Abstract
Although there has been much interest in the relation between brain size and cognition, few studies have investigated this relation within a genetic framework and fewer still in non-adult samples. We analyzed the genetic and environmental covariance between structural MRI data from four brain regions (Total Brain Volume, Neocortex, White Matter, and Prefrontal Cortex), and four cognitive measures (Verbal IQ (VIQ), Performance IQ (PIQ), Reading Ability, and Processing Speed), in a sample of 41 MZ twin pairs and 30 same-sex DZ twin pairs (mean age at cognitive test = 11.4 years; mean age at scan = 15.4 years). Multivariate Cholesky decompositions were performed with each brain volume measure entered first, followed by the four cognitive measures. Consistent with previous research, each brain and cognitive measure was found to be significantly heritable. The novel finding was the significant genetic but not environmental covariance between brain volumes and cognitive measures. Specifically, PIQ shared significant common genetic variance with all four measures of brain volume (rg = .58 –.82). In contrast, VIQ shared significant genetic influence with Neocortex volume only (rg= .58). Processing Speed was significant with Total Brain Volume (rg = .79), Neocortex (rg = .64), and White Matter (rg =.89), but not Prefrontal Cortex. The only brain measure to share genetic influence with Reading was Total Brain Volume (rg =.32), which also shared genetic influences with processing speed.
Keywords: twin design, MRI, brain volume, intelligence, processing speed, reading
The question of whether “smarter people have bigger brains” (intelligence is related to brain size) has been debated for over a century (Gould, 1981), even though it is widely accepted that species differences in brain size, especially relative to body size, relate to cognitive capacity (e.g. Jerison, 1989). More recent phenotypic research has consistently shown small/moderate correlations between brain size and IQ within our species. McDaniel (2005) conducted a meta-analysis of 37 neuroimaging studies which examined the relation between full brain volume and IQ and found an average correlation of .33. Behavioral genetic studies assessed the genetic and environmental influence on brain volume, finding high heritability of brain volume (ranging from .77 –.97; e.g., Baare et al. 2001; Giedd et al., 2007; Pennington et al., 2000; Posthuma et al.,2002; Thompson et al.,2001; van Leeuween et al., 2009), with little influence of shared environmental effects. Similarly, the moderate heritability of IQ (around .50), as well as significant environmental influences, have been well-established (see Plomin, DeFries, McClearn, & McGuffin, 2008 for review). However, few studies have directly tested whether the phenotypic relation between brain volume and IQ is mediated by genes, environments, or some combination. Posthuma and colleagues (Posthuma et al., 2002; Posthuma et al., 2003) have begun to investigate this question, finding significant genetic correlations between measures of brain volume and IQ measures, and van Leeuwen and colleagues have found similar results in a study of children (van Leeuween et al., 2009). However, studies assessing both cognitive measures and brain areas genetically thus far have been limited, so the current study seeks to expand our understanding of the relation between brain volume and aspects of cognition in a genetic framework.
In the first investigation of the genetic relation between brain volume and IQ, Posthuma et al. (2002) showed that both gray-matter volume and white-matter volume were genetically correlated with g (gray-matter rg = .29, white matter rg = .24), as assessed by the Wechsler Adult Intelligence Scale (WAIS) Full Scale IQ. A second study by these investigators explored these relations further by assessing the genetic relation between brain volume and the four factors of the WAIS (verbal comprehension, perceptual organization, processing speed, and working memory). They found that each of these factors differed in their patterns of genetic correlation with white and gray matter volumes, suggesting that subsets of IQ and cognition may be more genetically related to different areas of the brain (Posthuma et al., 2003). For instance, they found a significant genetic correlation between the WAIS Processing Speed Factor and white matter volume, but only a trend for such a relation with gray matter volume. Furthermore, processing speed has become increasingly recognized as an important factor related to IQ (Baker, Vernon, & Ho, 1991; Ho, Baker & Decker, 1988; Luciano, Smith, Wright, Geffen, Geffen, & Martin, 2001; Luciano, Wright, Smith, Geffen, Geffen, & Martin, 2001) and has been shown to share genetic overlap with IQ in an adult sample (e.g., Posthuma, de Geus, & Boomsma, 2001; Posthuma et al., 2002). In contrast, in a study of 9-year-old twins, van Leeuween et al. (2009) found no phenotypic or genetic relation between processing speed and brain volume measures. The present study aims to replicate Posthuma’s findings relating brain volume to IQ and, given the discrepant results relating brain volume to processing speed, to test more broadly for differential genetic relations between brain structures and other dimensions of cognition.
Posthuma et al.’s (2003) result for processing speed and white matter is theoretically interesting for several reasons. First, considerable work in adults supports a relation between white matter integrity and processing speed, both in cognitive aging and neurological diseases, such as multiple sclerosis (e.g., Anstey et al., 2007; Bunce et al., 2007; Filley, 2005; MacDonald, Nyberg, & Bäckman, 2006). Second, in child and adolescent development, there are robust increases in processing speed with age (Kail, 1991). Third, in the same developmental period, there are well known differences in the trajectory of white and gray matter volumes. Across middle childhood and adolescence, white matter volume increases because of myelination and gray matter volume decreases because of synaptic pruning (Lenroot & Giedd, 2007). So, it is plausible that both developmental and individual differences in processing speed relate to white matter volume and that these relations may be partly mediated genetically.
Other dimensions of cognition, such as crystallized intelligence (essentially, accumulated verbal knowledge), may relate more strongly to synaptic pruning in other brain structures, such as neocortical gray matter, because a different neural process (e.g. tuning of connections in neural networks) is critical for their development. We test this possibility by examining brain relations with Verbal IQ (VIQ), which mainly measures crystallized intelligence. In contrast to crystallized intelligence, fluid intelligence (novel problem solving) has been associated in previous neuroimaging work with the prefrontal cortex (e.g. Duncan et al., 2000; Jung & Haier, 2007). We will test this association between fluid intelligence and prefrontal cortex by examining brain relations with Performance IQ (PIQ), which is a stronger measure of fluid intelligence than is VIQ. Thus, the primary goal of this study is to test whether the overall genetic correlation between brain volume and IQ can be decomposed into different components with different etiologic and neural mechanisms.
