Abstract
We compared the differential effects of positional isomers of acetylsalicylic acid (o-ASA, m-ASA, and p-ASA) on cyclooxygenase (COX) inhibition, gastric prostaglandin E2 (PGE2), malondialdehyde, tumor necrosis factor-alpha (TNF-α) levels, superoxide dismutase (SOD) activity, human adenocarcinoma colon cancer cell growth inhibition, cell proliferation, apoptosis, and cell-cycle progression. We also evaluated the gastric toxicity exerted by ASA isomers. All ASA isomers inhibit COX enzymes, but only the o-ASA exerted an irreversible inhibitory profile. We did not observe a significant difference between ASA isomers in their ability to decrease the in vivo synthesis of PGE2 and SOD activity. Furthermore, all isomers increased the levels of gastric and TNF-α when administered orally at equimolar doses. We observed a dose-dependent cell growth inhibitory effect; the order of potency was p-ASA > m-ASA ≈ o-ASA. There was a dose-dependent decrease in cell proliferation and an increase in apoptosis, with a concomitant Go/G1 arrest. The ulcerogenic profile of the three ASA isomers showed a significant difference between o-ASA (aspirin) and its two positional isomers when administered orally at equimolar doses (1 mmol/kg); the ulcer index (UI) for o-ASA indicated extensive mucosal injury (UI = 38), whereas m-ASA and p-ASA produced a significantly decreased toxic response (UI = 12 and 8, respectively) under the same experimental conditions. These results suggest that the three positional isomers of ASA exert practically the same biologic profile in vitro and in vivo but showed different safety profiles. The mechanism of gastric ulcer formation exerted by aspirin and its two isomers warrants a more detailed and thorough investigation.
Introduction
2-Acetoxybenzoic acid (acetylsalicylic acid, ASA, aspirin) is one of the most widely used over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) to treat pain, fever, and inflammation. The major anti-inflammatory and analgesic mechanism of action of aspirin is the inhibition of cyclooxygenase (COX) enzymes (Vane, 1971). COX-1 and COX-2 enzymes catalyze the first step in the enzymatic transformation of arachidonic acid to prostaglandins (PGs), prostacyclin, and thromboxanes (Vane et al., 1998; Smith et al., 2000). COX-1 is generally regarded as a constitutive enzyme that is present in most tissues; it is involved in the physiologic production of PGs and provides maintenance functions such as cytoprotection in the stomach. In contrast, COX-2 has been regarded as an inducible enzyme (induced by cytokines, growth factor, interleukin-1-β, carrageenan) and is expressed in inflammatory cells (Masferrer et al., 1994).
Among all NSAIDs, aspirin is a unique nonselective irreversible COX inhibitor because of its ability to acetylate the Ser530 hydroxyl group in the primary active site of COX-1 and COX-2 (Awtry and Loscalzo, 2000). Acetylation of the weakly nucleophilic -OH of Ser530 by aspirin is thought to result from the initial binding of its carboxylate (COOH) to Arg120 near the mouth of the COX binding site, which positions the o-acetoxy moiety in close proximity to the Ser530 -OH, which it acetylates. Orally administered aspirin irreversibly acetylates Ser530 of COX-1 and COX-2, leading to complete inhibition of COX-1 activity; nevertheless, the acetylated COX-2 active site remains active, converting arachidonic acid to 15-(R)-hydroxyeicosatetraenoic acid (15-R-HETE), which is the precursor to “aspirin-triggered” endogenous anti-inflammatory lipoxins (Serhan and Chiang, 2002; Yasuda et al., 2008). Consequently, acetylation of COX enzymes by aspirin produces a desirable pharmacological profile, decreasing COX-1–derived PGs and thromboxanes and stimulating the biosynthesis of COX-2–derived endogenous anti-inflammatory lipoxins. These are two of the essential effects that make aspirin an attractive drug in our program tool chest.
Early observations showed that COX-2 is overexpressed in most premalignant and malignant neoplasms, and all essential features of carcinogenesis (mutagenesis, mitogenesis, angiogenesis, reduced apoptosis, metastasis, and immunosuppression) are linked to COX-derived PG biosynthesis (Qiao et al., 1995; Sheng et al., 1998; Kashfi and Rigas, 2005a; Harris, 2007). Thus, it was reasonable to consider that COX-2 was a suitable molecular target for cancer treatment or cancer prevention, and NSAIDs may be useful in the treatment of COX-expressing tumors. Based on these hypotheses, NSAIDs (including selective COX-2 inhibitors) have been extensively studied as chemotherapeutic and chemopreventive agents. Evidence suggests that NSAIDs decrease the incidence of, or mortality from, breast cancer (Holmes et al., 2010; Bardia et al., 2011) colon cancer (Chan et al., 2007; Cole et al., 2009; Gao et al., 2009; Grau et al., 2009; Cooper et al., 2010), esophageal cancer (Liu et al., 2009; Pandeya et al., 2010), non-small cell lung cancer (Van Dyke et al., 2008), and others. However, there is conflicting evidence showing that the COX inhibitory potency of NSAIDs does not always correlate with their anticancer activity, suggesting that NSAIDs may exert their chemotherapeutic and chemopreventive properties by multiple mechanisms of action other than, or in addition to, COX inhibition (Kashfi and Rigas, 2005a,b; Grosch et al., 2006).
Despite the wide variety of desirable pharmacological effects exerted by aspirin, there remains a significant risk of gastrointestinal bleeding, produced (presumably) by the inhibition of cytoprotective COX-mediated gastric PG synthesis, even with low prophylactic doses of this drug (Yeomans et al., 2009). This mechanism-based toxicity is the main reason why the use of aspirin (and NSAIDs in general) has been correlated with a relatively high incidence of adverse gastrointestinal side effects (Singh and Triadafilopoulos, 1999; Tenenbaum, 1999; Aalykke and Lauritsen, 2001; Fiorucci and Del Soldato, 2003; Schaffer et al., 2006) and has led some clinicians (and patients) to reduce their use (Scheiman and Fendrick, 2007).
In the last 15 years, several research groups have reported extensive structure-activity relationships on a wide variety of COX pharmacophores. In this regard, the attention was essentially focused on developing potent and selective COX-2 inhibitors based on the assumption that COX-2–derived PGs were uniquely and directly responsible for the inflammatory processes and COX-1–derived PGs were mainly involved in cytoprotection; however, recent reports have challenged these “traditional” roles for COX-1 and COX-2 enzymes, emphasizing the importance of re-evaluating their roles in the inflammatory process, (Rouzer and Marnett, 2009), as well as their contribution in the underlying mechanisms of NSAID-induced side effects.
Considering the simplicity of aspirin’s chemical structure, it is surprising that there are no reports in the literature describing the structure-activity relationships for the other two positional isomers of aspirin, namely, the meta- and para-acetylsalicylic acid isomers. Therefore, as part of an ongoing research work aimed to develop new anticancer agents derived from aspirin, we now report a comprehensive biologic evaluation of ASA positional isomers, comparing their anti-inflammatory profile, their differential effects on human adenocarcinoma colon cancer cell (HT-29) growth inhibition, cell proliferation, cell cycle, and apoptosis. We also evaluated the gastric ulcerogenic profile of aspirin’s positional isomers, as well as a comprehensive molecular modeling (docking) study for the three isomers (o-, m-, and p-) of ASA on COX-2.
Materials and Methods
Reagents.
