Abstract
Normal blood supply to the cochlea is critically important for establishing the endocochlear potential and sustaining production of endolymph. Abnormal cochlear microcirculation has long been considered an etiologic factor in noise-induced hearing loss, age-related hearing loss (presbycusis), sudden hearing loss or vestibular function, and Meniere's disease. Knowledge of the mechanisms underlying the pathophysiology of cochlear microcirculation is of fundamental clinical importance. A better understanding of cochlear blood flow (CoBF) will enable more effective management of hearing disorders resulting from aberrant blood flow. This review focuses on recent discoveries and findings related to the physiopathology of the cochlear microvasculature.
Keywords: Cochlear microvessels, hearing loss, diseases, measurements
Introduction
Normally functioning cochlear microcirculation is critically important for maintaining ion and fluid balance in the inner ear, as sensory hair cells are strikingly vulnerable to ischemia (Nuttall, 1999b; Wangemann, 2002b). The inability to measure cochlear blood flow (CoBF) in humans has limited the investigation in human subjects, but numerous studies using different animal models have aptly demonstrated physiological changes with the alteration of CoBF, including changes in leukocyte dynamics. Vascular permeability and deformation have been shown to be contributing factors in various hearing disorders including presbycusis, noise-induced hearing loss, and ear hydrops (Brown et al., 1995; Chen et al., 2005a; Gratton et al., 1996a; Gratton et al., 1997; Hawkins, 1971; Kellerhals, 1972; Lamm et al., 1998; Mazurek et al., 2006; Miller et al., 2003; Nuttall, 1999a; Ohlemiller, 2009; Prazma et al., 1990; Seidman et al., 1999a; Shi et al., 2003). In humans, compelling clinical evidence has associated blood risk factors and myocardial disease with hearing and vestibular abnormalities (Aimoni et al., 2010; Mitchell et al., 2009). Capillary and stria vascularis degeneration have also been shown in presbycusis patients (Nelson et al., 2006; Wagenaar et al., 2000). In addition, the incidence of hearing loss in patients with various systemic autoimmune diseases is quite high, reported to be between 15% – 75% (Barkhuizen et al., 2006; Mouadeb et al., 2005). One mechanism for hearing loss is disruption of the vascular barrier in the stria vascularis (Cadoni et al., 2002; Fattori et al., 2001; Naarendorp et al., 1998; Ottaviani et al., 1999), with subsequent loss of endocochlear potential (Lin et al., 1997; Ruckenstein et al., 1999). Study of the vascular system in the inner ear has a long and rich history, which has been well-documented in previous reviews (Axelsson, 1988; Axelsson et al., 1986; Kimura, 1986; Lawrence, 1980; Miller et al., 1988; Miller et al., 1995a; Nakashima et al., 2003; Nuttall, 1988; Seidman et al., 1999b; Sillman et al., 1989; Wangemann, 2002a). Animal models of cochlear microcirculation have provided a good understanding of cell-mediated CoBF homeostasis, and further studies will extend this basic understanding to clinical studies, which directly address vascular-related hearing disorders. This review focuses on the microvasculature, and in particular on recent findings that show CoBF regulation at the microvessel level. The review introduces a new view of the blood-labyrinth barrier (BLB), which has ramifications for treatment of clinical hearing disorders such as noise-induced hearing loss, presbycusis, and sudden hearing loss, or ear hydrops associated with the dysfunction of cochlear blood supply. The microvasculature is a key component of tissue and organ health (Klijin et al., 2008; Lockhart et al., 2009), and understanding the role of the microvasculature in the BLB and CoBF is the foundation for preventing, diagnosing, and treating many hearing disorders.
