechanism of this nongenomic vasodilatory effect of TES remains unclear. We and others have demonstrated that TES relaxes larger arteries in vitro (Yue et al., 1995; Chou et al., 1996; Costarella et al., 1996; Deenadayalu et al., 2001; Ding and Stallone, 2001; English et al., 2002; Tep-areenan et al., 2002); however, far fewer studies have investigated TES signaling in smaller, resistance vessels.
Gonadal steroids may influence cardiovascular function by modulating the production of reactive oxygen and nitrogen species (White et al., 2005; Iliescu et al., 2007; Sullivan et al., 2007). We and others have linked TES-induced relaxation of some vessels to the production of nitric oxide (NO) (Chou et al., 1996; Costarella et al., 1996; Tep-areenan et al., 2002; Deenadayalu et al., 2012). Interestingly, peroxynitrite (formed rapidly from the combination of superoxide with NO) lowers resistance in the mesenteric vasculature (Graves et al., 2005); however, whether peroxynitrite can mediate the vascular effects of androgens remains unknown. The present study provides the initial evidence that androgens induce vasodilation of microvascular resistance vessels via an acute, nongenomic mechanism dependent on peroxynitrite formed from the combination of TES-generated NO and superoxide.
Materials and Methods
Animals.
All procedures and protocols were approved by the Georgia Health Sciences University Committee on the Use of Animals in Research and Education and conformed to the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult male Sprague-Dawley rats (10–14 weeks old) were obtained from Harlan (Indianapolis, IN). Animals were anesthetized with 5–10% isoflurane (confirmed by lack of reflex responses), followed by exsanguination. The mesenteric bed was quickly removed, and mesenteric microvessels were isolated under stereomicroscope by carefully removing fat and connective tissue and placed into ice-cold dissociation buffer consisting of the following (in millimolars):118.06 NaCl, 48 KCl, 1.18 MgCl2, 26.18 NaHCO3, 1.17 KH2PO4, 2.45 CaCl2 and 11 glucose.
Microvascular Reactivity Studies.
Segments (2 mm long) of primary or secondary branches of the mesenteric artery were mounted on fine tungsten wires (40-mm diameter) on a Mulvany-Halpern myograph (Model 610 M; Danish Myo Technology, Aarhus, Denmark) (Mulvany and Halpern, 1977) and stabilized for 40 minutes in Krebs’ solution of the following composition (in millimolars): 122 NaCl, 4.7 KCl, 15.5 NaHCO3, 1.2 KH2PO4, 1.2 MgCl2, 1.8 CaCl2, 11.5 glucose, pH 7.2 (37°C). Vessels were stretched to a force of 3.0–3.5 mN to simulate physiologic tone and contracted with 10 μM phenylephrine to stabilize the preparations. After washout and re-equilibration (30 minutes), vessels were again contracted with phenylephrine, and a complete concentration-relaxation relationship was obtained for cumulative addition of TES. Vasodilator responses were expressed as the percentage of relaxation of phenylephrine-induced tone. Various inhibitors were added 30 minutes before the agonist and remained in the medium throughout the test period.
Detection of NO, Superoxide, and Peroxynitrite.
We used the fluorescent indicator dichlorofluorescein (DCF) as a general marker of cellular oxidation (El-Remessy et al., 2007), and dihydroethidium (DHE) was used to detect superoxide generation (White et al., 2005). Briefly, serial cryosections (10 µm) were preincubated for 30 minutes at room temperature in phosphate-buffered saline in the presence or absence of each of the following inhibitors: polyethylene glycol-superoxide dismutase (PEG-SOD; 400 U/ml), uric acid (UA; 1 mM), and NG-nitro-L-arginine methyl ester (L-NAME; 100 μM). Sections were then incubated with DCF or DHE (10 µM) in the presence or absence of the same inhibitors for 1 hour at 37°C in the dark. The NO indicator dye 4-amino-5-methylamino-2’,7’-difluorescein (DAF-FM) was also used (2 µM; 30 minutes), with L-NAME (100 μM) used to control for nonspecific fluorescence (White et al., 2002). Fluorescent images were obtained with a laser-scanning confocal microscope (Microradiance CSLM; Bio-Rad, Hercules, CA) at a magnification of ×200. Fluorescence was quantified with Image-J software (NIH, Bethesda, MD).
Cyclic Nucleotide Measurements.
cGMP was measured by enzyme immunoassay (Biomol, Farmingdale, NY) (White et al., 2000). Mesenteric vessels were preincubated for 30 minutes at 37°C in buffer containing inhibitors, whereas control vessels were treated with vehicle only. Tissues were then treated with either 1 nM, 100 nM, or 1 µM TES. Reactions were terminated after 5 or 15 minutes or 30 minutes by removing the buffer and adding 0.5 ml of 0.1 N HCl for 30 minutes at room temperature. Results were expressed as femtomoles nucleotide per cell number.
Xanthine Oxidase Assay.
Mesenteric vessels were preincubated for 30 minutes (37°C) in buffer containing inhibitors, whereas control vessels were treated with vehicle only. Tissues were then treated with 1 nM, 100 nM, or 1 µM TES in the absence or presence of inhibitor. Reactions were terminated after 30 minutes, and tissue was frozen (−80°C) until use. Tissues were homogenized in 100 mM Tris-HCl, pH 7.5, with protease inhibitors, and centrifuged (10,000g) for 15 minutes (40°C), and the supernatant was assayed for xanthine oxidase (XO) activity. XO first produces hydrogen peroxide, which then reacts with 10-acetyl-3,7-dihydroxyphenoxazine in the presence of horseradish peroxidase to produce the highly fluorescent compound resorufin.
Electronic Paramagnetic Resonance Measurement of Superoxide.
Superoxide levels in vessels were quantified by electronic paramagnetic resonance (EPR) using the spin-trap compound 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine HCl (Lakshminrusimha et al., 2007). Approximately 10 mm of artery was isolated and preincubated (30 minutes, 37°C) in buffer containing allopurinol (300 µM) and then treated with TES (100 nM or 1 μM). Control vessels were treated with vehicle only. Reactions were terminated by flash freezing in liquid nitrogen. The frozen tissue was pulverized and placed into EPR buffer [phosphate buffered saline supplemented with 5 μM diethydithiocarbamate (Sigma-Aldrich, St. Louis, MO) and 25 μM desferrioxamine (Sigma-Aldrich)] with 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine HCl (5 mg/ml). Samples were further incubated for 20 minutes on ice and centrifuged (14,000g; 15 minutes) at room temperature; 35 μl of supernatant from each sample was loaded into a 50-μl capillary tube and analyzed with a MiniScope MS200 ESR (Magnettech, Berlin, Germany). Resulting EPR spectra were analyzed using ANALYSIS v.2.02 software (Magnettech).