The three positional isomers of salicylic acid (o-SA, m-SA, p-SA), acetylsalicylic acid (o-ASA) and 4-(acetyloxy)-benzoic acid (p-ASA) were purchased from MP Biomedicals (Solon, OH); 3-(acetyloxy)benzoic acid (m-ASA) was prepared by acetylation of m-salicylic acid (Fig. 1). High-performance liquid chromatography–grade solvents and reagents acquired from Fisher Chemicals (Fair Lawn, NJ) were used; the colon adenocarcinoma HT-29 cell line (American Type Culture Collection HTB-38, Manassas, VA), McCoy’s 5A medium, and ovine COX-1 and COX-2 were obtained from Cayman Chemicals (Ann Arbor, MI).
Fig. 1.
Chemical structures of positional isomers of aspirin.
Animals.
All experimental procedures were approved by the institutional animal research committee at the City College of New York and performed in accordance with nationally approved guidelines for the treatment of laboratory animals. Male Wistar rats (five per group, obtained from Charles River Laboratories, Inc., Wilmington, MA) weighing 180–200 g were used. The rats were fed with standard laboratory chow and water; however, before each experiment, the rats were fasted for 48 hours but allowed to have free access to drinking water at all times. The experimental drugs were administered orally (gavage) by forming suspensions of o-ASA, m-ASA, or p-ASA (1 mmol/kg) in 1.0 ml of 1% carboxymethyl cellulose solution. The same volume of carboxymethyl cellulose solution was administered to the animals in the control group. Six hours after administration, the rats were euthanized by suffocation in a CO2 chamber and had their stomachs removed, cut along the greatest curvature, and rinsed with ice-cold distilled water. The ulcer index (UI) was determined for each experimental drug based on a previously reported procedure by Best et al. (1984). Additionally, tissues from the rat stomachs were excised and processed to measure the levels of PGE2 (prostaglandin E2) and malondialdehyde (MDA), as well as the activity of superoxide dismutase (SOD) enzyme. To determine plasma levels of tumor necrosis factor alpha (TNF-α), blood samples from each rat were taken by cardiac puncture using heparin-containing vials.
Determination of PGE2 Levels.
About 1 g of stomach tissue from each rat was removed, weighed, and placed in a test tube containing 5 ml of pH 7.4 phosphate buffer (0.1 M), EDTA (1 mM), and indomethacin (10 µM). Each tissue sample was homogenized and centrifuged for 10 minutes at 12,000g (4°C), and then the amount of PGE2 in supernatants (duplicate) was determined by an enzyme immunoassay kit (Cayman Chemical, Inc., Ann Arbor, MI) following the protocol described by the manufacturer. Briefly, the standard (50 μl) or homogenate (50 μl), enzymatic tracer (50 μl), and specific antiserum (50 μl) were mixed; after incubation for 17 hours (overnight) at 4°C, the plates were washed with wash buffer, and Ellman’s reagent (200 μl) was added to each well. The absorbance at 412 nm was measured after incubating the plate for 1 hour at room temperature. The amount of detected PG was expressed as picograms of PGE2 per milligram of protein; the protein levels were determined in a separate assay (Bio-Rad Laboratories, Hercules, CA).
Index of Lipid Peroxidation.
Approximately 25 mg of stomach tissue from each rat was snap-frozen and sonicated for about 15 seconds at 40 V, over ice, using 250 µl of radioimmunoprecipitation assay buffer (25 mM Tris-HCl, pH 7.6, 150 mM NaCl, 1% Tergitol-type NP-40 nonylphenoxypolyethoxyethanol, 1% sodium deoxycholate, and 0.1% SDS) and phenylmethylsulfonyl fluoride as protease inhibitor; then cell homogenates were centrifuged for 10 minutes at 1,600g (4°C). The amount of thiobarbituric acid-reactant substances in the supernatants were measured and stored on ice by a colorimetric kit (Cayman Chemical, Inc.) following the protocol described by the manufacturer. Briefly, the reaction of MDA with thiobarbituric acid at high temperature (90–100°C) in acidic conditions produced a chromophore, which absorbed visible light at 530–540 nm. The results were expressed as picomoles of MDA per gram of protein (as determined by a Bio-Rad assay).
Superoxide Dismutase Activity.
The activity of antioxidant enzymes (SOD) was determined in samples of gastric mucosal tissue isolated from each rat by a colorimetric assay kit (Cayman Chemical, Inc.) according to the protocol described by the manufacturer. Briefly, around 1 g of mucosal tissue was homogenized with 5 ml of 20 mM HEPES buffer (pH 7.2) containing EGTA (1 mM) and sucrose (300 mM). Then the cell homogenates were centrifuged at 1,500g for 10 minutes (4°C). The supernatants were removed and stored at −80°C until assayed. SOD activity was determined using a spectrophotometric method measuring absorbance at 460 nm; the results were expressed as units of SOD activity per milligram of protein. In this regard, one unit of SOD is defined as the amount of enzyme required to exhibit 50% dismutation of the superoxide radical.
Plasma TNF-α Levels.
Fresh samples of blood from animals were collected by cardiac puncture, receiving the fluid into heparin-containing vials. TNF-α plasma levels were determined using an enzyme immunoassay kit (R&D Systems, Minneapolis, MN) according to the protocol described by the manufacturer; the results were expressed as picograms of TNF-α/ml. Briefly, each sample (50 μl) was incubated with antibodies specific for rat TNF-α, and then the samples were washed three times with assay buffer. An enzyme-linked polyclonal antibody specific for rat TNF-α (conjugated to horseradish peroxidase) was added to the wells. After washing unbound antibody-enzyme complexes, a substrate solution containing tetramethylbenzidine and hydrogen peroxide were added to the wells. This enzymatic reaction yielded a blue product (oxidized tetramethylbenzidine) that turned yellow when we added dilute hydrochloride acid. The intensity of the color by spectrophotometry was determined measuring optical densities at 450 nm in a standard enzyme-linked immunosorbent assay plate reader. The sensitivity of this assay was determined (around 1.6 pg/ml) by adding 2 S.D. to the mean optical density value of 20× zero standard replicates and calculating the corresponding concentration. The results are expressed as picograms per milliliter.
Cell Growth Inhibition.
HT-29 human colon adenocarcinoma cells were cultured in McCoy’s 5A medium per American Type Culture Collection instructions; the cell growth inhibitory effect of o-ASA, m-ASA, and p-ASA [100 mM in dimethylsulfoxide (DMSO)] was measured using a colorimetric MTT assay kit (Roche, Indianapolis, IN) (Kashfi et al., 2002). To avoid interference from the solvent, the final concentrations of DMSO were adjusted to 1% in all media.
Assay for Apoptosis and Cell Proliferation.
HT-29 cells (approximately 0.5 × 106 cells/ml) were incubated with various concentrations of o-ASA, m-ASA, and p-ASA for 24 hours. The treated cells were washed and resuspended with 1× binding buffer (Annexin V binding buffer, 0.1 M HEPES/NaOH, pH 7.4, 1.4 M NaCl, 25 mM CaCl2; BD BioSciences, Pharmingen, San Diego, CA). Then 5 ml of Annexin V-FITC (final concentration: 0.5 mg/ml) was added, followed by propidium iodide (final concentration 20 mg/ml). Finally, the cells were incubated at room temperature for 15 minutes in the dark, transferring them to fluorescence-activated cell sorter tubes for subsequent analysis. The percentage of apoptotic cells was calculated using a Becton Dickinson LSR II, equipped with a single-argon ion laser. For each subset, about 10,000 events were analyzed. All the parameters were collected in list mode files, and the data were analyzed using the Flow Jo software Tree Star Inc. (Ashland, OR).