1. Features of cochlear microcirculation
1.1. Capillary networks of the cochlear lateral wall are distinctively layered in a parallel arrangement and anatomically distant from sensory hair cells in the cochlea
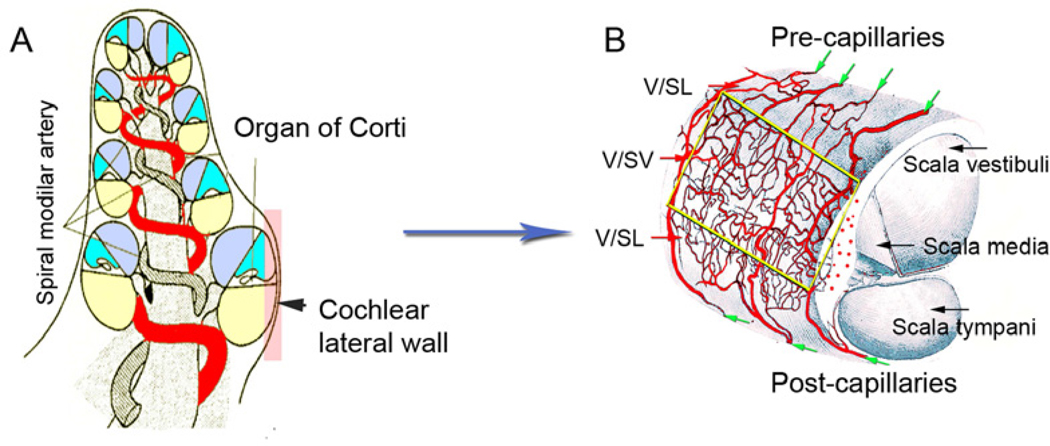
The main blood supply to the cochlea is the terminating spiral modiolar artery (SMA), a branch of the anterior inferior cerebellar artery (AICA) (see Figure 1). As shown in Figure 1A, the SMA branches from the AICA centrifugally and radiates over the scala vestibuli and across the spiral lamina. The spiral modiolar artery has radial branches to the lateral cochlear wall which form the two major capillary systems in the spiral ligament and stria vascularis. The two capillary systems form four distinct networks that are arranged in parallel in the cochlear lateral wall (Illustrated in Figure 1 B) (Axelsson, 1968). The networks are: (1) The supra-strial capillary network (arteriole system) of the spiral ligament. These microvessels, located above the attachment of Reissner’s membrane and just below the perilymphatic surface, are surrounded by a generous number of pre-capillaries. Location and arrangement is suggestive of a plasma filter for the perilymph. The perilymph may also originate in this network. (2) The post-strial capillary network (venous system) of the spiral ligament. Capillary branches from radiating arterioles passing down behind the stria turn longitudinally in the body of the prominence, and descend beneath the outer sulcus and insertion of the basilar membrance to join the collecting venules of the tympanic portion of the spiral ligament. (3) The ad-strial capillary network (true capillary system) of the spiral ligament. Most of the capillaries in the middle part of the spiral ligament that pass behind the stria run a more or less straight downward course in the spiral ligament until they reach the floor of the outer sulcus. A majority of the vessels turn longitudinally to join the venules in the wall of the scala tympani. (4) The capillaries of the stria vascularis. The largest branches of the radiating arterioles enter the stria vascularis just below the attachment of Reissner’s membrane, where they divide to form the strial network with its multiple anastomoses. The volume of cochlear blood flow is extremely small, with CoBF estimated on the order of 1/10 000 of total cardiac output in rodents such as guinea pigs or rats, and on the order of 1/1 000 000 of total cardiac output in humans (Nakashima et al., 2003). Strial capillaries are usually of larger diameter (12–16 micron) than spiral ligament capillaries (9–12 micron) (Miller et al., 1988). The strial capillaries are tightly packed with red blood cells. The flow is non-pulsatile and anatomically distant (> 100 micrometers) from sensory hair cells (Fig 1A and B), minimizing the acoustic perturbation of blood flow on hair cell transduction (Axelsson et al., 1990). The velocity of blood flow in the strial network is much slower than it is in the vessels of the spiral ligament. Themean velocity of blood flow in spiral ligament vessels was measured at 0.12 mm/s, while strial flow measured 0.08 mm/s. In a typical animal, blood velocity in various vessels of the ligament ranged between 0.09–0.18 mm/s, but only ranged 0.03–0.10 mm/s in strial vessels (Nuttall, 1987). The strial network is widest and most complex near the basal end and becomes narrower and simpler towards the apex.
Figure 1. Schematic view of CoBF supply.
A, The SMA, a major artery, supplies blood to the cochlea image from (Axelsson, 1968)]. B, A characterization of the vascular pattern on the outer wall of the cochlea is shown. Radiating arterioles arching over the roof of the scala vestibuli run in bony channels, branching as they emerge from the upper margin of the spiral ligament. Two distinct capillary networks in the spiral ligament and stria vascularis are apparent in the lateral wall. The networks parallel each other without cross connections [image adapted from (Mudry et al., 2009)]. V/SL: vessels of the spiral ligament; V/SV: vessels of the stria vascularis.
External wall vessels only form a single-layer capillary network at birth, but subsequently divide into two layers, constituting the microvessels of the stria vascularis and spiral ligament. This process occurs progressively from the basal turn toward the apical turn between days 5 and 8 in mice (Iwagaki et al., 2000). The cochlear vasculature tends to mature from the basal turn towards the apex (Iwagaki et al., 2000). In guinea pigs, the main stem of the inner ear vessel is formed by day 30 of fetal life, however, the peripheral capillary nets remain immature in form and vessel density is low (Nakai et al., 1986). It is also reported that BLB permeability is much greater before 14 days of birth in rats (Suzuki et al., 1998).
Pre-capillary and post-capillary vessels of the spiral ligament have vessel walls with smooth muscle cells, and regulation of lateral wall blood flow is largely considered to be a function of this network (Wangemann et al., 1996). In contrast, capillaries of the stria vascularis, formed in polygonal loops, are highly specialized vascular epithelia (Axelsson, 1968). Strial capillaries have a minor role in blood flow regulation, but a crucial one in maintaining the endocochlear potential, ion transport, and endolymphatic fluid balance essential for the ear’s sensitivity (Spicer et al., 2002a; Wangemann, 2002a).
