Abstract
Acoustic displays with difficult-to-execute sounds are often subject to strong sexual selection, because performance levels are related to the sender’s condition or genetic quality. Performance may also vary with age, breeding stage, and motivation related to social context. We focused on within-male variation in four components of trill performance in banded wren (Thryophilus pleurostictus) songs: note consistency, frequency bandwidth, note rate and vocal deviation. The latter is a composite measure reflecting deviation from the performance limit on simultaneously maximizing both frequency bandwidth and note rate. We compared the changes in these song parameters at three time scales: over the course of years, across the breeding season, and at different times of the day with contrasting agonistic contexts. Vocal deviation decreased and note consistency increased over years, suggesting that experience may improve individual proficiency at singing trills. Consistency also increased across the season, confirming that practice is important for this parameter. Although there was no significant seasonal change in vocal deviation, one of its components, note rate, increased during the season. Neither vocal deviation nor consistency varied with agonistic context. However, note rate increased during playback experiments simulating territorial intrusions compared to dawn chorus singing. The magnitude of a male’s increase in note rate was positively correlated with his aggressive behavior during the playback experiment. Thus consistency, bandwidth, and vocal deviation indicate age, whereas trill rate flexibly indicates the singer’s aggressive motivation. We also found evidence of a within-male trade-off between vocal deviation and consistency.
Keywords: motor performance, vocal deviation, trill note consistency, frequency bandwidth, trade-off, song quality, Thryophilus pleurostictus
Introduction
Displays with a significant motor component, such as vigorous and repetitive dance-like maneuvers and acoustic signals containing difficult-to-execute sounds, are often the targets of sexual selection (Bradbury and Vehrencamp 2011). The motor performance of displays is closely linked to whole-organism performance and engages a large number of genes and metabolic pathways (Byers et al. 2010). Display quality can therefore reflect aspects of the sender’s current condition, general health during development, and genetic quality. Furthermore, if displays are physically difficult to produce, then consistent repetition of those displays at the same high performance level can provide receivers with even more reliable information about sender quality (Botero and de Kort 2012). Choosy females in particular may benefit by preferring males that perform difficult motor displays well and consistently, exerting strong selection pressure on display form and repetition to reveal otherwise hidden correlates of underlying male quality. Although male receivers have access to additional signals and cues for directly assessing a rival’s size, strength, or motivation, they too may derive some information about a rival by attending to motor performance of displays (Bradbury and Vehrencamp 2011).
The songs of birds are typically loud and complex motor displays that require well-developed brain circuits and the coordination of vocal and respiratory muscles to produce (Jarvis 2004; Ashmore et al. 2005; Fletcher et al. 2006; Riede et al. 2006; Mendez et al. 2012). The patterned series of notes in birdsong not only identifies the species, but the fine structure of the notes can also reveal information about the singer’s natal origin (Poesel et al. 2012). Moreover, aspects of note structure associated with performance quality are correlated with male reproductive success in several species, suggesting that song performance matters to females (Drăgănoiu et al. 2002; Forstmeier et al. 2002; Ballentine et al. 2004; Janicke et al. 2008; Nemeth et al. 2012). Trilled song elements have been the focus of several studies because they may be particularly difficult to produce well (Podos 1997; Podos et al. 2009). To create the frequency-modulated syllables, the bird must force air through the syrinx and change the tension on the syringeal membranes rapidly while simultaneously modulating the volume of the upper vocal tract and beak gap to match the fundamental frequency (Goller and Suthers 1996; Hoese et al. 2000; Fletcher et al. 2006; Riede et al. 2006). Broadband frequency sweeps require more movement and are therefore more difficult to repeat at a rapid rate. Two features of trilled song elements are especially amenable to quantitative assessment by both animal receivers and researchers. The first is vocal deviation, measured as the deviation from the upper limit trade-off line between note repetition rate and frequency bandwidth (Podos 2001). High-performance trills by this measure are those closest to the limit and thus have the lowest deviations. The second feature is the consistency of the acoustic structure or frequency modulation pattern of repeatedly delivered notes (Sakata and Vehrencamp 2012). High-performance trills by this measure are those with more similar consecutive note shapes. Vocal deviation and consistency may be difficult to maximize simultaneously (Botero and de Kort 2012).
Vocal deviation and note consistency could vary on different time scales depending on what sender attributes they reflect: a) the sender’s intrinsic genetic quality (e.g., mutation load), b) the environmental conditions during the sender’s brain development and song learning phases (e.g., nutritional stress), c) the sender’s age (if singing skill improves with practice) and health, and d) current motivation (e.g., stage of breeding, presence of a female, or challenge by a rival). Evidence exists for some of these effects. Genetic evidence, such as documentation of significant heritability of song fine structure for benefits bestowed on offspring survival, has not yet been obtained for either vocal deviation or trill note consistency, but does exist for some other song features (Welch et al. 1998; Brandt and Greenfield 2004; Forstmeier et al. 2009; Simmons et al. 2010). Nutrition during development primarily affects song learning accuracy and complexity, but also affects song consistency in the zebra finch (Taeniopygia guttata) (Spencer et al. 2005; Holveck et al. 2008; Zann and Cash 2008). Song performance measures improve with age in several avian species (Kipper and Kiefer 2010). Plasma androgen levels affect trill performance in a Neotropical singing mouse (Scotinomys) (Pasch et al. 2011), and experimental manipulation of body condition with food deprivation reduces several aspects of singing performance in zebra finch males (Ritschard and Brumm 2012). Short-term changes in motivation have been shown to affect note-structure-based song performance measures in a few species: vocal deviation decreases (performance improves) during playback challenges in swamp sparrows (Melospiza georgiana) (DuBois et al. 2009), and consistency increases in the presence of a female in zebra finches and Bengalese finches (Lonchura striata) (Sakata et al. 2008).
Male banded wrens (Thryophilus pleurostictus) are vigorous songsters that sing for both territorial defense and mate attraction. Each male possesses a repertoire of 15–25 distinct song types that terminate in a loud song-type-specific trill. Vocal deviation of the trill of a given song type differs among males, and males behave more cautiously toward playback of a high performance trill relative to playback of a low performance trill (Illes et al. 2006; de Kort et al. 2009a). The consistency of the repeated trill notes increases with age over the first few years of life, and males behave more cautiously toward the highly consistent trills of older males compared to the inconsistent trills of young males (de Kort et al. 2009b). Females also appear to attend to trill fine structure, since males that obtained extra-pair fertilizations had both lower vocal deviation and greater note consistency than the males they cuckolded (Cramer et al. 2011). In this study we investigated how trill note consistency, vocal deviation, and its components (frequency bandwidth and note rate), changed within males on three time scales: 1) between years, 2) over the course of the breeding season, and 3) during dawn chorus singing compared to mid-morning aggressive interactions stimulated by song playback. A priori, we would expect males to increase their performance with age and during playback experiments, and to decrease their performance during the season. We also assessed whether individual male changes in trill components were associated with potential male quality traits such as age, body condition, survivorship, reproductive success, and aggressive behavior. Finally, we tested the prediction of a trade-off between consistency and vocal deviation. By testing whether trill performance components are fixed traits, or whether they can be modulated on a short time scale depending on context and motivation, we provide insight into the nature of the constraints operating on these sexually-selected traits that may influence their potential as honest indicators of male quality.
