Abstract
Increased sympathetic nervous system activity contributes to deoxycorticosterone acetate (DOCA)-salt hypertension in rats. ATP and norepinephrine (NE) are coreleased from perivascular sympathetic nerves. NE acts at prejunctional α2-adrenergic receptors (α2ARs) to inhibit NE release, and α2AR function is impaired in DOCA-salt rats. Adenosine, an enzymatic ATP degradation product, acts at prejunctional A1 adenosine receptors (A1Rs) to inhibit NE release. We tested the hypothesis that prejunctional A1R function is impaired in sympathetic nerves supplying mesenteric arteries (MAs) and veins (MVs) of DOCA-salt rats. Electrically evoked NE release and constrictions of blood vessels were studied in vitro with use of amperometry to measure NE oxidation currents and video microscopy, respectively. Immunohistochemical methods were used to localize tyrosine hydroxylase (TH) and A1Rs in perivascular sympathetic nerves. TH and A1Rs colocalized to perivascular sympathetic nerves. Adenosine and N6-cyclopentyl-adenosine (CPA, A1R agonist) constricted MVs but not MAs. Adenosine and CPA (0.001–10 µM) inhibited neurogenic constrictions and NE release in MAs and MVs. DOCA-salt arteries were resistant to adenosine and CPA-mediated inhibition of NE release and constriction. The A2A adenosine receptor agonist CGS21680 (C23H29N7O6.HCl.xH2O) (0.001–0.1 μM) did not alter NE oxidation currents. We conclude that there are prejunctional A1Rs in arteries and both pre- and postjunctional A1Rs in veins; thus, adenosine selectively constricts the veins. Prejunctional A1R function is impaired in arteries, but not veins, from DOCA-salt rats. Sympathetic autoreceptor dysfunction is not specific to α2ARs, but there is a more general disruption of prejunctional mechanisms controlling sympathetic neurotransmitter release in DOCA-salt hypertension.
Introduction
Adenosine is an ATP precursor and metabolite and an intercellular signaling molecule (Olah and Stiles, 2000; Tabrizchi and Bedi, 2001). In the cardiovascular system, adenosine plays an important role in controlling cardiac output and vascular function (Shryock and Belardinelli, 1997; Tabrizchi and Bedi, 2001). Considerable progress has been made in understanding adenosine receptor subtypes and their signaling mechanisms in the cardiovascular system. However, because of the many effects of adenosine, our understanding of adenosine receptor function in the sympathetic nerves supplying arteries and veins is less well developed, particularly in hypertension. This issue is important in mesenteric arteries (MAs) and veins (MVs), because they are densely innervated by sympathetic nerves (King et al., 2007). Sympathetic nerves supply blood vessels at the adventitial-medial border in arteries, and they distribute more deeply into the media for veins (Birch et al., 2008). This differential anatomic arrangement suggests that prejunctional regulation of transmitter release may also differ in arteries and veins. Differential regulation of arterial versus venous neuroeffector transmission may also be important, because sympathetic nervous system control of the mesenteric circulation contributes significantly to blood pressure regulation by two mechanisms. First, sympathetic nerves regulate resistance in small MAs (Rothe, 1983). Second, sympathetic nerves regulate capacitance of MVs, which hold a large fraction of total blood volume and provide 60–70% of venous return to the heart (Martin et al., 1998). Venous constriction shifts blood volume from the high compliance capacitance veins to the low compliance resistance arteries, causing an increase in arterial pressure (Fink, 2009).
Adenosine receptors are G-protein–coupled receptors (Olah and Stiles, 2000). There are four types of adenosine receptors (A1, A2A, A2B, and A3) (Tabrizchi and Bedi, 2001) that are distinguished on the basis of their ability to inhibit or stimulate adenylyl cyclase and by different agonist and antagonist binding profiles (Olah and Stiles, 2000). We focused on A1 adenosine receptors (A1Rs), which couple to the inhibitory G protein, Gi, leading to inhibition of adenylyl cyclase (Ralevic and Burnstock, 1998; Tabrizchi and Bedi, 2001). Activation of A1Rs in the nervous system inhibits Ca2+ currents, resulting in suppression of neurotransmitter release (Ralevic and Burnstock, 1998). Sympathetic nerves supplying MAs release norepinephrine (NE), ATP, and/or β-NAD (Smyth et al., 2004). NE, ATP, and β-NAD can act on postjunctional α1-adrenergic receptors and P2X1 purinoceptors, respectively, to cause arterial smooth muscle contraction (Smyth et al., 2004; Demel and Galligan, 2008). NE and ATP/β-NAD release is regulated by prejunctional α2-adrenergic receptors (α2ARs) and A1Rs (Illes et al., 1988; Rongen et al., 1996; Ralevic, 2000; Demel and Galligan, 2008). The action of ATP is quickly terminated by enzymatic degradation to ADP, AMP, and adenosine as a final product (Todorov et al., 1997; Westfall et al., 2002). Adenosine binds to prejunctional A1Rs to inhibit NE and ATP/β-NAD release. Long-term treatment of rats with a nonselective adenosine receptor antagonist, 1,3-dipropyl-8-sulphophenylxanthine (DPSPX), increased blood pressure and purinergic and adrenergic neurotransmission (Guimaraes et al., 2003). This result suggests that adenosine could regulate blood pressure in part by acting at prejunctional adenosine receptors. Data from animal models and from human studies showed that increased sympathetic activation contributes to hypertension (Anderson et al., 1989; Schlaich et al., 2004).
The deoxycorticosterone acetate (DOCA)–salt rat model of hypertension mimics excessive aldosterone-induced hypertension. This model is salt-sensitive, with low renin, and is driven by increased sympathetic nerve activity (Schenk and McNeill, 1992). Previous work has shown that augmented sympathetic activity in DOCA-salt hypertensive rats is attributable in part to disruption of prejunctional α2AR function (deChamplain et al., 1987; Luo et al., 2004). However, it is not known whether impairment of A1R function also occurs in hypertension or whether A1Rs regulate NE release from perivenous sympathetic nerves. Thus, the purpose of this study was to determine whether the disruption of prejunctional autoreceptors is specific to α2ARs or whether there is a more general disruption of prejunctional autoreceptor function in hypertension. Furthermore, we tested the hypothesis that dysfunction of prejunctional A1Rs contributes to increased NE release from sympathetic nerves in DOCA-salt hypertensive rats. Finally, there have been a number of studies of adenosine receptor function on periarterial sympathetic nerves, but interactions of adenosine with sympathetic nerves supplying MVs in normotensive or hypertensive animals have not been studied.
Materials and Methods
DOCA-Salt Hypertensive Rats.
Animals use protocols were approved by the Institutional Animal Care and Use Committee at Michigan State University. Adult male Sprague-Dawley rats (250–275 g) were obtained from Charles River Laboratories (Portage, MI), and they were acclimated 2–3 days before entry into experimental protocols. Rat chow (Harlan/Teklad 8640 Rodent Diet; Harlan Laboratories, Indianapolis, IN) and tap water were provided ad libitum. Rats were housed in temperature- and humidity-controlled room with 12:12-hour dark-light cycle.