In addition to its important influence in IQ, processing speed has also been shown to have a strong influence on reading ability (e.g., Badian, 1993; Catts, Gillispie, Leonard, Kail, & Miller, 2002; Fry & Hale, 1996; Manis, Doi, & Bhadha, 2000; Nicolson & Fawcett, 1990; Shanahan et al., 2006; Wolf & Bowers, 1999), and has been found to share genetic overlap with reading (e.g., Betjemann et al., 2008, Byrne et al., 2006; Compton et al., 2001, Petrill et al., 2006; Samuelsson et al., 2005). Reading has consistently also been found to have high genetic correlations with IQ (e.g., Gayán& Olson, 2003). In clinical research, processing speed has also become a primary candidate for the overlap between Reading Disability and Attention-Deficit/Hyperactivity Disorder, both phenotypically (e.g., Shanahan et al., 2007; Shanahan et al., 2006;Willcutt et al., 2005) and genetically (e.g., Betjemann et al., 2008; E. G. Willcutt, personal communication). Phenotypic studies have also showna relation between reading ability and brain volume (e.g., Pennington et al., 1999; Phinney et al., 2007), and although reading is known to be highly heritable, (see Fisher & DeFries, 2002; Olson, 2004; and Pennington & Olson, 2005, for reviews), the relation between brain volume and reading has not been investigated in a genetic framework. Hence, in the current study we expand our investigation of the genetic relation between brain volume and cognitive abilities to include reading.
Previous studies investigating the genetic covariation between brain volume and IQ have used measures of both gray matter and white matter; however, none have examined this overlap with more specific cortical brain structures. Thompson et al.(2001) postulated that the frontal regions may be the most highly linked to IQ, and also showed that the volume of the frontal lobes was the most highly heritable of the brain area volumes they investigated (also see Peper et al.,2007). However, bivariate genetic analyses were not done, leaving open the question of the genetic relationship between the frontal lobes and IQ. Consequently, this paper investigates the relation between our cognitive measures and prefrontal cortex volume, in addition to measures of neocortical gray matter, white matter, and total brain volume.
In summary, the current study expands on the previous genetic investigations of cognitive ability and brain volume. We investigate the genetic and environmental overlap between four measures of brain volume (total brain volume, neocortex, white matter, and prefrontal cortex) and four cognitive measures (VIQ, PIQ, processing speed, and reading), and predict these relations will be differential, for the reasons just discussed.
Method
Participants
Data analyzed in the present study came from a subset of twin pairs who were tested as part of the Colorado Learning Disabilities Research Center (CLDRC; DeFries et al. 1997; Olson, 2004). Twins were identified through school records from 27 different Colorado front-range school districts and were invited to participate if one or both twins were identified by school records or parent report to have a school history of reading problems. A comparison group of twin pairs with no history of reading problems was also invited to participate. All included twins had a Full-Scale IQ score of 85 or above, spoke English as their first language, and showed no evidence of serious neurological or emotional problems. For the behavioral/cognitive assessment, twins completed a full day of testing at the University of Colorado at Boulder. The current subset of twins includes 41 MZ twin pairs and 30 same-sex DZ twin pairs who ranged in age during cognitive testing from 8.1 to 17.9, with a mean age of 11.4 years. Imaging occurred at a later date. The age of participants at the time of MRI scan ranged from 11.7 to 23.7, with a mean age of 15.4 years (SD = 2.69). Sixty of the twin pairs were recruited as affected, and 11 were recruited as controls; 93 (65.5%)of the 142 individual twins had a school history of reading problems. All twin pairs included in these analyses were from predominantly middleclass homes, and the racial makeup was similar to that of the Colorado front-range area. Genders were about equal, with 52% males and 48% females.
Cognitive/Behavioral Measures
Verbal IQ(VIQ) and Performance IQ (PIQ)
Standard scores from the Wechsler Intelligence Scale for Children – Revised (WISC-R; Wechsler, 1974) were used for VIQ and PIQ.
Reading Composite
A composite score of Reading Ability was computed for each participant from the Peabody Individual Achievement Test (Dunn & Markwardt, 1970). Scores were created using discriminant weights from an earlier analysis of PIAT reading recognition, reading comprehension, and spelling subtest data in children with a history of reading difficulties and those without (e.g., DeFries, 1985).
Processing Speed Composite
A composite of four Processing Speed (PS) measures was computed by using the mean of age-standardized scores from each of the following four measures: 1) Identical Pictures task (Ekstrom, French, Harman & Derman, 1976) where participants match identical items from a series of similar distracter drawings, 2) Colorado Perceptual Speed task (DeFries & Baker, 1983; DeFries, Singer, Foch, & Lewitter, 1978)where participants circle the exact copy of the group of letters or numbers on the left from among the four choices to the right, 3) Coding subtest from the Wechsler Intelligence Scale for Children-Revised (WISC-R; Wechsler, 1974)in which the subject is presented with a novel code for each of the nine digits (e.g. a dash with a dot over it corresponds to the digit 1), and is asked to draw the correct code for as many randomly ordered digits as they can complete in two minutes, and 4) Rapid Naming (modeled after Denckla & Rudel, 1974), where participants are presented with pages of randomly ordered numbers and letters, and they name as many as possible in 15 seconds. Although these four tasks vary in both the stimuli presented (pictures vs. alphanumermic characters) and the response required (marking, drawing, or naming), they are moderately correlated (rs= .4 to .8, median r= .6 in Shanahan et al. 2006) and thus appear to tap a latent construct of processing speed.
Magnetic Resonance Imaging
Brain volume measures included Total Brain Volume, Neocortical Gray Matter, White Matter, and Prefrontal Cortex. For additional details beyond the following paragraphs regarding the MRI acquisition and analysis, please see Phinney et al. (2007).
Acquisition and Morphometric Procedures
All MR images were acquired on the General Electric 1.5-T Signa MR system (5X) (Milwaukee, WI, USA) located at the University of Colorado Health Sciences Center. After standard sagittal scout and coronal T2-weighted sequences, a coronal T1-weighted, three-dimensional (3D) spoiled gradient echo (SPGR) pulse sequence was performed with the following parameters: repetition time, 40 msec; echo time, 8 msec; flip angle, 40°; field of view, 24 cm; slice thickness, contiguous 3.0 mm; matrix, 256 × 256; averages, 1; imaging time, 10.5 minutes. All MR images in the current analyses were read clinically as within normal limits.