Materials and methods
Study area
The banded wren inhabits tropical dry deciduous forest on the Pacific slope of Central America from southern Mexico to northern Costa Rica. We studied a population in Santa Rosa National Park, Área de Conservación Guanacaste, Costa Rica (10° 49′ N, 85° 38′ W). For a detailed description of the habitat and study population, see Molles and Vehrencamp (1999) and Trillo and Vehrencamp (2005). Banded wrens breed from late April to September, extra-pair paternity is rare (Cramer et al. 2011), and pairs are territorial year-round, although most singing activity, including dawn chorusing, ceases in the non-breeding season. Males are the primary singers, but females occasionally deliver male-like and easily distinguished songs (Molles and Vehrencamp 1999). Individual birds in the study were color-banded for identification and regularly censused for breeding activity and survival. Eight adult males in this study were banded as nestlings in previous years so their absolute ages were known, but most birds were of unknown age. Captured birds were measured using standard techniques and sexed based on these measurements, presence or absence of a brood patch, and subsequent singing and territorial behavior. Body condition was computed as the season-corrected ratio of weight to tarsus length (×100). We selected songs for this study from recordings made during the 2003–2006 breeding seasons using a Sennheiser ME67 directional microphone and a Marantz PMD 690 digital solid-state recorder at a sampling rate of 48 kHz and 16 bits per sample. Recordists were usually able to approach singing birds to within 10 m, but this distance varied as birds moved around their territories. We are not aware of any biases in recording distance as a function of year, season, bird’s age, or context (dawn chorus singing versus playback experiments). Audio files were saved in WAVE format, scanned for wren songs using the energy detector in SyrinxPC (John Burt, Seattle, Washington; www.syrinxpc.com), and reviewed by the recordist to annotate all song types, calls, and voice comments.
Trill parameter measurements
We targeted song types with broadband unimodally up-sweeping trill notes, since these types of trills are more likely to be subject to performance constraints than trills with chevron-shaped notes (Podos et al. 2009), and also are more amenable to accurate measurement of note consistency and bandwidth than narrowband or down-sweeping notes, which are very short and rapidly repeated in this species. Based on these criteria and on frequency of use within the study population, we have included seven specific song types for analysis (i.e., song types 103, 202, 206, 213, 220, 242, and 303; for spectrograms illustrating some of these types, see Fig. 2 in Trillo and Vehrencamp (2005)). Males differed in their song-type repertoires and also exhibited different subtype variants. Thus for each male and temporal/contextual comparison we extracted trills of the same song type from the contrasting conditions (clarified below) so that direct within-male and within-song-type comparisons could be made. Where enough high-quality recordings were available, we aimed to analyze 2–3 song types per male for each comparison, with 10–12 songs per bird, song type, and condition within a comparison. On average, we obtained 1.88 (range 1–4) song types per male and 9.2 (range 5–23) songs per male, song type, and condition (usually from a single day’s recording session). We used SyrinxPC (spectrogram settings: FFT size = 512, window type = Blackman) to clip trills of target song types from recordings, selectively filter out background noise, and adjust the waveforms of entire trills by values larger than or less than 100% to visually similar amplitudes (recordings were made with different gain levels) so that trill types could be accurately classified and poor quality samples with excessive reverberation could be identified and rejected. We quantified both vocal deviation and consistency for each of the remaining trills.
To quantify vocal deviation, we used Avisoft SASLab Pro v. 4.39 (Avisoft Bioacoustics, Berlin, Germany; spectrogram settings: FFT size = 512, frame size = 100%, window type = Hamming, overlap = 75%, frequency resolution = 94 Hz, temporal resolution = 2.67 msec) and its band-pass time domain filter (FIR) to remove interfering background noise below 1 kHz and above 6 kHz. We then used the automatic parameter measurement tool to delineate each trill note (additional filtering between notes was sometimes needed to accurately delineate notes) and to measure each note’s minimum and maximum frequency at an amplitude threshold of -22 dB below the peak amplitude in a given song; this arbitrary threshold value provided the best delineation of the banded wren’s up-sweeping trill notes. For each trill, we computed bandwidth by averaging the frequency bandwidth (maximum minus minimum frequency) of all notes in the trill, and note rate as the number of notes per second. We divided the number of notes in the trill minus 1 by the time from the beginning of the first note to the beginning of the last note to avoid confounding note rate with the number of notes in the trill. We calculated an upper-bound regression line representing the performance limit using a distribution of bandwidth versus note repetition rate according to the methods described in (Blackburn et al. 1992; Janicke et al. 2008). The trill rate data were divided into bins of 0.5 notes/sec in width. The song in each bin with the highest trill rate was then used to construct the upper bound regression line. To compute the vocal deviation distance, we calculated the orthogonal distance of each trill from the upper bound regression line. Note that a lower deviation score is closer to the trade-off limit, indicating a higher trill performance.
To quantify trill note consistency, all trills for a given bird, song type, and comparison were opened as a single file stream in XBAT Revision 5 (Bioacoustics Research Program (2012); spectrogram settings FFT size = 512, window size = 1.0, window type = Hanning, overlap = 75%, frequency resolution = 92.6 Hz, temporal resolution = 2.6 msec). Using the template detector feature, a single trill note was selected as the template and all trill notes in the file were detected and boxed as identical-sized events. A few birds produced a conspicuously different first note in their trills, which was omitted from all trills in the sample so that the consistency measure would not be unduly influenced by this note. The trill notes were extracted as separate sound clips and subjected to spectral cross correlation (SPCC) using a custom Matlab™ routine called SoundXT v. 2.0 (K. A. Cortopassi 2005, personal communication). SPCC provides a measure of similarity between notes by comparing the spectral information in a spectrogram display (Cortopassi and Bradbury 2006). A SPCC coefficient of 1 implies that two notes are identical. We reduced background noise using a threshold setting of 25% of peak amplitude, which was visually similar to the amplitude threshold used for the vocal deviation measurements. Each trill note was compared with all other notes within the same trill, which resulted in a triangular cross-correlation matrix. The values in the correlation matrix were averaged to yield a mean consistency value for each trill. Trills that contained any obvious background sounds within the frequency range of the wren trill notes were omitted (5.5% of the trills on average), resulting in slightly smaller samples sizes for the consistency analyses compared to the vocal deviation analyses.
Temporal and contextual comparisons
We constructed separate datasets using different subsets of males to compare within-male consistency, vocal deviation, frequency bandwidth, and note rate as they changed across three sets of conditions. We refer to the two contrasting conditions in each comparison as states.