1.2. The cochlear capillary network is densely populated with pericytes
The capillary networks of the cochlear lateral wall include a rich population of pericytes (Shi et al., 2008). Pericytes are smooth-muscle-like cells and also are considered as pluripotential progenitor cells. Pericytes are generally situated on microvessels, such as arterioles and venules, and particularly on the smallest capillaries, where there is little or no smooth muscle (Gerhardt et al., 2003; Hirschi et al., 1996; Thomas, 1999). Pericytes typically have a prominent nucleus and relatively little cytoplasm, and display nearby short processes as well as several long processes which embrace the abluminal endothelium wall (Diaz-Flores et al., 1991). The long cytoplasmic processes of pericytes, often in contact with numerous endothelial cells, serve to integrate signals along the length of the vessel (Bergers et al., 2005) (Figure 2 showing cochlear pericytes).
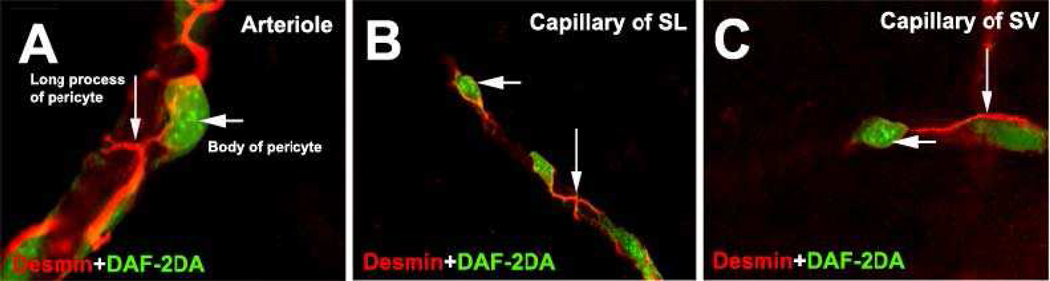
Figure 2. Cochlear pericytes on cochlear microvessels in adult guinea pig.
Pericytes are idenified with double-staining for desmin (red), a pericyte marker protein, and nitric oxide (DAF-2DA, green). A: an arteriole; B: a capillary of the spiral ligament (SL); C: a capillary of the stria vascularis (SV). Pericytes have a body (short arrows) and many primary processes (long arrows) which tightly embrace the endothelial tube. Pericytes on the outer wall of vessels have a characteristic “bump on a log” shape.
Pericytes show considerable morphological heterogeneity in the capillary beds of different tissues as well as wide differences in distribution density. For example, the ratio of pericytes to endothelial cells varies from 1:1 in retina, 1:5 in brain, 1:10 in lung, to 1:100 in skeletal muscle (Frank et al., 1987; Shepro et al., 1993). In the cochlea, the ratio of pericytes to endothelial cells in the stria vascularis and spiral ligament are approximately 1:2 (Shi et al., 2008), similar to that in the retina. The cochlear capillary system has a relatively high population of pericytes. The morphology of pericytes also differs depending on where they are found. The majority of cochlear pericytes on true capillaries have a polygonal cell body and long, slender processes (see Figure 3, Panels A–C), while most pericytes in the pre-capillary areas have prominent soma and band-like processes which completely encircle the vessel (see Figure 3, Panels D–F). Most pericytes in the post-capillary venule areas have flattened cell bodies and, likewise, circumferential band-like vessel-enshrouding processes (see Figure 3, Panels G–I). Pericytes on branch points have spindle-cell bodies and long processes distributed over the two branches (see Figure 3, Panels J–L). Pericytes on the vessels of the spiral ligament express contractile proteins, including α-SMA, desmin, F-actin, and tropomyosin (Shi et al., 2008), and exhibit vasocontractility (Dai et al., 2009). In contrast, pericytes on the vessels of the stria vascularis, lacking expression of α-SMA or tropomyosin, express abundant desmin structural proteins.