The levels of proliferating cell nuclear antigen (PCNA) was determined using an enzyme-linked immunosorbent assay Kit (Calbiochem, La Jolla, CA) in accordance with the manufacturer’s protocol. Briefly, HT-29 cells were incubated with serum-free media for 24 hours to remove the effect of endogenous growth factors. Then the cells (1×106 cells/ml) were treated with various concentrations (1–5 mM) of o-ASA, m-ASA, and p-ASA. After this, the cells (approximately 1 × 106 cells/ml) were suspended in suspension buffer (5 mM EDTA, 0.2 mM phenylmethyl sulfonyl fluoride, 1 μg/ml pepstatin, 0.5 μg/ml leupeptin, and 50 mM Tris-HCl, pH 8.0). The samples of the suspension were pipetted into the wells of the plate containing rabbit polyclonal antibody (specific for the human PCNA protein) included with the kit. Then mouse monoclonal antibody clone PC10 (detector antibody) was added to each well, and the mixture was incubated for 2 hours at room temperature. After washing the wells, horseradish peroxidase streptavidin solution was added, and the plate was incubated for 30 minutes at room temperature; the chromogenic substrate tetramethylbenzidine was added, and the plates were incubated again for a further 30 minutes. Finally, the stop solution was added and the absorbance was measured in each well at 450 nm.
Cell-Cycle Analysis.
Cell-cycle phase distributions of control were obtained and HT-29 cells were treated by using a Coulter Profile XL equipped with a single argon ion laser. For each subset, more than 10,000 events were analyzed. All the parameters were collected in list mode files. The data were analyzed on a Coulter XL Elite workstation using the software programs Multigraph and Multicycle. Cells (approximately 0.5 × 106) were fixed in 100% methanol for 10 minutes at −20°C, pelleted (5,000g ×10 minutes at 4°C), resuspended, and incubated in phosphate-buffered saline containing 1% fetal bovine serum/0.5% NP-40 on ice for 5 minutes. After that, the cells were washed again with phosphate-buffered saline/1% fetal bovine serum containing 40 mg/ml propidium iodide (used to stain DNA) and 200 mg/ml RNase type IIA. The analysis was carried out within the next 30 minutes by flow cytometry. The percentage of cells in G0/G1, G2/M, and S phases were determined from DNA content histograms.
Cyclooxygenase Inhibition Assay.
Ovine COX-1 or COX-2 (200 U) were incubated in the presence of o-ASA, m-ASA, or p-ASA (1–5 mM in DMSO) in 600 µl of reaction buffer [100 mM Tris-maleate buffer, pH 6.5, 0.1% Tween-20, gelatin at 1 mg/ml, hematin (3 µM), and tetramethylparaphenylinediamine (100 µM; Sigma-Aldrich, St. Louis, MO)]. The concentration of DMSO was always 10% of the final volume. After incubating the samples for 30 minutes at 4°C, arachidonic acid (100 µM) was added to start the enzymatic reaction and incubated for 5 minutes at 25°C. The activity of the COX enzymes was measured by monitoring the oxidation of tetramethylparaphenylinediamine at 600 nm in a microtiter plate. The percent inhibition was determined as follows: [(IA−D)/IA] × 100, where IA = average absorbance from the 100% initial activity wells, and D = average absorbance from the corresponding drug wells. Additionally, the reversibility of COX inactivation exerted by ASA isomers was assessed by carrying out a microdialysis procedure. In this regard, COX-1 or COX-2 (2000 U) was incubated in the presence of o-ASA, m-ASA, or p-ASA (1–5 mM in DMSO) in 1000 μl of reaction buffer for 30 minutes at 4°C. After incubation, the samples were loaded into microdialysis chambers (10-kDa molecular mass cutoff membrane; Amicon Inc., Billerica, MA) and dialyzed against 500 μl of inhibitor-free reaction buffer for 2 hours After dialysis, the remaining enzymatic activity was determined as described previously (without adding more inhibitor).
Ulcerogenicity.
Two different experiments were conducted to compare the gastric toxicity exerted by the three aspirin isomers, namely the UI and the erosion index (EI) assays. The UI was determined as described by Best et al. (1984); o-ASA, m-ASA, or p-ASA (1 mmol/kg) was administered by gavage suspended in 1% carboxymethyl cellulose solution (1.0 ml). Animals in the control group received an equivalent volume of vehicle. UI measured clearly visible ulcers (elongated, hemorrhagic lesions varying in length), whereas EI measured less noticeable microhemorrhagic lesions, which were observed only by using a magnifying lens.
Docking Protocol.
A series of molecular modeling (docking) experiments were performed using Discovery Studio Client v2.5.0.9164 (2005–2009; Accelrys Software, Inc., San Diego, CA), running on a HP xw4600 workstation (processor ×86 family 6 model 23 stepping 10 Genuine Intel 2999 MHz). The coordinates were obtained for the X-ray crystal structure of the enzyme COX-1 and COX-2 from the RCSB Protein Data Bank (PDB); hydrogens ions were added after download. The ligand molecules were constructed using the Build Fragment tool and minimizing the energy for 1000 iterations until reaching a convergence equal to or lower than 0.01 kcal/mol Å. The docking experiment on COX-1 (PDB ID 1prh) was carried out by suitably positioning the energy-minimized ligand in the active site while carefully monitoring nonbonded interactions of the ligand-enzyme assembly and any side-chain bumps; in contrast, the docking experiment on COX-2 was carried out by superimposing the energy minimized ligand on SC-558 in the PDB ID 1cx2, at which point we deleted the structure of SC-558. In all experiments, the resulting ligand-enzyme complex was docked using the Libdock command (protocol of Discovery Studio) after defining subsets of the enzyme within a 10 Å radial distance from the ligand. In this regard, the force-field Chemistry HARvard Macromolecular Mechanics was used for all docking experiments. Subsequently, a molecular dynamics simulation was conducted with the corresponding ligand-enzyme complex using a simulation protocol at a temperature of 300 K, a 100-step equilibration/1000 iterations, a time step equal to 1 femtosecond (fs), and a distance-dependent dielectric constant 4r. Finally (by 1000 iterations), the optimal binding orientation of each ligand-enzyme complex was minimized by using the conjugate gradient method until reaching a convergence = 0.001 kcal/mol Å. The different intermolecular energies (Eintermolecular) between the ligand and the enzyme were evaluated and compared and, expressed in kcal/mol.
Statistics.
All data are presented as the mean ± S.E.M., with sample sizes of at least five rats per group (unless otherwise specified). Comparisons between groups were performed using a one-way analysis of variance followed by the Student’s t test.
Results
Determination of PGE2 Levels.
We observed that all three isomers of ASA (1 mmol/kg) decreased the COX-mediated PGE2 synthesis from 73 ± 4.4 pg/mg protein in the control group to about 10 pg/mg protein in animals treated with o-ASA, m-ASA, or p-ASA. This represents about an 86% reduction in PGE2 levels in gastric tissue compared with the control group; however, we did not find a statistically significant difference between the three ASA isomers, since m-ASA (9.7 ± 1.0 pg/mg protein) and p-ASA (10.7 ± 1.5 pg/mg of protein) decreased the biosynthesis of PGE2 to the same extent as compared with o-ASA (11.8 ± 1.2 pg/mg of protein). These results are shown in Fig. 2A.
Fig. 2.
Effects of positional isomers of aspirin on gastric PGE2 level, lipid peroxidation (MDA), SOD, and plasma TNF-α. Four groups of rats were treated with vehicle, o-ASA, m-ASA, and p-ASA, and their stomachs were removed and processed as described in Materials and Methods. All three drugs caused a significant reduction in gastric mucosal PGE2 levels (A). Results are mean ± S.E.M. of five rats in each group; *P < 0.01 versus vehicle group. All three compounds caused an almost 6-fold increase in MDA levels (B). Results are mean ± S.E.M. for five rats in each group; *P < 0.01 versus vehicle group. All three compounds caused a significant reduction in SOD activity (C). Results are mean ± S.E.M. of five rats; *P < 0.05 versus vehicle group. All three compounds caused a significant rise in plasma TNF-α (D). Results are mean ± S.E.M. for three rats in each group; *P < 0.01 versus vehicle.
