Abstract
The subarachnoid space, where cerebrospinal fluid (CSF) flows over the brain and spinal cord, is lined on one side by arachnoid barrier (AB) cells that form part of the blood-CSF barrier. However, despite the fact that drugs are administered into the CSF and CSF drug concentrations are used as a surrogate for brain drug concentration following systemic drug administration, the tight-junctioned AB cells have never been examined for whether they express drug transporters that would influence CSF and central nervous system drug disposition. Hence, we characterized drug transporter expression and function in AB cells. Immunohistochemical analysis showed P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) in mouse AB cells but not other meningeal tissue. The Gene Expression Nervous System Atlas (GENSAT) database and the Allen Mouse Brain Atlas confirmed these observations. Microarray analysis of mouse and human arachnoidal tissue revealed expression of many drug transporters and some drug-metabolizing enzymes. Immortalized mouse AB cells express functional P-gp on the apical (dura-facing) membrane and BCRP on both apical and basal (CSF-facing) membranes. Thus, like blood-brain barrier cells and choroid plexus cells, AB cells highly express drug transport proteins and likely contribute to the blood-CSF drug permeation barrier.
Introduction
The blood-brain barrier (BBB) (endothelial cells in the veins, arteries, and capillaries of the brain and spinal cord) and the blood–cerebrospinal fluid barrier (BCSFB) [comprising choroid plexus (CP) epithelial cells and arachnoid barrier (AB) epithelial cells of the meninges) consist of tight-junctioned cells that form physical barriers to central nervous system (CNS) and cerebrospinal fluid (CSF) drug penetration (Fig. 1) (Saunders et al., 2008). The BBB and CP also express drug transporters that affect drug penetration into the brain (Redzic, 2011). To the best of our knowledge, AB cells have not been characterized previously for drug transporter expression.
Fig. 1.
AB cell–CSF interface. Meninges comprising pia mater (on the brain surface and lining one side of the CSF in the subarachnoid space), AB cells (sandwiching one side of the CSF space), and the dura mater containing fenestrated (leaky) capillaries. (A) Expression and location of drug transporters determined from this manuscript. (B) Coronal section of the brain showing the three drug transporter barrier cells, BBB, CP, and AB cells, relative to the CSF produced by the CP and circulating in the subarachnoid space.
The AB cells make up part of the three-layered meninges that cover the brain and spinal cord (Fig. 1). The meninges consist of an outermost dura layer (adjacent to the skull) and the two innermost layers (leptomeninges), comprising the arachnoid mater, which includes the tight-junctioned AB cells that sit adjacent to the CSF in the subarachnoid space; and the innermost pia mater, consisting of non-tight-junctioned cells lining the brain surface. The AB cell layer has numerous tight junctions and functions as the physiologic barrier between the CSF in the subarachnoid space and the fenestrated capillaries in the dura (Schachenmayr and Friede, 1978; Vandenabeele et al., 1996).
Knowledge of drug transporter expression and localization in AB cells bordering the CSF in the subarachnoid space is important for two reasons. First, a growing number of drugs, including chemotherapies, are administered intrathecally (into the CSF of the subarachnoid space) by intralumbar injection (Stapleton and Blaney, 2006). For example, a regimen of 13–28 intralumbar chemotherapy injections is standard therapy to eradicate CSF lymphoblasts in childhood acute lymphoblastic leukemia patients because they are at high risk for CNS relapse (Pui et al., 2009). This drug therapy has now fully replaced the more neurotoxic cranial irradiation. Intrathecal chemotherapy is also used to treat some types of brain and leptomeningeal tumors and to treat other diseases such as meningitis. It would be expected that drug transporters expressed in AB cells would influence the concentration of intrathecally administered drugs that remain in the CSF, their rate of egress from the CSF, and hence, the concentration available to the CNS. Second, the concentration of drugs in the CSF is often used as a surrogate for that of unbound drug in the interstitial fluid in the brain and, hence, available to drug targets (Kodaira et al., 2011). Thus, identification of transporters localized in the AB cells adjacent to the CSF space is necessary to correctly interpret exposure-response relationships for CNS-acting drugs. The goal of this study was to determine whether drug transporters are expressed in AB cells and evaluate their cellular localization and function.
Materials and Methods
Mice.
Animal studies were conducted under protocols approved by the St. Jude Children’s Research Hospital Committee on the Use and Care of Animals. Female (12- to 16-week-old) P-glycoprotein (P-gp) wild-type (WT) and knockout (KO) [CF-1 mice (Charles River Laboratories, Wilmington, MA) deficient in Pgp] (Pippert and Umbenhauer, 2001) and C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were maintained in a pathogen-free facility. Abcg2-EGFP (enhanced green fluorescent protein) mice have been previously described (Tadjali et al., 2006).
Immunohistochemistry on Tissues.
Mouse tissue was isolated from P-gp WT and KO and Abcg2-EGFP mice. Human brain tissue was obtained from normal brain at autopsy of anonymous donors within 24 hours from the time of death. Tissue was fixed with 10% formalin for 24 hours and paraffin sections processed by heat-induced epitope retrieval. Slides were blocked with 5% normal goat serum/phosphate-buffered saline (PBS) for 30 minutes, followed by overnight incubation with rabbit anti-P-gp (1:4000) we developed (raised against amino acids 555–575 of human P-gp and immunopurified with the same peptide) or with mouse anti-P-gp antibody JSB-1 (1:20) (Abcam, Cambridge, MA) or with the rat monoclonal anti-BCRP (breast cancer resistance protein) IgG BXP-53 (Kamiya Biomedical, Seattle, WA) at 4°C or with anti-GFP (green fluorescent protein) IgG (Life Technologies, Grand Island, NY) followed by appropriate secondary antibodies, and detected with the streptavidin-biotin immunoperoxidase method and diaminobenzidine substrate for visualization. After counterstaining with hematoxylin, the slides were mounted. For negative control, the primary antibody was omitted.
Bcrp Gene Expression Analysis—Allen Brain Atlas.
Bcrp mRNA expression pattern was analyzed in the Allen Mouse Brain Atlas in situ hybridization database [Lau et al., 2008; Allen Mouse Brain Atlas (http//mouse.brain-map.org)].
P-gp (Abcb1a) Gene Expression Analysis—Gene Expression Nervous System Atlas.
Localization of P-gp (Abcb1a) expression in mouse AB tissue was analyzed in the Gene Expression Nervous System Atlas (GENSAT, http://www.gensat.org) database (Gong et al., 2003) in Abcb1a-EGFP bacterial artificial chromosome reporter mice in which the EGFP reporter gene is inserted immediately upstream of the coding sequence for P-gp and brain tissue from the mice is immunostained with an anti-GFP antibody.