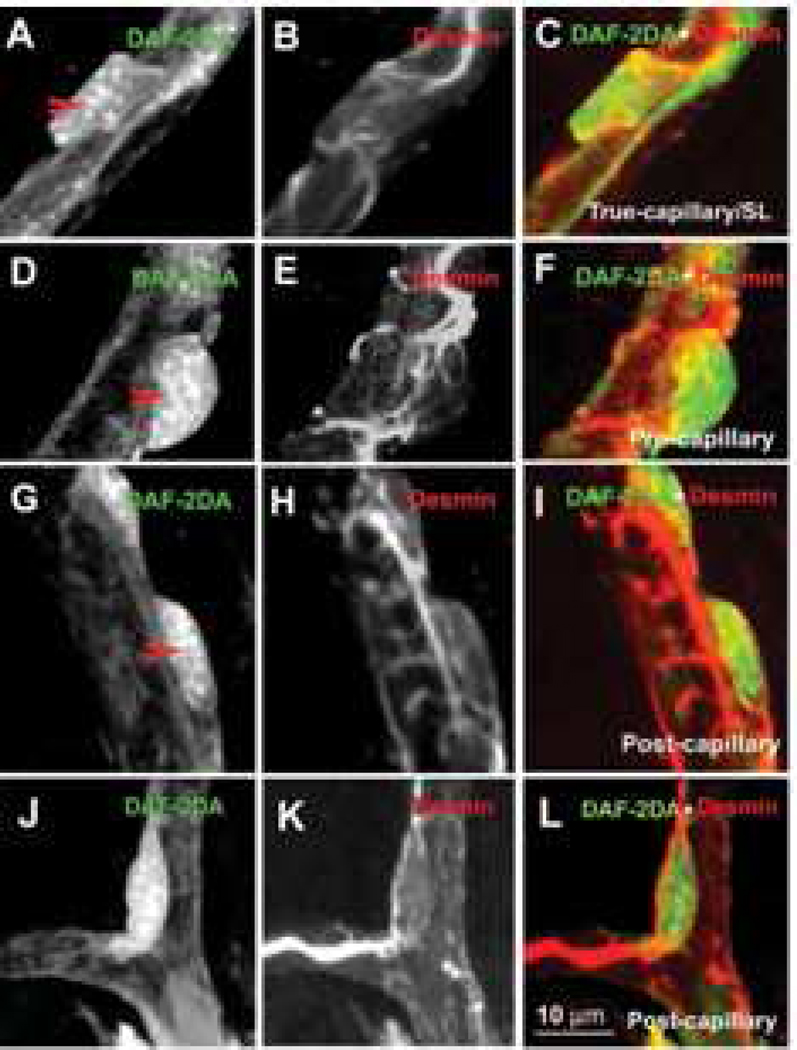
Figure 3. Shapes of pericytes on different cochlear microvessels.
The pericytes were double-labeled with a pericyte marker protein: desmin (red), combined with fluorescent indicator for intracellular nitric oxide DAF-2DA (green). Panels A–C show the morphology of a pericyte on a true capillary. The pericyte has a polygonal-shaped cell body (Panel A, 10 sections; interval: 1 µm), relatively few long longitudinal processes, and short, fine circumferential projections (Panel B, 10 sections; interval: 1 µm). Panel C is a merged image of Panels A and B. Panels D–F show the morphology of a pericyte on a precapillary. The pericyte has a “bump-shaped” soma (Panel D, 11 sections; interval: 1 µm) and relatively large processes that encircle the capillary (Panel E, 10 sections; interval: 1 µm). Panel F is a merged image of Panels D and E. Panels G–I show the morphology of a pericyte on a postcapillary. These pericytes have a flattened cell body (Panel G, 11 sections; interval: 1 µm) and short processes encircling the vessel (Panel H, 11 sections; interval: 1 µm). Panel I is a merged image of Panels G and H. Panels J–L show the morphology of a pericyte on a branch point of the postcapillary. The pericyte has a spindle-shaped cell body (Panel J, 10 sections; interval: 1 µm) and long processes distributed over the two branches (Panel K, 10 sections; interval: 1 µm). Panel L is a merged image of Panels J and K
Pericytes in the kidney, retina, liver, and lung not only play a role in regulating capillary blood flow, but also in blood vessel formation, immune response, and regulation of endothelial activity via different cell factors and signaling agents, including neuromodulators, vasoactive peptides, metabolic factors, growth factors, and cytokines (Allt et al., 2001; Betsholtz et al., 2005; del Zoppo et al., 2006; Donoghue et al., 2006; Kim et al., 2006; Nehls et al., 1993; Pallone et al., 2001; von Tell et al., 2006; Yamagishi et al., 2005). In addition, pericytes exhibit multipotent stem cell activity and can differentiate into a variety of different cell types, including macrophages and phagocytes, fibroblasts, and smooth muscle cells (Dore-Duffy et al., 2006; Sims, 2000). Moreover, various vascular diseases have been found to be associated with pericyte pathology (von Tell et al., 2006). Pericytes are receiving increased attention in microcirculation studies. However, cochlear pericytes have traditionally received little attention and the specific role of pericytes in cochlear homeostasis is largely unknown.
1.3 Autoregulation
Another feature of cochlear microcirculation is its strong autoregulation (Brechtelsbauer et al., 1995; Brown et al., 1994; Laurikainen et al., 1993; Miller et al., 1995a; Nakashima, 1999; Nakashima et al., 2003). A significant decrease in systemic blood pressure only causes a slight change in CoBF (Albera et al., 2003; Degoute et al., 1997; Tono et al., 1998). The rapid recovery of CoBF that occurs during occlusion of the anterior inferior cerebellar artery, the main blood supply to the ear, is a further indication of the autoregulation (Nakashima, 1999; Ren et al., 1993). Moreover, Suzuki et al. (1993) found that when cerebrospinal fluid pressure is increased, CoBF is not correspondingly decreased by the elevation in fluid pressure.