Index of Lipid Peroxidation.
We determined the in vitro modulatory effects of o-ASA, m-ASA, and p-ASA (1 mmol/kg) on the peripheral markers of oxidative stress and lipid peroxidation, namely, MDA. As shown in Fig. 2B, the three positional isomers of aspirin increased the gastric tissue concentration of MDA. In this regard, we observed a significant increase in MDA levels from 8.4 ± 3.0 nmol/mg in the control group to 59.4 ± 2.1 nmol/mg for o-ASA, 63.0 ± 2.0 nmol/mg for m-ASA, and 66.6 ± 2.6 nmol/mg protein for p-ASA. This represents about a 7-fold increase in gastric MDA compared with the control group. According to these results, there appears to be a direct relationship between the chemical structure of ASA isomers and MDA levels. As we increased the distance between the acetoxy group and the carboxylic acid moiety present in aspirin (o-ASA), the concentration of MDA also increased (p-ASA > m-ASA ≈ o-ASA); however, this change was not statistically significant among ASA isomers and a definite conclusion in this regard cannot be made.
Superoxide Dismutase Activity.
We also determined the in vitro modulatory effects of o-ASA, m-ASA, and p-ASA (1 mmol/kg) on the activity of the antioxidant enzyme SOD. As shown in Fig. 2C, the three positional isomers of aspirin decreased the catalytic activity of SOD expressed in samples of gastric tissue. We observed a significant decrease in SOD activity from 2.9 ± 0.3 U/mg protein in the control group to 1.1 ± 0.4 U/mg for o-ASA, 1.0 ± 0.2 U/mg for m-ASA, and 0.8 ± 0.3 U/mg for p-ASA. Although there appears to be a downward trend in SOD enzymatic activity as a function of positional isomerism, a relationship between these two entities cannot be established.
Plasma TNF-α Levels.
The oral administration of o-ASA, m-ASA, or p-ASA (1 mmol/kg) produced a significant increase in the expression of the proinflammatory mediator TNF-α compared with that obtained in samples of gastric mucosa from control rats. In this regard, we determined the basal levels of TNF-α in animals receiving vehicle (control group) to be 5.2 ± 1.3 pg/ml, whereas the levels of this protein in treatment groups increased to 194.9 ± 3.1 pg/ml (o-ASA), 181.5 ± 4.3 pg/ml (m-ASA), and 187.7 ± 2.9 pg/ml (p-ASA). Based on these results, all three positional isomers of ASA were equally potent in increasing plasma TNF-α levels (Fig. 2D).
Cancer Cell Growth Inhibition.
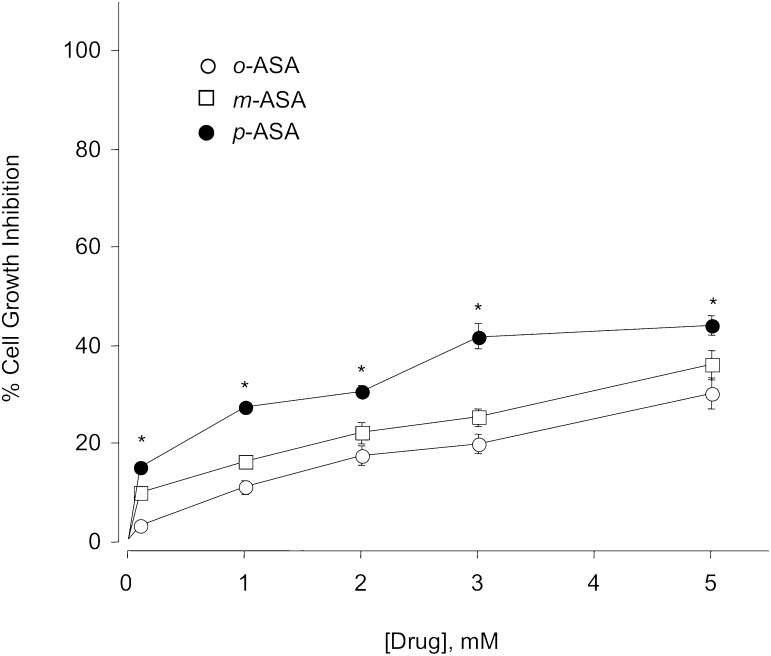
When we incubated the human colon adenocarcinoma HT-29 cells (0.5 × 106 cells/well) with o-ASA, m-ASA, or p-ASA, we observed a concentration-dependent cell growth inhibitory effect. All drugs were incubated at the same concentrations (0, 1, 2, 3, and 5 mM) during a 24-hour incubation period. Under these conditions, we observed that all ASA isomers exerted a modest inhibition in cell growth (28–43%) compared with control cells treated with vehicle (DMSO) only. According to our results, there was a direct and statistically significant relationship between the chemical structure of ASA isomers and cell growth inhibition. As we increased the distance between the acetoxy group and the carboxylic acid moiety present in aspirin (o-ASA), there was a small but significant increase in cancer cell growth inhibition (p-ASA > m-ASA ≈ o-ASA). The most potent isomer was p-ASA, which decreased cell growth by about 43% compared with vehicle-treated cells at the highest test compound concentration used in this experiment (5 mM). These results are graphically represented in Fig. 3.
Fig. 3.
Positional isomers of aspirin inhibit the growth of HT-29 human colon cancer cells. Cells were treated with various concentrations of o- m- p- ASA as described in Materials and Methods. Cell numbers were determined at 24 hours. The IC50 for cell growth inhibition was >5 mM for all three positional isomers of ASA; however, p-ASA was significantly more potent than the o- and m- isomer at all concentrations. Results represent means ± S.E.M. of three different experiments performed in triplicate. *P < 0.05 versus o-ASA and m-ASA.
Assay for Apoptosis.
Resistance against apoptosis is critical for cancer cell survival; therefore, we determined the percent of cells undergoing apoptosis on incubation with o-ASA, m-ASA, and p-ASA at different concentrations (3 and 5 mM) by the Annexin V assay. According to our results, we observed a dose-dependent induction of apoptosis in HT-29 cells exerted by all ASA isomers; the percent of cells undergoing apoptosis increased from 0 in control cells to about 15% in cells treated with ASA isomers at 3 mM, whereas the percentage of apoptotic cells was significantly higher (about 40%) in cells incubated in the presence of 5 mM o-ASA, m-ASA, or p-ASA. The distance between the acetoxy group and the carboxylic acid moiety present in ASA isomers was not a determinant for inducing apoptosis (Fig. 4A).
Fig. 4.
Positional isomers of aspirin induce apoptosis and inhibit proliferation of HT-29 cells. Cells were treated with o-ASA, m-ASA, or p-ASA at the concentration indicated for 24 hours, after which the cells were stained with annexin and propidium iodide and subjected to flow cytometric analysis as described in Materials and Methods. The percentage of apoptotic cells increased in a concentration-dependent manner (A). Cells were treated with o-ASA, m-ASA, or p-ASA at the concentrations indicated for 24 hours, after which PCNA expression was determined by flow cytometry and expressed as a percentage positive cells as described in Materials and Methods (B). Results are mean ± S.E.M. of three different experiments. *P < 0.05 compared with untreated cells.
Cell Proliferation.