We compared male songs from two age states: younger and older. We assessed the effect of age by comparing trill parameters using dawn chorus recordings of the same bird in subsequent years. We controlled for seasonal effects by selecting recordings within two weeks of the same date in the two comparison years. For a few males and song types, we lacked good quality recordings in the year after the first recording and used songs from the next year, giving an age difference of two years. Male age was therefore relative rather than absolute. However, 8 of the 17 males in this comparison were first banded as nestlings. For these known-aged males, we used songs recorded in their first and second years after hatching. Some of the remaining 9 males were new to the study area and could have been one-year-old birds when first recorded, but others were known to be older. Since males had to survive at least two years to be included in this age analysis, our results apply only to a subset of males that could be of higher quality than males that died after one year. The age dataset included trills from 793 songs and a mean of 2.47 song types per male.
We compared male songs from two seasonal states: early and later in the season. During the 2005 breeding season, each bird was recorded three to four times between late April and early August from about 05:00 to 07:00 hr during and after dawn chorus. We selected an early season recording from late April or May and a mid-season recording from June or July for this comparison. Breeding stage was carefully monitored for all pairs in 2005, and we knew which birds were still alive the following spring and which had disappeared later in the breeding season or during the subsequent dry season. The season dataset included a total of 955 high-quality songs from 30 males and a mean of 1.8 types per male.
We compared male songs from two social context states: dawn chorus singing and a mid-morning simulated territorial intrusion. Between 07:30 to 09:30 on 2–29 July 2005, each male was subjected to a 4-min playback experiment (see Vehrencamp et al. (2007) for experimental details). The recordings made during and 11 min after the playback (using the same equipment and sampling rate as described earlier) provided the songs for the aggressive context, and time spent within 15 m of the speaker during and after the playback provided our measure of aggressive response. A dawn chorus recording of the same male, recorded within two weeks of the playback experiment to control for seasonal effects, provided the songs for the less threatening context. In order to evaluate a potential confounding effect of time of day on this comparison, we included the start time for each song in this dataset. We obtained 344 high-quality songs from 14 males and 1.36 types per male for the social context comparison.
Statistical analyses
To assess the within-bird differences in trill note consistency, vocal deviation, frequency bandwidth, and note rate between the two states in each comparison (age, season, and social context), we employed an ANOVA model with the two-state categorical variable and bird ID as fixed variables. No interaction term between bird and state was included, so that the test would strictly compare the mean values of the two states within males. We also included song type nested within bird in the model to control for each male’s unique song type variants. All variables were transformed to normalize the distribution of residuals as required for parametric analysis; specific transformations for each variable are given in the summary table of results.
To assess whether the magnitude of changes in males’ trill performance components were associated with their individual characteristics, we computed the mean difference between states for each male, song type, trill component, and comparison, and used these values as the dependent variable to evaluate possible correlations with male quality measures and other environmental and social predictors. The independent variables differed as appropriate for each comparison. For the age comparison we included: body condition in the older year, whether the bird was a known-aged one-year-old adult or an unknown-aged bird (yes or no), minimum age in the younger year (known age for males banded as nestlings and number of years since first banding for other adults), and survival to the beginning of the subsequent breeding season after the older year (0 or 1). For the season comparison we included: condition, minimum age, survival, number of nests built, number of clutches laid, whether any young were successfully fledged (yes or no), the number of days between the early and late season samples, date of the late-season sample, nesting stage (building, laying, incubating, nestlings/fledglings, none, or unpaired), and aggressive response during the playback experiment. For the social context comparison we included: condition, minimum age, survival, number of nests, number of clutches, successful fledging, mean time of dawn chorus sample songs, playback start time, whether most singing occurred during or post playback, whether the analyzed song type was present in the playback stimulus set (yes or no), and aggressive response (time within 15 m of the speaker). The set of predictor variables was first submitted to a stepwise analysis with the trill component as the dependent variable; bird ID was forced into the model and the minimum BIC criterion was used in forward selection to add any other significant independent variables. This reduced subset of variables was then analyzed in a mixed model with bird ID included as a random variable. Because we did not capture and measure some of the birds and thus had missing data for condition, when condition was not significant in the first run we reran the stepwise analysis without condition to obtain a larger sample size. We provide the statistical results of the final reduced models for these 12 analyses.
To evaluate the potential trade-off between consistency and vocal deviation, we used the method of van de Pol and Wright (2009), which employs within-subject centering to separate the within- and between-subject levels of correlation between two measured variables. We pooled the data from all three datasets (age, season, and context). We computed a new variable with the mean vocal deviation values within each bird, song type and state (representing the between-subject effect), and a second derived variable of the difference between each vocal deviation observation and male-state mean value (representing the centered, or within male effect). We therefore controlled for male, song type and age/season/context effects and looked for trade-offs within a recording session. We then ran a mixed model analysis with consistency as the dependent variable and mean bird-state vocal deviation, centered bird-state performance, and bird ID (random) as independent variables.
The JMP® Pro 9.0.2 statistical package (SAS Institute, Inc.) was used for all of the aforementioned analyses. We corrected the p values for multiple tests within each dataset (age, season, and context) using the false discovery rate method (Benjamini and Hochberg 1995) computed with the R Package (http://stat.ethz.ch/R-manual/R-devel/library/stats/html/p.adjust.html).
Results
Variation between years
Vocal deviation decreased and note consistency increased with age (Table 1a and Fig. 1). The vocal deviation effect was associated with a highly significant increase in frequency bandwidth, despite a slight but significant decrease in note rate. In our analyses of the male characteristics associated with differences in magnitudes of change, the only consistent factor was male age (Table 2a). The changes in all four performance measures were much greater for the subset of known-aged birds that were recorded in their first and second years compared to changes in performance components for the subset of unknown-aged birds. More specifically, note consistency increased by 2% for known-aged birds (from 0.875±0.0022 to 0.890±0.0023, least squares means) versus 0% for unknown-aged birds, and vocal deviation improved 13% for known-aged birds (from 0.927 to 0.808) versus 1% improvement for the unknown-aged birds. Similarly, bandwidth increased 5.4% for known-aged birds (from 3459 to 3647 Hz) versus 0.6% for unknown-aged birds, and note rate decreased 1.0% for known-aged birds and 0.2% in unknown-aged birds. Condition met the minimum BIC criterion for inclusion in the mixed model analyses for vocal deviation and bandwidth, but had no significant effect in the final models; the direction of the trends were the opposite from that expected, with higher-condition birds showing a reduction in performance.
Table 1.