Creation of Immortalized Mouse AB Cells.
Leptomeningeal tissue was isolated from Abcg2-GFP male and female mouse embryos (P3), combined, and placed in cold dissociation media (DM) containing 90 mM Na2SO4, 30 mM K2SO4, 0.25 mM CaCl2, 5.8 mM MgCl2, 10 mM glucose, and 1 mM HEPES. Leptomeningeal tissue was centrifuged at 800g for 10 minutes and dissociated for 25 minutes in 37°C DM with 0.0467% collagenase followed by rapid pipetting through a 1-ml pipet, washed in DM, and pelleted. Cells were cultured until confluent on poly-d-lysine–coated dishes in Dulbecco’s modified Eagle’s medium with 1% fetal bovine serum/9% horse serum, amphotericin B, penicillin-streptomycin (Life Technologies), and glutamine (Murphy et al., 1991). Cells were trypsinized and GFP+/CD31− cells were sorted by fluorescence-activated cell sorter and cultured. At 60% confluence, cells were transduced with an ecotropic lentivirus (manufactured by the St. Jude Vector Core Laboratory) containing the lentiviral plasmid pLOX (containing the LoxP sequence in the LTR)-T-ag (SV40 large T antigen)-IRES (internal ribosomal entry)-TK (herpes simplex virus 1thymidine kinase) (Salmon et al., 2000) obtained from the nonprofit plasmid repository (Addgene, Cambridge, MA). Four days posttransduction, cells were transferred to poly-d-lysine–coated plates and individual colonies were clonally selected and characterized.
Immunohistochemistry Analysis of Immortalized Mouse AB Cells.
Immortalized AB cells were either cultured on 3.0-μm polyester transwell dishes (Corning Inc., Corning, NY) to confluence or cultured on 35-mm poly-d-lysine glass-bottom dishes (BD Biosciences, San Jose, CA) overnight. Cells were fixed with 3.7% formaldehyde in PBS, pH 7.4, for 15 minutes at room temperature followed by three 5-minute washes in PBS. Cells were blocked for 1 hour with 2% normal goat serum in PBS followed by a 30-minute block in 5% bovine serum albumin in PBS, incubated for 1 hour with primary antibodies in 2% normal goat serum: our rabbit anti-P-gp at 1:500, rat anti-BCRP (BXP-53; Kamiya) at 1:20, rabbit anti-cytokeratin (a “pan” cytokeratin antibody) (Cat. #20622; DAKO, Carpinteria, CA) at 1:20, rabbit anti-desmoplakin (Cat. #sc-33555; Santa Cruz Biotechnology, Santa Cruz, CA) at 1:50, or rabbit anti-vimentin (ab92547; Abcam) at 1:50. Cells were washed and appropriate secondary antibodies (Alexa Fluor 555–conjugated anti-rat and anti-rabbit; Life Technologies) were added at a concentration of 1:500 in 2% normal goat serum for 1 hour. Z-stack images were acquired using the Marianas system (Intelligent Imaging Innovation, Denver, CO), and xz and yz images were acquired to visualize the apical versus basal membrane localization of transporters. Following immunohistochemistry staining (IHC), the plasma membrane was stained with wheat germ agglutinin conjugated to Alexa 594 at a concentration of 5 μg/ml (Invitrogen, Grand Island, NY) for 10 minutes at 22°C. ProLong Gold with 4′,6-diamindino-2-phenylindole (Invitrogen) was used for mounting.
MDR1 (P-gp)–EGFP Lentivirus Transduction of AB Cells.
The human MDR1-EGFP cDNA (including 62 bases of the 5′ untranslated region) was amplified by polymerase chain reaction (PCR) from MDR1-pEGFP-N1 (provided by Dr. Basil D. Roufogalis, University of Sydney, Australia) (Fu et al., 2004) using the following primers: Forward Primer (with SacII restriction site italicized): 5′ TCCCCGCGGCGTGTACGGTGGGAGGTCTA 3′; and Reverse Primer: 5′ GGGAGGTGTGGGAGGTTTT 3′. The human MDR1-EGFP cDNA was PCR amplified, purified, and ligated (T4 DNA ligase; Promega, Madison, WI) into the restriction-digested (SacII and NotI) Lenti CL20c (CMV Lenti20c)-MSCV (murine stem cell virus enhancer)-EGFP vector (Dr. John Gray, St. Jude) and transformed into One Shot TOP10 Competent Cells (Invitrogen). Clones were screened by SacII and NotI restriction digestion and CL20c–MSCV–P-gp–EGFP-positive clones verified by DNA sequencing. The P-gp–EGFP lentiviral particles were manufactured by Dr. John Gray (St. Jude Vector Core). Briefly, human embryonic kidney 293T cells were calcium phosphate cotransfected with the plasmids CL20c–MSCV–P-gp–EGFP, gagpol (CAGG-HIV gpco; CMV-beta-actin hybrid promoter driving a codon optimized human immunodeficiency virus gagpol), envelope (CAGG-VSVG; CMV-beta-actin hybrid promoter driving the Indiana strain of Vesicular Stomatis Virus glycoprotein G), and revtat (CAG-RTR2; CMV promoter driven construct expressing both human immunodeficiency virus rev and tat genes), and 3 days later supernatant was harvested, filtered, and lentiviral particles titered. Primary mouse AB cells at 60% confluence were transduced with P-gp–EGFP lentiviral particles, and 48 hours later confocal images were captured by confocal analysis on the Marianas system.
In Vitro Accumulation Assay.
AB cells (50,000 cells/well) were plated on poly-d-lysine–coated 24-well plates for 18 hours, treated with inhibitors [5 μM cyclosporin A or 5 μM fumitremorgin C (FTC) or 1 μM elacridar (Toronto Research Chemicals, Toronto, Canada)] for 30 minutes, and then with calcein AM (1 μM) or mitoxantrone (5 μM) or rhodamine 800 (0.1 μM) (Santa Cruz Biotechnology) for 1 hour. Cells were washed with ice-cold 1× PBS and harvested. The cellular fluorescence signal of calcein AM (excitation, 490 nm; emission, 520 nm), mitoxantrone (excitation, 607 nm; emission, 684 nm), and rhodamine 800 (excitation, 682 nm; emission, 712 nm) were measured with a Synergy H4 Hybrid Multi-Mode Microplate Reader (Biotek, Winooski, VT).
Daunomycin Accumulation Assay in AB Cell Cultures.