Position Normalization and Image Segmentation
All 3D SPGR scans were analyzed blind to diagnosis or twin/cotwin status according to a standard morphometric protocol, which includes positional normalization and image segmentation. On each normalized T-1 weighted 3D MRI slice, gray and white matter were segmented using intensity contour mapping and differential intensity contour algorithms (Kennedy et al., 1989; Filipek et al., 1989).
Morphometric Analyses
The acquired images were analyzed into volumes using two different methods: segmentation and parcellation, described below. Segmentation analysis yielded three of the four volumes used here: total white matter, total neocortex (gray matter only), and total cerebral volume, which was the combination of the first two volumes plus subcortical gray matter structures. The gray vs. white matter segmentation described above permitted the identification of all voxels containing neocortical gray matter, so their sum defined the volume of total neocortex. After neocortical voxels were accounted for, what remained in the total cerebrum was white matter plus subcortical gray matter. During the segmentation analysis, all subcortical structures were outlined by hand for each scan image, and their volumes were subtracted from the remainder above, leaving the volume of total white matter.
Parcellation
This method was utilized only for the prefrontal cortex volume (gray matter only) used in this study. Once the images have been segmented into gray matter and white matter compartments, the parcellation method specifies anatomic landmarks along the Y axis. These specific points in turn specify coronal image planes that determine the anterior and posterior boundaries of parcellation units (PU). The rostral and caudal limits of each of the PU are set automatically from the coordinates entered for points along the Y axis. The second step, the division of the cortex into PU, involves dividing the cortical ribbon into segments with cursor drawn parcellation lines, corresponding to medial and lateral boundaries of PU. The third and final step is to assign PU names to the segments of the cortical ribbon in the coronal image planes. This is accomplished by matching PU name in the PU-Table (Caviness et al., 1996) with the corresponding parcellated segment in the cortical ribbon. When there is an unambiguous match between the PU labels and the number of regions to be labeled, the algorithm automatically makes an initial naming assignment. The operator then verifies or corrects the assignment (Caviness et al., 1996).
Prefrontal cortex was defined by a sum of the frontal pole, pars triangularis, and frontal gyrus (for structural definitions see Caviness et al., 1996 and Kennedy et al., 1998). The prefrontal cortex (PFC) structures were measured through use of major cortical landmarks such as the conjunction of major fissures. For each of these landmarks the point along the Y axis was specified within the fissure just at the point where conjunction of fissures is explicit. Landmarks may also be set based on the conjunction of a secondary or tertiary fissure with a major fissure of the convexity or the medial hemispheric surface. Again the point on the Y axis was set at the point of fissure conjunction. Caviness et al. (1996) report reliability for voxel assignment to PU within the overall neocortex at 80.2%. In sum, there were four brain volumes utilized in these analyses: total cerebral volume, total white matter, total neocortex (gray matter only), and prefrontal cortex (gray matter only). So, prefrontal cortex was a subset of total neocortex, and total neocortex and total white matter were distinct volumes, but both were a subset of total cerebral volume. So, the analyses included the entire brain except the cerebellum and brainstem.
Standardizing Scores
Standardized scores of the brain area volumes from the structural MRI scans were used in the present analyses. Since some pairs of twins were recruited as controls and some as affected, as described above, all scores were converted to z-scores controlling for age, age-squared, and gender, standardized within their respective groups. (Because both members of each MZ and DZ twin pair are members of the same group, any differences between the group means would result in over-estimates of shared environmental influences if the data were combined without regard to group membership. Consequently, to adjust for possible differences between the group means, the twin data were standardized within group. This standardization was done within the greater sample of 1274 twins.) The distribution of each variable was then assessed for outliers, defined as scores that fell more than three standard deviations from the mean of each group, and more than 0.5 standard deviations beyond the next most extreme score. Outliers were replaced by bringing them in to 0.5 SD beyond the next most extreme data point.
Multivariate Genetic Analyses
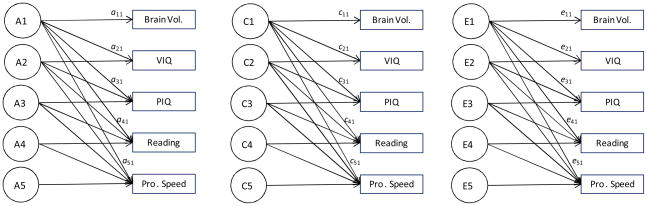
Variance in the measures was partitioned into genetic, shared environmental, and nonshared environmental factors. Four separate multivariate Cholesky decompositions were performed using the Mx statistical modeling package (Neale, Boker, Xie, & Maes, 2002). As shown in Figure 1, five variables were entered in each Cholesky: the first variable entered was always one brain area measure, and then the four cognitive measures were entered as the second through fifth variables.1
Figure 1.
Behavioral Genetic Cholesky model of brain volume and cognitive measures.
Results
The mean age-based standard scores for each standardized measure are presented in Table 1. While IQ scores are in the average range, reading scores are slightly below average, which would be expected since a significant portion of the sample had a history of reading problems. Phenotypic correlations of the standardized scores for all cognitive and brain variables are presented in Table 2. As can be seen, all brain volumes were significantly correlated with one another, some quite highly, and all cognitive variables were also significantly correlated with one another. There was a variable pattern in the strength of the phenotypic correlations between the cognitive and brain variables. The most consistent finding we observe is that brain volumes are more highly correlated with PIQ than with other cognitive measures. For example, the correlation with Total Brain Volume is significantly higher for PIQ than for VIQ (Fisher’s Z = 2.56, p < .05), and the PIQ correlation is also higher than VIQ with Neocortex (Fisher’s Z = 3.35, p < .05) and PFC (Fisher’s Z = 2.57, p < .05). White Matter showed the opposite pattern, being more highly correlated with VIQ than PIQ, but the difference between these was not significant (Fisher’s Z <1). Neocortex is additionally more highly correlated with PIQ than with both Reading (Fisher’s Z = 2.17, p < .05) and Processing Speed (Fisher’s Z = 2.34, p < .05), and Prefrontal Cortex also has a higher correlation with PIQ than with Reading (Fisher’s Z = 2.27, p < .05).
Table 1.