To elucidate the mechanism underlying the effect of ASA isomers on cancer cell growth, we also determined the modulatory effect exerted by o-ASA, m-ASA, and p-ASA on cell proliferation. In this regard, we measured the expression of PCNA in human adenocarcinoma HT-29 cells incubated for 24 hours in the presence of ASA isomers (1, 3, and 5 mM). We observed that all ASA isomers exerted a dose-dependent decrease in cancer cell proliferation by reducing PCNA expression in HT-29 cells from 100% in the control group to 57–87% in treated cells (Fig. 4B). At 1 mM, the three ASA isomers exerted a nonsignificant reduction in PCNA expression; however, this decrease was significant in cells treated with 3 mM or 5 mM o-ASA, m-ASA, or p-ASA. The modulation of cell proliferation induced by ASA isomers was not conclusively correlated with structural differences in these molecules; it was only at the highest test compound concentration (5 mM) that we observed a modest nonsignificant correlation (o-ASA > m-ASA > p-ASA) between them.
Cell-Cycle Analysis.
We incubated HT-29 cells in the presence of the three positional isomers of ASA for 24 hours, analyzing the end results by flow cytometry. We observed that o-ASA, m-ASA, and p-ASA induced a similar dose-dependent accumulation of HT-29 cells in the Go/G1 phase relative to the population of cells in Go/G1 phase in the untreated controls (40.3% ± 0.9%). For example, treatment of HT-29 cells with o-ASA increased the relative population of HT-29 cells in Go/G1 phase from 0 to 69.3% ± 0.6% at 3 mM and 86.3% ± 0.8% at 5 mM. The phase-specific cell-cycle arrest exerted by the m-ASA (66.7% ± 0.62% at 3 mM, 82.7% ± 0.8% at 5 mM), and p-ASA (62.4% ± 0.6% and 87.2% ± 0.8% at 3 and 5 mM, respectively). These results are represented graphically in Fig. 5, A–C.
Fig. 5.
Effects of positional isomers of aspirin on cell cycle in HT-29 cells. Cells were treated for 24 hours with various concentrations of o-ASA, m-ASA, or p-ASA, and their cell-cycle phase distribution was determined by flow cytometry as described in Materials and Methods. Results are representative of two different experiments. This study was repeated twice, generating results within 10% of those presented here.
Cyclooxygenase Inhibition Assay.
We determined the percent of enzyme inhibition exerted by o-ASA, m-ASA, and p-ASA isomers following two different protocols. In the first protocol, we carried out a concentration-dependent (1, 3, 5 mM) COX inhibition (Table 1), whereas in the second protocol, we measured the percent of COX-1 inhibition exerted by m-ASA at different time points (2, 5, 15, 30, and 120 minutes) at 1 mM (Table 2). To estimate the extent of irreversible acetylation exerted by the different ASA isomers, we determined the percent of COX inhibition after filtration of the corresponding inhibitor solution by microdialysis.
TABLE 1.
Inhibition of COX-1 and COX-2 by positional isomer of aspirin before and after dialysis
Ovine COX-1 or COX-2 was incubated in the presence of o-ASA, m-ASA, or p-ASA together with arachidonic acid and enzyme activity determined before and after microdialysis as detailed in Materials and Methods. Results are mean ± S.E.M. for five to seven different determinations with enzymes assays being done in triplicates.
| Agent | COX-1 Inhibition (%) |
COX-2 Inhibition (%) |
||
|---|---|---|---|---|
| Before | After | Before | After | |
| Dialysis | Dialysis | Dialysis | Dialysis | |
| o-ASA 1 mM | 72 ± 3 | 72 ± 4 | ND | ND |
| 3 mM | 87 ± 3 | 86 ± 3 | 83 ± 2 | 81 ± 2 |
| 5 mM | 93 ± 4 | 95 ± 3 | 95 ± 2 | 95 ± 3 |
| m-ASA 1 mM | 54 ± 2† | 38 ± 2*,¶ | ND | ND |
| 3 mM | 63 ± 3† | 45 ± 3*,¶ | 80 ± 2 | 56 ± 3*,¶ |
| 5 mM | 88 ± 2 | 70 ± 2*,¶ | 99 ± 3 | 76 ± 4*,¶ |
| p-ASA 1 mM | 48 ± 2† | 29 ± 1*,¶ | ND | ND |
| 3 mM | 71 ± 3† | 54 ± 3*,¶ | 80 ± 3 | 38 ± 2*,¶ |
| 5 mM | 94 ± 3 | 73 ± 3*,¶ | 94 ± 4 | 67 ± 4*,¶ |
| Indomethacin | ||||
| 1 µM | 69 ± 2 | 24 ± 1* | 67 ± 2 | 32 ± 2* |
ASA, acetyl salicylic acid (aspirin); COX, cyclooxygenase; ND, not done.
P < 0.05 compared with before dialysis.
P < 0.05 compared with o-ASA at same concentration before dialysis.
P < 0.05 compared with o-ASA at same concentration after dialysis.
TABLE 2.
Time course inhibition of COX-1 by m-ASA
Effect of m-ASA on COX-1 enzyme activity was determined as a function of time before and after dialysis as detailed in Materials and Methods. Results are mean ± S.E.M. for five different determinations with enzymes assays being done in triplicates.
| Time, min |
m-ASA, 1 mM |
|
|---|---|---|
| COX-1 Inhibition (%) |
||
| Before Dialysis | After Dialysis | |
| 2 | 5.0 ± 1 | 4.2 ± 1 |
| 5 | 18 ± 2 | 13 ± 1 |
| 15 | 46 ± 2 | 20 ± 2* |
| 30 | 54 ± 3 | 34 ± 1* |
| 120 | 43 ± 2 | 27 ± 1* |
ASA, acetyl salicylic acid (aspirin); COX, cyclooxygenase.
P < 0.05 compared with before dialysis.
Results obtained in the first protocol showed a concentration-dependent COX inhibition exerted by o-ASA, m-ASA, and p-ASA. In this regard, we observed that aspirin (o-ASA) exerted a marked inhibitory profile on COX-1 by inhibiting the catalytic activity of this enzyme (72–93% inhibition) within the range of concentrations used (1–5 mM), and this profile did not change after replacing the medium by microdialysis (Table 1). However, the inhibitory profile exerted by the other two ASA isomers was significantly lower at 1 mM (o-ASA = 54%, m-ASA = 48%) and 3 mM (o-ASA = 63%, m-ASA = 71%). In contrast, we observed that at 5 mM, the three ASA isomers exerted the same inhibitory profile (93% ± 4%, 88% ± 2%, and 94% ± 3% inhibition for o-ASA, m-ASA, and p-ASA respectively). Nevertheless, unlike the o-ASA isomer, the m-ASA and p-ASA compounds exerted a significantly lower COX-1 inhibitory profile after microdialysis (Table 1).
In regard to COX-2 inhibition, we observed that before microdialysis, the three ASA isomers exerted the same degree of enzyme inhibition. For example, the o-ASA isomer inhibited COX-2 by 83% ± 2% and 95% ± 2% at 3 and 5 mM, respectively, whereas the m-ASA and p-ASA isomers inhibited this enzyme by 80% ± 2%, 80% ± 3% at 3 mM, and 99% ± 3%, 94% ± 4% at 5 mM. However, as noted for COX-1 inhibition, only the o-ASA isomer exerted the same degree of COX-2 inhibition before and after microdialysis, since the other two isomers exerted a significantly lower inhibitory profile after microdialysis. For example, the m-ASA isomer inhibited COX-2 by 56% ± 3% at 3 mM after the microdialysis protocol, compared with 80% ± 2% before microdialysis at the same concentration. The same trend was observed for this compound at 5 mM (99% ± 3% and 76% ± 4% inhibition before and after dialysis, respectively).