2. Regulation of CoBF in the inner ear
Sound stimulation of the inner ear imposes an energy demand that requires efficacious delivery of oxygen and glucose. A well-regulated cochlear blood flow (CBF) is needed to meet these requirements while also effectively clearing away metabolites. Regulation of CoBF, under the prevailing model, is hypothesized to include both local auto-regulatory and central control through neural pathways. The model incorporates neural- and autocrine/paracrine-based regulation of vasoconstriction and dilation at the level of artery and arterioles, as well as at the level of capillaries.
2.1 Regulation of CoBF by smooth muscle cells
Contraction of the smooth muscle cells in the spiral modiolar artery is hypothesized to be tightly regulated to meet the demand of cochlear tissues (Wangemann, 2002b). Contraction of the smooth muscle cells of the vascular wall reduces its luman diameter with the effect of decreasing blood flow, while relaxation of the smooth muscle cells increases blood flow. Smooth muscle cell contractility is signaled both with central neural and local metabolic signals. Sympathetic (peptidergic and adrenergic) nerve fibers have been found in the spiral modiolar artery of the gerbil and guinea pig (Brechtelsbauer et al., 1990; Carlisle et al., 1990; Rauchegger et al., 1981). Norepinephrine-induced vasoconstriction in the spiral modiolar artery is mediated by α1A-adrenergic receptors (Gruber et al., 1998). Stimulation applied in the sympathetic ganglia, stellate ganglion, or superior cervical chain in the guinea pig has been shown to alter CoBF in situ (Laurikainen et al., 1994; McLaren et al., 1993; Ren et al., 1993). In addition, distribution of vasoactive intestinal peptide (VIP), neuropeptide Y (NPY), substance P (SP), and calcitonin gene-related peptide (CGRP) are also found in the spiral modiolar artery (Carlisle et al., 1990; Qiu et al., 2001). These findings support a hypothesis that CoBF is controlled by neuronal signals at the level of the artery (Gruber et al., 1998; Herzog et al., 2002; Sadanaga et al., 1997; Scherer et al., 2005; Wangemann, 2002b; Wangemann et al., 1998; Wonneberger et al., 2000).
2.2 Regulation of CoBF by pericytes
Capillary-mediated local control of perfusion was first reported by (Wangemann et al., 1996). Recent findings on the vascular capillaries in the brain and retina highlight the role of pericytes in controlling capillary blood flow and maintaining microvascular homeostasis (Peppiatt et al., 2006). Microvessels in the spiral ligament contain a high density of pericytes, spaced approximately 2–25 µm apart as compared to 100 µm on true capillaries (Shi et al., 2008). The pericytes express contractile proteins, including