The surgical procedures and drug treatment protocols for producing sham control and DOCA-salt hypertensive rats have been described in detail previously (Luo et al., 2004). In brief, DOCA-salt rats underwent uninephrectomy and DOCA implantation, and sham rats were only uninephrectomized. Rats were anesthetized using isoflurane inhalation (4% with O2). After recovery from the surgical procedures, rats were housed under standard conditions for 4 weeks. DOCA-implanted rats received standard pelleted rat chow and drinking solution containing 1% NaCl + 0.2% KCl in distilled water, and sham rats received standard pelleted rat chow and distilled water. Blood pressure was measured using tail-cuff plesmythography 3–5 days before experimentation. Rats with mean arterial pressure ≥150 mmHg were considered to be hypertensive.
Tissue Preparation for In Vitro Studies.
Four weeks after DOCA-salt or sham surgery, rats were euthanized with a lethal injection of pentobarbital (100 mg/kg i.p.). The mesentery was removed and transferred to a silicone elastomer-lined Petri dish with Krebs solution: 117 mol/l NaCl, 4.7 mol/l KCl, 2.5 mol/l CaCl2, 1.2 mol/l MgCl2, 25 mol/l NaHCO3, 1.2 mol/l NaHPO4, and 11 mol/l glucose. Sections of tertiary MAs (145–250 µm outside diameter) and MVs (190–390 µm outside diameter) were dissected and transferred to a recording chamber. The tissue was pinned flat with 50-µm diameter stainless steel pins. MAs and MVs were cleaned of adipose and connective tissue under a dissecting microscope. The chamber was mounted on the stage of an inverted microscope, and tissues were superfused continuously at flow rate of 3 ml/min with warmed (36°C), oxygenated (95% O2, 5% CO2) Krebs solution. Tissues were allowed to equilibrate for 30 minutes before beginning experiments. Video images were obtained using a black and white video camera (Hitachi model KP-111; Hitachi, Yokohama, Japan) connected to the microscope and fed to a Picolo frame grabber board (Euresys Inc., Itasca, IL) mounted in a personal computer. Video images were analyzed using Diamtrak edge tracking software (Adelaide, SA, Australia). Diameter changes of 1 μm can be resolved.
Drug-Induced Constrictions.
Drugs were applied using a 3-way stopcock system so that the superfusing Krebs solution could be changed to one containing a known drug concentration. Blood vessels were initially constricted with three consecutive applications of NE (10 µM for arteries and 1 µM for veins) at 15-minute intervals to verify viability and response stability. Responses greater than 20% of initial diameter in arteries and 30% in veins were considered to be acceptable, and only those blood vessels were used for further experiments. Adenosine (0.001–100 µM) and N6-cyclopentyl-adenosine (CPA; 0.00001–10 µM) were added noncumulatively. Successive concentrations were applied at 45-minute intervals. Antagonists were applied for 30 minutes before testing the effect of agonists. Increasing concentrations of the A1R antagonist, 1,3-dipropl-8-cyplopentylxanthine (DPCPX), were applied cumulatively with a 15-minute incubation period for each concentration.
Transmural Stimulation of Perivascular Nerves.
Perivascular sympathetic nerves were stimulated using Ag/AgCl wire electrodes placed parallel to the length of the blood vessel. Parameters for nerve stimulation were 30 stimuli (0.5 millisecond duration) at 10 Hz, 60–80 V. Constrictions caused by electrical stimulation were blocked by the Na+ channel blocker, tetrodotoxin (0.3 μM).
Amperometric Detection of Norepinephrine.
Construction of carbon fiber microelectrodes was described in detail previously (Park et al., 2007). The microelectrode was positioned parallel to the blood vessel so that NE flux from nearby release site could be sensed by the electrode surface. The microelectrode was pressed gently against the vessel to enable the microelectrode to maintain contact with the vessel during stimulation-evoked constrictions. A platinum wire counter electrode and a commercial “no leak” Ag-AgCl (3 M KCl, model EE009; Cypress System Inc., Lawrence, KS) reference electrode also were mounted in the chamber to complete the electrochemical cell. Continuous amperometric measurements were made using an Omni 90 analog potentiostat (Cypress Systems Inc.), a Minidigi analog-to-digital converter, and a computer running Axoscope 9.0 (Molecular Devices, Sunnyvale, CA). Data were obtained at a 100-Hz sampling rate. An applied potential of 600 mV was used to detect NE currents, because this is the oxidation potential for NE at a mass-transfer limited rate (Park et al., 2007). Currents were low pass filtered with at a time constant of 200 milliseconds. Data were stored on the computer hard drive for further analysis. A bipolar focal stimulation electrode positioned along the surface of the vessel was used to excite perivascular nerves. The electrode was placed at a distance of 200 µm from the carbon fiber microelectrode to minimize the stimulus artifact in the current recording.
Immunohistochemistry.
Fixation and staining techniques have been described in detail previously (Demel and Galligan, 2008), and only modifications are described here. Arteries and veins were incubated in Zamboni fixative for 2 hours at 4°C and washed 3 times at 10-minute intervals with 0.1 M phosphate-buffered saline. Permeation and nonspecific binding blockade: tissues were incubated with a mixture of 4% goat-sheep serum in phosphate-buffered saline with 0.1%Triton-X100 (Sigma-Aldrich, St. Louis, MO) for 1 hour at room temperature. Three different primary rabbit polyclonal antibodies (1:200 dilution) against A1Rs from three commercial suppliers were tested (rabbit polyclonal, 1:200; Santa Cruz Biotechnology, Santa Cruz, CA; Sigma-Aldrich; and EMD Millipore Chemicals, Billerica, MA) and anti-tyrosine hydroxylase (TH) (mouse monoclonal, 1:100; EMD Millipore). To control for nonspecific binding, control vessels were incubated without primary antibodies. Omission of primary antibodies from the protocol prevented all tissue labeling. Fluorescein isothiocyanate–conjugated goat anti-mouse IgG (1:50) and Cy3-conjugated sheep anti-rabbit IgG (1:400; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were used to visualize TH and A1R staining, respectively. Specimens were viewed under a confocal microscope (Leica model DMLFSA; Leica Microinstrument Inc., Buffalo Grove, IL).
Drugs.
All drugs were obtained from Sigma-Aldrich. All drugs, except DPCPX, were dissolved in de-ionized water as a concentrated stock solution kept at 0°C. DPCPX was made as a stock solution in ethanol. Control experiments with the highest concentration of ethanol (0.01% vol/vol) did not affect neurogenic or agonist-induced blood vessel contraction (unpublished data).
Statistics.
Data are reported as mean ± S.E.M., and n values are the number of animals. Differences between groups were assessed using Student’s t test. Differences in agonist concentration-response curves were assessed first using two-way analysis of variance and Bonferonni’s test for multiple comparisons (GraphPad Prism 5.0; GraphPad Software, La Jolla, CA). Adenosine and CPA IC50 and maximum effect values (Emax) were determined from individual concentration-response curves with use of a nonlinear fitting routine with a logistic equation (Origin 8.0; OriginLab, Northampton, MA). Mean IC50 and Emax values were compared using Student’s t test. IC50 values were expressed as the negative log of the drug concentration that causes 50% of the maximum inhibitory response (pIC50). P < 0.05 was considered to be statistically significant.
Results
Adenosine and CPA Constricted Mesenteric Veins but Not Arteries.