Western Blotting.
Expression of NOS or protein kinase B (Akt) was detected using specific antibodies (1:1000; BD Biosciences, Franklin Lakes, NJ) as described previously (Han et al., 2007). Protein concentrations were determined by Bio-Rad DC protein assay. Blots were visualized with an enhanced chemiluminescence system (GE Healthcare, Piscataway, NJ). Phosphorylation of Akt was determined by immunoblotting with antibody-recognizing phosphorylated (ser 473) kinase (1:1000; BD Biosciences). Phospho-Akt immunoblots were stripped and immunoblotted for total Akt (1:1000; Sigma-Aldrich).
Nitrotyrosine Formation.
Relative amounts of proteins nitrated on tyrosine were measured using slot-blot techniques (El-Remessy et al., 2003). Briefly, cellular protein samples were immobilized onto polyvinylidene difluoride membranes by using a slot-blot microfiltration unit (Bio-Rad). A dilution series of peroxynitrite-modified bovine serum albumin (Cayman Chemical, Ann Arbor, MI) was loaded to generate a standard curve, and nitrotyrosine was detected using a monoclonal anti-nitrotyrosine antibody (Cayman Chemical) followed by peroxidase-labeled goat anti-mouse IgG and electrogenerated chemiluminescence (ECL). Relative levels of nitrotyrosine immunoreactivity were determined by densitometry.
Statistical Analysis.
All data were expressed as the mean ± S.E. Significance between two groups was evaluated by Student's t test. A multivariate (two groups by three drug concentrations) repeated measures analysis of variance was used to compare control groups to drug-treated groups (SAS, Cary, NC; version 9.2). A probability of less than 0.05 indicated a significant difference.
Results
TES Induces Nongenomic Vasodilation of Microvessels.
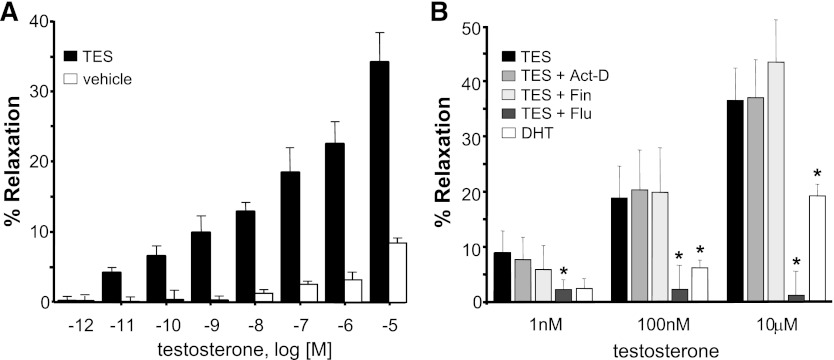
TES produced a concentration-dependent relaxation of mesenteric microvessels, whereas vehicle alone (<0.1% ethanol) had minimal effect on vessel tension over the same concentration range (Fig. 1A). On average, TES produced a maximal relaxation (10 μM) of 38.3 ± 3.6% (n = 11). Pretreating vessels with 80 μM actinomycin D, an inhibitor of protein synthesis, had no effect on the magnitude of TES-induced relaxation (1 nM, 100 nM, or 10 μM TES) (Fig. 1B). Maximal relaxation in the presence of actinomycin D was 37.0 ± 6.8% (n = 5). Because aromatase is expressed within the vascular wall (Diano et al., 1999), it was possible that TES could be converted to estrogen. However, dihydrotestosterone, a nonaromatizable androgen, also relaxed mesenteric microvessels, albeit to a lesser extent (Fig. 1B). On average, 10 μM dihydrotestosterone produced 19.2 ± 2.2% relaxation (n = 5; P < 0.01). Further, TES-induced relaxation was unaltered by treating vessels with 75 nM finasteride, an inhibitor of 5α reductase (n = 5; Fig. 1B). In contrast, TES-induced relaxation was attenuated by 10 μM flutamide, an antagonist of the androgen receptor. Flutamide abolished TES-induced relaxation at all concentrations (n = 5; P < 0.001; Fig. 1B). Similarly, cyproterone (androgen receptor partial agonist) inhibited TES-induced relaxation (unpublished data). On average, 10 μM cyproterone inhibited the relaxation response to 10 μM TES by 22.7 ± 2.9% (n = 5; P < 0.001).
Fig. 1.
TES induces a rapid, nongenomic vasodilation of rat mesenteric microvessels. (A) Mean concentration-response relationship for TES-induced vasodilation (black bars) or vehicle (0.1% ethanol; white bars). Each bar represents the mean response of 6–11 arteries ± S.E. (B) Comparison of vasodilatory response to TES (n = 6–11) in the absence or presence of either 80 μM actinomycin D (n = 5), 75 nM finasteride (Fin) (n = 5), or 10 μM flutamide (Flu) (n = 5). Responses to dihydrotestosterone (DHT) (n = 5) are also summarized. Each bar represents the mean response ± S.E. *P < 0.05 compared with TES response.
TES-Induced Vasodilation Involves Peroxynitrite Generation.