α-smooth muscle actin and tropomyosin, and exhibit vasocontractility under both in vivo and in vitro conditions (Dai et al., 2009). The contractility of pericytes could affect flow resistance of the vascular network, and may profoundly alter overall blood flow. In particular, cochlear pericyte long processes span considerable distances (~ 60 µm) within the microcirculatory network and touch each other on the surface of microvessels, which may set the stage for signal integration. Pericytes may be functionally linked to form a “pumping system” to regulate blood flow.
2.3 Regulation of CoBF by fibrocytes
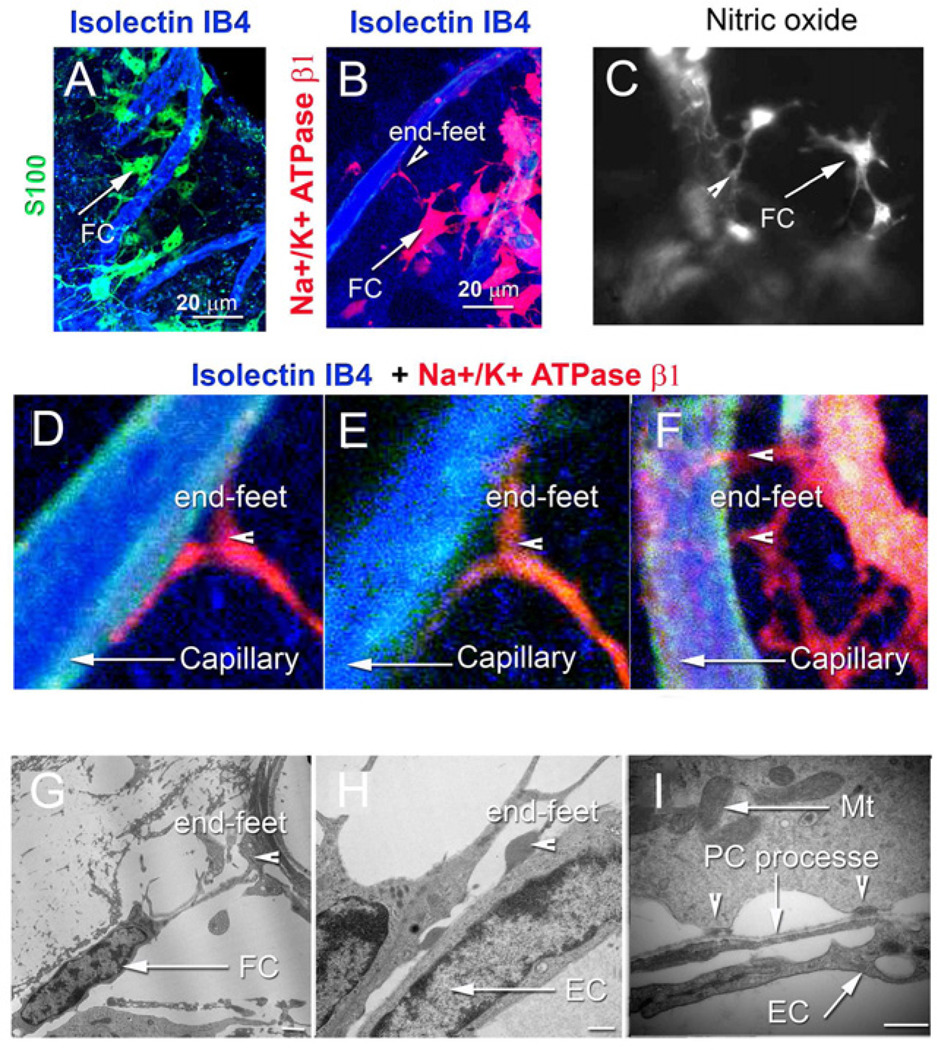
Recent experiments have also shown that CoBF is modulated by lateral fibrocyte input (Dai and Shi, 2011, an illustration in Figure 4). Fibrocytes in the cochlear lateral wall are divided into five types (I–V) based on morphological appearance, immunostaining pattern, and general location (Spicer et al., 1991; Suko et al., 2000). Fibrocytes have long been regarded as simple supporting cells; however, recent evidence suggests other functional roles under both physiological and pathological conditions (Adams, 2009; Doherty et al., 2004; Hirose et al., 2003; Moon et al., 2006; Nakashima, 1999; Nakashima et al., 2003; Qu et al., 2007; Spicer et al., 1991; Spicer et al., 2002b; Trowe et al., 2008; Wangemann, 2002c; Wu et al., 2003). In particular, fibrocytes participate in ion transport. They facilitate generation of the endocochlear potential by recycling K+ from hair cell transduction, through gap junctions to strial intermediate cells and marginal cells, into the endolymph.
Figure 4. Morphological details of fibro-vascular coupling is shown in confocal and TEM images.
(A) Type V fibrocytes positive for S100 (green) abut capillary walls labeled by isolectin IB4 (blue). (B) Type V fibrocytes are positive for Na+/K+ ATPase β1 (red). (C) Type V fibrocytes also contain high levels of NO, as detected with the intracellular NO indicator, DAF-2DA (gray). (D) Magnification of panel B shows foot processes in contact with a capillary. (E) A multiple-foot process of a fibrocyte abuts capillary wall. (F) A high magnification image shows a fibrocyte end-foot structure at the soma of a pericyte. The soma of pericytes were labeled by an antibody for NG2, (red), and processes were labeled with an antibody for the structural protein, desmin (blue). Capillary walls are labeled by phalloidin (green). (G) and (H) Fibrocytes contact capillaries with enlarged endings. (I) The endings display electron-dense membrane regions rich in mitochondria. Abbreviations: FC, fibrocyte; EC, endothelial cells; PC, pericyte; Mt, mitochondria. Calibration bars in H and I are 500 nm.