Main effects of (a) age, (b) season, and (c) social context on within-male changes in trill consistency, vocal deviation, bandwidth, and note rate. Columns show the dependent variable with the power exponent used for transformation, sample sizes of birds and songs, F and p values for each test, and least squares means for each state in the comparison. Significant results, shown in bold, remained significant after correction for multiple tests. Bird ID was significant in all cases (p < 0.0001)
| (a) Age | N birds (songs) | F | p | Younger LSM (±SE) | Older LSM (±SE) |
|---|---|---|---|---|---|
| Consistency ^3 | 17 (788) | 8.632 | 0.0034 | 0.877 (0.0015) | 0.896 (0.0015) |
| Vocal deviation ^0.8 | 17 (793) | 11.74 | 0.0006 | 0.939 (0.0115) | 0.881 (0.0117) |
| Bandwidth (Hz) ^2.2 | 17 (793) | 38.29 | <0.0001 | 3496 (11.61) | 3591 (11.77) |
| Note rate ^0.05 | 17 (793) | 16.02 | <0.0001 | 4.929 (0.0057) | 4.901 (0.0058) |
| (b) Season | N birds (songs) | F | p | Early LSM (±SE) | Later LSM (±SE) |
|---|---|---|---|---|---|
| Consistency ^5 | 30 (919) | 8.584 | 0.0035 | 0.864 (0.0017) | 0.871 (0.0016) |
| Vocal deviation ^0.6 | 30 (955) | 0.031 | 0.879 | 0.985 (0.0125) | 0.992 (0.0118) |
| Bandwidth (Hz) ^3.3 | 30 (955) | 1.079 | 0.299 | 3620 (12.24) | 3594 (11.48) |
| Note rate ^0.1 | 30 (955) | 23.94 | <0.0001 | 4.681 (0.0055) | 4.714 (0.0051) |
| (c) Social context | N birds (songs) | F | p | Dawn chorus LSM (±SE) | Playback LSM (±SE) |
|---|---|---|---|---|---|
| Consistency ^3 | 14 (332) | 1.027 | 0.312 | 0.881 (0.0023) | 0.878 (0.0025) |
| Vocal deviation ^0.6 | 14 (344) | 0.879 | 0.349 | 1.131 (0.0163) | 1.159 (0.0177) |
| Bandwidth (Hz) ^2.2 | 14 (344) | 9.097 | 0.0028 | 3370 (15.52) | 3296 (16.86) |
| Note rate ^0.1 | 14 (344) | 41.28 | <0.0001 | 4.809 (0.0084) | 4.887 (0.0091) |
Fig. 1.
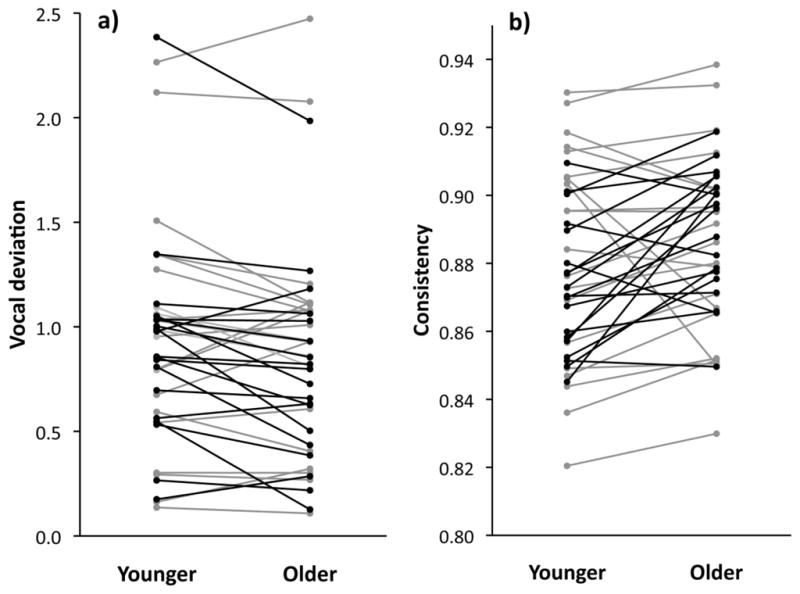
Change in (a) vocal deviation and (b) consistency for males when they are younger versus older. Lines connect mean values for the same individual. Black lines denote birds of known age recorded in their first and second year, gray lines indicate males of unknown age recorded one or two years apart
Table 2.
Male quality correlates of (a) age, (b) season, and (c) social context changes in trill performance components. Tables show reduced set of variables after stepwise analysis, the df, F, p and direction for each variable, and the adjusted R2 for the whole model. The only significant p-value after subtablewise correction for multiple tests is shown in bold
| Performance component (N) | Variables in reduced model | df | F | p | Direction | Adj. R2 |
|---|---|---|---|---|---|---|
| (a) Age changes | ||||||
|
| ||||||
| Consistency (61) | Known age | 1, 12.9 | 4.47 | 0.055 | yes>no | 0.301 |
|
| ||||||
| Vocal deviation (38) | Survival | 1, 14.5 | 2.76 | 0.118 | − | 0.280 |
| Condition | 1, 11.8 | 4.70 | 0.051 | + | ||
|
| ||||||
| Bandwidth (38) | Known age | 1, 12.9 | 5.39 | 0.037 | yes>no | 0.473 |
| Survival | 1, 13.4 | 3.96 | 0.127 | + | ||
| Condition | 1, 13.1 | 3.93 | 0.069 | − | ||
|
| ||||||
| Note rate (61) | Minimum age | 1, 13.3 | 3.20 | 0.096 | + | 0.161 |
|
| ||||||
| (b) Season changes | ||||||
|
| ||||||
| Consistency (52) | Aggressive resp | 1, 21.3 | 3.76 | 0.066 | − | 0.458 |
|
| ||||||
| Vocal deviation (52) | Aggressive resp | 1, 27.7 | 3.25 | 0.083 | − | 0.580 |
|
| ||||||
| Bandwidth (52) | Aggressive resp | 1, 27.9 | 4.18 | 0.050 | + | 0.085 |
| Nest stage | 1, 29.8 | 4.97 | 0.034 | b&l>i&n&u* | ||
|
| ||||||
| Note rate (52) | Successful nest | 1, 22.6 | 3.99 | 0.058 | − | 0.600 |
| Nest stage | 1, 47.1 | 4.87 | 0.032 | b&n&u>l&i* | ||
|
| ||||||
| (c) Social context changes | ||||||
|
| ||||||
| Consistency (19) | No variables added | |||||
|
| ||||||
| Vocal deviation (19) | Aggressive resp | 1, 13.2 | 2.49 | 0.138 | − | 0.858 |
|
| ||||||
| Bandwidth (19) | No variables added | |||||
|
| ||||||
| Note rate (19) | Aggressive resp | 1, 11.0 | 14.65 | 0.003 | + | 0.922 |
| Type in stimulus | 1, 12.7 | 4.17 | 0.063 | yes>no | ||
| DC mean time | 1, 11.3 | 3.91 | 0.073 | − | ||
| Minimum age | 1, 11.4 | 3.32 | 0.095 | + | ||
b=building, l=laying, i=incubating, n=nestlings/fledglings, u=unpaired
Variation across the season
Note consistency increased during the breeding season (Table 1b). Vocal deviation did not differ between early and late season recordings, but note rate increased as the season progressed. The magnitude of a bird’s seasonal change in consistency, vocal deviation, frequency bandwidth, and note rate was not correlated with body condition or with survival to the next breeding season (Table 2b). However, changes in three of the performance measures were associated with the male’s aggressive response to playback (playback experiments occurred during the middle of the breeding season when the late season recordings were usually made). More aggressive birds tended to show a stronger seasonal improvement in bandwidth and vocal deviation, and a greater decrease in consistency.