The second protocol showed that m-ASA decreased the enzymatic activity of COX-1 in a time-dependent manner. However, this time-dependent inhibition was observed only within the first 30 minutes because at the last time point (120 minutes), we observed an 11% decrease in potency (43% inhibition compared with 54% at 30 minutes). As noted for the concentration-dependent experiments described previously herein, in the time-dependent protocol, we also observed a significant decrease in potency after removal of the inhibitor by microdialysis (Table 2).
Ulcerogenicity.
We carried out two different assays to evaluate the toxic side effects exerted by an acute oral equimolar (1 mmol/kg) dose of o-ASA, m-ASA, and p-ASA isomers. In the first experiment, we determined macroscopic ulcerative lesions in the glandular region of the rat stomach (UI; Fig. 6A); in the second one, we determined smaller points (erosions) on the stomach epithelial layer (EI; Fig. 6B), which are only visible using a magnifying lens. In this regard, animals in the control group showed a normal glandular region with no ulcers or erosions on the luminal surface (UI = EI = 0). As expected, the administration of o-ASA resulted in extensive mucosal injury (UI = 45 ± 3.1) to the glandular portion of the gastric fundus; however, the administration of m-ASA (UI = 14 ± 3.0) and p-ASA (UI = 17 ± 4.1) produced a significantly decreased ulcerogenicity compared with the o-isomer. On the other hand, when we compared these drugs in the EI assay, we observed an opposite trend where aspirin (EI = 28 ± 1.7) was the least toxic compound. The positional isomers of aspirin m-ASA (EI = 46 ± 4.0) and p-ASA (EI = 41 ± 4.1) were significantly more aggressive in this assay compared with the o-ASA isomer.
Fig. 6.
m-ASA and p-ASA cause less gastric damage compared with o-ASA. Drugs were administered orally at equimolar doses (1 mmol/kg) and effects on the stomach were evaluated as indicated in Materials and Methods. o-ASA caused severe gastric damage, UI = 45 ± 3.1 mm, whereas both m-ASA and p-ASA were relatively gastric damage sparing, UI = 14 ± 3.0 mm and 17 ± 4.1 mm for m-ASA and p-ASA, respectively (A). All three drugs also caused erosions of the gastric mucosa, but the damage was less with o-ASA compared with that of m-ASA and p-ASA (B). Results are mean ± S.E.M. for five rats in each group, *P < 0.05 compared with the vehicle group; †P < 0.05 compared with o-ASA.
Docking Results.
We carried out a comprehensive molecular modeling (docking) study to evaluate the differential binding interactions observed between o-ASA, m-ASA, and p-ASA isomers within the active sites of COX-1 and COX-2. These results are summarized below.
Docking Aspirin in COX-1.
The most stable enzyme-ligand complex (Fig. 7A) showed that the central phenyl ring was oriented in a hydrophobic region at the center of the COX active site, surrounded by Leu352, Leu384, Tyr385, Trp387, Ile523, and Met522 (distance < 5 Å). The C-1 carboxylate of aspirin was oriented toward the mouth of the active site closer to polar amino acids such as Arg120 and Tyr355. The carboxylate (COOH) underwent two electrostatic interactions with both hydrogen atoms of NH2 of Arg120 (distance <4 Å), and was about 4.9 Å away from OH of Tyr355. As expected, the C-2 acetoxy substituent (OCOCH3) was oriented 4.20 Å away from OH of Ser530 (reported acetylation site for aspirin) in the COX-1 active site. Moreover, the acetoxy CH3 group underwent nonpolar binding interactions (<5 Å) with Val349 and Leu531. This indicates that the C-2 acetoxy group in aspirin is suitably positioned to irreversibly acetylate Ser530.
Fig. 7.
Docking of positional isomers of aspirin to the active site of cyclooxygenase-1 and -2. Hydrogen atoms are not shown for clarity.
Docking Aspirin in COX-2.
The most stable enzyme-ligand complex (Fig. 7D) showed that the central phenyl ring of aspirin was oriented in a region at the center of the active site surrounded by Val349, Leu352, Leu384, Trp387, Met522, Val523, and Ala527 (distance <5 Å). The C-1 carboxylate was oriented close to the mouth of the COX-2 active site, and the carboxylate COOH was about 6.4 Å away from NH2 of Arg120 and about 5.3 Å away from OH of Tyr355. The C-2 acetoxy (OCOCH3) substituent was oriented toward Tyr385 and Ser530; the carbonyl (C = O) was about 3.4 Å away from OH of Ser530. The C = O group also formed a hydrogen bond with OH of Tyr385 (distance = 3.07 Å), which plays a role in the aspirin-mediated acetylation of Ser530.
Docking ASA Isomers in COX-1.
The most stable enzyme-ligand complexes (Fig. 7, B and C) showed that the central phenyl rings were oriented in a hydrophobic region at the center of the active site surrounded by Leu352, Phe518, Ile523, Met522, and Ala527 (distance <5 Å). The C-1 carboxylate of both m-ASA and p-ASA was oriented toward the mouth of the active site closer to the polar amino acids Arg120 and Tyr355. As noted for aspirin, the carboxylate (COOH) moieties in m-ASA and p-ASA were about 3.6 and 3.5 Å away from OH of Tyr355, respectively, undergoing electrostatic interactions with both hydrogen atoms of NH2 of Arg120 (distance <2.5 Å). The C-3 acetoxy group (OCOCH3) was oriented toward the apex of the COX-1 active site (Leu352, Typ385, and Trp387), which locates their C = O ester group near (about 4.3–5.5 Å away) from OH of Ser530. This suggests that despite the meta and para relationships between the carboxy and the ester groups in these ASA isomers, the acetoxy moiety is still located near polar amino acids at the active site and favors the orientation of the acetoxy groups toward Ser530, the acetylation site for aspirin.
Docking ASA Isomers in COX-2.
We observed that the central phenyl rings were also oriented in a hydrophobic region at the center of the COX-2 active site surrounded by Val349, Leu352, Ser353, and Val523 (around 5 Å away). The C-1 carboxylate of the two aspirin derivatives was oriented close to the mouth of the COX-2 active site; the carboxylate COOH moieties formed a hydrogen bond with OH of Tyr355 (distance = 3.2 and 3.4 Å for m- and p-ASA, respectively) and were about 4.1–5.8 Å away from Arg120. Because of the presence of a small Val523 in the COX-2 active site, we observed that the phenyl ring with the C-1 acid substituent in m-ASA was able to interact with polar amino acid residues (His90 and Arg513) present in the secondary pocket of COX-2; this was not the case for p-ASA. These observations indicate that the COOH group in m-ASA acted as an anchor orienting the acetoxy group toward Ser530, the acetylation site of aspirin. However, for both isomers, the C-3 acetoxy substituent (OCOCH3) was oriented toward the apex of the active site in a hydrophobic region comprising Leu384, Tyr385, Trp387, Phe518, and Met522 (distance <5 Å), and the distances between the acetoxy group (OCOCH3) in m-ASA and p-ASA and the OH of Ser530 (the acetylation site of aspirin), were about 6.75 and 5.6 Å, respectively (see Fig. 7, E and F).
Discussion
The major pharmacological mechanism of action exerted by NSAIDs is the inhibition of COX-1 and COX-2 enzymes. Aspirin is the only NSAID that irreversibly inhibits both enzymes, decreasing the amount of proinflammatory PGs; consequently, aspirin also decreases the production of cytoprotective PGE2 in vivo (Ligumsky et al., 1982; Lichtenberger et al., 2007) When administered at equimolar doses (1 mmol/kg orally), o-ASA, m-ASA, and p-ASA exerted the same biologic profile, which suggests that all ASA isomers inhibited, to the same extent, COX-derived PGE2 synthesis in gastric tissue. In the present study, we did not evaluate the anti-inflammatory profile of ASA isomers, but according to these results, it is reasonable to assume that regardless of positional isomerism, the ASA COX pharmacophore is equally effective in decreasing the biosynthesis of PGs locally and systemically.