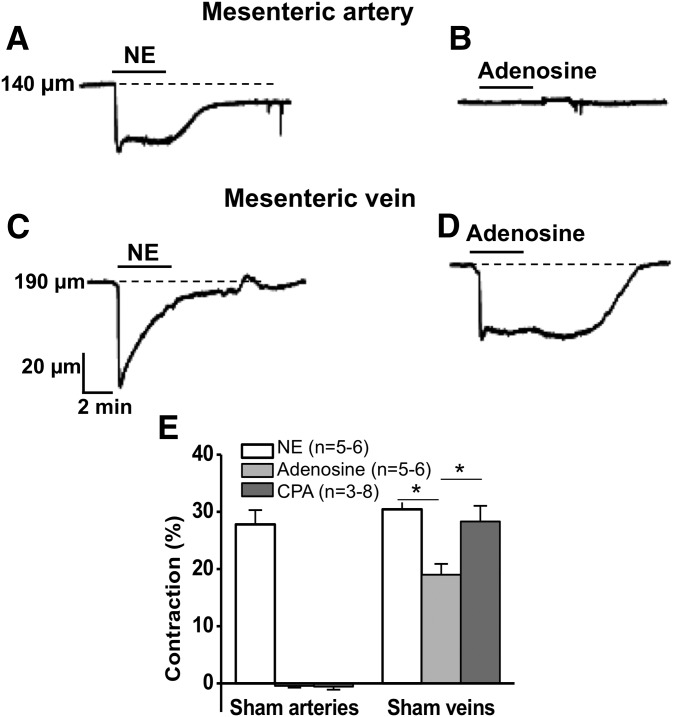
Adenosine and the selective A1R agonist, CPA, were tested for their constrictor effects in MAs and MVs. NE produced a sustained constriction of arteries, and neither adenosine nor CPA constricted arteries (Fig. 1, A and B). In sham arteries, adenosine (10 μM) and CPA (1 μM) caused changes in diameter of only 0.4% ± 0.3% (n = 6) and 0.8% ± 0.5% (n = 8), respectively (Fig. 1E). Adenosine and CPA caused sustained constrictions of veins, and NE produced a transient constriction of veins (Fig. 1, C and D). In sham veins, adenosine and CPA caused decreases in diameter of 19% ± 2% (n = 5) and 28% ± 3% (n = 3) (Fig. 1E), respectively. Similar data were obtained in DOCA-salt MAs and MVs.
Fig. 1.
NE, adenosine, and CPA induced constrictions of sham MAs and MVs. (A–D) Representative traces of NE and adenosine-induced contractions. NE contracted MA (A) and adenosine had no effect (B). Arterial constriction was sustained throughout the period of NE application (A) and venous constriction showed rapid desensitization to NE (C). (D) Adenosine produced a sustained constriction in MV. (E) Different contractile responses in MA and MV caused by NE (10 μM arteries, 1 μM veins), adenosine (10 μM), and CPA (1 μM). Data are mean ± S.E.M. Data were analyzed using one-way analysis of variance (ANOVA) and Dunnett’s post-hoc test. *Significantly different between groups (P < 0.05).
A1Rs Were Localized to Periarterial and Perivenous Sympathetic Nerves.
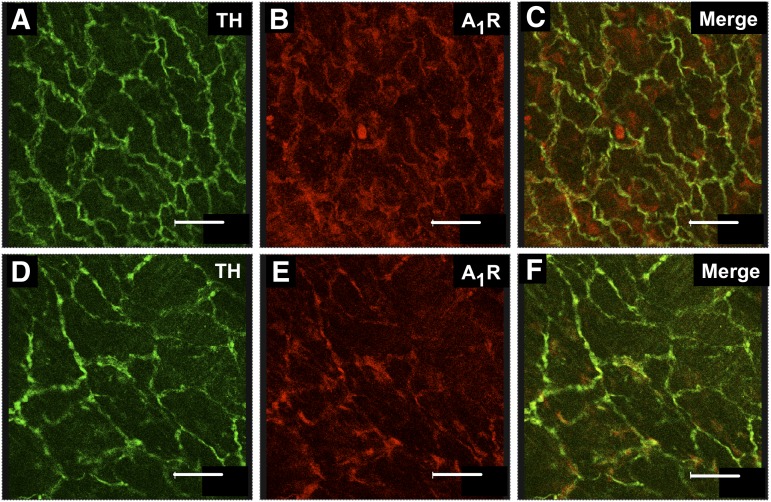
Antibodies raised against A1Rs and TH, a marker for sympathetic nerves, were used to show that immunoreactivity for A1Rs, and TH is coexpressed in nerve fibers supplying MAs and MVs (Fig. 2, A–F). Labeling of A1Rs was much less intense, compared with that for TH. This observation was consistent for all A1R antibodies tested. It is possible that A1Rs are expressed at low levels relative to TH, and this accounts for the low intensity labeling. It is also clear that immunoreactivity for A1Rs is present in non-TH–containing structures, which could be primary afferent nerve fibers, smooth muscle cells, or fibroblasts present in the adventitial layer.
Fig. 2.
A1Rs are localized to perivascular sympathetic nerves. Periarterial sympathetic nerves were labeled with an anti-TH antibody in arteries from sham (A) and DOCA-salt (D) rats. The same blood vessels were labeled using an anti-A1R antibody (EMD Millipore) (B and E), and the merged images are shown in (C and F). The merged images show colocalization of TH and A1Rs (yellow labeling). Scale bar = 35 μm.
Adenosine and CPA Inhibited Neurogenic Constriction of Mesenteric Arteries.
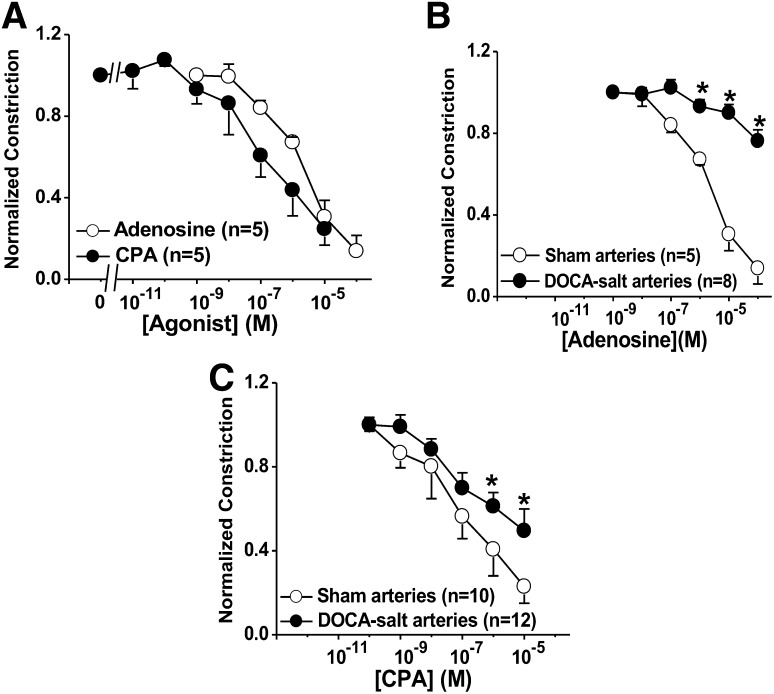
The actions of adenosine and CPA on neurogenic constrictions in MVs were not studied, because these agonists constricted veins directly (Fig. 1E). However, both adenosine and CPA caused concentration-dependent inhibition of neurogenic constriction in sham arteries (Fig. 3A). The concentration-response curve for CPA was left-shift, compared with that for adenosine. The pIC50 values for CPA and adenosine were 7.0 ± 0.4 and 5.6 ± 0.2, respectively (n = 5 for each, P < 0.05). We next compared adenosine and CPA concentration-response curves for inhibition of neurogenic constrictions in arteries from sham and DOCA-salt hypertensive rats. Adenosine and CPA concentration response curves were both right shifted in DOCA-salt arteries, compared with those obtained in sham arteries (Fig. 3, B and C). The rightward shift was greater for adenosine, compared with that for CPA.