AB cells (50,000) were plated onto the center well of 35-mm poly-d-lysine–coated glass-bottom dishes and cultured overnight. Cells were preincubated with 5 μM cyclosporine for 1 hour; then 0.5 μM daunomycin was added for 30 minutes. Images were captured using the Marianas system, which incorporates a Zeiss Axioplan microscope, a Yokogawa CSUX spinning disk confocal scanhead, and a Photometrics Cascade II CCD camera. Daunomycin was excited at 488 nm and the emission was collected through a 617/73 bandpass filter, using a 63× Plan-Neofluar (NA 1.4) objective. The intensity of the intracellular staining was quantified using SlideBook software.
Mouse Leptomeningeal Tissue Microarray Analysis.
Mouse leptomeningeal tissue from 20–30 neonatal mice (2 days after birth) was isolated and combined, and total RNA was prepared using QIAzol Lysis Reagent (Qiagen, Valencia, CA). To remove any genomic DNA contamination, total RNA was treated with the RNase-free DNase (Qiagen) and further purified using RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. The quality of total RNA was monitored by Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). First-strand cDNA was synthesized by use of SuperScript II Reverse Transcriptase (Invitrogen) and T7-Oligo (dT) Primer (Affymetrix, Santa Clara, CA). In vitro transcription reactions were performed using a GeneChip IVT Labeling Kit. The labeled cRNA was hybridized to a GeneChip Mouse Genome 430 2.0 Array (Affymetrix). After washing, the arrays were scanned with the GeneChip Scanner 3000 (Affymetrix). Affymetrix MicroArray Suite software (version 5.0) was used to calculate the signal and P values and to set the algorithm’s absolute call flag, which indicates the reliability of the data points according to P (present), M (marginal), and A (absent). The data on the chip were scaled to the 500-target intensity value. The prescaled chip data from the experiment were normalized using GeneSpring GX (version 7.3; Agilent Technologies). Normalization with default parameters in GeneSpring software (per chip: normalize to 50th percentile; per gene: normalize to median) was used. Mouse expression values of <1.0 × 102 are considered low.
Quantitative Real-Time PCR Analysis.
Total cellular RNA was extracted from C57BL/6 mouse tissues using TRIzol (Life Technologies), and cDNA was synthesized from 1 μg of total RNA according to the manufacturer's instructions using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Quantitative real-time PCR was performed with specific primers (Supplemental Table 1) using the SYBR Green Master Mix (Life Technologies) according to the manufacturer's instructions and was carried out in an ABI PRISM 7900HT System (Applied Biosystems, Foster City, CA). PCR conditions include initial activation step at 95°C for 15 minutes, followed by 40 cycles in which each cycle consisted of denaturation at 92°C for 30 seconds, annealing for 30 seconds, and synthesis at 72°C for 60 seconds. Specificity of amplification was confirmed in each case by performing melt curve analysis. To minimize the effect of sample-handling differences, all results were calculated as ΔCt [the Ct (cycle threshold) value for any gene of interest − Ct of the endogenous housekeeping gene glyceraldehyde-3-phosphate dehydrogenase].
Results
P-gp Is Expressed at the Apical Membrane of AB Cells of Mouse, Monkey, and Human Leptomeninges.
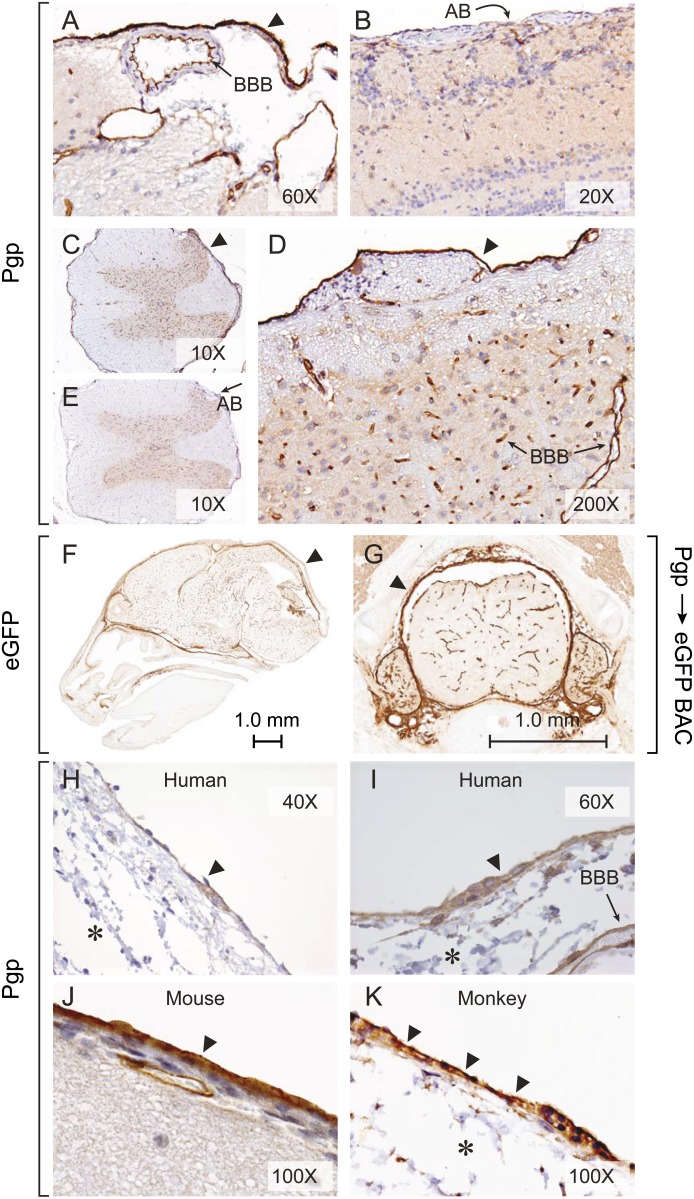
IHC revealed P-gp in endothelial cells that comprise the BBB in the veins, arteries, and capillaries throughout the brain and spine of P-gp WT but not KO mice (Fig. 2, A-E). P-gp was also expressed in AB cells in the brain and spinal cord, and P-gp’s staining intensity was at least equal to that in the endothelial cells comprising the BBB. Further confirmation of P-gp leptomeningeal expression came from evaluating a bacterial artificial chromosome transgenic mouse that used the endogenous mouse Abcb1a promoter to drive EGFP reporter expression. This mouse was previously generated as part of the GENSAT project. IHC for EGFP in mouse brain (Fig. 2F) and spinal cord (Fig. 2G) found P-gp expression in the endothelial cells in the veins, arteries, and capillaries of the brain and spinal cord and in the leptomeninges. The GENSAT site identifies P-gp in pia cells; however, this is incorrect, because an extensive review of numerous slides of mouse brain revealed P-gp immunostaining only in AB cells, not pia cells (Fig. 2, A, C, and D).