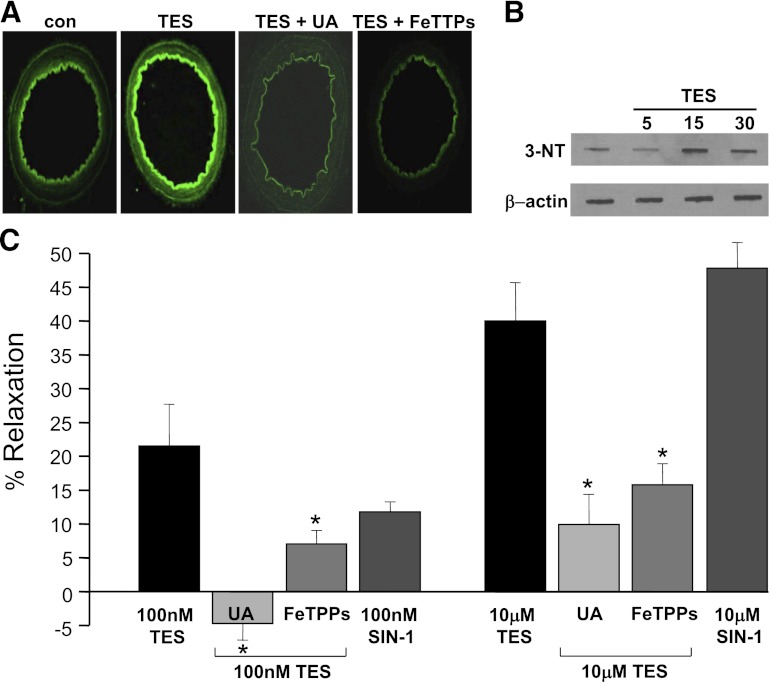
As illustrated in Fig. 2A, 100 nM TES (30 minutes) stimulated DCF fluorescence in resistance arteries. On average, TES increased fluorescence intensity by >50% in microvessels (n = 3; P < 0.05). Interestingly, TES-induced fluorescence was prevented by first treating vessels with either 1 mM UA (peroxynitrite scavenger) or 10 μM 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinato iron III chloride (FeTPPs), a peroxynitrite decomposition catalyst (Fig. 2A). On average, UA or FeTPPs inhibited TES-induced fluorescence by 75 and 95%, respectively (n = 3; P < 0.05). In addition, membrane-permeable superoxide dismutase, PEG-SOD + catalase also abolished the effect of TES on oxidant production (n = 3; unpublished data).
Fig. 2.
TES-induced vasodilation involves peroxynitrite. (A) Representative images of TES (100 nM)-stimulated DCF fluorescence (n = 3) in cross-sections of mesenteric microvessels in the absence or presence of 1 mM UA (n = 3) or 10 μM FeTTPs (n = 3). (B) Representative immunoblots illustrating a rapid, time-dependent increase in 3-nitrotyrosine formation stimulated by 100 nM TES (minutes of treatment indicated above top panel; n = 3). Protein loading controls (β-actin) are illustrated in the lower panel. (C) Summary of TES-induced vasodilation (100 nM or 10 μM) in the absence or presence of 1 mM UA (n = 4–5) or 10 μM FeTTPs (n = 4–5). Average vasodilatory effect of 100 nM or 10 μM SIN-1 is also illustrated (n = 5). Each bar represents the mean response ± S.E. *P < 0.05 compared with TES alone. con, control.
Peroxynitrite can modify proteins by interacting with and nitrating tyrosine residues to form 3-nitrotyrosine (3-NT), and we found that acute (15–30 minutes) treatment with 100 nM TES increased detection of tyrosine-nitrated proteins by nearly 30% (n = 3; Fig. 2B). Further, scavenging of peroxynitrite attenuated TES-induced vasodilation. A summary diagram illustrating the effect of UA or FeTPPs on the response of microvessels to TES is illustrated in Fig. 2C. On average, the maximal relaxant effect of 10 μM TES was only 9.9 ± 3.5% in the presence of 1 mM UA or 15.8 ± 3.1% in the presence of 10 μM FeTPPs (n = 4–5 for each treatment; P < 0.001). Lastly, we found that the effect of TES could be mimicked by 5-amino-3-(4-morpholinyl)-1,2,3-oxadiazolium chloride (SIN-1), a peroxynitrite generator (Kohr et al., 2010) (Fig. 2C). SIN-1 (10 μM)-induced relaxation (47.8 ± 3.3%; n = 5) was similar to that observed with 10 μM TES; however, SIN-1 may not be a “pure” peroxynitrite donor (Paolocci et al., 2000).
TES Stimulates NO Production in Microvessels.
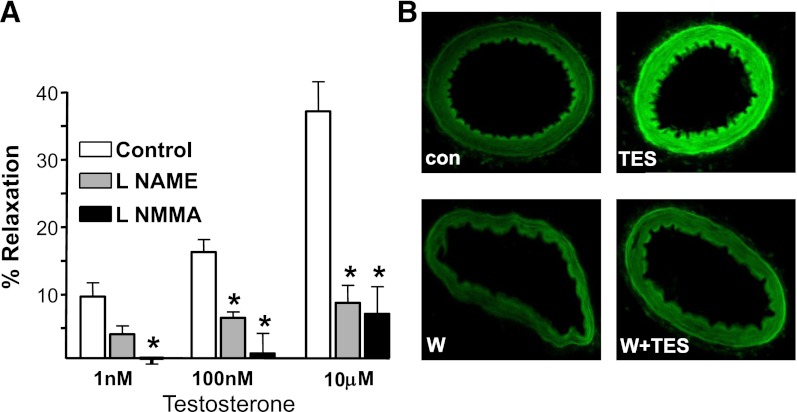
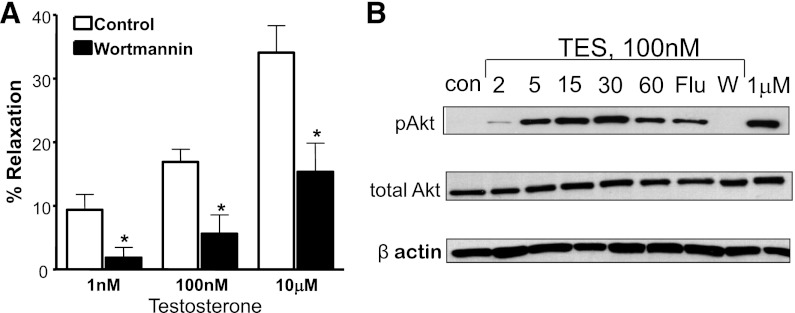
Peroxynitrite results from the rapid combination of NO with superoxide, and we found that inhibiting nitric oxide synthase (NOS) activity attenuated TES-induced relaxation. NG-monomethyl-L-arginine (10 μM) nearly abolished relaxation to maximal levels of 10 μM TES (6.6 ± 4.0%; n = 5; P = 0.002; Fig. 3A). Similarly, pretreating microvessels with 10 μM Nω-nitro-L-arginine methyl ester (L-NAME) also attenuated the relaxation response to 10 μM TES (8.3 ± 2.6%; n = 6; P < 0.001; Fig. 3A). NG-monomethyl-L-arginine and L-NAME are nonselective NOS inhibitors, and we detected expression of both NOS-3 / eNOS and NOS-1 / nNOS in mesenteric microvessels (unpublished data). Subsequent fluorescence studies confirmed TES-stimulated NO production. As illustrated in Fig. 3B (upper panels), 100 nM TES significantly increased DAF-2 fluorescence after a 30-minute exposure (n = 3). On average, TES increased NO-stimulated fluorescence by >60% (P < 0.01; n = 3) in these vessels. Because DAF-2 is not completely specific for NO, we included control experiments using L-NAME. In the presence of L-NAME, TES had no effect on fluorescence (unpublished data). Further, Wortmannin [an inhibitor of the phosphoinositide 3 (PI3) kinase-Akt signaling cascade] also attenuated TES-induced NO fluorescence. In the presence of 50 nM Wortmannin, 100 nM TES had no effect on DAF-2 fluorescence (Fig. 3B, lower panels; n = 4).