Means (with Standard Deviations) of Standard Scores* on cognitive measures
| Measure | Mean Standard Score |
|---|---|
| WISC Verbal IQ (VIQ) | 101.67 (12.34) |
| WISC Performance IQ (PIQ) | 103.76 (11.5) |
| PIAT Word Recognition | 92.67 (12.26) |
| PIAT Comprehension | 95.91 (12.37) |
Scores presented are the published Standard Scores by Age
Table 2.
Phenotypic correlations between standardized scores (corrected for Age and Age2) for all cognitive and brain volume measures in all participants (n=142).†
| Tot Brain Vol | Neocortex | White Mat. | PFC | VIQ | PIQ | Reading | Proc. Speed | |
|---|---|---|---|---|---|---|---|---|
| Total Brain Volume | -- | |||||||
| Neocortex | .85** | -- | ||||||
| White Matter | .69** | .25* | -- | |||||
| Prefrontal Cortex | .65** | .79* | .21 | -- | ||||
| VIQ | .14 | .01 | .27** | .06 | -- | |||
| PIQ | .42** | .39* | .24* | .35** | .30** | -- | ||
| Reading Score | .22* | .15* | .18* | .09 | .42** | .31** | -- | |
| Processing Speed | .26* | .13 | .28** | .19 | .18* | .52** | .42** | -- |
Correlation coefficients presented are computed with all participants, however when both twins are included this violates the independence of the data points. Therefore, the twin pairs were also split randomly and the correlations were re-done in both halves of the split sample, and the significance of those split halves is presented here.
Indicates correlation is significant at the p < .05 level in both halves of split sample;
indicates significance at the p < .05 level in one of the two halves of the split sample.
The overall proportions of variance in each measure due to genetic and environmental influences are presented in Table 3 with 95% confidence intervals. All cognitive and brain volume measures were found to be significantly heritable, as indicated by confidence intervals above zero. Nonshared environment was also significant for all measures, where as shared environmental influences were significant for only the Neocortex and Processing Speed measures.
Table 3.
Twin correlations for MZ and DZ pairs, and univariate estimates of heritability, shared environment, and nonshared environment computed from Cholesky models, with 95% confidence intervals in parentheses
| Measure | rMZ | rDZ | Heritability (a2) | Shared Environment (c2) | Nonshared Environment (e2) |
|---|---|---|---|---|---|
| Total Brain Volume | .95 | .48 | 0.80 (.49, .96) | 0.15 (0, .47) | 0.05 (.03, .09) |
| Neocortex | .87 | .68 | 0.30 (.07, .60) | 0.57 (.27, .77) | 0.14 (.08, .23) |
| White Matter | .65 | .14 | 0.60 (.19, .76) | 0.04 (0, .41) | 0.36 (.23, .56) |
| Prefrontal Cortex | .76 | .46 | 0.38 (.04, .77) | 0.34 (0, .67) | 0.28 (.17, .43) |
| VIQ* | .78 | .24 | 0.66 (.24, .82) | 0.08 (0, .46) | 0.26 (.16, .43) |
| PIQ* | .68 | .19 | 0.66 (.28, .80) | 0.03 (0, .35) | 0.31 (.19, .50) |
| Reading Score* | .59 | −.06 | 0.72 (.39, .85) | 0.01 (0, .18) | 0.27 (.15, .58) |
| Processing Speed* | .77 | .60 | 0.40 (.13, .75) | 0.42 (.08, .67) | 0.19 (.12, .31) |
Estimates for the behavioral measures are the means of the estimates from the four separate Cholesky decompositions
Standardized Cholesky path coefficients from the brain volume measures to the four cognitive measures are presented in Table 4. While the full Cholesky model computes path coefficients for all five of the genetic, shared environmental, and nonshared environmental factors in each model, for the current study the paths of primary interest were those shared between the initial brain volume measures and the cognitive measures. So, in the interest of space, only the Cholesky path coefficients for those initial factors (A1, C1, and E1)are presented in Table 4. Paths from genetic factor A1 were significant between Total Brain Volume and PIQ (a31 = .57), Reading (a41 = .27), and Speed (a51 = .48), but not VIQ (a21 = .23). In contrast, the Neocortex volume shared genetic influence with VIQ (a21 = .45) as well as PIQ (a31 = .67) and Speed (a51 = .40), but not Reading (a41 = .12). White Matter volume shared strong common genetic influence with Speed (a51 = .58), and PIQ (a31 = .47). Finally, the PFC shared a genetic factor with PIQ (a31 = .59), but with no other cognitive measure. After the paths for the first genetic factors (presented in Table 4) shared with brain volume, the unique path for VIQ (a22) was also significant in each of the four Choleskys (path coefficients ranged .64 –.80). No additional genetic paths were significant. There were no significant shared environmental paths common between brain and cognitive measures, nor were any of the nonshared environmental paths shared with brain volume significant. Unique nonshared environmental paths for each measure were significant, and there was significant common nonshared environmental variance between VIQ, PIQ, and reading in all analyses except the Total Brain Volume analysis.
Table 4.