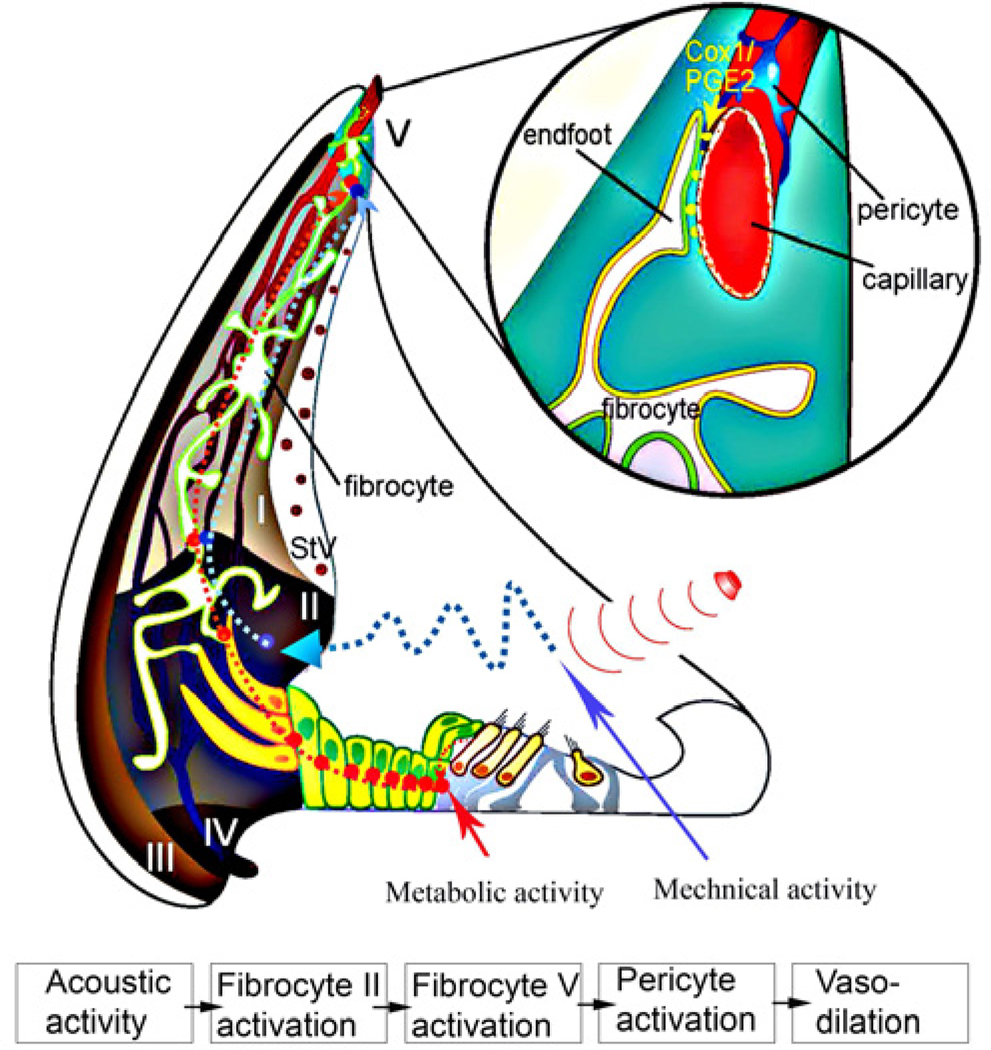
Normal hearing requires tight control over the supply of oxygen and glucose. In the brain and retina, “neuro-vascular units” (NVUs) provide direct and fast control of local blood flow. Activation of smooth muscle cells and pericytes, mediated by brain astrocytes and retinal glial cells, enable these tissues to accommodate the metabolic demand. Type V fibrocytes resemble astrocytes and glial cells. The fibrocytes are in morphological association with pre-capillaries of the spiral ligament through “end-feet” structures similar to astrocyte/pericyte junctions in NVUs (See Figure 5) (Dai et al., 2011). Fibrocyte activation significantly affects capillary diameter and blood flow velocity by initiating COX-1 activity and release of several vasoactive metabolites of arachidonic acid (Dai et al., 2011). The mechanism is analogous to the NVU for regulation of blood flow in brain.
Figure 5. A working model of fibro-vascular coupled signaling in the inner ear.
Cochlear blood flow is anatomically distant from sensory hair cells, but the cells are morphologically coupled to supporting and fibrocytes by gap junctions. Mechanical activity (red line) or metabolic activity (red dotted line) increases COX-1 enzymatic activity in type V fibrocytes, but the exact pathway is unknown. Activation of COX-1 may result in conversion of arachidonic acid into metabolic intermediates such as PGE2. The PGE2 diffuses into the perivascular space and elicits vasodilatation through the mediation of fibrocyte-coupled pericyte activity.
2.4 CoBF regulation by local metabolites
Multiple metabolic factors, including ATP, NO, lactate, PGE and K+, are involved in local blood perfusion.
2.4.1 Nitric oxide (NO)
NO is a potent vasodilator and regulator of vascular tone, and thus is an agent controlling organ blood flow (Brechtelsbauer et al., 1994; Feletou et al., 1996; Nelson et al., 1995). Nitric oxide synthase (NOS) has been found in a variety of cochlear cell types in several animal models including the mouse and guinea pig (Chen et al., 2005b; Konishi et al., 1998; Ruan, 2002; Shi et al., 2002). Direct NO production is found in cochlear vascular and smooth muscle cells (Chen et al., 2005b; Ruan, 2002; Shi et al., 2002; Shi et al., 2001). The NO causes smooth muscle and pericyte relaxation by activating cGMP and affecting its downstream target, protein kinase G (PKG) (Haefliger et al., 1994; Tian et al., 1999). NO also directly inhibits voltage-gated calcium channels, to the effect of relaxing smooth muscle cells (Sakagami et al., 2001), and activates ATP-sensitive K+ channels in endothelial and smooth muscle cells of the spiral modiolar artery, causing hyperpolarization and smooth muscle relaxation (Jiang et al., 2004; Si et al., 2002). Through one of these several pathways, pharmacological intervention of NO production offers a viable strategy for modulating regional blood flow in the cochlea.