Fig. 3.
TES stimulates NO production in microvessels. (A) Comparison of vasodilatory response to TES in the absence (control) or presence of either 100 μM L-NAME (n = 6) or 100 μM NG-monomethyl-L-arginine (L-NMMA) (n = 5). Each bar represents the mean response ± S.E. *P < 0.05 compared with TES response. (B) upper panels: Representative images of DAF-2 fluorescence detected in cross-sections of mesenteric microvessels before (con) and 30 minutes after exposure to 100 nM TES (n = 3). Lower panels: DAF-2 fluorescence in the presence of 50 nM Wortmannin (W) alone or Wortmannin + 100 nM TES (n = 3).
TES Generates Superoxide in Microvessels via Xanthine Oxidase Activity.
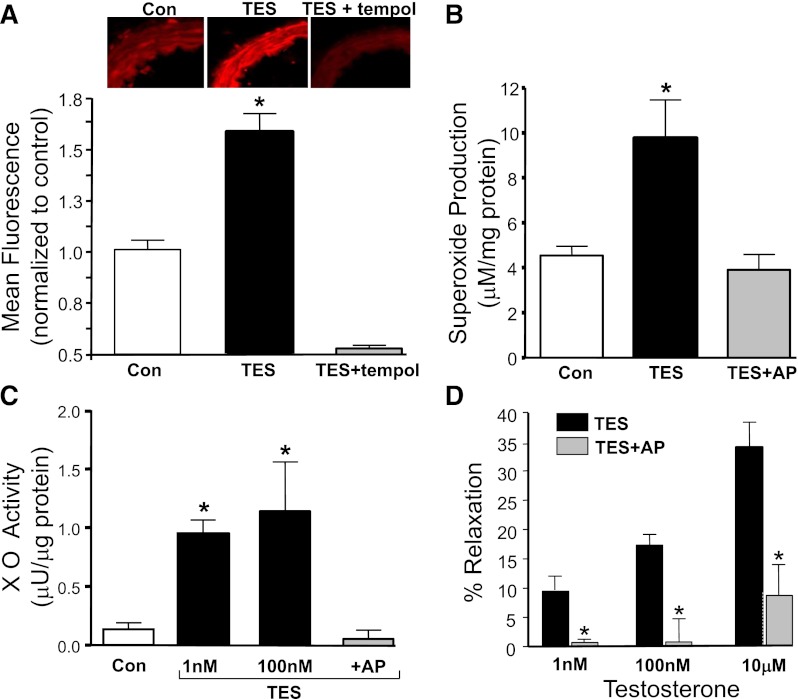
TES-enhanced DHE fluorescence in microvessels (Fig. 4A). On average, 100 nM TES increased DHE fluorescence by nearly 60% (n = 3; P < 0.05) over control levels. This response was abolished by 300 μM tempol (SOD mimetic; n = 4; Fig. 4A) or by 250 U/ml of PEG-SOD (n = 4; unpublished data). We then used EPR to quantify the effect of TES on superoxide production, and these studies revealed that TES (1 μM, 30 minutes) more than doubled superoxide production in mesenteric microvessels (from 4.53 ± 0.41 to 9.75 ± 1.69 µM/µg of protein; Fig. 4B; n = 4; P < 0.01).
Fig. 4.
TES increases superoxide production via XO activity. (A) Representative images of DHE fluorescence of mesenteric microvessels in the absence (con) or presence of 100 nM TES (30 minute; n = 4) or TES + 300 μM tempol (n = 4). Each bar represents the mean fluorescence ± S.E. under each condition. *P < 0.05 compared with control levels. (B) Electron paramagnetic resonance measurement of superoxide levels in the absence (con) or presence of 1 μM TES (30 minutes) or TES + 300 μM allopurinol (AP; n = 4). Each bar represents the mean ± S.E. under each condition. *P < 0.05 compared with control levels. (C) XO activity in the absence (con) or presence of either 1 nM or 100 nM TES (n = 3) or in the presence of 100 nM TES + 300 μM allopurinol (AP; n = 3). Each bar represents the mean ± S.E. under each condition. *P < 0.05 compared with control levels. (D) Comparison of vasodilatory response to TES in the absence or presence of 300 μM allopurinol (AP; n = 6). Each bar represents the mean response ± S.E. *P < 0.05 compared with TES response.
EPR studies further demonstrated that TES-stimulated superoxide production from microvessels was completely abolished by 300 μM allopurinol, an inhibitor with selectivity for xanthine oxidase (XO; 3.9 ± 0.68 µM/µg of protein; n = 4; Fig. 4B). Subsequent biochemical measurements revealed that TES stimulated XO activity in a concentration-dependent fashion (Fig. 4C). On average, XO activity (0.13 ± 0.05 µU/µg of protein) was increased to 0.95 ± 0.11 µU/µg of protein by 1 nM TES and to 0.72 ± 0.1 µU/µg of protein by 100 nM TES (n = 3; P < 0.001). As expected, TES-stimulated XO activity was abolished by 300 μM allopurinol (0.05 ± 0.07 µU/µg of protein). Isometric tension studies confirmed that allopurinol was highly effective at inhibiting TES-induced vasodilation TES (Fig. 4A). On average, 10 μM TES produced only 8.7 ± 5.3% relaxation in the presence of 300 μM allopurinol (n = 6; P < 0.001). In contrast, TES-induced relaxation was unaffected by 300 μM apocyanin (NADPH oxidase inhibitor; unpublished data) or 3 μM antimycin (mitochondrial electron transport inhibitor; unpublished data).
TES Signaling Involves the PI3 Kinase-Akt Cascade.
Experiments using Wortmannin (Fig. 3B) suggested involvement of PI3 kinase activity in the response to TES. Similarly, we found that 50 nM Wortmannin attenuated TES-induced vasodilation. On average, 50 nM Wortmannin attenuated the relaxation response to 10 μM TES by 40% (n = 5; P < 0.001; Fig. 5A). In addition, immunoblot data detected TES-stimulated phosphorylation of Akt. TES (100 nM) produced a time-dependent increase in Akt phosphorylation, which reached a maximal level at 30 minutes (>40-fold increase in band density compared with control levels; P < 0.001; n = 3; Fig. 5B). Interestingly, this increase in Akt phosphorylation was completely abolished by 50 nM Wortmannin (n = 3) and was also attenuated significantly by 10 μM flutamide (P < 0.05; n = 3). In contrast, the amount of total Akt detected in these experiments did not vary.