Fig. 3.
Adenosine and CPA concentration-response curves (CRCs) for inhibition of neurogenic constrictions of sham and DOCA-salt MAs. (A) Adenosine and CPA inhibited neurogenic constriction in sham arteries. The CPA curve was significantly left-shifted, compared with the adenosine CRC (P < 0.05). (B and C) Adenosine- and CPA-induced inhibition of neurogenic constriction were reduced in DOCA-salt arteries, compared with sham arteries. Data are mean ± S.E.M. Data were analyzed using two-way analysis of variance (ANOVA) and Bonferoni’s post-hoc test. *Significantly different from sham arteries (P < 0.05).
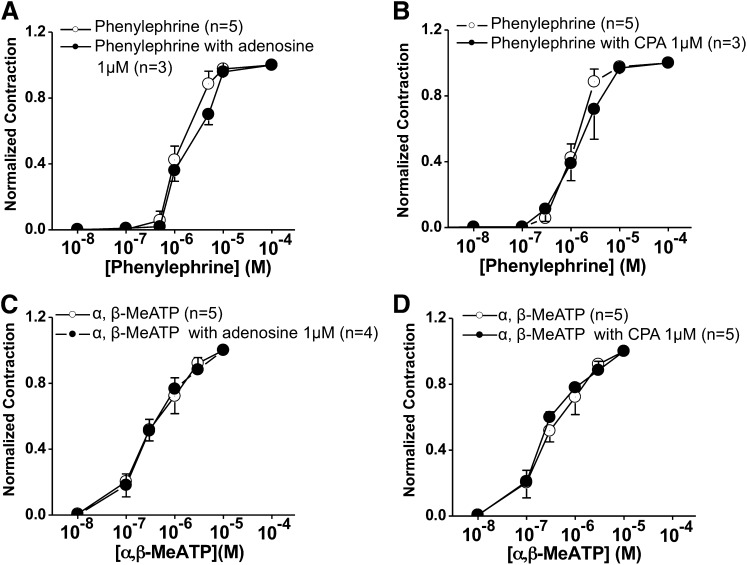
Control experiments were performed to determine whether inhibition of neurogenic constrictions by adenosine or CPA was attributable to pre- or postjunctional effects. The selective α1-adrenergic receptor agonist, phenylephrine, and the P2X receptor agonist, α,β-methylene ATP caused concentration-dependent constrictions of MAs. Neither adenosine (1 μM) nor CPA (1 μM) altered phenylephrine or α,β-methylene ATP-induced constrictions (Fig. 4, A–D). This indicates that inhibition of the neurogenic constriction caused by adenosine, and CPA was mediated prejunctionally.
Fig. 4.
CPA and adenosine do not inhibit constrictions caused by phenylephrine (α1-adrenergic receptor agonist) and α,β-MeATP (P2X receptor agonist). Phenylephrine concentration response curves (CRCs) were not altered by adenosine (A) or CPA (B) in sham MA. α,β-MeATP CRCs were not altered by adenosine (C) or by CPA (D) in sham MA. Data are mean ± S.E.M.; n values represent the number of animals for each experiment. Data were analyzed using two-way analysis of variance (ANOVA). There were no differences detected.
A1Rs but Not A2ARs Mediate Inhibition of NE Release.
Neurogenic constriction was used as an indirect measure of NE release, which could be performed only in arteries, because both adenosine and CPA constricted veins directly. In the next experiments, we made more direct measures of NE release with use of continuous amperometry with carbon-fiber microelectrodes. This allowed measurement of NE release in real time near the surface of arteries and veins. CPA was used in these studies, because it is an A1R-selective agonist and actions at other adenosine receptors would not complicate our data interpretation. In addition, adenosine could not be used in these studies, because it interacted with the carbon fiber electrode, making the electrode baseline current unstable.
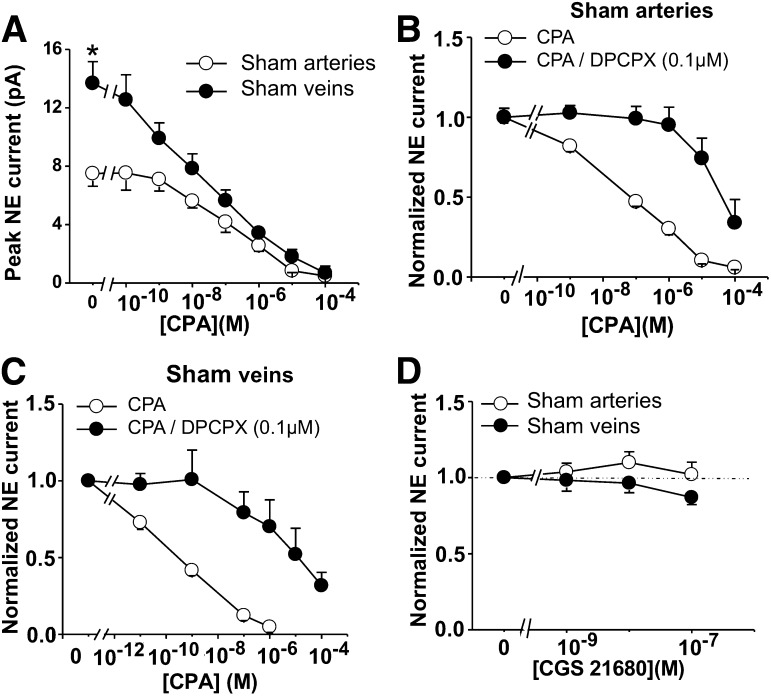
Short trains of stimulation evoked an oxidation current for which the time course of the neurogenic constriction in MAs was tracked (Fig. 5, A and B). CPA produced a concentration-dependent inhibition of the oxidation current and the neurogenic constriction (Fig. 5, A–D). Similar data were obtained in MVs (where only the oxidation current was monitored as a result of the direct constrictor effect of CPA), but peak NE currents in veins were substantially larger, compared with arteries (13.7 ± 1.5 versus 7.5 ± 0.9 pA; P < 0.05) (Fig. 6A). Furthermore, DPCPX (0.1 μM), a selective A1R antagonist, caused a rightward shift in the CPA concentration-response curve in sham arteries and veins (Fig. 6, B and C). The pIC50 values for CPA in sham arteries in the absence and presence of DPCPX were 6.6 ± 0.2 and 4.7 ± 0.2, respectively (P < 0.05). The pIC50 values for CPA in sham veins in the absence and presence of DPCPX were 9.6 ± 0.2 and 5.2 ± 0.4, respectively (P < 0.05).
Fig. 5.