Standardized Cholesky path coefficients from initial genetic (A1), shared environmental (C1) and nonshared environmental (E1) factors shared between brain area variables and cognitive measures, from four separate Cholesky decompositions
| Genetic Path Coefficients | |||||
|---|---|---|---|---|---|
| Path | TBV | NeoC | WMat | PFC | |
| a11 | .89 (.70, .98)* | .54(.27, .77)* | .77(.44, .87)* | .62(.19, .88)* | |
| VIQ | a21 | .23 (−.09, .48) | .45 (.01, .77)* | .27 (−.11, .66) | .41 (−.11, .82) |
| PIQ | a31 | .57 (.32, .78)* | .67 (.27, .87)* | .47 (.19, .85)* | .59 (.12, .87)* |
| Reading | a41 | .27 (.04, .49)* | .12 (−.30, .51) | .22 (−.10, .64) | .15 (−.43, .56) |
| Processing Speed | a51 | .48 (.22, .70)* | .40 (.01, .72)* | .58 (.25, .83)* | .24 (−.24, .71) |
| Shared Environmental Path Coefficients | |||||
| Path | TBV | NeoC | WMat | PFC | |
| c11 | .39 (0, .68)† | .75(.52, .88)* | .19(0, .64)† | .59(0, .82)† | |
| VIQ | c21 | −.16 (−.60, .55) | −.31 (−.58, .03) | .15 (−.61, .64) | −.32 (−.66, .21) |
| PIQ | c31 | −.18 (−.55, .49) | −.02 (−.31, .29) | −.23 (−.61, .58) | −.02 (−.44, .40) |
| Reading | c41 | −.04 (−.35, .31) | .10 (−.16, .34) | −.10 (−.45, .37) | −.01 (−.32, .31) |
| Processing Speed | c51 | −.37 (−.80, .80) | −.13 (−.48, .21) | −.63 (−.80, .80) | .06 (−.71, .74) |
| Nonshared Environmental Path Coefficients | |||||
| Path | TBV | NeoC | WMat | PFC | |
| e11 | .22 (.17, .29)* | .37(.29, .48)* | .60(.48, .75)* | .53(.41, .66)* | |
| VIQ | e21 | .05 (−.12, .21) | .05 (−.11, .22) | .01 (−.14, .17) | .02 (−.13, .19) |
| PIQ | e31 | −.04 (−.22, .14) | .12 (−.04, .31) | −.09 (−.25, .08) | −.01 (−.18, .17) |
| Reading | e41 | −.02 (−.22, .18) | .04 (−.17, .23) | .03 (−.13, .24) | .02 (−.15, .22) |
| Processing Speed | e51 | −.01 (−.14, .13) | .05 (−.08, .20) | −.05 (−.16, .08) | .05 (−.09, .19) |
TBV= Total Brain Volume, NeoC= Neocortex, WMat= White Matter, and PFC= Prefrontal Cortex
Indicates path coefficient is significant, as indicated by 95% confidence interval above zero.
Diagonals of the matrices were bound to above zero, so confidence intervals could not go below zero for these values, but they should be assumed to be non-significant.
Estimates of the genetic correlations between brain volume and cognitive measures are presented in Table 5. The patterns of significance in the correlations are identical to those of the path coefficients presented above. Significant genetic correlations were found between Total Brain Volume and PIQ (.71), Speed (.79), and Reading (.32), but not VIQ (.28). In contrast, the Neocortex volume shared genetic influence with VIQ (.58)as well as PIQ (.82)and Speed (.64), but not Reading (.14). White Matter volume shared strong common genetic influence with Speed (.89), and additionally with PIQ (.58). Finally, the PFC shared a genetic factor with PIQ (.72), but no other cognitive measure. None of the environmental correlations (either shared or nonshared) between brain and cognitive measures were significant.
Table 5.
Genetic correlations with Brain Area volumes from four separate Cholesky decompositions
| Total Brain Volume | Neocortex | White Matter | Prefrontal Cortex | |
|---|---|---|---|---|
| VIQ | .28 (−.11, .68) | .58 (.02, .98)* | .32 (−.15, .85) | .52 (−.14, .99) |
| PIQ | .71 (.41, 1.0)* | .82 (.36, 1.0)* | .58 (.23, 1.0)* | .72 (.16, 1.0)* |
| Reading | .32 (.04, .61)* | .14 (−.28, .64) | .26 (−.12, .78) | .17 (−.54, .66) |
| Processing Speed | .79 (.36, 1.0)* | .64 (.02, .97)* | .89 (.41, 1.0)* | .38 (−.38, .98) |
indicates correlationis significant, as indicated by 95% confidence interval above zero
Discussion
While we know that IQ is significantly influenced by both genetics and environment (e.g., Plomin et al., 2008), the results in the current study suggest that the correlation between IQ and brain volume is due substantially to genetic influences. This finding is supported by the few previous behavior genetic studies of brain volume and IQ, which have also found that phenotypic overlap between IQ and brain volume was significant only for genetic factors (Posthuma et al. 2002; Posthuma et al., 2003).
Previous research also has shown that Full Scale IQ is related to both gray-and white-matter brain volumes (Posthuma et al., 2002). The current results indicate that when IQ is partitioned into Verbal and Performance subscales, however, VIQ and PIQ components of IQ are not related to brain areas in the same way. In the phenotypic correlations, all brain volumes except white matter are more highly related to PIQ than the VIQ. More specifically in the genetic results, PIQ shared significant genetic influence with both gray-matter and white-matter volumes, while VIQ shared significant genetic influence with gray matter but not white matter. While the confidence intervals of these comparisons do overlap, so we cannot say that they are significantly different per se, the pattern of findings is consistent with the hypothesized relation, suggested in the Introduction, between crystallized intelligence and cortical networks. Also, as suggested earlier, PIQ is more strongly related to prefrontal cortex in both the phenotypic correlations and the genetic results, consistent with previous research demonstrating a relation between fluid intelligence and prefrontal cortex. These findings fit well with those of Posthuma et al. (2003), who showed that each of four IQ dimensions differed in the pattern of genetic influence with gray and white matter.
Furthermore, we were interested in how more specific measures of cognitive function, processing speed and reading, were related to brain volume. Similar to the findings with PIQ, processing speed also shared significant genetic variance with both gray matter and white matter. Our finding that speed overlaps with the same brain areas as PIQ is reasonable given findings that speed has been found to be more correlated with fluid than crystallized intelligence, both phenotypically and genetically (i.e., Luciano et al., 2004). This finding is somewhat in contrast to the results of Posthuma et al. (2003) however, who found that the WAIS processing speed factor was significantly related to white matter but not gray matter. Both studies, however, agree in finding a stronger relation between PS and white matter than with other brain regions. As discussed earlier, this is consistent with prior literature that has shown a strong relation between white matter integrity and PS, but is inconsistent with the findings of van Leeuween and colleagues (2009), who found no relation between processing speed and brain volume. The different measures of PS used in the two studies could contribute to this discrepancy in results: our PS score is computed from four separate tests of processing speed, while the PS subscale of the WISC used by van Leeuween and colleagues is a combination of only digit-symbol substitution and symbol search tasks. Since measures of response speed are known to be less reliable in children, and since the WISC PS score is less reliable than other WISC composite scores (Wechsler, 2003), it is possible that the current study’s older participants, combined with a more robust PS measure, allowed us to find significant relations between PS and brain volume where van Leeuween et al. did not.
In contrast, reading skills shared genetic overlap with Total Brain Volume only. While previous phenotypic results using an overlapping sample to the current study showed some significant relations between brain area volumes and reading skill, these findings were not extremely strong (Phinney et al. 2007), lending support to the current findings.