Fig. 2.
P-gp is highly expressed in brain and spinal cord AB cells. Immunochemical staining using an anti-P-gp antibody shows P-gp expression (solid arrowhead) in AB cells and in blood vessel endothelial cells of the BBB in the brain and spine, respectively, of P-gp WT mice (A, C, D) and its absence in P-gp KO mice (B, E). GFP immunostaining (solid arrowhead) in the meninges of BAC (bacterial artificial chromosome) abcb1a-EGFP reporter mice at embryonic day 15.5 in a sagittal head section (F) and in adult spine (G). (images from GENSAT). P-gp immunostaining (solid arrowhead) in human AB cells using JSB-1 (H) and rabbit anti-P-gp antibodies (I). P-gp immunostaining (solid arrowhead) is more intense on the apical membranes of mouse (J) and monkey (K) AB cells. Arachnoid reticular cell layer and trabeculae (asterisk) visible in human and monkey (H, I, K) meninges.
IHC of human meninges with JSB-1 monoclonal antibody (used for P-gp clinical diagnostics) (Fig. 2H) and with rabbit P-gp IgG (Fig. 2I) revealed an identical pattern of P-gp immunostaining in the BBB and ABp cells. Monkey AB cells were also positive for P-gp (Fig. 2K). There was no staining for P-gp in the arachnoid reticular cell layer or trabecular cells or in the pia mater in monkey or human tissue. Compared with the closely associated pia-arachnoidal membranes in the mouse, the human (and monkey) arachnoid membrane is characterized by a relatively thick reticular cell layer with trabecular cells (strands of connective tissue connecting pia and AB) that clearly separates the AB cell layer from the pia mater.
The anatomy of the AB layer shows a continuous basal lamina on its inner surface facing the CSF and the innermost, loosely organized arachnoid reticular cell layer, which traverses the subarachnoid space between the AB cells and the pia cells on the surface of the brain (Schachenmayr and Friede, 1978; Vandenabeele et al., 1996). By definition, the surface of the AB cell forming the base and contacting the basement membrane (and facing the CSF) is the basal surface. Increased magnification of P-gp IHC of mouse and monkey brain (Fig. 2, J and K) demonstrated prominent staining on the apical membrane of AB cells facing the dura.
The localization and expression of P-gp in CP and ependymal cells is controversial (Rao et al., 1999; Gazzin et al., 2008; Roberts et al., 2008). Immunostaining of P-gp WT and KO mouse CP revealed an identical nonspecific punctate and granular staining pattern throughout the cytoplasm (Fig. 3, A and B) of mice with both genotypes. At a lower concentration of primary antibody (Fig. 3C), P-gp was stained in the BBB, but staining was absent in the ependymal cells and only weakly present in some CP cells.
Fig. 3.
P-gp expression in mouse CP. Immunostaining for P-gp in CP of P-gp WT (A, C) and KO (B) mice.
BCRP Is Highly Expressed in Mouse AB Cells.
BCRP immunostaining was readily detected in mouse AB cells and blood vessel endothelium (Fig. 4A). The expression pattern of BCRP was confirmed using a mouse containing the Abcg2–IRES (internal ribosomal entry)–EGFP allele (IRES-EGFP is inserted downstream of the Abcg2 stop codon). This allelic modification results in expression of the EGFP reporter gene under control of the Abcg2 transcriptional regulatory elements, with coexpression of a functional WT BCRP protein (Tadjali et al., 2006). IHC detected EGFP expression in the mouse AB cells but not in pia cells (Fig. 4, B and C). Final verification of Bcrp’s mRNA expression pattern comes from the Allen Mouse Brain Atlas in situ hybridization database (Fig. 4, D and E). Expression of Abcg2 is higher in the arachnoid meningeal tissue than in virtually all other brain regions. However, whether the same is true in AB tissue from other species, including humans, remains to be determined.
Fig. 4.
BCRP expression in mouse AB cells. (A) Immunostaining of BCRP in AB cells of mouse meninges with anti-BCRP IgG (BXP-53). Immunostaining of GFP in AB cells of Abcg2-promoter–EGFP reporter mice at low (B) and high (C) magnification. In situ hybridization (ISH) for Bcrp expression in a sagittal section of mouse brain at low (D) and high (E) magnification. Images from the Allen Institute for Brain Science (http://mouse.brain-map.org).
Establishment of Immortalized Mouse AB Cell Lines.
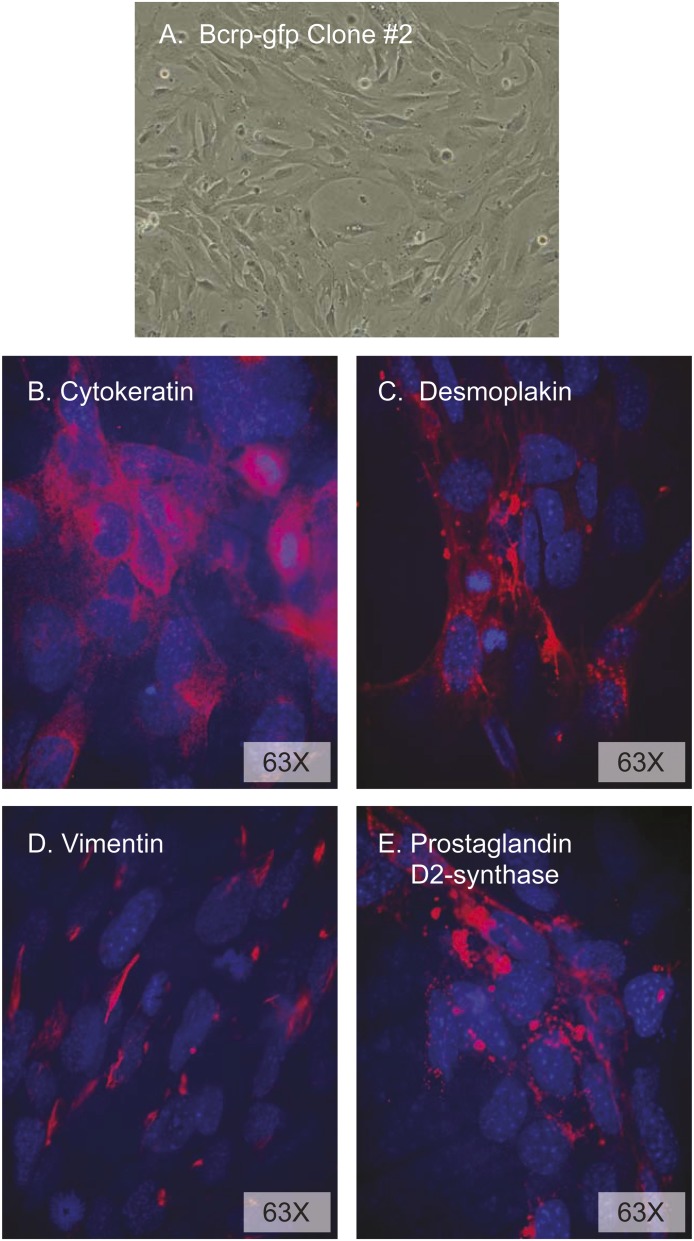
The leptomeninges were dissected from Abcg2-IRES-EGFP mice (3 days after birth), cells were isolated by collagenase digestion and then cultured for 4 days, and AB cells were isolated by fluorescence-activated cell sorter (cells positive for GFP and negative for the blood vessel endothelial marker CD31). Since primary AB cultures rapidly senesce after four or five passages, lentivector transfer of pLOX-Tag-iresTK was used to immortalize the primary cells (Salmon et al., 2000). The morphology of the primary (not shown) and SV40 Tag immortalized cells was identical, with large nuclei and a more spindle-like appearance at low density (Fig. 5A) and a cobblestone appearance at higher density. The AB cells had strong positive immunostaining for cytokeratin (cytoplasmic and displaying long filaments and a basketlike structure indicative of cytokeratin organization) (Fig. 5B), desmoplakin (with the strongest expression between cells being indicative of its role as an obligate component of functional desmosomes) (Fig. 5C), and the intermediate filament protein vimentin (primarily seen in juxtanuclear knots) (Fig. 5D). The coexpression of these three proteins identifies them as AB cells (Murphy et al., 1991; Janson et al., 2011) and eliminates the possibility that the cells are contaminated with endothelial cells, fibroblasts, astrocytes, or macrophages (Murphy et al., 1991). The cells were also stained intensely positive for prostaglandin D2 synthase (Fig. 5E), a protein known to be highly expressed in AB cells (Beuckmann et al., 2000; Fujimori et al., 2007).