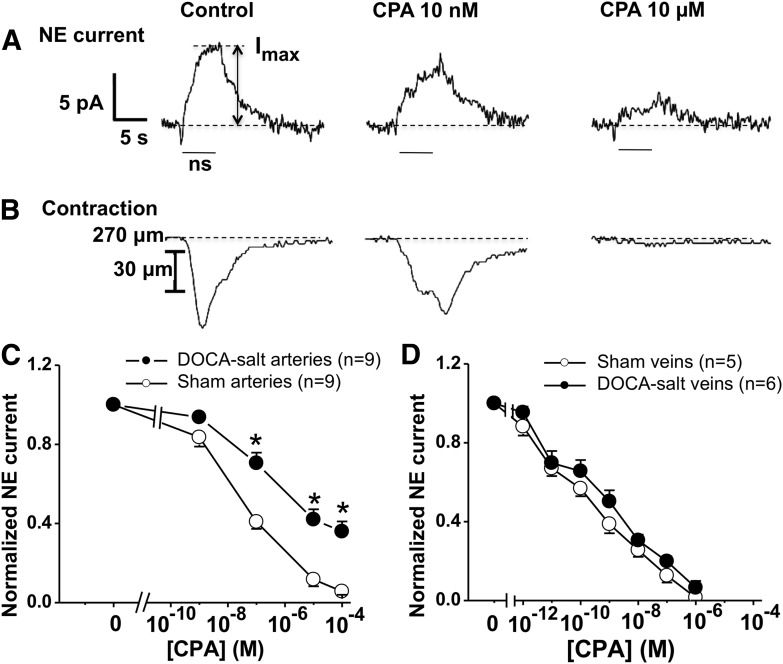
CPA-mediated inhibition of NE oxidation currents and constrictions in sham, but not DOCA-salt MAs. Electrically evoked NE release (60 stimuli, 10 Hz frequency, 0.5 millisecond pulse duration, 60–80 V intensity) was detected by amperometry with a carbon fiber microelectrode. CPA was then added in increasing concentrations. Periarterial sympathetic nerves were stimulated and oxidation currents (A), and constrictions (B) were inhibited by CPA. Duration of nerve stimulation (ns) is indicated by the bars. Imax indicates amplitude of NE oxidation current. (C) The CPA CRC was right shifted in DOCA-salt arteries, compared with sham arteries. (D) There were no differences in CPA CRCs between sham and DOCA-salt veins. Data are mean ± S.E.M. *Significantly different from sham arteries (P < 0.05). Data were analyzed using two-way analysis of variance (ANOVA) and Bonferoni’s post hoc test.
Fig. 6.
CPA, but not CGS21680, inhibits norepinephrine (NE) oxidation currents in sham MAs and MVs. (A) Nerve stimulation was used to evoke NE oxidation currents, which were inhibited by CPA in sham arteries and veins. *Baseline oxidation currents were significantly larger in veins, compared with arteries. (B and C) CPA concentration-response curves for inhibition of NE oxidation currents in arteries were right-shifted by the A1R antagonist DPCPX in arteries (B) and veins (C). (D) Increasing concentrations of A2A adenosine receptor agonist CGS 21680 did not significantly change amplitude of NE oxidation currents in sham MAs or MVs. Data were analyzed using one-way analysis of variance (ANOVA) and Dunnett’s post hoc test. Data are mean ± S.E.M.
Amperometric measurement of NE release after brief electrical stimulation (60 stimuli at 10 Hz) showed that the A2AR agonist CGS21680 (C23H29N7O6.HCl.xH2O) in a range of concentrations selective for the A2AR (1–100 nM) did not alter significantly NE oxidation currents (Fig. 6D).
Impaired Function of Prejunctional A1Rs in DOCA-Salt Mesenteric Arteries but Not Veins.
The data presented above indicate that adenosine and CPA act prejunctionally to inhibit NE oxidation currents and neurogenic constrictions. The next study determined whether this mechanism was impaired in DOCA-salt hypertension. CPA produced a concentration-dependent inhibition of NE oxidation currents in arteries and veins, but DOCA-salt arteries were less sensitive to the inhibitory effects of CPA, compared with sham arteries (Fig. 5C; P < 0.05). In DOCA-salt arteries, the maximum inhibition of NE release produced by CPA (100 µM) was reduced, and the pIC50 value was decreased, compared with sham artery values (Table 1). The effects of CPA on NE oxidation currents were not different in sham and DOCA-salt veins (Fig. 5D; Table 1).
TABLE 1.
Analysis of concentration-response curves for the effects of the A1R agonist, CPA, on norepinephrine oxidation currents recorded from the surface of mesenteric arteries (MAs) and veins (MVs) from sham and DOCA-salt rats
pIC50 values and maximum inhibition were determined from nonlinear curve fits of concentration-response curves from individual preparations. Mean values were then compared using Student’s t test.
| Mesenteric arteries | N | pIC50 | % Max Inhibition |
|---|---|---|---|
| Sham | 9 | 7.6 ± 0.2 | 94 ± 2.0 |
| DOCA-salt | 9 | 6.4 ± 0.3* | 64 ± 4.2* |
| Sham | 5 | 8.5 ± 0.5 | 95 ± 3.6 |
| DOCA-salt | 6 | 8.6 ± 0.2 | 99 ± 1.4 |
A1R, A1 adenosine receptors; CPA, N6 cyclopentyl-adenosine; DOCA, deoxycorticosterone acetate; pIC50, negative log of the half maximal inhibitory drug concentration.
Significantly different from sham values (P < 0.05).
Role of Endogenous Adenosine in Modulation of Adrenergic Transmission.
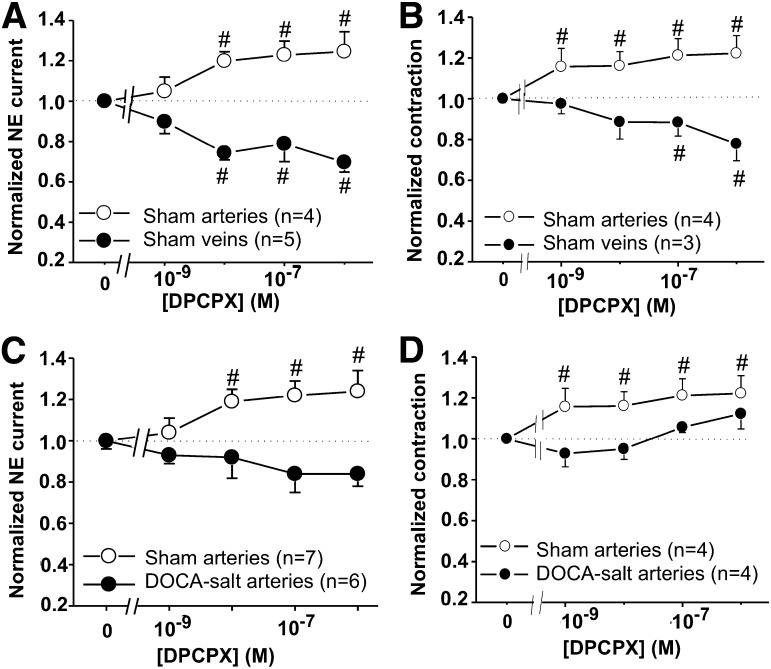
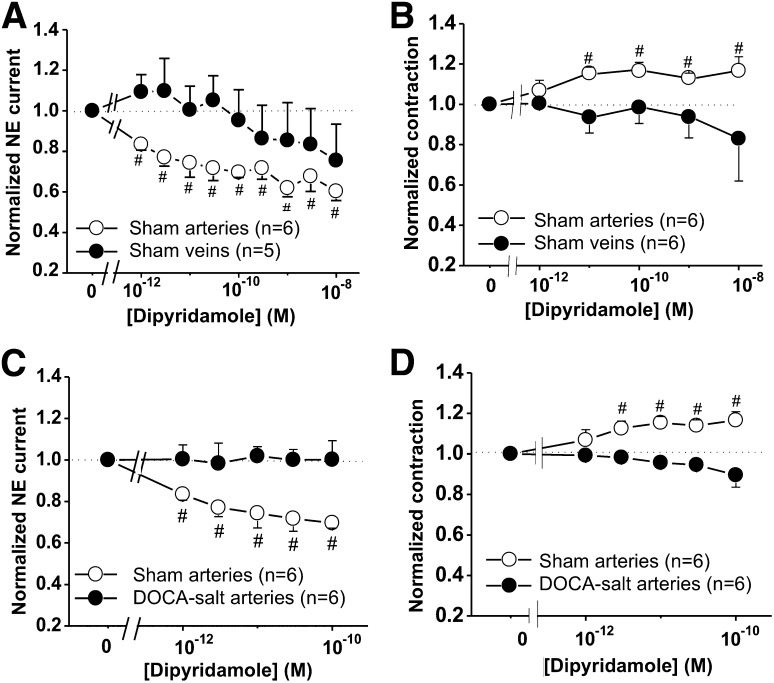
We next determined the role of endogenous adenosine as a neuromodulator of sympathetic neurotransmission in MAs and veins. DPCPX, a selective A1R antagonist, was used to block A1R autoinhibition. DPCPX caused an increase in NE current and neurogenic contraction in concentration-dependent manner in arteries (Fig. 7, A and B). Conversely, DPCPX caused a reduction of NE current and neurogenic contraction in concentration-dependent manner in veins (Fig. 7B). DPCPX did not change NE oxidation currents and neurogenic contractile response in DOCA-salt arteries (Fig. 7, C and D). Furthermore, dipyridamole, an adenosine transporter blocker, was used to produce an accumulation of endogenous adenosine, providing a greater A1R activation. Dipyridamole produced a decrease in the NE current in a concentration-dependent manner in arteries, but not in veins (Fig. 8A). Although dipyridamole inhibited NE oxidation currents, it produced an increase in neurogenic contraction of arteries, but not veins (Fig. 8B). Dipyridamole did not change NE oxidation currents and neurogenic contraction in DOCA-salt arteries (Fig. 8, C and D).