The current results present an interesting picture of the relation between specific cognitive functions and different brain structures. The overlap between these factors appears to be entirely due to genetic influences, despite environmental influence on the individual measures. Nonetheless, it is important to realize that this pattern of genetic but not environmental correlations can be interpreted in several ways. Posthuma et al. (2003) distinguished four possibilities: 1) pleiotropy, 2) unidirectional causation from brain to cognition, 3) unidirectional causation from cognition to brain, and 4) reciprocal causation between brain and cognition. These possibilities relate to the four possible interpretations of any correlation: 1) a third variable influences both A and B, 2) A causes B, 3) B causes A, and 4) reciprocal causation between A and B. The first possibility, pleiotropy, would indicate that the same genes influence both brain volume and cognition, which do not directly influence each other. The second possibility corresponds to a simple innatist model which holds that brain volume directly mediates the relation between genes and cognition. That is, genes determine brain size early in life, perhaps prenatally, and these innate differences in brain size contribute to individual differences in cognitive development in a fairly direct way. The third possibility corresponds to an emergentist model in which cognitive development affects brain development through a protracted postnatal developmental process. This possibility is consistent with a G-E correlation process, in which genetic differences in a neural parameter are correlated with environmental differences that affect postnatal brain developmental processes, like synaptic pruning and myelination. Finally the fourth possibility, reciprocal causation, combines the second and third possibilities. In sum, the overall point is that a genetic correlation between a given brain structure and dimension of cognition, such as those reported here, leaves open the developmental process underlying that correlation. Moreover, the particular developmental process may well vary across different domains of cognition and particular brain structures.
In a more recent paper by van Leeuwen and colleagues (van Leeuwen et al., 2009, also see De Moor, Boomsma, Stubbe, Willemsen, & De Geus, 2008), the authors argue that if the causal path runs from cognition to brain, then there should be both environmental and genetic correlations between cognition and brain, since there are significant environmental and genetic effects on cognition, which would then be passed on to brain in the causal chain. Since they found only genetic but not environmental correlations between brain volumes and cognition in this study, they argue that only a causal path from brain to cognition or pleiotropy (possibilities 2and 1above) are consistent with their data. In the current results, since we also found significant genetic but not environmental correlations between brain and cognition, the most likely causal models are either a causal path from brain to cognition or pleiotropy. The same argument could be made from Posthuma et al. (2003)’s study with adults, which also did not find both environmental and genetic correlations between any cognitive and brain volume measures.
Due to the small sample size in the present study, the power to detect significance was not high; thus some of the non-significant paths between brain volume and cognitive variables could be significant in a larger sample. Furthermore, although we show a differential pattern of significance across bivariate relations, it is important to note that that most of the confidence intervals of these comparisons overlap, so we cannot say that they are significantly different from one another. Thus, the specific differences between measures should be interpreted with caution, and future research with larger samples should be done to replicate these findings. Results also showed that there were almost no additional significant genetic or environmental influences on any of the cognitive measures after that shared with brain volume. There was a significant independent genetic factor for VIQ alone in each model. While the other genetic paths were not significant, estimates for the unique paths for PIQ and reading were moderately high, suggesting that with a larger sample size these might become significant. Interestingly, after the variance shared with brain volume, the additional common genetic path coefficients shared between the cognitive measures were quite low, suggesting that the genetic variation shared with brain volume accounts for most of the overlap between the cognitive measures. There were independent nonshared environmental factors for each cognitive measure; however these estimates include test error. While this could imply that almost all genetic and environmental influences on cognition are shared with brain development, due to the size of the sample in this study, it is possible that we do not have sufficient power to detect those additional influences here. Further studies with larger sample sizes will be needed to explore this further.
Additionally, one factor to consider when generalizing the current results is that our sample was overselected for reading problems. However, although pairs with at least one twin referred for having reading problems do have a lower VIQ than the control pairs, the mean VIQ of the entire sample is actually 101.7, which is very close to the population mean. The standard deviation of the VIQ scores is 12 points, so there is not an issue with restricted range in the sample, either. Therefore, we do not believe that there is a problem with generalizing our findings to an unselected population, but further research is needed to address this question.
Finally, although we did not find significant environmental factors shared between cognition and brain volume, we do not dismiss this possibility in future research. While previous studies support the genetic basis for overlap between brain volume and IQ, based on what we know about brain plasticity, it seems likely that learning would be an environmental influence on cognition that would be mediated by an increase in synaptic density in the cortex, and quite possibly an increase in white matter myelination as well. Thus, while it seems possible that environmental learning could increase brain volume, it may be difficult to observe those differences either in predominantly middle class samples or with the magnet used in the current study. It is possible that in future research with different samples or larger magnets, an environmental correlation between cognition and brain maybe detected.
Acknowledgments
Initial work on this paper was done when R. Betjemann was a postdoctoral trainee at the Institute for Behavioral Genetics, University of Colorado, Boulder, CO. This training was funded by NIMH training grant T32 MH016880-25.
This project was also funded by NIH grant HD027802 to the Colorado Learning Disabilities Research Center, of which B. Pennington, J. DeFries, and E. Willcutt are Co-PIs.
Footnotes
Cholesky decompositions were also done with each cognitive variable entered in the first position, and the four brain volumes entered as the second through fifth variables, and the pattern of results was the same as what is presented here.