Variation between social contexts
Neither vocal deviation nor note consistency differed between dawn chorus and playback singing (Table 1c). However, the analyses of vocal deviation components revealed that frequency bandwidth was significantly lower, and trill note rate was significantly higher (Fig. 2), during playback experiments compared to dawn chorus. The difference in frequency bandwidth with context may have been due to time-of-day effects, since bandwidth decreased gradually with time and playbacks occurred later than dawn chorus recordings. However, the difference in trill rate was likely due to contextual differences rather than time of day (see Online Resource 1A). The magnitude of change in trill note rate in the aggressive context versus dawn chorus was positively correlated with time spent close to the speaker during the playback experiment (r = 0.623, n = 19, p = 0.004; Table 2c and Fig. 3). Furthermore, there was a trend for trill note rate to increase even more if the analyzed song type was present in the stimulus set. These results were not affected by breeding stage at the time of the playback experiment or with male condition. Neither consistency nor frequency bandwidth showed a correlation with aggressive response, but vocal deviation showed a negative correlation trend (r = −0.412, p = 0.08), probably due to its partial dependence on note rate.
Fig. 2.
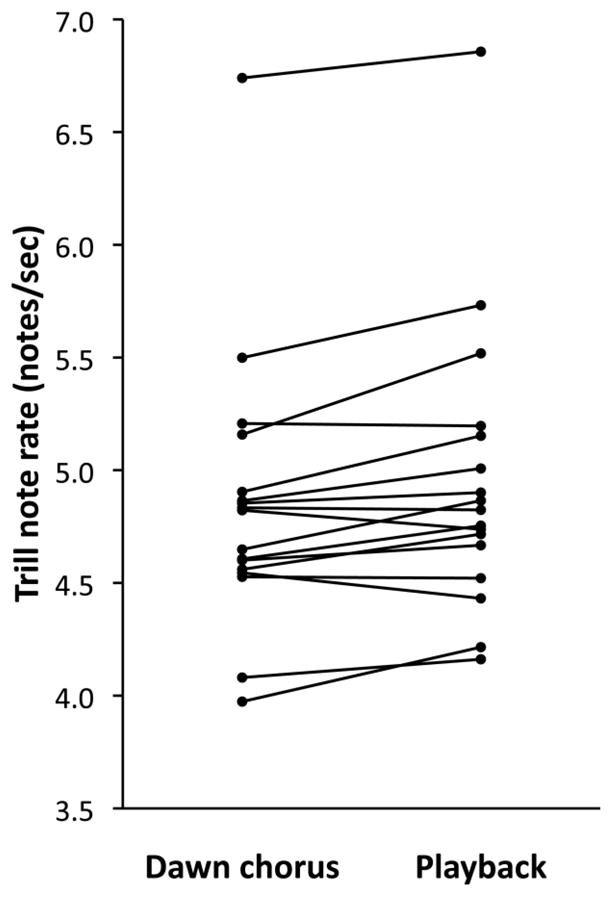
Differences in trill note rate during dawn chorus singing versus during playback experiments. Lines connect mean values for the same individual
Fig. 3.
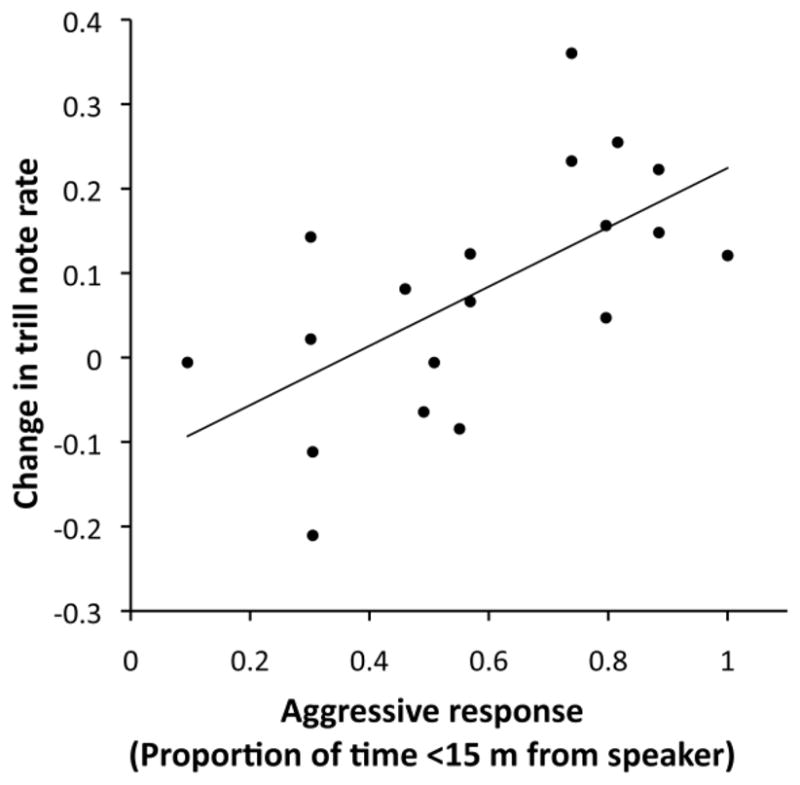
Correlation between the change in trill note rate (notes/sec) during playback relative to dawn chorus singing versus aggressive response during playback (proportion of time within 15 meters of the speaker during and after playback). Four males are represented twice with different song types
Trade-off analysis between consistency and vocal deviation
In our bird-state centered analysis of the potential short-term trade-off between consistency and vocal deviation, we found a highly significant positive correlation within bird-state (F1,1557 = 25.54, p < 0.0001), as well as a significantly positive between bird-state correlation (F1,193.7 = 24.24, p < 0.0001). Note that a positive correlation between these two variables indicates a trade-off. Thus even within a recording session, an attempt by the bird to improve its vocal deviation performance score results in a reduction in trill note consistency, and vice versa. Similarly, males with a vocal deviation score closer to the limit had lower trill consistency, controlling for song type and context. For a more extensive examination of this trade-off using other methods of analysis, see Online Resource 1B.
Discussion
Banded wren trill notes showed within-male variation in structural parameters at all three time-scales. Performance on both vocal deviation and note consistency improved across years, while consistency and note rate increased during the season. Note rate increased in a more aggressive context. The changes are often small in magnitude yet highly significant. We address the possible signaling implications of these changes at different time scales below.