Fig. 5.
Characterization of mouse immortalized AB cell cultures. (A) Phase-contrast microscopy of AB cells at early passage. Confocal analysis of mouse AB cells in transwell culture incubated with antibodies against cytokeratin (B), desmoplakin (C), vimentin (D), and prostaglandin D2 synthase (E) and revealed (red staining) with appropriate secondary antibodies. Cell nuclei were counterstained with DAPI (4′,6-diamindino-2-phenylindole).
P-gp and BCRP Immunolocalization and Function in Cultured AB Cells.
The subcellular distribution of P-gp and BCRP was determined in AB cells in transwell culture. Examination of the cells at a plane perpendicular to the transwell culture dish showed endogenous P-gp most highly expressed at the apical membrane (Fig. 6A). Further confirmation that P-gp is apically expressed was determined by transducing the cells in Fig. 6B with a lentivirus expressing a P-gp–EGFP fusion protein (Fu et al., 2004). Coexpression of the P-gp–EGFP (green) with a plasma membrane stain (red) to the apical membrane was revealed by yellow costaining (Fig. 6B). We first attempted to verify transporter function by measuring directional transport of a P-gp or BCRP substrate across mouse AB cells in transwell culture. Unfortunately, we were unable to sustain high transepithelial electrical resistance values to generate consistent transport results in transwell culture. To verify P-gp transporter function, AB cells were incubated with the P-gp substrate daunomycin (Fig. 6C) or calcein AM (Fig. 6D) or rhodamine 800 (On et al., 2011) in the presence or absence of the inhibitor cylosporin A [at a concentration (1 μM) that should inhibit only P-gp] or elacridar (a dual P-gp/BCRP inhibitor). The intracellular accumulation of each P-gp substrate was reproducibly increased in the presence of the P-gp inhibitor. Pretreatment with neither the BCRP inhibitor FTC nor the multidrug resistance–associated protein inhibitor MK571 had any effect on retention of rhodamine 800 (unpublished data).
Fig. 6.
P-gp and BCRP localization and function in immortalized mouse AB cells. Confocal analysis of endogenous P-gp (A) and BCRP (E and F) [immunostained in red with nucleic acids stained with DAPI(4′,6-diamindino-2-phenylindole)] in cultured AB cells. The top view of the cells is the largest panel and the upper and left panels show an optical section perpendicular to the plane of the cell layer. (B) Confocal analysis of AB cells transduced for 48 hours with a P-gp–EGFP (green) fusion lentivirus followed by plasma membrane chemical staining (red) shows apical costaining (yellow) of P-gp–EGFP and plasma membrane. Daunomycin (C) and calcein AM (1 μM) and rhodamine 800 (Ro800; 0.1 μM) (D) fluorescence in AB cells cultured in the absence or presence of cyclosporin A (CsA) or 1 μM elacridar (ECD). (G) Mitoxantrone fluorescence in AB cells in the absence or presence of 5 μM FTC. Cellular fluorescence values for substrate alone (set at 1) (± S.D.) were compared with values in the presence of inhibitors using a Student’s t test (two-sided). **P < 0.01 vs. substrate alone.
In contrast to P-gp, endogenous BCRP immunostaining was equal at the apical and basal membranes (with some cytoplasmic staining) of all AB cells on polylysine glass dishes (Fig. 6E) or in transwell culture (Fig. 6F) Preincubation of AB cells with the BCRP inhibitor FTC increased intracellular mitoxantrone fluorescence up to 2.5-fold (Fig. 6G). Thus, immortalized cultures of mouse AB cells retain membrane expression of functional P-gp and BCRP.
Microarray Analysis of Drug Detoxification Genes in AB Leptomeninges.
Mouse leptomeningeal mRNA expression of drug uptake and efflux transporters, cytochrome P450s, the nuclear hormone receptors PXR and CAR, and the AB marker prostaglandin D2 synthase was analyzed by microarray and compared with that of normal adult human arachnoid mRNA expression (Gene Expression Omnibus) (Table 1). Both MDR1 and BCRP were highly expressed in human and mouse tissue. The monocarboxylate transporter MCT1 was the most highly expressed drug transporter in mouse, but its expression was lower in human tissue. This transporter is also known to interact with drugs such as simvastatin and valproate (Anderson and Thwaites, 2010). SLCO1C1, an organic anion transporter highly expressed at the BBB (and previously called BBB-specific anion transporter 1), was also expressed in mouse leptomeningeal tissue. Methotrexate SLC uptake transporters (OAT1, OAT3, and the reduced folate carrier SLC19A1) (VanWert and Sweet, 2008; Zhao et al., 2011) were expressed in AB tissue from both species. Human leptomeninges had detectable expression of the nuclear hormone receptor PXR (NR1I2), while CAR (Nr1I3) was more highly expressed in mouse leptomeninges. CYP1B1 and CYP4A were the most abundant cytochrome P450 enzymes in AB cells from both species. Quantitative real-time PCR of mouse leptomeninges, CP, and whole brain pooled from day 2 neonatal mice was used to evaluate and confirm microarray gene expression data (Table 1). The lower the ΔCt value, the higher the expression of the gene of interest. In total, the results showed that, like BBB and CP (Decleves et al., 2011), AB cells express a variety of drug detoxification genes. Moreover, the expression pattern of transporters in the epithelial AB cells is closer to that of the other BCSFB cells—the epithelial CP cells (e.g., higher expression of Oat1/OAT1 and Oat3/OAT3)—rather than BBB blood vessel endothelial cells.