Fig. 7.
Role of endogenous adenosine by A1R antagonist (DPCPX) in adrenergic transmission of sham and DOCA-salt rats. Dose-response curves of DPCPX were performed under a 10 Hz stimulus train to evoke NE oxidation currents and neurogenic contraction. (A) DPCPX increased NE current and contraction (B) in concentration-dependent manner in sham MAs, but not in MVs. DPCPX did not alter the amplitude of NE oxidation currents (C) and neurogenic contraction (D) in DOCA-salt arteries. Data are mean ± S.E.M. #Significantly different from baseline values. Data were analyzed using one-way analysis of variance (ANOVA) and Dunnett’s post hoc test. N indicates the number of animals in the experiment.
Fig. 8.
The adenosine transporter blocker, dipyridamole, reduced NE oxidation currents. The effects of dipyridamole were tested NE oxidation currents evoked by a 10 Hz stimulus train. (A) Dipyridamole caused a reduction of NE current in sham MAs, but not in MVs. (B) Dipyridamole produced an increase in neurogenic contraction in sham MAs, but not in MVs. (C and D) Dipyridamole did not alter the amplitude of NE oxidation currents (C) and neurogenic contraction (D) in DOCA-salt arteries. Data are mean ± S.E.M. #Significantly different from baseline values. Data were analyzed using one-way analysis of variance (ANOVA) and Dunnett’s post hoc test. N indicates the number of animals in the experiment.
Discussion
Adenosine Acts at A1Rs to Constrict Veins but Not Arteries.
Adenosine modulates venous tone by constricting venous smooth muscle and by inhibiting NE release from perivenous sympathetic nerves. Adenosine does not constrict arteries, but it inhibits NE release from periarterial sympathetic nerves. Adenosine-induced constriction of veins was mimicked by the A1R agonist, CPA. It is not surprising that adenosine or CPA did not constrict arteries, because previous work has shown that adenosine dilates mesenteric arterioles (Mian and Marshall, 1995), and this effect is mediated by A2Rs in the rat stomach (Nagata et al., 1996) and rabbit MAs (de Brito et al., 2002). We did not detect a vasodilation in our studies, because we did not preconstrict the blood vessels. Previous studies showed that adenosine dilates rat MVs in situ (Mian and Marshall, 1995). In these studies, the veins were blood perfused, and our veins were maintained in a physiologic buffer solution in vitro. Methodological differences might contribute to these differences in results. Because the focus of our study was adenosine modulation of sympathetic neuroeffector transmission, we did not pursue this issue.
A1Rs Couple to Inhibition of NE Release.
Our neurogenic contraction and amperometry data are consistent with previous findings showing that adenosine, a degradation product of ATP metabolism in the neuroeffector junction (Todorov et al., 1997; Tabrizchi and Bedi, 2001; Westfall et al., 2002), modulates sympathetic neurotransmission in the mesenteric circulation (Illes et al., 1988; Ralevic, 1995; Diniz et al., 2004; Donoso et al., 2006), in the rat tail artery (Bucher et al., 1992; Diniz et al., 2004) in rat caudal arteries (Shinozuka et al., 1988), and in human forearm blood vessels (Rongen et al., 1996).
We show that A1Rs are localized to sympathetic nerves supplying rat MAs and MVs. Sympathetic nerves were identified by localizing TH in nerve fibers. These data are consistent with our conclusion that A1Rs modulate NE release in the mesenteric circulation. This differs from the conclusion of Donoso et al. (2006), who concluded that A1Rs do not couple to inhibition of NE release in the rat mesentery. Their conclusion is based in part on reverse-transcription polymerase chain reaction studies, which did not detect A1R transcripts in the mesentery. However, mRNA encoding the A1R protein would be found in highest concentrations in the cell body of sympathetic neurons in prevertebral ganglia and not in nerve terminals. Immunohistochemistry detects A1R protein, and these methodological differences would account for differences in conclusions about the role of the A1R in perivascular sympathetic nerves. We also show that there are TH-negative nerve fibers expressing A1R immunoreactivity. These A1Rs may be localized to sensory nerves (Burnstock and Wood, 1996). We did not measure mRNA or protein levels of prejunctional A1R in mesenteric blood vessels, because A1Rs are expressed by sympathetic and primary afferent nerves, smooth muscle cells, and fibroblasts (Ginés et al., 2000). Measurements of A1R expression in blood vessel wall protein extracts would not distinguish A1R protein from nerves versus other cell types. Our immunohistochemical results revealed that there was no obvious difference in A1R expression by periarterial sympathetic nerve fibers (costained with the TH antibody) from sham and DOCA-salt rats.
Neurogenic contraction permits studies of the prejunctional A1R in arteries, but not in veins, because adenosine and CPA constricted veins. Neurogenic constrictions of MAs are mediated by NE acting at α1-adrenergic receptors and a purine acting at P2X1 receptors on vascular smooth muscle cells (Dunn et al., 1999). We confirmed that A1R stimulation does not directly affect arterial smooth muscle reactivity, by showing that constrictions caused by phenylephrine and α,β-methylene ATP were unaffected by adenosine and CPA. However, adenosine and CPA decreased nerve-mediated arterial constrictions, indicating that A1R activation inhibits neurotransmitter release.
We showed that inhibition of neurogenic constriction by adenosine is more sensitive to impairment in DOCA-salt hypertension, compared with CPA. This may be attributable to different potencies of adenosine analogs acting at A1Rs. In addition, adenosine may act the A3 adenosine receptor, which also couples to inhibition of NE release (Donoso et al., 2006). Further studies are needed to clarify this issue.