Between-year changes in all four trill performance components were most pronounced for known-aged birds recorded in their first and second years of adult life. Note consistency increased by 2% for this subset of birds. Consistency also increased for most birds as the season progressed (Table 1b). These results lend further support to the observation that consistency increases with practice and is related to a bird’s age (de Kort et al. 2009b; that study focused on nine males including the eight studied here but examined a different sample of songs). Two studies on different species have documented a similar increase in note consistency with age (Botero et al. 2009; Rivera-Gutierrez et al. 2010). The mixed direction of changes in consistency for unknown-aged birds suggests that consistency may plateau or decrease in older birds, as described for the great tit (Parus major) (Rivera-Gutierrez et al. 2012). Performance for the vocal deviation parameter also improved across years and was more pronounced for known first-year males (13%) compared to unknown-aged males (1%). Similar changes have been documented in the swamp sparrow (Ballentine 2009). These age differences in trill performance measures are detectable by receivers: banded wrens responded differentially to playback treatments of the same song type recorded from the same male in his first and third years of life (de Kort et al. 2009b). Territorial male banded wrens and other songbird species also responded differentially to treatments in which consistency and vocal deviation had been manipulated (Illes et al. 2006; Cramer and Price 2007; de Kort et al. 2009a,b; Rivera-Gutierrez et al. 2011; DuBois et al. 2011). Furthermore, females in several species show mating preferences for song features correlated with male age such as song consistency, vocal performance, and repertoire size (Akçay and Roughgarden 2007; Kipper and Kiefer 2010).
Short-term changes in trill parameters were most pronounced for trill note rate. Nearly all birds showed a higher trill note rate during the more aggressive context (Fig. 2). Moreover, the magnitude of the increase in trill note rate was strongly correlated with the bird’s aggressive response to the playback. In addition, males tended to increase their trill note rate even further if the playback stimulus contained the measured song type; type matching is an aggressive challenge signal that elicits an approach response by receivers (Vehrencamp et al. 2007). Male swamp sparrows increase both the note rate and the bandwidth of their trills during playback experiments compared to solo singing (DuBois et al. 2009). However, in follow-up experiments to test the signal value of these within-male changes, DuBois et al. (2011) found no differential behavioral responses and concluded that the changes were too small to affect the outcome of aggressive interactions. Playback experiments with note rate treatments in the banded wren (Illes et al. 2006) increased the rates by 25–30%, far beyond the maximum 7% note rate increase observed in this study, so we cannot directly assess the salience of these short-term note rate changes. Nevertheless, birds have excellent auditory temporal resolution abilities (Lohr et al. 2006; Gall et al. 2012), and selection should favor attending to these subtle cues in song structure that are linked to a bird’s subsequent aggressive behavior.
Our seasonal (intermediate-term) comparison revealed an increase in consistency, possibly due to additional singing practice. We also found an increase in dawn chorus note rate during the season, which could be related to increased aggression levels consistent with the social context comparison results. Another playback experiment on the banded wren that spanned several months of the breeding season showed a seasonal increase in aggressive responses (SR de Kort et al., unpublished). Furthermore, birds that engaged in daytime boundary disputes also interacted more intensely during the dawn chorus, frequently matching and overlapping each other’s songs (Burt and Vehrencamp 2005). Thus the seasonal increase in dawn chorus trill note rate could reflect heightened levels of boundary disputes in the middle, compared to early in the breeding season. This interpretation is further bolstered by our finding that the only consistent predictor of the magnitude of seasonal increase in trill note rate was the bird’s aggressive response to playback; these experiments were performed in the middle of the breeding season around the same time as the late season sample. A non-exclusive alternative hypothesis for the seasonal changes in note rate and bandwidth could be that these two components of vocal deviation are directed at different listeners (Collins et al. 2009). Trill rate maximization may be important for male receivers during aggressive encounters, while bandwidth maximization may be more important early in the season when most birds are courting. We obtained some support for this explanation: males that were courting their mates (nest-building and laying stages) during the late season sample showed a seasonal increase in bandwidth, whereas non-courting males tended to decrease their bandwidth (Table 2b).
We did not measure song amplitude in this study, another component of song performance that has recently been shown to affect responses to playback and some relationships with male quality and reproductive success (Lampe et al. 2010; Ritschard et al. 2010; Brumm and Ritschard 2011; Ritschard et al. 2012). Nevertheless, amplitude could be associated with some of the effects we found in this study. The slower trill note rate found in older males (Table 1a) could enable the birds to increase their bandwidth and their amplitude, as found for song rate in the rock sparrow (Petronia petronia) (Nemeth et al. 2012). The slower trill note rate during the first half hour of dawn chorus could be associated with higher amplitude singing, based on an assessment by ear. However, banded wren males do not seem to increase their song amplitude during intense boundary disputes, preferring instead to increase their trill note rate.
We found no associations between the magnitude of individual males’ seasonal or yearly changes in trill performance components and their condition or survival. If anything, males in poor condition tended to show increases in several of the performance measures (see Table 2). These trends are consistent with earlier findings of a terminal investment effect on song rates during playback, in which males that subsequently died sang songs at higher rates than males that survived (Hall et al. 2009). Our condition index does not seem to reflect the probability of mortality in this long-lived tropical bird (75% annual survival rate). Cardoso et al. (2012) likewise found no associations between components of trill performance and condition in dark-eyed juncos (Junco hymenalis). A direct effect of health and condition on song performance parameters has only been described in a few species (Pasch et al. 2011; Ritschard and Brumm 2012), but senescence is associated with decreased song performance in two avian species (Rivera-Gutierrez et al. 2012; Cooper et al. 2012).
Song performance parameters should not only be lower in poor quality and unhealthy individuals, but even healthy and high quality individuals should exhibit tradeoffs between trill components. In principle, physiological constraints should prevent simultaneous maximization of frequency bandwidth and note rate, and it should also be very difficult for singers to maximize both vocal deviation and trill consistency (Podos et al. 2009; Botero and de Kort 2012). We found a significant within-male trade-off between note consistency and vocal deviation, but found only weak evidence for a tradeoff between frequency bandwidth and note rate (Online Resource 1B).
In conclusion, vocal deviation and trill consistency are two closely interlinked song performance components that can reveal hidden aspects of a sender’s quality separately and together. Displaying individuals may be selected to increase performance on both components simultaneously, but the physical constraints on maximizing both parameters make overall trill performance a good indicator of intrinsic quality. More specifically for the banded wren: 1) trill note consistency indicates age and experience but seems to be independent of motivational state, 2) frequency bandwidth varies primarily as a function of age and possibly with nesting stage, and 3) trill note rate shows seasonal and short-term variation as a function of aggressive motivation.
Supplementary Material
Acknowledgments
We thank the Área de Conservación Guanacaste for permission to work in Santa Rosa National Park and the park staff, especially Roger Blanco and María Marta Chavarría, for logistical support in Costa Rica. Anya Illes, Erin Eldermire, Jessica Niederer, Stephanie Lessard-Pilon, Elizabeth Ochoa, and Kate Neville assisted with the field recording. Jack Bradbury provided comments on the manuscript. This work was funded by NIMH grant R01-MH60461.
Footnotes
Conflicts of interest The authors declare that they have no conflict of interest.