The enzymatic inhibition of COX enzymes exerted by aspirin (IC50 values) reported in the literature varies considerably, depending on the experimental conditions used for the particular assay. In the present study, we worked with an in vitro system with purified ovine COX-1 and COX-2 resuspended in a reaction buffer with no cell fractions, thus excluding enzymatic degradation of ASA isomers by nonspecific esterases. The irreversible inhibition of COX enzymes exerted by o-ASA is demonstrated by comparing the activity of COX-1 and COX-2 before and after microdialysis, which after 30 minutes of incubation eliminates any unbound drug from the enzyme suspension by filtration through a semipermeable membrane. We observed that only the o-ASA isomer showed the same degree of inhibition before and after microdialysis, whereas the m-ASA and p-ASA isomers showed lower inhibitory profiles after the drug was removed. This may suggest that either the m-ASA and p-ASA exert a lower degree of acetylation in the active site compared with o-ASA, or they reversibly inhibit COX-1 and COX-2 enzymes. In this regard, we used indomethacin as reference drug. After dialysis, the potency of indomethacin decreased considerably, from 69 to 24%, on COX-1, and from 67 to 32% inhibition on COX-2, which clearly showed a reversible pattern of enzyme inhibition.
To investigate the potential irreversible inhibition of COX enzymes exerted by the three ASA isomers, we carried out a series of molecular modeling (docking) studies to evaluate their differential binding interactions within the active site of COX enzymes. As expected, the acetyl group in o-aspirin was oriented toward the Ser530 residue in both proteins; however, we observed that despite a few differences in orientation between m- and p-ASA isomers, these compounds also adopt relatively favorable conformations which may lead to acetylation of Ser530. This observation is supported by the bioequivalent in vivo profile (decrease in PGE2 levels) exerted by all ASA isomers when administered orally to rats.
The significant increase in the levels of MDA in samples of gastric tissue suggests that o-ASA, m-ASA, and p-ASA induced oxidative stress to the same extent. According to our results, changes in the relative position of the carboxylic acid group relative to the acetoxy group did not alter the well established tendency of aspirin (Chattopadhyay et al., 2010) to induce an intracellular oxidative environment. This observation correlates well with the observed decrease in the activity of SOD in gastric tissue. In a previous study, our group reported the inhibitory activity of aspirin on this antioxidant enzyme (Chattopadhyay et al., 2010); according to the results obtained in the present work, the other two ASA isomers are equally suited to decrease the catalytic activity of SOD. Furthermore, we observed that all ASA isomers exerted an upregulation of the proinflammatory mediator TNF-α, which has been reported to trigger the adherence and activation of leukocytes, leading to the release of oxygen-derived free radicals (oxygen superoxide) and proteases, producing epithelial injury (Perini et al., 2004). However, despite these observations suggesting that all ASA isomers would exert the same degree of gastric toxicity, we were surprised to see that, according to the ulcer index assay, the o-ASA was significantly more ulcerogenic than the m-ASA or p-ASA isomers. This piece of information is interesting, considering that all isomers decreased PGE2 levels, increased cellular oxidative stress as determined by MDA, and inhibited the antioxidant enzyme SOD to the same extent. If we analyze the results obtained in the UI assay, we could speculate that the position of the acetyl group (COCH3), relative to that of the carboxylic acid (COOH) moiety in acetylsalicylic acids, plays a major role in the ulcerogenic response. However, when we compared the erosion index for all ASA isomers, we observed that the m-ASA and p-ASA isomers are significantly more toxic than o-ASA, producing more epithelial gastric erosions at equimolar doses. This structure-activity relationship may be useful in future studies to fine tune the pharmacological and toxicological profile of ASA derivatives and warrants further studies.
The use of NSAIDs has been correlated with a lower incidence of colon cancer among regular users of these drugs (Chan et al., 2007; Cole et al., 2009; Gao et al., 2009; Grau et al., 2009; Cooper et al., 2010). We know that NSAIDs induce apoptosis in many cells and in response to different stimuli. Proposed mechanisms for the proapoptotic effects include activation of caspases, induction of cytochrome c release, regulation of protein kinase C, inhibition of nuclear factor-κB, and suppression of activator protein-1 (Wong et al., 2004). In the current study, we determined that there was a significant difference between ASA isomers in their ability to inhibit cancer (HT-29) cell growth in vitro, where p-ASA was the most potent isomer, followed by the m-ASA and the o-ASA. This biologic profile correlated with a significant decrease in cell proliferation and induction of apoptosis at 3 and 5 mM. Further studies confirmed that all ASA isomers dose-dependently induced a cell-cycle arrest in HT-29 cells, producing an increased percentage of cells in the Go/G1 phase, relative to the population of cells in the untreated control. In this regard, we did not find any significant difference between ASA isomers, which suggests that the anticancer and chemopreventive effects of acetylsalicylic acids are independent from the relative position of COOH and COCH3 moieties in the pharmacophore.
In conclusion, the observed structure-activity relationships among ASA isomers suggests that m-ASA and p-ASA should be considered equivalent to aspirin from a pharmacological point of view because despite differences observed in the in vitro assays, all ASA isomers decreased PGE2 levels to the same extent in vivo. Moreover, despite the marked similarities in the synthesis of MDA, expression of TNF-α, and inhibition of antioxidant SOD in gastric tissue, there is a significant difference between different ASA isomers in their ability to produce gastric ulcers when administered orally, which warrants further studies to explore the potential use of relatively safer ASA analogs possessing either a meta- or para- relationship between the COOH and COCH3 moieties.
Abbreviations
- ASA
acetylsalicylic acid (aspirin)
- COX
cyclooxygenase
- DMSO
dimethylsulfoxide
- EI
erosion index
- 15-R-HETE
15-(R)-hydroxyeicosatetraenoic acid
- HT-29
human adenocarcinoma colon cancer cell
- MDA
malondialdehyde
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PCNA
proliferating cell nuclear antigen
- PG
prostaglandin
- PGE2
prostaglandin E2
- SOD
superoxide dismutase
- TNF-α
tumor necrosis factor-alpha
- UI
ulcer index
Authorship Contributions
Participated in research design: Kashfi, Kodela, Velázquez-Martínez.
Conducted experiments: Kodela, Chattopadhyay, Goswami, Gan, Rao, Nia.
Performed data analysis: Kashfi, Velázquez-Martínez, Chattopadhyay.
Wrote or contributed to the writing of the manuscript: Kashfi, Rao, Chattopadhyay, Velázquez-Martínez.
Footnotes
This work was supported in part by the National Institutes of Health National Cancer Institute through a subcontract from ThermoFisher [Contract #FBS-43312-26]; and by the National Institutes of Health [Grant R24 DA018055].