Measurement of neurogenic constrictions is an indirect measure of neurotransmitter release. Amperometry directly assesses adenosine receptor modulation of NE release from sympathetic nerves. Our amperometry data confirm previous work showing that A1Rs mediate prejunctional inhibition of sympathetic neuroeffector transmission in the mesentery, but we expand on these findings in two ways. First, previous work used increases in vascular perfusion pressures, arterial contractions, or overflow of 3H-NE after long trains of electrical stimulation to assess A1R-mediated modulation of NE release. We used microelectrodes to measure NE release from a few nerve fibers on the blood vessel surface. This technique allows measurements in real time and also permits use of short trains of stimulation that more closely mimic sympathetic nerve activity in vivo. Second, we showed that endogenous adenosine can inhibit NE release, because the A1R antagonist, DPCPX, increased NE oxidation currents and neurogenic contraction, and the adenosine uptake inhibitor, dipyridamole, decreased NE oxidation currents. Even though dipyridamole produced a reduction in NE current, there was no change in the neurogenic contraction, indicating an additional effect of dipyridamole at the neurovascular junction. This result supports our use of amperometry to directly assess NE release in real time from perivascular sympathetic nerves. Indirect measures of neurotransmitter release using blood vessel constriction as a measure are contaminated by potential drug- or disease- (hypertension) induced alterations in smooth muscle reactivity. Postjunctional factors are not an issue with amperometric measures of NE release. In addition, enhancement of NE release by endogenously released adenosine could be attributable to an action at facilitatory A2A receptors on sympathetic nerve terminals (Fresco et al., 2002; Diniz et al., 2004). Previous work has shown that prejunctional A2ARs couple to increased or decreased NE release from perivascular sympathetic nerves (Diniz et al., 2004). These investigators used long trains of electrical stimulation, and NE was measured in overflow solutions offline. We used amperometry to measure NE release in real time near a few release sites on a single blood vessel with brief trains of electrical stimulation that more closely mimic sympathetic nerve activity in vivo. Under these conditions, we found that the A2AR agonist CGS21680 did not alter significantly NE oxidation currents. The function of facilitatory A2A receptors may be most prominent when A1Rs are blocked.
Impaired Prejunctional A1R Function in DOCA-Salt Mesenteric Arteries.
Increased sympathetic tone and impaired α2-AR autoreceptor function contributes to DOCA-salt hypertension (deChamplain et al., 1987; Luo et al., 2004; Demel and Galligan, 2008). Before this study, it was not clear whether A1R function is also impaired in DOCA-salt hypertension. We use “impaired” to refer to hypertension-associated changes in the A1R that leads to reduced modulation of NE release. Changes could include receptor downregulation, change in affinity, or change in coupling efficiency to downstream signaling mechanisms. We found that CPA concentration-response curves for inhibition of neurogenic arterial constrictions and NE release from periarterial nerves are right-shifted in DOCA-salt hypertension, suggesting reduced A1R function. Impaired A1R function also affects the inhibitory actions of endogenously released adenosine, because increases in NE oxidation currents caused by DPCPX (A1R antagonist) and reductions in NE oxidation currents caused by dipyridamole (adenosine uptake inhibitor) were also reduced in DOCA-salt arteries. These results indicate that prejunctional α2ARs are not selectively affected in DOCA-salt hypertension, but there is a more general disruption of prejunctional mechanisms controlling neurotransmitter release form periarterial sympathetic nerves. Indeed, there is evidence that α2ARs and A1Rs interact to modulate NE released from sympathetic nerves supplying the rat tail artery (Bucher et al., 1992).
A new finding is that A1R modulation of NE release was impaired in periarterial but not perivenous sympathetic nerves in DOCA-salt hypertension. Similar artery/vein differences have been established for α2AR function DOCA-salt hypertension (Luo et al., 2004; Park et al., 2010). α2AR function is partly restored in DOCA-salt rats treated with apocynin, an NADPH oxidase inhibitor (Demel et al., 2010). NADPH oxidase produces O2- in the vasculature (Szasz et al., 2007), and this enzyme is also localized to perivascular sympathetic nerves (Cao et al., 2009). Increased O2- levels in arteries are associated with higher arterial pressures, and elevated O2- could disrupt signaling mechanisms linking α2AR and A1R to inhibition of NE release. Venous pressure is not increased in DOCA-salt hypertension (Fink, 2009); thus, levels of O2- would not be elevated in veins, and this might spare autoreceptor function. There are also likely to be artery/vein differences in enzymes that produce and degrade O2- (Szasz et al., 2007). This difference could also contribute to maintained autoreceptor function in perivenous sympathetic nerves in DOCA-salt hypertension.
We identified the challenges to mechanistic studies (i.e., A1R expression in nerve terminals, coupling to effectors, receptor affinity) above. However, future work could use Cre-Lox-recombinase approaches for selective silencing or overexpression of A1Rs in arterial versus venous sympathetic nerves in mice. Alternatively, adenovirus transfection of sympathetic neurons could be used to overexpress A1Rs or rescue A1Rs in periarterial sympathetic neurons in DOCA-salt hypertensive rats.
Abbreviations
- α2AR
α2-adrenergic receptors
- A1R
A1 adenosine receptors
- CGS21680
C23H29N7O6.HCl.xH2O
- CPA
N6 cyclopentyl-adenosine
- DOCA
deoxycorticosterone acetate
- DPCPX 1
3-dipropyl-8-cyplopentylxanthine
- DPSPX
1,3-dipropyl-8-sulphophenylxanthine
- MA
mesenteric artery
- MV
mesenteric vein
- NE
norepinephrine
- pIC50
negative log of the half maximal inhibitory drug concentration
- TH
tyrosine hydroxylase
Authorship Contributions
Participated in research design: Sangsiri, Dong, Swain, Galligan, Xu.
Conducted experiments: Sangsiri, Dong, Xu.
Contributed new reagents or analytic tools: Swain, Dong.
Performed data analysis: Sangsiri, Dong, Galligan, Xu, Swain.
Wrote or contributed to the writing of the manuscript: Sangsiri, Galligan, Xu, Swain.
Footnotes
This work was supported by funding from Thai Government Science and Technology Scholarship [Grant TS-146]; an American Heart Association, Mid-West Affiliate predoctoral fellowship [Grant 09PRE2180037] (to S.S.); and the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL070687 (to J.J.G.) and HL84258 (to G.M.S.)].