Fig. 5.
TES activates PI3 kinase-Akt signaling in mesenteric microvessels. (A) TES-induced microvascular relaxation in the absence (control) or presence of 50 nM Wortmannin (n = 5). Each bar represents the mean response ± S.E. *P < 0.001 compared with TES alone. (B) Representative time-course (2–60 minute, as labeled) immunoblot of 100 nM TES-stimulated phosphorylation of Akt (p-Akt; top panel; n = 3). The effect TES treatment was also measured in the presence of 10 μM flutamide (Flu) or 50 nM Wortmannin (W), as was the effect of 1 μM TES alone (right lane). Middle panel reveals total Akt. β-actin was detected to control for protein loading (lower panel).
cGMP Is Elevated by TES in Microvessels.
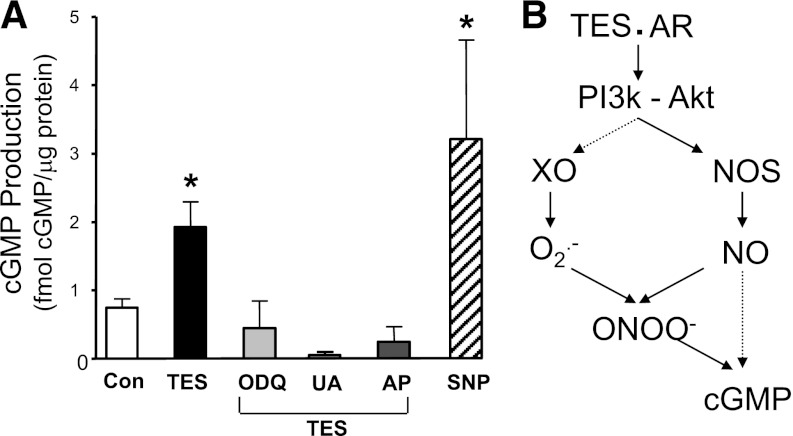
As illustrated in Fig. 6A, 100 nM TES more than doubled cGMP production (from 0.75 ± 0.12 to 1.92 ± 0.37 fmole cGMP per μg of protein; n = 3; P < 0.001) in mesenteric microvessels. This response to TES was attenuated by 10 μM 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, an inhibitor of guanylyl cyclase activity, 1 mM UA, or 300 μM allopurinol (n = 3 determinations for each inhibitor). Sodium nitroprusside (10 μM) was used as a positive control for cGMP production (n = 3; P < 0.05). In a related functional study, 10 μM 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one reduced 10 μM TES-induced relaxation to only 20.1 ± 7.3% (n = 5; P < 0.05; unpublished data).
Fig. 6.
TES stimulates cGMP production in mesenteric microvessels. (A) Summary of cGMP production in the absence (con) or presence of 100 nM TES and in the presence of TES + 10 μM ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one) (n = 3), 1 mM UA (n = 3), or 300 μM allopurinol (AP; n = 3). Sodium nitroprusside (SNP; 10 μM; n = 3) was used as a positive control. Each bar represents the mean response ± S.E. *P < 0.05 compared with control. (B) Proposed model of nongenomic TES signaling in rat mesenteric microvessels.
Discussion
The present findings provide evidence for a novel mechanism of androgen action in the microvasculature (Fig. 6B). We propose that TES activates the androgen receptor (or an AR-like protein) and stimulates activity of the PI3 kinase-Akt signaling cascade. Subsequently, activity of both XO and NOS (i.e., eNOS, nNOS) are enhanced to generate superoxide and NO, respectively. These compounds rapidly combine to form peroxynitrite, which enhances production of cGMP in mesenteric microvessels to promote vasodilation. Our detection of TES-induced production of peroxynitrite, superoxide, and NO in microvessels strongly supports this model. Further, TES-induced vasodilation was nearly abolished by agents that prevent peroxynitrite accumulation or inhibit XO activity. Because reduction of peroxynitrite levels or XO inhibition also attenuated TES-stimulated cGMP production, we propose that TES-stimulated cGMP production involves peroxynitrite generation in these microvessels.
Previous studies report that TES increases global oxidative stress, which might contribute to higher blood pressure in men (Iliescu et al., 2007; Sullivan et al., 2007). In agreement, we found that TES increases oxidant production (i.e., superoxide and peroxynitrite) in isolated microvessels from male animals. Interestingly, we found that TES-stimulated oxidant production induced microvascular dilation, which could lower peripheral vascular resistance. Our findings point to peroxynitrite as a novel mediator of TES action in the microcirculation. Detection of TES-stimulated peroxynitrite precursors and tyrosine-nitrated proteins in microvessels, coupled with the observation that TES-induced vasodilation was attenuated by lowering peroxynitrite, indicates an essential role for peroxynitrite in the acute vasodilatory effect of androgens in microvessels. Although our understanding of how peroxynitrite influences vascular function is rudimentary at best, previous studies have demonstrated peroxynitrite-induced vasodilation. For example, systemic injection of peroxynitrite lowers mesenteric vascular resistance and mean arterial pressure in male rats (Graves et al., 2005). Further, peroxynitrite is a potent pulmonary vasodilator in these animals (Casey et al., 2012). Although these studies support our findings of peroxynitrite-mediated vasodilation, we can find no previous studies linking peroxynitrite with the vasodilatory effects of androgens.
Because peroxynitrite is formed from superoxide and NO, we hypothesized that TES enhanced superoxide production in mesenteric microvessels. Accordingly, we detected TES-stimulated production of superoxide and then quantified this stimulation by using EPR techniques. There are several potential sources of superoxide in blood vessels; however, we found that inhibitors of NADPH oxidase or mitochondrial electron transport did not alter responses to TES. In contrast, TES-stimulated superoxide generation was abolished by allopurinol, a selective inhibitor of XO, which is a source of superoxide in vascular tissues (Berry and Hare, 2004). Moreover, allopurinol attenuated TES-induced vasodilation. These findings point to XO as a novel target of TES action and a source of TES-stimulated oxidant production in the microvasculature. Although circulating XO binds to vascular endothelium, XO mRNA has been detected within large arteries and veins (Szasz et al., 2008). The present study now demonstrates XO activity intrinsic to the microvasculature and, to our knowledge, is the first to provide direct evidence that androgens can stimulate XO activity. Such stimulation is consistent with earlier reports that mature male rats exhibit 59% more hepatic XO activity compared with female rats (Levinson and Chalker, 1980).