TABLE 1.
Absorption, distribution, metabolism, and excretion gene expression in mouse brain and human arachnoid tissue
| Mouse Leptomeninges | ×102a | (ΔCt)b | Human Arachnoid | ×103c | Examples of Human Substrates/Ligands | ||
|---|---|---|---|---|---|---|---|
| Mouse LM | Mouse CP | Mouse Brain | |||||
| L-Ptgds | 73.4 | −6.01 (1) | 1.34 (1) | 1.75 (1) | L-PTGDS | 1371 | |
| Beta-actin | 59.1 | Zona Occludens 1 | 78.2 | ||||
| Slc16a1/Mct1 | 27.9 | CYP1B1 | 73.4 | Retinal, arachidonic acid | |||
| Slco1c1/Oatp-f | 17.3 | CYP4A11 | 38.8 | Fatty acids (medium chain) | |||
| Cyp1b1 | 12.9 | 2.63 (4) | 7.04 (4) | 9.38 (7) | ABCB1/MDR1 | 28.5 | Hydrocortisone |
| Slc22a6/Oat1 | 10.9 | 0.28 (2) | 8.30 (6) | 10.25 (8) | SLC22A2/OCT1 | 24 | Nucleoside analogs |
| Abcg2/Bcrp | 10.8 | 3.22 (5) | 7.61 (5) | 6.54 (2) | SLC22A6/OAT1 | 15.8 | Methotrexate |
| Slc22a8/Oat3 | 9.2 | 1.80 (3) | 4.78 (2) | 7.61 (4) | SLC22A8/OAT3 | 6.9 | Methotrexate |
| Abcb1a/Mdr1a | 6.2 | 3.89 (6) | 6.15 (3) | 7.58 (3) | CYP3A5 | 6.4 | Tacrolimus |
| Abcc4/Mrp4 | 2.1 | 6.16 (7) | 8.49 (7) | 9.28 (6) | ABCG2/BCRP | 4.7 | Topotecan |
| Slco1a4/Oatp2 | 1.6 | CYP2D6 | 4.4 | Tamoxifen | |||
| Nr1i3/Car | 1.1 | 15.96 (9) | 12.60 (9) | 16.51 (9) | SLC16A1/MCT1 | 4.4 | Lactate, glucose |
| Abcb6/Prp | 1.1 | ABCB6/PRP | 4.1 | Porphyrin | |||
| Slc16a11/Mct11 | 0.9 | SLC19A1/RFC1 | 2.8 | Methotrexate | |||
| Abcc1/Mrp1 | 0.9 | 7.18 (8) | 9.54 (8) | 8.43 (5) | SLC16A11 | 1.5 | Monocarboxylates |
| Cyp2d22 | 0.6 | NR1I2/PXR | 1.2 | Rifampin | |||
| Abcc3/Mrp3 | 0.2 | CYP2B6 | 1.2 | Cyclophosphamide | |||
Mouse leptomeninges from day 2 neonates; mouse expression (normalized signal intensity values) <1.0 × 102 are considered low.
The ΔCt (cycle threshold) values for genes of interest (rank order in parentheses) obtained from quantitative real-time polymerase chain reaction analysis of mouse leptomeninges (LM), choroid plexus (CP), and whole brain pooled from day 2 neonates.
Human arachnoid membrane expression: average normalized signal intensity value from two normal human arachnoid membranes (Gene Expression Omnibus dataset GSE19727).
Discussion
Almost 2 decades after it was first demonstrated that BBB-expressed P-gp has a protective role in excluding drugs from the brain (Schinkel et al., 1994) and that CP P-gp functions as a drug permeability barrier (Rao et al., 1999), we report that AB cells represent another blood-CSF drug transport barrier expressing functional drug transporters such as P-gp and BCRP. Transporter expression in AB cells in the cranial and spinal meninges makes mechanistic sense because, like CP, the fenestrated capillaries in the dura lack barrier properties and the barrier function shifts to the AB epithelial layers. Moreover, Affymetrix GeneChip array analysis of mouse and human AB tissue revealed expression of additional uptake and efflux transporters and showed that AB cells have a cytochrome P450 enzymatic barrier.
There are four distinct blood-CSF interfaces: the CP, the AB, the ependymal cells separating the ventricular system from the extracellular fluid of the brain, and the pia leptomeningeal cells on the brain surface (Johanson et al., 2011). Our studies suggest that P-gp in the AB membrane is a primary drug efflux barrier at the blood-CSF interface. First, we and others (Roberts et al., 2008) did not detect P-gp in either pia leptomeningeal cells or ependymal cells. Ependymal cell P-gp immunostaining has been reported (King et al., 2001), but it is difficult to reconcile why ependymal cells, which lack tight junctions to restrict the free exchange of drugs between the CSF and brain tissue, would express a drug transporter. In addition, the expression level and cellular localization of P-gp in CP has been reported to be cytoplasmic (Miller, 2004), or at the subapical membrane (Rao et al., 1999), or low to nonexistent (Baehr et al., 2006; Roberts et al., 2008). In contrast, P-gp immunostaining in AB was at least equivalent to its immunostaining intensity at the BBB. Drug transporter expression in AB cells has likely been missed to date because the AB layer is frequently detached from the brain by skull removal. This occurs because, even within days after birth, the dura increasingly attaches to the skull. The result is that if care is not taken, when the skull is removed from the brain, tissue above the CSF space (arachnoid tissue and dura) is retained with the skull.
Creation of an immortalized AB cell model was essential to elucidation of AB function. These cells polarize and retain key morphologic AB features including cytokeratin, desmoplakin, and vimentin and high expression of prostaglandin D2 synthase. They also preserve P-gp and BCRP transporter localization and function, as shown by export of transporter typical substrates such as mitoxantrone, calcein AM, rhodamine 800, and daunomycin. Most importantly, cultured AB cells expressed P-gp and BCRP at the membrane. P-gp was expressed on the apical surface of cultured AB cells, consistent with a more intense P-gp apical immunostaining on AB tissue from mouse and monkey brain. Although the apical surface of many cells faces a lumen, the AB apical surface faces the dura, and the basal surface faces the CSF.