References
- Aalykke C, Lauritsen K. (2001) Epidemiology of NSAID-related gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol 15:705–722 [DOI] [PubMed] [Google Scholar]
- Awtry EH, Loscalzo J. (2000) Aspirin. Circulation 101:1206–1218 [DOI] [PubMed] [Google Scholar]
- Bardia A, Olson JE, Vachon CM, Lazovich D, Vierkant RA, Wang AH, Limburg PJ, Anderson KE, Cerhan JR. (2011) Effect of aspirin and other NSAIDs on postmenopausal breast cancer incidence by hormone receptor status: results from a prospective cohort study. Breast Cancer Res Treat 126:149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best R, Lewis DA, Nasser N. (1984) The anti-ulcerogenic activity of the unripe plantain banana (Musa species). Br J Pharmacol 82:107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AT, Ogino S, Fuchs CS. (2007) Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med 356:2131–2142 [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M, Velazquez CA, Pruski A, Nia KV, Abdellatif KR, Keefer LK, Kashfi K. (2010) Comparison between 3-nitrooxyphenyl acetylsalicylate (NO-ASA) and O2-(acetylsalicyloxymethyl)-1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate (NONO-ASA) as safe anti-inflammatory, analgesic, antipyretic, antioxidant prodrugs. J Pharmacol Exp Ther 335:443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BF, Logan RF, Halabi S, Benamouzig R, Sandler RS, Grainge MJ, Chaussade S, Baron JA. (2009) Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst 101:256–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K, Squires H, Carroll C, Papaioannou D, Booth A, Logan RF, Maguire C, Hind D, Tappenden P. (2010) Chemoprevention of colorectal cancer: systematic review and economic evaluation. Health Technol Assess 14:1–206 [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Del Soldato P. (2003) NO-aspirin: mechanism of action and gastrointestinal safety. Dig Liver Dis 35 (Suppl 2):S9–S19 [DOI] [PubMed] [Google Scholar]
- Gao F, Liao C, Liu L, Tan A, Cao Y, Mo Z. (2009) The effect of aspirin in the recurrence of colorectal adenomas: a meta-analysis of randomized controlled trials. Colorectal Dis 11:893–901 [DOI] [PubMed] [Google Scholar]
- Grau MV, Sandler RS, McKeown-Eyssen G, Bresalier RS, Haile RW, Barry EL, Ahnen DJ, Gui J, Summers RW, Baron JA. (2009) Nonsteroidal anti-inflammatory drug use after 3 years of aspirin use and colorectal adenoma risk: observational follow-up of a randomized study. J Natl Cancer Inst 101:267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grösch S, Maier TJ, Schiffmann S, Geisslinger G. (2006) Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst 98:736–747 [DOI] [PubMed] [Google Scholar]
- Harris RE. (2007) Cyclooxygenase-2 (cox-2) and the inflammogenesis of cancer. Subcell Biochem 42:93–126 [DOI] [PubMed] [Google Scholar]
- Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. (2010) Aspirin intake and survival after breast cancer. J Clin Oncol 28:1467–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashfi K, Rigas B. (2005a) Is COX-2 a ‘collateral’ target in cancer prevention? Biochem Soc Trans 33:724–727 [DOI] [PubMed] [Google Scholar]
- Kashfi K, Rigas B. (2005b) Non-COX-2 targets and cancer: expanding the molecular target repertoire of chemoprevention. Biochem Pharmacol 70:969–986 [DOI] [PubMed] [Google Scholar]
- Kashfi K, Ryan Y, Qiao LL, Williams JL, Chen J, Del Soldato P, Traganos F, Rigas B. (2002) Nitric oxide-donating nonsteroidal anti-inflammatory drugs inhibit the growth of various cultured human cancer cells: evidence of a tissue type-independent effect. J Pharmacol Exp Ther 303:1273–1282 [DOI] [PubMed] [Google Scholar]
- Lichtenberger LM, Romero JJ, Dial EJ. (2007) Surface phospholipids in gastric injury and protection when a selective cyclooxygenase-2 inhibitor (Coxib) is used in combination with aspirin. Br J Pharmacol 150:913–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligumsky M, Grossman MI, Kauffman GL., Jr (1982) Endogenous gastric mucosal prostaglandins: their role in mucosal integrity. Am J Physiol 242:G337–G341 [DOI] [PubMed] [Google Scholar]
- Liu JF, Jamieson GG, Wu TC, Zhu GJ, Drew PA. (2009) A preliminary study on the postoperative survival of patients given aspirin after resection for squamous cell carcinoma of the esophagus or adenocarcinoma of the cardia. Ann Surg Oncol 16:1397–1402 [DOI] [PubMed] [Google Scholar]
- Masferrer JL, Zweifel BS, Manning PT, Hauser SD, Leahy KM, Smith WG, Isakson PC, Seibert K. (1994) Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci USA 91:3228–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandeya N, Webb PM, Sadeghi S, Green AC, Whiteman DC, Australian Cancer Study (2010) Gastro-oesophageal reflux symptoms and the risks of oesophageal cancer: are the effects modified by smoking, NSAIDs or acid suppressants? Gut 59:31–38 [DOI] [PubMed] [Google Scholar]
- Perini R, Fiorucci S, Wallace JL. (2004) Mechanisms of nonsteroidal anti-inflammatory drug-induced gastrointestinal injury and repair: a window of opportunity for cyclooxygenase-inhibiting nitric oxide donors. Can J Gastroenterol 18:229–236 [DOI] [PubMed] [Google Scholar]
- Qiao L, Kozoni V, Tsioulias GJ, Koutsos MI, Hanif R, Shiff SJ, Rigas B. (1995) Selected eicosanoids increase the proliferation rate of human colon carcinoma cell lines and mouse colonocytes in vivo. Biochim Biophys Acta 1258:215–223 [DOI] [PubMed] [Google Scholar]
- Rouzer CA, Marnett LJ. (2009) Cyclooxygenases: structural and functional insights. J Lipid Res 50 (Suppl):S29–S34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer D, Florin T, Eagle C, Marschner I, Singh G, Grobler M, Fenn C, Schou M, Curnow KM. (2006) Risk of serious NSAID-related gastrointestinal events during long-term exposure: a systematic review. Med J Aust 185:501–506 [DOI] [PubMed] [Google Scholar]
- Scheiman JM, Fendrick AM. (2007) Summing the risk of NSAID therapy. Lancet 369:1580–1581 [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N. (2002) Lipid-derived mediators in endogenous anti-inflammation and resolution: lipoxins and aspirin-triggered 15-epi-lipoxins. Scientific World J 2:169–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. (1998) Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res 58:362–366 [PubMed] [Google Scholar]
- Singh G, Triadafilopoulos G. (1999) Epidemiology of NSAID induced gastrointestinal complications. J Rheumatol Suppl 56:18–24 [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. (2000) Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69:145–182 [DOI] [PubMed] [Google Scholar]
- Tenenbaum J. (1999) The epidemiology of nonsteroidal anti-inflammatory drugs. Can J Gastroenterol 13:119–122 [DOI] [PubMed] [Google Scholar]
- Van Dyke AL, Cote ML, Prysak G, Claeys GB, Wenzlaff AS, Schwartz AG. (2008) Regular adult aspirin use decreases the risk of non-small cell lung cancer among women. Cancer Epidemiol Biomarkers Prev 17:148–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane JR. (1971) Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 231:232–235 [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. (1998) Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 38:97–120 [DOI] [PubMed] [Google Scholar]
- Wong BC, Jiang XH, Lin MC, Tu SP, Cui JT, Jiang SH, Wong WM, Yuen MF, Lam SK, Kung HF. (2004) Cyclooxygenase-2 inhibitor (SC-236) suppresses activator protein-1 through c-Jun NH2-terminal kinase. Gastroenterology 126:136–147 [DOI] [PubMed] [Google Scholar]
- Yasuda O, Takemura Y, Kawamoto H, Rakugi H. (2008) Aspirin: recent developments. Cell Mol Life Sci 65:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans ND, Hawkey CJ, Brailsford W, Naesdal J. (2009) Gastroduodenal toxicity of low-dose acetylsalicylic acid: a comparison with non-steroidal anti-inflammatory drugs. Curr Med Res Opin 25:2785–2793 [DOI] [PubMed] [Google Scholar]