As expected, we detected TES-stimulated NO production in microvessels and found that inhibition of NOS activity attenuates TES-induced vasodilation. These findings are consistent with previous studies indicating that NO mediates TES-induced vasodilation of the intact mesenteric bed (Tep-areenan et al., 2002). Similarly, TES-induced relaxation of rat aorta (Costarella et al., 1996), canine coronary arteries (Chou et al., 1996), and airway smooth muscle (Kouloumenta et al., 2006) also involves NO production; however, the mechanisms whereby TES stimulates NOS activity are unknown. Interestingly, we found that inhibition of PI3 kinase activity strongly attenuated both TES-stimulated NO production and TES-induced vasodilation. These pharmacological studies suggested that the effect of TES on microvessels involved activity of the PI3 kinase-Akt signaling cascade. This hypothesis was confirmed more directly by demonstrating TES-stimulated phosphorylation (i.e., activation) of Akt in microvessels. Our findings are consistent with a previous report that TES enhances PI3 kinase-Akt signaling and NO production in human vascular endothelial cells (Yu et al., 2010). Similarly, 17β-estradiol enhances activity of the PI3 kinase-Akt signaling system to stimulate NO production acutely from either eNOS expressed in human endothelial cells (Haynes et al., 2000) or nNOS expressed in porcine or human coronary smooth muscle (Han et al., 2007). Therefore, our findings with TES are consistent with the idea that PI3 kinase-Akt constitutes an important transduction mechanism mediating nongenomic, steroid-induced NO production in the vasculature. In contrast, TES-induced relaxation of rabbit coronary (Yue et al., 1995), rabbit carotid (Marrachelli et al., 2010), or human radial (Seyrek et al., 2007) arteries is largely NO-independent. Thus, there is a significant degree of vascular heterogeneity regarding the reliance of TES signaling on NO production. Nonetheless, our findings that TES stimulates production of both NO and superoxide are entirely consistent with our model of TES-stimulated peroxynitrite production in microvessels.
At present, the mechanism of how peroxynitrite promotes vasodilation is unclear; however, it is known that peroxynitrite can stimulate soluble guanylyl cyclase (sGC) activity in endothelial cells, probably via S-nitrosation, to produce cGMP (Mayer et al., 1995). In the present study, TES more than doubled cGMP levels in microvessels, and either UA (peroxynitrite scavenger) or allopurinol (XO inhibitor) attenuated both TES-stimulated cGMP production and TES-induced microvascular dilation. Taken together, these findings strongly suggest that TES elevates peroxynitrite formation, leading to enhanced sGC activity and cGMP production in microvessels. Support for this conclusion is derived from reports that TES stimulates cGMP production in coronary arteries (Deenadayalu et al., 2001) and that peroxynitrite relaxes aortic rings by generating cGMP (Li et al., 2005). These findings support the concept that the vasodilatory effect of TES is mediated via peroxynitrite-induced cGMP production (at least in some arteries). It is also possible that sGC could be stimulated directly by NO or Akt; however, the fact that inhibiting superoxide or peroxynitrite accumulation attenuated TES-stimulated cGMP production (and TES-induced vasodilation) points to peroxynitrite as playing a significant role in this vasodilatory mechanism. At this time, however, signaling events downstream from peroxynitrite remain to be elucidated. For example, we have reported that TES stimulates the opening of large-conductance, calcium-activated potassium (BKCa) channels in coronary artery smooth muscle cells via cGMP/protein kinase G (Deenadayalu et al., 2001, 2012), and this channel also mediates a substantial portion of TES-induced vasodilation of the intact rat mesenteric arterial bed (Tep-areenan et al., 2002). Thus, it is quite possible that BKCa channels are molecular effectors involved in peroxynitrite-induced relaxation of mesenteric microvessels in our study; however, direct patch-clamp experiments will be required to confirm this possibility. In addition, Katori et al. (2006) demonstrated that infusion of peroxynitrite in vivo leads to arterial dilation mediated by decomposition products (i.e., NO2− and NO3−). Although the present findings have not investigated the potential role of peroxynitrite decomposition products, we previously demonstrated that TES enhances nitrite production in coronary arteries (Deenadayalu et al., 2012). Further studies are required to determine whether similar decomposition products might mediate the vasodilatory effect of peroxynitrite in mesenteric microvessels.
It is generally thought that the acute effects of androgens on vascular function do not involve the classic AR. For example, flutamide did not affect TES-induced relaxation of rabbit coronary arteries (Yue et al., 1995) and TES relaxed aorta from rats without a functional AR (Costarella et al., 1996). Somewhat surprisingly, we found that TES-induced vasodilation and Akt phosphorylation were attenuated by AR antagonists, suggesting that AR activation may be an initial signaling event in TES-induced dilation of these microvessels. This conclusion is supported by previous studies indicating that flutamide inhibits acute TES effects on calcium activity in other cells (Gorczynska and Handelsman, 1995; Lyng et al., 2000; Er et al., 2007). Similarly, inhibiting AR expression (via siRNA techniques) or activation (with flutamide) attenuates acute TES-induced NO production from endothelial cells (Yu et al., 2010). Therefore, the AR (or an AR-like protein) may indeed mediate some rapid responses to TES. Regardless, it is clear that the nongenomic vascular signaling mechanisms of TES are complicated and require further research to elucidate.
In summary, our findings provide evidence for a novel mechanism of TES action in the microvasculature: TES-stimulated oxidant production resulting in vasodilation involving peroxynitrite formation, a mechanism dependent on superoxide generated from XO and NO generated from eNOS or nNOS, culminating in the production of cGMP. TES can acutely stimulate NOS activity via activation of the PI3 kinase-Akt signaling cascade that appears to be initiated by activation of the classic AR or an AR-like protein. There is evidence that exogenous TES can exert a protective effect on exercise-induced myocardial ischemia (Mathur et al., 2009), but mechanisms underlying this protective effect remain unclear. Based on our findings, it could be speculated that TES-induced peroxynitrite formation might contribute to this vasodilatory response; however, further studies are required to substantiate a protective role for TES-induced peroxynitrite production in other vascular beds.
Acknowledgments
The authors gratefully acknowledge the superb technical assistance of Handong Ma.