Ethical standards The experimental methods for this study complied with current rules and regulations within the USA.
Contributor Information
S. L. Vehrencamp, Email: slv8@cornell.edu.
J. Yantachka, Email: jeyantac@syr.edu.
M. L. Hall, Email: hall.m@unimelb.edu.au.
S. R. de Kort, Email: s.dekort@mmu.ac.uk.
References
- Akçay E, Roughgarden J. Extra-pair paternity in birds: review of the genetic benefits. Evol Ecol Res. 2007;9:855–868. [Google Scholar]
- Ashmore RC, Wild JM, Schmidt MF. Brainstem and forebrain contributions to the generation of learned motor behaviors for song. J Neurosci. 2005;25:8543–8554. doi: 10.1523/JNEUROSCI.1668-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballentine B, Hyman J, Nowicki S. Vocal performance influences female response to male bird song: an experimental test. Behav Ecol. 2004;15:163–168. [Google Scholar]
- Ballentine B. The ability to perform physically challenging songs predicts age and size in male swamp sparrows, Melospiza georgiana. Anim Behav. 2009;77:973–978. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J Roy Stat Soc B. 1995;57:289–300. [Google Scholar]
- Blackburn TM, Lawton JH, Perry JN. A method of estimating the slope of upper-bounds of plots of body size and abundance in natural animal assemblages. Oikos. 1992;65:107–112. [Google Scholar]
- Botero CA, de Kort SR. Learned signals and consistency of delivery: a case against receiver manipulation in animal communication. In: Stegmann U, editor. Animal Communication Theory: Information and Influence. Cambridge University Press; Cambridge: 2012. in press. [Google Scholar]
- Botero CA, Rossman RJ, Caro LM, Stenzler LM, Lovette IJ, de Kort SR, Vehrencamp SL. Syllable type consistency is related to age, social status and reproductive success in the tropical mockingbird. Anim Behav. 2009;77:701–706. doi: 10.1016/j.anbehav.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL. Principles of Animal Communication. 2. Sinauer Associates, Inc; Sunderland, MA: 2011. [Google Scholar]
- Brandt LSE, Greenfield MD. Condition-dependent traits and the capture of genetic variance in male advertisement song. J Evol Biol. 2004;17:821–828. doi: 10.1111/j.1420-9101.2004.00716.x. [DOI] [PubMed] [Google Scholar]
- Bioacoustics Research Program. XBAT R6: eXtensible BioAcoustics Tool. Cornell Lab of Ornithology; Ithaca, NY: 2012. p. 14850. Available from www.birds.cornell.edu/brp/software/xbat-introduction. [Google Scholar]
- Brumm H, Ritschard M. Song amplitude affects territorial aggression of male receivers in chaffinches. Behav Ecol. 2011;22:310–316. [Google Scholar]
- Burt JM, Vehrencamp SL. Dawn chorus as an interactive communication network. In: McGregor PK, editor. Animal Communication Networks. Cambridge University Press; Cambridge, UK: 2005. pp. 320–343. [Google Scholar]
- Byers J, Hebets E, Podos J. Female mate choice based upon male motor performance. Anim Behav. 2010;79:771–778. [Google Scholar]
- Cardoso GC, Atwell JW, Hu Y, Ketterson ED, Price TD. No correlation between three selected trade-offs in birdsong performance and male quality for a species with song repertoires. Ethology. 2012;118:584–593. doi: 10.1111/j.1439-0310.2012.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SA, de Kort SR, Perez-Tris J, Telleria JL. Migration strategy and divergent sexual selection on bird song. Proc R Soc Lond B. 2009;276:585–590. doi: 10.1098/rspb.2008.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BG, Mendez JM, Saar S, Whetstone AG, Meyers R, Goller F. Age-related changes in the Bengalese finch song motor program. Neurobiol Aging. 2012;33:564–568. doi: 10.1016/j.neurobiolaging.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortopassi KA, Bradbury JW. Contact call diversity in wild orange-fronted parakeet pairs, Aratinga canicularis. Anim Behav. 2006;71:1141–1154. [Google Scholar]
- Cramer ERA, Price JJ. Red-winged blackbirds Ageliaus phoeniceus respond differently to song types with different performance levels. J Avian Biol. 2007;38:122–127. [Google Scholar]
- Cramer ERA, Hall ML, de Kort SR, Lovette IJ, Vehrencamp SL. Infrequent extra-pair paternity in the banded wren, a synchronously breeding tropical passerine. Condor. 2011;113:637–645. [Google Scholar]
- de Kort SR, Eldermire ERB, Cramer ERA, Vehrencamp SL. The deterrent effect of bird song in territory defense. Behav Ecol. 2009a;20:200–206. doi: 10.1093/beheco/arn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kort SR, Eldermire ERB, Valderrama S, Botero CA, Vehrencamp SL. Trill consistency is an age-related assessment signal in banded wrens. Proc R Soc Lond B. 2009b;276:2315–2321. doi: 10.1098/rspb.2009.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drăgănoiu TI, Nagle L, Kreutzer M. Directional female preference for an exaggerated male trait in canary (Serinus canaria) song. Proc R Soc Lond B. 2002;269:2525–2531. doi: 10.1098/rspb.2002.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois AL, Nowicki S, Searcy WA. Swamp sparrows modulate vocal performance in an aggressive context. Biol Lett. 2009;5:163–165. doi: 10.1098/rsbl.2008.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois AL, Nowicki S, Searcy WA. Discrimination of vocal performance by male swamp sparrows. Behav Ecol Sociobiol. 2011;65:717–726. [Google Scholar]
- Fletcher NH, Riede T, Suthers RA. Model for vocalization by a bird with distensible vocal cavity and open beak. J Acoust Soc Am. 2006;119:1005–1011. doi: 10.1121/1.2159434. [DOI] [PubMed] [Google Scholar]
- Forstmeier W, Burger C, Temnow K, Deregnaucourt S. The genetic basis of zebra finch vocalizations. Evolution. 2009;63:2114–2130. doi: 10.1111/j.1558-5646.2009.00688.x. [DOI] [PubMed] [Google Scholar]
- Forstmeier W, Kempenaers B, Meyer A, Leisler B. A novel song parameter correlates with extra-pair paternity and reflects male longevity. Proc R Soc Lond B. 2002;269:1479–1485. doi: 10.1098/rspb.2002.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall MD, Henry KS, Lucas JR. Two measures of temporal resolution in brown-headed cowbirds (Molothrus ater) J Comp Physiol A. 2012;198:61–68. doi: 10.1007/s00359-011-0687-9. [DOI] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Role of syringeal muscles in controlling the phonology of bird song. J Neurophysiol. 1996;76:287–300. doi: 10.1152/jn.1996.76.1.287. [DOI] [PubMed] [Google Scholar]
- Hoese WJ, Podos J, Boetticher NC, Nowicki S. Vocal tract function in birdsong production: Experimental manipulation of beak movements. J Exp Biol. 2000;203:1845–1855. doi: 10.1242/jeb.203.12.1845. [DOI] [PubMed] [Google Scholar]