P-gp expression on the apical membrane of AB cells is likely an important component of the blood-CSF barrier preventing entry into the CSF of systemic drugs that easily leave the leaky fenestrated dural capillaries. In addition, it would enhance removal of drugs that get into the CSF either by secretion from the brain interstitial fluid compartment or following intrathecal drug administration. Although our studies were performed in mice, anecdotal evidence supporting a role for apical membrane P-gp having a barrier function comes from a magnetic resonance imaging study of human brain 90 minutes after systemic administration of 99mTc-sestamibi (Rao et al., 1999), a P-gp substrate. There was an intense magnetic resonance imaging signal localized in the CP (but none in the CSF or brain parenchyma) and around the brain periphery. Our results suggest that the peripheral signal may represent 99mTc-sestamibi that left the fenestrated dural capillaries and infiltrated the dura and that AB P-gp is preventing it from penetrating into the CSF.
The dual expression of BCRP on both apical and basal surfaces of AB cells is unique—there are no other cell types where BCRP is reportedly expressed on both cell membranes. This implies that BCRP has two distinct functions in the AB cells. BCRP on the apical AB membrane likely forms part of the blood-CSF drug transport barrier preventing CSF penetration by systemic drugs. Conversely, BCRP on the basal AB membrane would increase the CSF retention of substrates such as topotecan and methotrexate that can be administered intrathecally.
The finding that BCRP faces the CSF subarachnoid space in both types of blood-CSF barrier cells, CP (Zhuang et al., 2006) and AB, suggests that BCRP may have an important function in secreting some factor produced in these cells into the CSF. The CSF is a regulator of neurogenesis and behavior (Zappaterra and Lehtinen, 2012), and CP and AB cells produce micronutrients, neurotrophins, growth factors, retinoic acid, and prostaglandins that are secreted into the CSF. Although BCRP is known to transport endogenous vitamins such as riboflavin (vitamin B2), folic acid (folate, vitamin B9), and vitamin K3 (Vlaming et al., 2009), the physiologic function and endogenous substrate secreted into the CSF by BCRP remain to be determined.
Our studies have strong implications for using CSF as a surrogate for brain exposure following systemic administration of BCRP and P-gp substrates (Xiao et al., 2012). For P-gp substrates, conventional knowledge posits that CSF drug concentration is only influenced by BBB and CP P-gp (Kodaira et al., 2011). Our studies put forth a new paradigm: CSF drug concentration is strongly influenced by AB P-gp and BCRP. Pharmacokinetic quantification of brain drug exposure by using CSF will only be as good as the models, which currently do not incorporate the cranial and spinal AB surface area and drug transporters or the apical or basal expression of these transporters. Moreover, by our calculations the total meningeal surface area (0.27 m2) [= cranial meninges (0.17 m2) + spinal meninges (0.02 m2) + arachnoid granulations (0.078 m2)] actually exceeds the CP surface area (0.021 m2). Given the clear evidence for intersite differences in CNS pharmacokinetics of P-gp and BCRP substrates and the difficulty in modeling the impact of the drug transporters on drug concentration in each compartment, improved models are needed to quantitatively evaluate the individual contributions of P-gp and BCRP at the BBB, CP, and AB to brain and CSF concentrations.
In conclusion, AB cell expression of drug transporters adds to the increasing recognition of the complexity of transporters of the BBB and BCSFB. This finding is important for two reasons. 1) A growing number of drugs, including chemotherapeutics, are administered into the CSF by intrathecal injection. It would be expected that drug transporters expressed in AB cells would influence the rate of drug egress from the CSF and the concentration of drug that remains in the CSF. 2) The concentration of drugs in the CSF is often used as a surrogate for the unbound drug in the interstitial fluid in the brain. Hence, identification of transporters localized in the AB cells adjacent to the CSF space is necessary to correctly interpret exposure-response relationships for brain and CSF concentrations of P-gp and BCRP substrates, which include many chemotherapeutics.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the technical support of St. Jude Children’s Research Hospital: Small Animal Imaging Resource, the Cellular Imaging Shared Resource, the Veterinary Pathology Core, the Vector Production Core, and the Hartwell Center for oligonucleotide synthesis; and Drs. Richard and Michelle Smeyne of St. Jude for training on isolation of mouse leptomeningeal tissue.
Abbreviations
- AB
arachnoid barrier
- BBB
blood-brain barrier
- BCRP
breast cancer resistance protein
- BCSFB
blood–cerebrospinal fluid barrier
- CFS
cerebrospinal fluid
- CNS
central nervous system
- CP
choroid plexus
- Ct
cycle threshold
- DM
dissociation media
- EGFP
enhanced green fluorescent protein
- FTC
fumitremorgin C
- GENSAT
Gene Expression Nervous System Atlas
- GFP
green fluorescent protein
- IHC
immunohistochemistry staining
- IRES
internal ribosomal entry
- KO
knockout
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- P-gp
P-glycoprotein
- WT
wild type
Authorship Contributions
Participated in research design: Yasuda, Cline, Sorrentino, Ekins, Schuetz.
Conducted experiments: Yasuda, Cline, Onciu, Thirumaran, Fujimori.
Contributed new reagents or analytic tools: Fatima, Sorrentino, Urade.
Performed data analysis: Yasuda, Cline, Vogel, Onciu, Fujimori, Schuetz.
Wrote or contributed to the writing of the manuscript: Yasuda, Cline, Vogel, Ekins, Urade, Fujimori, Schuetz.
Footnotes
The work was supported by a National Institutes of Health Cancer Center Support Grant [Grant P30 CA21765]; by Cancer Center Developmental Funds; and by the American Lebanese Syrian Associated Charities.