References
- Anstey K, Mack H, Christensen H, Li S, Reglade-Meslin C, Maller J, et al. Corpus callosum size, reaction time speed and variability in mild cognitive disorders and in a normative sample. Neuropsychologia. 2007;45:1911–1920. doi: 10.1016/j.neuropsychologia.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Baare WFC, Pol HE, Boomsma DI, Posthuma D, de Geus EJC, Schnack HG, et al. Quantitative Genetic Modeling of Variation in Human Brain Morphology. Cerebral Cortex. 2001;11:816–824. doi: 10.1093/cercor/11.9.816. [DOI] [PubMed] [Google Scholar]
- Badian NA. Phonemic awareness, naming, visual symbol processing, and reading. Reading and Writing: An Interdisciplinary Journal. 1993;5:87–100. [Google Scholar]
- Baker LA, Vernon PA, Ho H. The genetic correlation between intelligence and speed of information processing. Behavior Genetics. 1991;21:351–367. doi: 10.1007/BF01065972. [DOI] [PubMed] [Google Scholar]
- Betjemann RS, Willcutt EG, Olson RK, Keenan JM, DeFries JC, Wadsworth SJ. Word reading and comprehension: Stability, overlap and independence. Reading and Writing: An Interdisciplinary Journal. 2008;21:539–558. [Google Scholar]
- Bunce D, Anstey K, Christensen H, Dear K, Wen W, Sachdev P. White matter hyperintensities and within-person variability in community-dwelling adults aged 60–64 years. Neuropsychologia. 2007;45:2009–2015. doi: 10.1016/j.neuropsychologia.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Byrne B, Olson RK, Samuelsson S, Wadsworth S, Corley R, DeFries JC, Willcutt E. Genetic and environmental influences on early literacy. Journal of Research in Reading. 2006;29(1):33–49. [Google Scholar]
- Catts HW, Gillispie M, Leonard LB, Kail RV, Miller CA. The role of speed of processing, rapid naming, and phonological awareness in reading achievement. Journal of Learning Disabilities. 2002;35:509–524. doi: 10.1177/00222194020350060301. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Meyer J, Makris N, Kennedy DN. MRI based topographic parcellation of human neocortex: An anatomically specified method with estimate of reliability. Journal of Cognitive Neuroscience. 1996;8:566–587. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- Compton DL, Davis CJ, DeFries JC, Gayan J, Olson RK. Genetic and environmental influences on reading and RAN: An overview of results from the Colorado Twin Study. In: Wolf M, editor. Conference proceedings of the Dyslexia Research Foundation Conference in Extraordinary Brain Series: Time, fluency, and developmental dyslexia. Baltimore MD: York Press; 2001. pp. 277–303. [Google Scholar]
- De Moor MHM, Boomsma DI, Stubbe JH, Willemsen G, De Geus EJC. Testing causality in the association between regular exercise and symptoms of anxiety and depression. Archives of General Psychiatry. 2008;65:897–905. doi: 10.1001/archpsyc.65.8.897. [DOI] [PubMed] [Google Scholar]
- DeFries JC. Colorado Reading Project. In: Gray DB, Kavanagh JF, editors. Biobehavioral measures of dyslexia. Parkton, MD: York Press; 1985. pp. 107–122. [Google Scholar]
- DeFries JC, Baker LA. Colorado Family Reading Study: Longitudinal Analyses. Annals of Dyslexia. 1983;33:153–162. [Google Scholar]
- DeFries JC, Filipek PA, Fulker DW, Olson RK, Pennington BF, Smith SD. Colorado Learning Disabilities Research Center. Learning Disabilities. 1997;8:7–19. [Google Scholar]
- DeFries JC, Singer SM, Foch TT, Lewitter FI. Familial Nature of Reading Disability. British Journal of Psychiatry. 1978;132:361–367. doi: 10.1192/bjp.132.4.361. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Rudel R. Rapid “automatized” naming of pictured objects, colors, letters, and numbers by normal children. Cortex. 1974;10:186–202. doi: 10.1016/s0010-9452(74)80009-2. [DOI] [PubMed] [Google Scholar]
- Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN, Emslie H. A neural basis for general intelligence. Science. 2000;289:457–60. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Markwardt FC. Examiner’s manual: Peabody individual achievement test. Circle Pines, MN: American Guidance Service; 1970. [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Derman D. Manual for kit of factor-referenced cognitive tests 1976. Education Testing Service; Princeton, NJ: 1976. [Google Scholar]
- Filipek PA, Kennedy DN, Caviness VS, Rossnick SL, Spraggins TA, Starewicz PM. MRI-based morphometry: Development and application to normal subjects. Annals of Neurology. 1989;25:61–67. doi: 10.1002/ana.410250110. [DOI] [PubMed] [Google Scholar]
- Filley CM. White matter and behavioral neurology. Annals of the New York Academy of Sciences. 2005;1064:162–183. doi: 10.1196/annals.1340.028. [DOI] [PubMed] [Google Scholar]
- Fisher SE, DeFries JC. Developmental dyslexia: genetic dissection of a complex cognitive trait. Nature Reviews Neuroscience. 2002;3:767–780. doi: 10.1038/nrn936. [DOI] [PubMed] [Google Scholar]
- Fry AE, Hale S. Processing speed, working memory, and fluid intelligence: Evidence for a developmental cascade. Psychological Science. 1996;7:237–241. [Google Scholar]
- Gayán J, Olson RK. Genetic and environmental influences on individual differences in printed word recognition. Journal of Experimental Child Psychology. 2003;84:97–123. doi: 10.1016/s0022-0965(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Geidd JN, Schmitt JE, Neale MC. Structural brain magnetic resonance imaging of pediatric twins. Human Brain Mapping. 2007;28:474–481. doi: 10.1002/hbm.20403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SJ. The mismeasure of man. New York: WW Norton; 1981. [Google Scholar]
- Ho HZ, Baker LA, Decker SN. Covariation between Intelligence and speed of cognitive processing: Genetic and environmental influences. Behavior Genetics. 1998;18:247–261. doi: 10.1007/BF01067845. [DOI] [PubMed] [Google Scholar]
- Jerison HJ. Brain size and the evolution of the mind. New York: American Museum of Natural History; 1989. [Google Scholar]
- Jung RE, Haier RJ. The parieto-frontal integration theory of intelligence: Converging neuroimaging evidence. Behavioral and Brain Sciences. 2007 doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Kail R. Development of processing speed in childhood and adolescence. Advances in Child Develeopment and Behavior. 1991;23:151–185. doi: 10.1016/s0065-2407(08)60025-7. [DOI] [PubMed] [Google Scholar]
- Kennedy DN, Filipek PA, Caviness VS., JR Anatomic segmentation and volumetric calculations in nuclear magnetic resonance imaging. IEEE Trans Medical Imaging. 1989;8:1–7. doi: 10.1109/42.20356. [DOI] [PubMed] [Google Scholar]