- Hall ML, Molles LE, Illes AE, Vehrencamp SL. Singing in the face of death: male banded wrens Thryophilus pleurostictus sing more to playback in their last breeding season. J Avian Biol. 2009;40:217–224. doi: 10.1111/j.1600-048X.2009.04540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holveck MJ, de Castro ACV, Lachlan RF, ten Cate C, Riebel K. Accuracy of song syntax learning and singing consistency signal early condition in zebra finches. Behav Ecol. 2008;19:1267–1281. [Google Scholar]
- Illes AE, Hall ML, Vehrencamp SL. Vocal performance influences male receiver response in the banded wren. Proc R Soc Lond B. 2006;273:1907–1912. doi: 10.1098/rspb.2006.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke T, Hahn S, Ritz MS, Peter HU. Vocal performance reflects individual quality in a nonpasserine. Anim Behav. 2008;75:91–98. [Google Scholar]
- Jarvis ED. Brains and birdsong. In: Marler P, Slabbekoorn H, editors. Nature’s Music: the science of birdsong. Elsevier Academic Press; Boston: 2004. pp. 226–271. [Google Scholar]
- Kipper S, Kiefer S. Age-related changes in birds’ singing styles: On fresh tunes and fading voices? Adv Stud Behav. 2010;41:77–118. [Google Scholar]
- Lampe HM, Balsby TJS, Espmark YO, Dabelsteen T. Does twitter song amplitude signal male arousal in redwings (Turdus iliacus)? Behaviour. 2010;147:353–365. [Google Scholar]
- Lohr B, Dooling RJ, Bartone S. The discrimination of temporal fine structure in call-like harmonic sounds by birds. J Comp Psychol. 2006;120:239–251. doi: 10.1037/0735-7036.120.3.239. [DOI] [PubMed] [Google Scholar]
- Mendez JM, Mindlin GB, Goller F. Interaction between telencephalic signals and respiratory dynamics in songbirds. J Neurophysiol. 2012;107:2971–2983. doi: 10.1152/jn.00646.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molles LE, Vehrencamp SL. Repertoire size, repertoire overlap, and singing modes in the Banded Wren (Thryothorus pleurostictus) Auk. 1999;116:677–689. [Google Scholar]
- Nemeth E, Kempenaers B, Matessi G, Brumm H. Rock sparrow song reflects male age and reproductive success. PLoS ONE. 2012;7:e43259. doi: 10.1371/journal.pone.0043259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasch B, George AS, Campbell P, Phelps SM. Androgen-dependent male vocal performance influences female preference in Neotropical singing mice. Anim Behav. 2011;82:177–183. [Google Scholar]
- Podos J. A performance constraint on the evolution of trilled vocalizations in a songbird family (Passeriformes:Emberizidae) Evolution. 1997;51:537–551. doi: 10.1111/j.1558-5646.1997.tb02441.x. [DOI] [PubMed] [Google Scholar]
- Podos J. Correlated evolution of morphology and vocal signal structure in Darwin’s finches. Nature. 2001;409:185–188. doi: 10.1038/35051570. [DOI] [PubMed] [Google Scholar]
- Podos J, Lahti DC, Moseley DL. Vocal performance and sensorimotor learning in songbirds. Adv Stud Behav. 2009;40:159–195. [Google Scholar]
- Poesel A, Nelson DA, Gibbs HL. Song sharing correlates with social but not extrapair mating success in the white-crowned sparrow. Behav Ecol. 2012;23:627–634. [Google Scholar]
- Riede T, Suthers RA, Fletcher NH, Blevins WE. Songbirds tune their vocal tract to the fundamental frequency of their song. P Natl Acad Sci USA. 2006;103:5543–5548. doi: 10.1073/pnas.0601262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritschard M, Brumm H. Zebra finch song reflects current food availability. Evol Ecol. 2012;26:801–812. [Google Scholar]
- Ritschard M, Riebel K, Brumm H. Female zebra finches prefer high-amplitude song. Anim Behav. 2010;79:877–883. [Google Scholar]
- Ritschard M, van Oers K, Naguib M, Brumm H. Song amplitude of rival males modulates the territorial behaviour of great tits during the fertile period of their mates. Ethology. 2012;118:197–202. [Google Scholar]
- Rivera-Gutierrez HF, Pinxten R, Eens M. Multiple signals for multiple messages: great tit, Parus major, song signals age and survival. Anim Behav. 2010;80:451–459. [Google Scholar]
- Rivera-Gutierrez HF, Pinxten R, Eens M. Songs differing in consistency elicit differential aggressive response in territorial birds. Biol Lett. 2011;7:339–342. doi: 10.1098/rsbl.2010.0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Gutierrez HF, Pinxten R, Eens M. Tuning and fading voices in songbirds: age-dependent changes in two acoustic traits across the life span. Anim Behav. 2012;83:1279–1283. [Google Scholar]
- Sakata JT, Hampton CM, Brainard MS. Social modulation of sequence and syllable variability in adult birdsong. J Neurophysiol. 2008;99:1700–1711. doi: 10.1152/jn.01296.2007. [DOI] [PubMed] [Google Scholar]
- Sakata JT, Vehrencamp SL. Integrating perspectives on vocal performance and consistency. J Exp Biol. 2012;215:201–209. doi: 10.1242/jeb.056911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons LW, Tinghitella RM, Zuk M. Quantitative genetic variation in courtship song and its covariation with immune function and sperm quality in the field cricket Teleogryllus oceanicus. Behav Ecol. 2010;21:1330–1336. [Google Scholar]
- Spencer KA, Wimpenny JH, Buchanan KL, Lovell PG, Goldsmith AR, Catchpole CK. Developmental stress affects the attractiveness of male song and female choice in the zebra finch (Taeniopygia guttata) Behav Ecol Sociobiol. 2005;58:423–428. [Google Scholar]
- Trillo PA, Vehrencamp SL. Song types and their structural features are associated with specific contexts in the banded wren. Anim Behav. 2005;70:921–935. doi: 10.1016/j.anbehav.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pol MV, Wright J. A simple method for distinguishing within- versus between-subject effects using mixed models. Anim Behav. 2009;77:753–758. [Google Scholar]
- Vehrencamp SL, Hall ML, Bohman ER, Depeine CD, Dalziell AH. Song matching, overlapping, and switching in the banded wren: the sender’s perspective. Behav Ecol. 2007;18:849–859. doi: 10.1093/beheco/arm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch AM, Semlitsch RD, Gerhardt HC. Call duration as an indicator of genetic quality in male gray tree frogs. Science. 1998;280:1928–1930. doi: 10.1126/science.280.5371.1928. [DOI] [PubMed] [Google Scholar]
- Zann R, Cash E. Developmental stress impairs song complexity but not learning accuracy in non-domesticated zebra finches (Taeniopygia guttata) Behav Ecol Sociobiol. 2008;62:391–400. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