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Anderson CM, Thwaites DT. (2010) Hijacking solute carriers for proton-coupled drug transport. Physiology (Bethesda) 25:364–377 [DOI] [PubMed] [Google Scholar]
- Baehr C, Reichel V, Fricker G. (2006) Choroid plexus epithelial monolayers—a cell culture model from porcine brain. Cerebrospinal Fluid Res 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckmann CT, Lazarus M, Gerashchenko D, Mizoguchi A, Nomura S, Mohri I, Uesugi A, Kaneko T, Mizuno N, Hayaishi O, et al. (2000) Cellular localization of lipocalin-type prostaglandin D synthase (beta-trace) in the central nervous system of the adult rat. J Comp Neurol 428:62–78 [DOI] [PubMed] [Google Scholar]
- Decleves X, Jacob A, Yousif S, Shawahna R, Potin S, Scherrmann JM. (2011) Interplay of drug metabolizing CYP450 enzymes and ABC transporters in the blood-brain barrier. Curr Drug Metab 12:732–741 [DOI] [PubMed] [Google Scholar]
- Fu D, Bebawy M, Kable EP, Roufogalis BD. (2004) Dynamic and intracellular trafficking of P-glycoprotein-EGFP fusion protein: implications in multidrug resistance in cancer. Int J Cancer 109:174–181 [DOI] [PubMed] [Google Scholar]
- Fujimori K, Watanabe M, Urade Y, Ishikawa K. (2007) Increased production of lipocalin-type prostaglandin D synthase in leptomeningeal cells through contact with astrocytes. Neurosci Lett 423:133–137 [DOI] [PubMed] [Google Scholar]
- Gazzin S, Strazielle N, Schmitt C, Fevre-Montange M, Ostrow JD, Tiribelli C, Ghersi-Egea JF. (2008) Differential expression of the multidrug resistance-related proteins ABCb1 and ABCc1 between blood-brain interfaces. J Comp Neurol 510:497–507 [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, et al. (2003) A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425:917–925 [DOI] [PubMed] [Google Scholar]
- Janson C, Romanova L, Hansen E, Hubel A, Lam C. (2011) Immortalization and functional characterization of rat arachnoid cell lines. Neuroscience 177:23–34 [DOI] [PubMed] [Google Scholar]
- Johanson C, Stopa E, McMillan P, Roth D, Funk J, Krinke G. (2011) The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread. Toxicol Pathol 39:186–212 [DOI] [PubMed] [Google Scholar]
- King M, Su W, Chang A, Zuckerman A, Pasternak GW. (2001) Transport of opioids from the brain to the periphery by P-glycoprotein: peripheral actions of central drugs. Nat Neurosci 4:268–274 [DOI] [PubMed] [Google Scholar]
- Kodaira H, Kusuhara H, Fujita T, Ushiki J, Fuse E, Sugiyama Y. (2011) Quantitative evaluation of the impact of active efflux by p-glycoprotein and breast cancer resistance protein at the blood-brain barrier on the predictability of the unbound concentrations of drugs in the brain using cerebrospinal fluid concentration as a surrogate. J Pharmacol Exp Ther 339:935–944 [DOI] [PubMed] [Google Scholar]
- Lau C, Ng L, Thompson C, Pathak S, Kuan L, Jones A, Hawrylycz M. (2008) Exploration and visualization of gene expression with neuroanatomy in the adult mouse brain. BMC Bioinformatics 9:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS. (2004) Confocal imaging of xenobiotic transport across the choroid plexus. Adv Drug Deliv Rev 56:1811–1824 [DOI] [PubMed] [Google Scholar]
- Murphy M, Chen JN, George DL. (1991) Establishment and characterization of a human leptomeningeal cell line. J Neurosci Res 30:475–483 [DOI] [PubMed] [Google Scholar]
- On NH, Chen F, Hinton M, Miller DW. (2011) Assessment of P-glycoprotein activity in the blood-brain barrier (BBB) using near infrared fluorescence (NIRF) imaging techniques. Pharm Res 28:2505–2515 [DOI] [PubMed] [Google Scholar]
- Pippert TR, Umbenhauer DR. (2001) The subpopulation of CF-1 mice deficient in P-glycoprotein contains a murine retroviral insertion in the mdr1a gene. J Biochem Mol Toxicol 15:83–89 [DOI] [PubMed] [Google Scholar]
- Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, Ribeiro RC, Rubnitz JE, Raimondi SC, Onciu M, et al. (2009) Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 360:2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, Piwnica-Worms D. (1999) Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci USA 96:3900–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redzic Z. (2011) Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS 8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LM, Black DS, Raman C, Woodford K, Zhou M, Haggerty JE, Yan AT, Cwirla SE, Grindstaff KK. (2008) Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience 155:423–438 [DOI] [PubMed] [Google Scholar]
- Salmon P, Oberholzer J, Occhiodoro T, Morel P, Lou J, Trono D. (2000) Reversible immortalization of human primary cells by lentivector-mediated transfer of specific genes. Mol Ther 2:404–414 [DOI] [PubMed] [Google Scholar]
- Saunders NR, Ek CJ, Habgood MD, Dziegielewska KM. (2008) Barriers in the brain: a renaissance? Trends Neurosci 31:279–286 [DOI] [PubMed] [Google Scholar]
- Schachenmayr W, Friede RL. (1978) The origin of subdural neomembranes. I. Fine structure of the dura-arachnoid interface in man. Am J Pathol 92:53–68 [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP, et al. (1994) Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 77:491–502 [DOI] [PubMed] [Google Scholar]
- Stapleton S, Blaney S. (2006) New agents for intrathecal administration. Cancer Invest 24:528–534 [DOI] [PubMed] [Google Scholar]
- Tadjali M, Zhou S, Rehg J, Sorrentino BP. (2006) Prospective isolation of murine hematopoietic stem cells by expression of an Abcg2/GFP allele. Stem Cells 24:1556–1563 [DOI] [PubMed] [Google Scholar]
- Vandenabeele F, Creemers J, Lambrichts I. (1996) Ultrastructure of the human spinal arachnoid mater and dura mater. J Anat 189:417–430 [PMC free article] [PubMed] [Google Scholar]
- VanWert AL, Sweet DH. (2008) Impaired clearance of methotrexate in organic anion transporter 3 (Slc22a8) knockout mice: a gender specific impact of reduced folates. Pharm Res 25:453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaming ML, Lagas JS, Schinkel AH. (2009) Physiological and pharmacological roles of ABCG2 (BCRP): recent findings in Abcg2 knockout mice. Adv Drug Deliv Rev 61:14–25 [DOI] [PubMed] [Google Scholar]
- Xiao G, Black C, Hetu G, Sands E, Wang J, Caputo R, Rohde E, Gan LS. (2012) Cerebrospinal fluid can be used as a surrogate to assess brain exposures of breast cancer resistance protein and P-glycoprotein substrates. Drug Metab Dispos 40:779–787 [DOI] [PubMed] [Google Scholar]
- Zappaterra MW, Lehtinen MK. (2012) The cerebrospinal fluid: regulator of neurogenesis, behavior, and beyond. Cell Mol Life Sci 69:2863–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Diop-Bove N, Visentin M, Goldman ID. (2011) Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr 31:177–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y, Fraga CH, Hubbard KE, Hagedorn N, Panetta JC, Waters CM, Stewart CF. (2006) Topotecan central nervous system penetration is altered by a tyrosine kinase inhibitor. Cancer Res 66:11305–11313 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.