References
- Anderson EA, Sinkey CA, Lawton WJ, Mark AL. (1989) Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension 14:177–183 [DOI] [PubMed] [Google Scholar]
- Birch DJ, Turmaine M, Boulos PB, Burnstock G. (2008) Sympathetic innervation of human mesenteric artery and vein. J Vasc Res 45:323–332 [DOI] [PubMed] [Google Scholar]
- Bucher B, Corriu C, Stoclet JC. (1992) Prejunctional opioid p-receptors and adenosine A1-receptors on the sympathetic nerve endings of the rat tail artery interact with the alpha 2-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol 345:37–43 [DOI] [PubMed] [Google Scholar]
- Burnstock G, Wood JN. (1996) Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol 6:526–532 [DOI] [PubMed] [Google Scholar]
- Cao X, Demel SL, Quinn MT, Galligan JJ, Kreulen D. (2009) Localization of NADPH oxidase in sympathetic and sensory ganglion neurons and perivascular nerve fibers. Auton Neurosci 151:90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito MTV, Canto A, Correia JHD, Cunha RA, Marques MC. (2002) Adenosine A(2A) receptors in portal hypertension: their role in the abnormal response to adenosine of the cranial mesenteric artery in rabbits. Br J Pharmacol 135:1324–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- deChamplain J, Bouvier M, and Drolet G (1987) Abnormal regulation of the sympathoadrenal system in deoxycorticosterone acetate-salt hypertensive rats. Can J Physiol Pharmacol 65:1605–1614. [DOI] [PubMed]
- Demel SL, Dong H, Swain GM, Wang X, Kreulen DL, Galligan JJ. (2010) Antioxidant treatment restores prejunctional regulation of purinergic transmission in mesenteric arteries of deoxycorticosterone acetate-salt hypertensive rats. Neuroscience 168:335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demel SL, Galligan JJ. (2008) Impaired purinergic neurotransmission to mesenteric arteries in deoxycorticosterone acetate-salt hypertensive rats. Hypertension 52:322–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz C, Fresco P, Leal S, Gonçalves J. (2004) Adenosine receptors involved in modulation of noradrenaline release in isolated rat tail artery. Eur J Pharmacol 504:17–25 [DOI] [PubMed] [Google Scholar]
- Donoso MV, Aedo F, Huidobro-Toro JP. (2006) The role of adenosine A2A and A3 receptors on the differential modulation of norepinephrine and neuropeptide Y release from peripheral sympathetic nerve terminals. J Neurochem 96:1680–1695 [DOI] [PubMed] [Google Scholar]
- Dunn WR, Brock JA, Hardy TA. (1999) Electrochemical and electrophysiological characterization of neurotransmitter release from sympathetic nerves supplying rat mesenteric arteries. Br J Pharmacol 128:174–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GD. (2009) Arthur C. Corcoran Memorial Lecture. Sympathetic activity, vascular capacitance, and long-term regulation of arterial pressure. Hypertension 53:307–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresco P, Diniz C, Queiroz G, Gonçalves J. (2002) Release inhibitory receptors activation favours the A2A-adenosine receptor-mediated facilitation of noradrenaline release in isolated rat tail artery. Br J Pharmacol 136:230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginés S, Hillion J, Torvinen M, Le Crom S, Casadó V, Canela EI, Rondin S, Lew JY, Watson S, Zoli M, et al. (2000) Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc Natl Acad Sci USA 97:8606–8611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães S, Morato M, Sousa T, Albino-Teixeira A. (2003) Hypertension due to blockade of adenosine receptors. Pharmacol Toxicol 92:160–162 [DOI] [PubMed] [Google Scholar]
- Illes P, Jackisch R, Regenold JT. (1988) Presynaptic P1-purinoceptors in jejunal branches of the rabbit mesenteric artery and their possible function. J Physiol 397:13–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Osborn JW, Fink GD. (2007) Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension 50:547–556 [DOI] [PubMed] [Google Scholar]
- Luo M, Fink GD, Lookingland KJ, Morris JA, Galligan JJ. (2004) Impaired function of alpha2-adrenergic autoreceptors on sympathetic nerves associated with mesenteric arteries and veins in DOCA-salt hypertension. Am J Physiol Heart Circ Physiol 286:H1558–H1564 [DOI] [PubMed] [Google Scholar]
- Martin DS, Rodrigo MC, Appelt CW. (1998) Venous tone in the developmental stages of spontaneous hypertension. Hypertension 31:139–144 [DOI] [PubMed] [Google Scholar]
- Mian R, Marshall JM. (1995) The role of adenosine in mediating vasodilatation in mesenteric circulation of the rat in acute and chronic hypoxia. J Physiol 489:225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata H, Sekizuka E, Morishita T, Tatemichi M, Kurokawa T, Mizuki A, Ishii H. (1996) Adenosine A2-receptor mediates ethanol-induced arteriolar dilation in rat stomach. Am J Physiol 271:G1028–G1033 [DOI] [PubMed] [Google Scholar]
- Olah ME, Stiles GL. (2000) The role of receptor structure in determining adenosine receptor activity. Pharmacol Ther 85:55–75 [DOI] [PubMed] [Google Scholar]
- Park J, Galligan JJ, Fink GD, Swain GM. (2007) Differences in sympathetic neuroeffector transmission to rat mesenteric arteries and veins as probed by in vitro continuous amperometry and video imaging. J Physiol 584:819–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Galligan JJ, Fink GD, Swain GM. (2010) Alterations in sympathetic neuroeffector transmission to mesenteric arteries but not veins in DOCA-salt hypertension. Auton Neurosci 152:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V. (1995) Modulation by nicotinamide adenine dinucleotide of sympathetic and sensory-motor neurotransmission via P1-purinoceptors in the rat mesenteric arterial bed. Br J Pharmacol 114:1541–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V. (2000) Sympathoinhibition by adenosine A(1) receptors, but not P2 receptors, in the hamster mesenteric arterial bed. Eur J Pharmacol 387:287–293 [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492 [PubMed] [Google Scholar]
- Rongen GA, Lenders JWM, Lambrou J, Willemsen JJ, Van Belle H, Thien T, Smits P. (1996) Presynaptic inhibition of norepinephrine release from sympathetic nerve endings by endogenous adenosine. Hypertension 27:933–938 [DOI] [PubMed] [Google Scholar]
- Rothe CF. (1983) Reflex control of veins and vascular capacitance. Physiol Rev 63:1281–1342 [DOI] [PubMed] [Google Scholar]
- Schenk J, McNeill JH. (1992) The pathogenesis of DOCA-salt hypertension. J Pharmacol Toxicol Methods 27:161–170 [DOI] [PubMed] [Google Scholar]
- Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, Hastings J, Aggarwal A, Esler MD. (2004) Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension 43:169–175 [DOI] [PubMed] [Google Scholar]
- Shinozuka K, Bjur RA, Westfall DP. (1988) Characterization of prejunctional purinoceptors on adrenergic nerves of the rat caudal artery. Naunyn Schmiedebergs Arch Pharmacol 338:221–227 [DOI] [PubMed] [Google Scholar]
- Shryock JC, Belardinelli L. (1997) Adenosine and adenosine receptors in the cardiovascular system: biochemistry, physiology, and pharmacology. Am J Cardiol 79 (12A):2–10 [DOI] [PubMed] [Google Scholar]
- Smyth LM, Bobalova J, Mendoza MG, Lew C, Mutafova-Yambolieva VN. (2004) Release of beta-nicotinamide adenine dinucleotide upon stimulation of postganglionic nerve terminals in blood vessels and urinary bladder. J Biol Chem 279:48893–48903 [DOI] [PubMed] [Google Scholar]
- Szasz T, Thakali K, Fink GD, Watts SW. (2007) A comparison of arteries and veins in oxidative stress: producers, destroyers, function, and disease. Exp Biol Med (Maywood) 232:27–37 [PubMed] [Google Scholar]
- Tabrizchi R, Bedi S. (2001) Pharmacology of adenosine receptors in the vasculature. Pharmacol Ther 91:133–147 [DOI] [PubMed] [Google Scholar]
- Todorov LD, Mihaylova-Todorova S, Westfall TD, Sneddon P, Kennedy C, Bjur RA, Westfall DP. (1997) Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature 387:76–79 [DOI] [PubMed] [Google Scholar]
- Westfall DP, Todorov LD, Mihaylova-Todorova ST. (2002) ATP as a cotransmitter in sympathetic nerves and its inactivation by releasable enzymes. J Pharmacol Exp Ther 303:439–444 [DOI] [PubMed] [Google Scholar]