- Kennedy DN, Lange N, Makirs N, Bates J, Meyer J, Caviness VS., Jr Gyri of the human neocortex: An MRI-based analysis of volume and variance. Cerebral Cortex. 1998;8:372–384. doi: 10.1093/cercor/8.4.372. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. The structural development of the human brain as measured longitudinally. In: Coch D, Fischer KF, Dawson G, editors. Human Behavior, Learning and the Developing Brain. New York: The Guilford Press; 2007. pp. 50–73. [Google Scholar]
- Luciano M, Smith GA, Wright MJ, Geffen GM, Geffen LB, Martin NG. On the heritability of inspection time and its covariance with IQ: A twin study. Intelligence. 2001;29:443–457. [Google Scholar]
- Luciano M, Wright MJ, Geffen GM, Geffen LB, Smith GA, Martin NG. A genetic investigation of the covariation among inspection time, choice reaction time, and IQ subtest scores. Behavior Genetics. 2004;34:41–50. doi: 10.1023/B:BEGE.0000009475.35287.9d. [DOI] [PubMed] [Google Scholar]
- Luciano M, Wright MJ, Smith GA, Geffen GM, Geffen LB, Martin NG. Genetic covariance among measures of information processing speed, working memory, and IQ. Behavior Genetics. 2001;31:581–592. doi: 10.1023/a:1013397428612. [DOI] [PubMed] [Google Scholar]
- MacDonald S, Nyberg L, Bäckman L. Intra-individual variability in behavior: Links to brain structure, neurotransmission and neuronal activity. Trends in Neurosciences. 2006 Aug;29:474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Manis FR, Doi LM, Bhadha B. Naming speed, phonological awareness, and orthographic knowledge in second graders. Journal of Learning Disabilities. 2000;33:325–333. 374. doi: 10.1177/002221940003300405. [DOI] [PubMed] [Google Scholar]
- McDaniel MA. Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33:337–46. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 6. Richmond, VA: Department of Psychiatry; 2002. [Google Scholar]
- Nicolson RI, Fawcett AJ. Automaticity: a new framework for dyslexia research. Cognition. 1990;35:159–182. doi: 10.1016/0010-0277(90)90013-a. [DOI] [PubMed] [Google Scholar]
- Olson RK. SSSR, environment, and genes. Scientific Studies of Reading. 2004;8:111–124. [Google Scholar]
- Pennington BF, Filipek PA, Lefly D, Churchwell J, Kennedy DN, Simon JH, et al. Brain mophometry in reading-disabled twins. Neurology. 1999;53:723–729. doi: 10.1212/wnl.53.4.723. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Filipek PA, Lefly D, Chhabildas N, Kennedy DA, Simon JH, et al. A twin MRI study of size variations in the human brain. Journal of Cognitive Neuroscience. 2000;12:223–232. doi: 10.1162/089892900561850. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Olson RK. Genetics of dyslexia. In: Snowling M, Hulme C, editors. The Science of Reading: A Handbook. Oxford, England: Blackwells; 2005. pp. 453–472. [Google Scholar]
- Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Pol HEH. Genetic influences on human brain structure: A review of brain imaging studies in twins. Human Brain Mapping. 2007;28:464–473. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrill SA, Deater-Deckard K, Thompson LA, DeThorne L, Schatschneider C. Reading skills in early readers: Genetic and shared environmental influences. Journal of Learning Disabilities. 2006;39(1):48–55. doi: 10.1177/00222194060390010501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney E, Pennington BF, Olson RK, Filley CM, Filipek PA. Brain structure correlates of component reading processes: implications for reading disability. Cortex. 2007;43:777–791. doi: 10.1016/s0010-9452(08)70506-9. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. 5. New York: Worth Publishers; 2008. [Google Scholar]
- Posthuma D, Baare WFC, Pol HEH, Kahn RS, Boomsma DI, de Gues EJC. Brain volumes and the WAIS-III dimensions of Verbal Comprehension, Working Memory, Perceptual Organization, and Processing Speed. Twin Research. 2003;6:131–139. doi: 10.1375/136905203321536254. [DOI] [PubMed] [Google Scholar]
- Posthuma D, de Geus EJC, Baare WFC, Pol HEH, Kahn RS, Boomsma DI. The association between brain volume and intelligence is of genetic origin. Nature Neuroscience. 2002;5:83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Posthuma D, de Geus EJC, Boomsma DI. Perceptual speed and IQ are associated through common genetic factors. Behavioral Genetics. 2001;31:593–602. doi: 10.1023/a:1013349512683. [DOI] [PubMed] [Google Scholar]
- Samuelsson S, Byrne B, Quain P, Wadsworth S, Corley R, DeFries JC, Willcutt E, Olson RK. Environmental and genetic influences on preschool skills in Australia, Scandinavia, and the United States. Journal of Educational Psychology. 2005;97:705–722. [Google Scholar]
- Shanahan MA, McGrath L, Santerre-Lemmon L, Barnard H, Willcutt EG, Olson RK, Pennington BF. Shared cognitive deficits in Reading Disability and Attention-Deficit/Hyperactivity Disorder. Presented at the Biennial meeting of the Society for Research in Child Development; Boston, MA. Mar, 2007. [Google Scholar]
- Shanahan MA, Pennington BF, Yerys BE, Scott A, Boada R, Willcutt EG, Olson RK, DeFries JC. Processing Speed Deficits in Attention Deficit/Hyperactivity Disorder and Reading Disability. Journal of Abnormal Child Psychology. 2006;34:584–601. doi: 10.1007/s10802-006-9037-8. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, et al. Genetic influences on brain structure. Nature Neuroscience. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- van Leeuwen M, Peper JS, van den Berg SM, Brouwer RM, Hulshoff Pol HE, Boomsma DI. A genetic analysis of brain volumes and IQ in children. Intelligence. 2009;37:181–191. [Google Scholar]
- Wechsler D. Examiners’ manual: Wechsler Intelligence Scale for Children. 4. San Antonio, TX: The Psychological Corporation; 1974. [Google Scholar]
- Wechsler D. WISC-IV Technical and Interpretive Manual. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Willcutt EG, Pennington BF, Olson RK, Chhibildas N, Hulslander J. Neuropsychological analyses of comorbidity between reading disability and attention deficit hyperactivity disorder: In search of the common deficit. Developmental Neuropsychology. 2005;27:35–78. doi: 10.1207/s15326942dn2701_3. [DOI] [PubMed] [Google Scholar]
- Wolf M, Bowers G. The double-deficit hypothesis for the developmental dyslexias. Journal of Educational Psychology. 1999;91:415–438. [Google Scholar]