Abbreviations
- Akt
protein kinase B
- DAF-2 DA
4,5-diaminofluorescein diacetate
- DCF
dichlorofluorescein
- DHE
dihydroethidium
- DHT
dihydrotestosterone
- EPR
electron paramagnetic resonance
- FeTPP
tetrakis(4-sulfonatophenyl)porphyrinato iron III chloride
- L-NAME
Nω-nitro-L-arginine methyl ester
- NO
nitric oxide
- NOS
nitric oxide synthase
- PEG-SOD
polyethylene glycol-superoxide dismutase
- PI3 kinase
phosphoinositide 3-kinase
- sGC
soluble guanylyl cyclase
- SIN-1
5-amino-3-(4-morpholinyl)-1,2,3-oxadiazolium chloride
- TES
testosterone
- UA
uric acid
- XO
xanthine oxidase
Authorship Contributions
Participated in research design: Puttabyatappa, El-Remessy, Ergul, White.
Conducted experiments: Puttabyatappa, Kumar, White.
Contributed new reagents or analytic tools: Black.
Performed data analysis: Puttabyatappa, Kumar, Johnson, White.
Wrote or contributed to the writing of the manuscript: Puttabyatappa, Kumar, El-Remessy, Ergul, Owen, Stallone, White.
Footnotes
This work was supported in part by grants from the National Institutes of Health [Grants HL080402 (to J.N.S. and R.E.W.), NS070239 (to A.E.), EY022408 (to A.B.E.), HL06190, and HL67841 (to S.B.)]; Juvenile Diabetes Research Foundation [JDRF 2-2008-149] (to A.B.E.); Center for Chronic Disorders of Aging, endowed by the Osteopathic Heritage Foundation (to M.P.O.); and an American Heart Association Established Investigator Award (to A.E.).
References
- Berry CE, Hare JM. (2004) Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol 555:589–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S, Buckwalter JG. (2001) Testosterone supplementation in older men: a rational idea whose time has not yet come. J Androl 22:718–731 [PubMed] [Google Scholar]
- Casey DB, Pankey EA, Badejo AM, Bueno FR, Bhartiya M, Murthy SN, Uppu RM, Nossaman BD, Kadowitz PJ. (2012) Peroxynitrite has potent pulmonary vasodilator activity in the rat. Can J Physiol Pharmacol 90:485–500 [DOI] [PubMed] [Google Scholar]
- Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, Collins P, Chatterjee K. (1996) Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation 94:2614–2619 [DOI] [PubMed] [Google Scholar]
- Costarella CE, Stallone JN, Rutecki GW, Whittier FC. (1996) Testosterone causes direct relaxation of rat thoracic aorta. J Pharmacol Exp Ther 277:34–39 [PubMed] [Google Scholar]
- Deenadayalu V, Puttabyatappa Y, Liu AT, Stallone JN, White RE. (2012) Testosterone-induced relaxation of coronary arteries: activation of BKCa channels via the cGMP-dependent protein kinase. Am J Physiol Heart Circ Physiol 302:H115–H123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deenadayalu VP, White RE, Stallone JN, Gao X, Garcia AJ. (2001) Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol Heart Circ Physiol 281:H1720–H1727 [DOI] [PubMed] [Google Scholar]
- Diano S, Horvath TL, Mor G, Register T, Adams M, Harada N, Naftolin F. (1999) Aromatase and estrogen receptor immunoreactivity in the coronary arteries of monkeys and human subjects. Menopause 6:21–28 [PubMed] [Google Scholar]
- Ding AQ, Stallone JN. (2001) Testosterone-induced relaxation of rat aorta is androgen structure specific and involves K+ channel activation. J Appl Physiol 91:2742–2750 [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Abou-Mohamed G, Caldwell RW, Caldwell RB. (2003) High glucose-induced tyrosine nitration in endothelial cells: role of eNOS uncoupling and aldose reductase activation. Invest Ophthalmol Vis Sci 44:3135–3143 [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Al-Shabrawey M, Platt DH, Bartoli M, Behzadian MA, Ghaly N, Tsai N, Motamed K, Caldwell RB. (2007) Peroxynitrite mediates VEGF’s angiogenic signal and function via a nitration-independent mechanism in endothelial cells. FASEB J 21:2528–2539 [DOI] [PubMed] [Google Scholar]
- English KM, Jones RD, Jones TH, Morice AH, Channer KS. (2002) Testosterone acts as a coronary vasodilator by a calcium antagonistic action. J Endocrinol Invest 25:455–458 [DOI] [PubMed] [Google Scholar]
- Er F, Michels G, Brandt MC, Khan I, Haase H, Eicks M, Lindner M, Hoppe UC. (2007) Impact of testosterone on cardiac L-type calcium channels and Ca2+ sparks: acute actions antagonize chronic effects. Cell Calcium 41:467–477 [DOI] [PubMed] [Google Scholar]
- Gorczynska E, Handelsman DJ. (1995) Androgens rapidly increase the cytosolic calcium concentration in Sertoli cells. Endocrinology 136:2052–2059 [DOI] [PubMed] [Google Scholar]
- Graves JE, Lewis SJ, Kooy NW. (2005) Role of ATP-sensitive K+ -channels in hemodynamic effects of peroxynitrite in anesthetized rats. J Cardiovasc Pharmacol 46:653–659 [DOI] [PubMed] [Google Scholar]
- Han G, Ma H, Chintala R, Miyake K, Fulton DJ, Barman SA, White RE. (2007) Nongenomic, endothelium-independent effects of estrogen on human coronary smooth muscle are mediated by type I (neuronal) NOS and PI3-kinase-Akt signaling. Am J Physiol Heart Circ Physiol 293:H314–H321 [DOI] [PubMed] [Google Scholar]
- Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. (2000) Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res 87:677–682 [DOI] [PubMed] [Google Scholar]
- Iliescu R, Cucchiarelli VE, Yanes LL, Iles JW, Reckelhoff JF. (2007) Impact of androgen-induced oxidative stress on hypertension in male SHR. Am J Physiol Regul Integr Comp Physiol 292:R731–R735 [DOI] [PubMed] [Google Scholar]
- Katori T, Donzelli S, Tocchetti CG, Miranda KM, Cormaci G, Thomas DD, Ketner EA, Lee MJ, Mancardi D, Wink DA,, et al. (2006) Peroxynitrite and myocardial contractility: in vivo versus in vitro effects. Free Radic Biol Med 41:1606–1618 [DOI] [PubMed] [Google Scholar]
- Keating NL, O’Malley AJ, Smith MR. (2006) Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 24:4448–4456 [DOI] [PubMed] [Google Scholar]
- Khaw KT, Barrett-Connor E. (1988) Blood pressure and endogenous testosterone in men: an inverse relationship. J Hypertens 6:329–332 [PubMed] [Google Scholar]
- Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Day N. (2007) Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation 116:2694–2701 [DOI] [PubMed] [Google Scholar]
- Kohr MJ, Traynham CJ, Roof SR, Davis JP, Ziolo MT. (2010) cAMP-independent activation of protein kinase A by the peroxynitrite generator SIN-1 elicits positive inotropic effects in cardiomyocytes. J Mol Cell Cardiol 48:645–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouloumenta V, Hatziefthimiou A, Paraskeva E, Gourgoulianis K, Molyvdas PA. (2006) Non-genomic effect of testosterone on airway smooth muscle. Br J Pharmacol 149:1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminrusimha S, Wiseman D, Black SM, Russell JA, Gugino SF, Oishi P, Steinhorn RH, Fineman JR. (2007) The role of nitric oxide synthase-derived reactive oxygen species in the altered relaxation of pulmonary arteries from lambs with increased pulmonary blood flow. Am J Physiol Heart Circ Physiol 293:H1491–H1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DJ, Chalker D. (1980) Rat hepatic xanthine oxidase activity: age and sex specific differences. Arthritis Rheum 23:77–82 [DOI] [PubMed] [Google Scholar]
- Li J, Li W, Altura BT, Altura BM. (2005) Peroxynitrite-induced relaxation in isolated rat aortic rings and mechanisms of action. Toxicol Appl Pharmacol 209:269–276 [DOI] [PubMed] [Google Scholar]
- Lyng FM, Jones GR, Rommerts FF. (2000) Rapid androgen actions on calcium signaling in rat sertoli cells and two human prostatic cell lines: similar biphasic responses between 1 picomolar and 100 nanomolar concentrations. Biol Reprod 63:736–747 [DOI] [PubMed] [Google Scholar]
- Maggio M, Lauretani F, Ceda GP, Bandinelli S, Ling SM, Metter EJ, Artoni A, Carassale L, Cazzato A, Ceresini G,, et al. (2007) Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (InCHIANTI) study. Arch Intern Med 167:2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrachelli VG, Miranda FJ, Centeno JM, Salom JB, Torregrosa G, Jover-Mengual T, Pérez AM, Moro MA, Alborch E. (2010) Role of NO-synthases and cyclooxygenases in the hyperreactivity of male rabbit carotid artery to testosterone under experimental diabetes. Pharmacol Res 61:62–70 [DOI] [PubMed] [Google Scholar]
- Mathur A, Malkin C, Saeed B, Muthusamy R, Jones TH, Channer K. (2009) Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol 161:443–449 [DOI] [PubMed] [Google Scholar]
- Mayer B, Schrammel A, Klatt P, Koesling D, Schmidt K. (1995) Peroxynitrite-induced accumulation of cyclic GMP in endothelial cells and stimulation of purified soluble guanylyl cyclase. Dependence on glutathione and possible role of S-nitrosation. J Biol Chem 270:17355–17360 [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. (1977) Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41:19–26 [DOI] [PubMed] [Google Scholar]
- Paolocci N, Ekelund UE, Isoda T, Ozaki M, Vandegaer K, Georgakopoulos D, Harrison RW, Kass DA, Hare JM. (2000) cGMP-independent inotropic effects of nitric oxide and peroxynitrite donors: potential role for nitrosylation. Am J Physiol Heart Circ Physiol 279:H1982–H1988 [DOI] [PubMed] [Google Scholar]
- Rosano GM, Sheiban I, Massaro R, Pagnotta P, Marazzi G, Vitale C, Mercuro G, Volterrani M, Aversa A, Fini M. (2007) Low testosterone levels are associated with coronary artery disease in male patients with angina. Int J Impot Res 19:176–182 [DOI] [PubMed] [Google Scholar]
- Seyrek M, Yildiz O, Ulusoy HB, Yildirim V. (2007) Testosterone relaxes isolated human radial artery by potassium channel opening action. J Pharmacol Sci 103:309–316 [DOI] [PubMed] [Google Scholar]
- Sullivan JC, Sasser JM, Pollock JS. (2007) Sexual dimorphism in oxidant status in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 292:R764–R768 [DOI] [PubMed] [Google Scholar]
- Sullivan ML, Martinez CM, Gennis P, Gallagher EJ. (1998) The cardiac toxicity of anabolic steroids. Prog Cardiovasc Dis 41:1–15 [DOI] [PubMed] [Google Scholar]
- Szasz T, Thompson JM, Watts SW. (2008) A comparison of reactive oxygen species metabolism in the rat aorta and vena cava: focus on xanthine oxidase. Am J Physiol Heart Circ Physiol 295:H1341–H1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tep-areenan P, Kendall DA, Randall MD. (2002) Testosterone-induced vasorelaxation in the rat mesenteric arterial bed is mediated predominantly via potassium channels. Br J Pharmacol 135:735–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RE, Han G, Dimitropoulou C, Zhu S, Miyake K, Fulton D, Dave S, Barman SA. (2005) Estrogen-induced contraction of coronary arteries is mediated by superoxide generated in vascular smooth muscle. Am J Physiol Heart Circ Physiol 289:H1468–H1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RE, Han G, Maunz M, Dimitropoulou C, El-Mowafy AM, Barlow RS, Catravas JD, Snead C, Carrier GO, Zhu S, et al. (2002) Endothelium-independent effect of estrogen on Ca(2+)-activated K(+) channels in human coronary artery smooth muscle cells. Cardiovasc Res 53:650–661 [DOI] [PubMed] [Google Scholar]
- White RE, Kryman JP, El-Mowafy AM, Han G, Carrier GO. (2000) cAMP-dependent vasodilators cross-activate the cGMP-dependent protein kinase to stimulate BK(Ca) channel activity in coronary artery smooth muscle cells. Circ Res 86:897–905 [DOI] [PubMed] [Google Scholar]
- Yu J, Akishita M, Eto M, Ogawa S, Son BK, Kato S, Ouchi Y, Okabe T. (2010) Androgen receptor-dependent activation of endothelial nitric oxide synthase in vascular endothelial cells: role of phosphatidylinositol 3-kinase/akt pathway. Endocrinology 151:1822–1828 [DOI] [PubMed] [Google Scholar]
- Yue P, Chatterjee K, Beale C, Poole-Wilson PA, Collins P. (1995) Testosterone relaxes rabbit coronary arteries and aorta. Circulation 91:1154–1160 [DOI] [PubMed] [Google Scholar]