2.4.2 Prostaglandin
Prostaglandin E (PGE), a major arachidonic acid metabolite in a wide variety of tissues, has a complex and diverse pathophysiology in blood flow regulation (Yang, 2007). PGE signaling, mediated by four distinct E-prostanoid receptors (EP1–4), has been demonstrated in the stria vascularis, spiral ligament, and organ of Corti (Nakagawa, 2011). The EPs also have significant roles in blood flow regulation in other tissues (Gordon et al., 2007). EP2 and EP4 have been shown to mediate vasodilatation in several organs, with EP1 and EP3 shown to mediate vasoconstriction (Legler et al., 2010; Nakagawa, 2011). CoBF in animals, measured by laser Doppler anenometry, was increased by local administration of prostaglandin E1 (PGE1) (Sone et al., 2003; Tominaga et al., 2006). PGE2 also induces a dose-dependent increase in inner ear blood flow (Rhee et al., 1999; Umemura et al., 1997). The prostaglandins are generally shown to enhance autoregulation of the inner ear vessels (Nagahara et al., 1988). Indeed, PGE has been used to treat idiopathic sudden sensorineural hearing loss (Nishimura et al., 2002). However, further studies are needed to delineate the distinct regulatory roles of PGE signaling on CoBF.
2.4.3 Adenosine 5'-triphosphate (ATP)
ATP also plays a role in CoBF regulation. Extracellular ATP applied to vessels has been shown to produce a dose-dependent increase in CoBF (Munoz et al., 1999). ATP transiently increases intracellular Ca2+ in ECs. 1 mM ATP caused a 10% dilation in spiral ligament capillaries in vivo (Wu et al., 2010). The ATP-induced effect on CoBF involves P2X- and P2Y-subtype purinoceptors (Ren et al., 1997; Takago et al., 2001). Inhibition of P2X4 receptor significantly blocks ATP-induced vessel dilation (Wu et al., 2010). Humoral adenosine 5'-triphosphate (ATP), adenosine, and uridine 5'-triphosphate (UTP) have also been shown to have a role in controlling local blood flow in the stria vascularis (Munoz et al., 1999). Manipulations of the adenosine signaling system hold significant promise for therapeutic management of dysfunctional CoBF.
2.4.4 Lactate
Lactate, a major by-product of metabolism, is involved in the regulation of local blood flow in many tissues (Gordon et al., 2008; Lombard, 2006; Mendrinos et al., 2008). Cochlear perilymph has a three times higher concentration of lactate than blood and cerebrospinal fluid. This suggests the perilymph lactate is of intracochlear origin (Scheibe et al., 1976) and may rise to effective levels with sound stimulation. Different concentrations of extracellular lactate serve as dynamic signals for pericyte relaxation and contraction, which cause perturbations in intracellular Ca2+ by inhibiting the Na+/Ca2+ exchanger in the retinal capillary system (Lombard, 2006). Recently, we found that lactate also has a significant effect on regulation of CoBF. The effect on capillary diameter is mediated by an NO signaled coupling with fibrocytes (Dai et al., 2010). Few experiments have been done on lactate-based regulation of CoBF, and further study is needed, particularly considering the high concentration of lactate in the cochlea. Lactate may be an essential signal in the control of CoBF.
2.4.5 Potassium (K+)
Elevating the K+ concentration from 3.6 to 150 mM by superfusion of the spiral modiolar artery in vitro caused transient vasoconstriction (Wangemann et al., 1998). Pericytes can also “detect” extracellular K+ signals (Matsushita et al., 2006). Under both in vitro and in vivo conditions, we found that an extracellular K+ concentration of 10 mM induces pericyte contraction (Dai et al., 2009). Endolymphatic K+ recycling through sensory hair cells and non-sensory supporting cells has recently been shown to have an important role in maintaining normal hearing function (Fujimura et al., 2005; Marcus et al., 2002; Mistrik et al., 2009; Rickheit et al., 2008; Wangemann, 2002a). Although cochlear blood flow is anatomically distant from sensory hair cells, the cells are morphologically coupled to intermediate cells and fibrocytes by gap junctions (Ando et al., 1998; Takeuchi et al., 2001). K+ movement through gap junctions between hair cells and lateral wall supporting cells could produce a variable K+ concentration in the interstrial space. K+ cycling through the cochlear lateral wall may be regulate pericyte function. The outward ERG channel found in intermediate cells (Nie et al., 2005) is consistent with this regulation. The channel produces a marked K+ extrusion into the interstitial fluid which affects extracellular K+ concentration (Nie et al., 2005). Retinal pericytes, hyperpolarized or depolarized depending on the concentration of extracellular K+, cause pericyte relaxation or contraction (Cao et al., 2006; Matsushita et al., 2006; Quignard et al., 2003). Whether cochlear K+ recycling regulates pericyte function to control capillary diameter has not been determined.
3. Blood-labyrinth barrier in the stria vascularis
3.1. The physical structure of the blood-labyrinth barrier
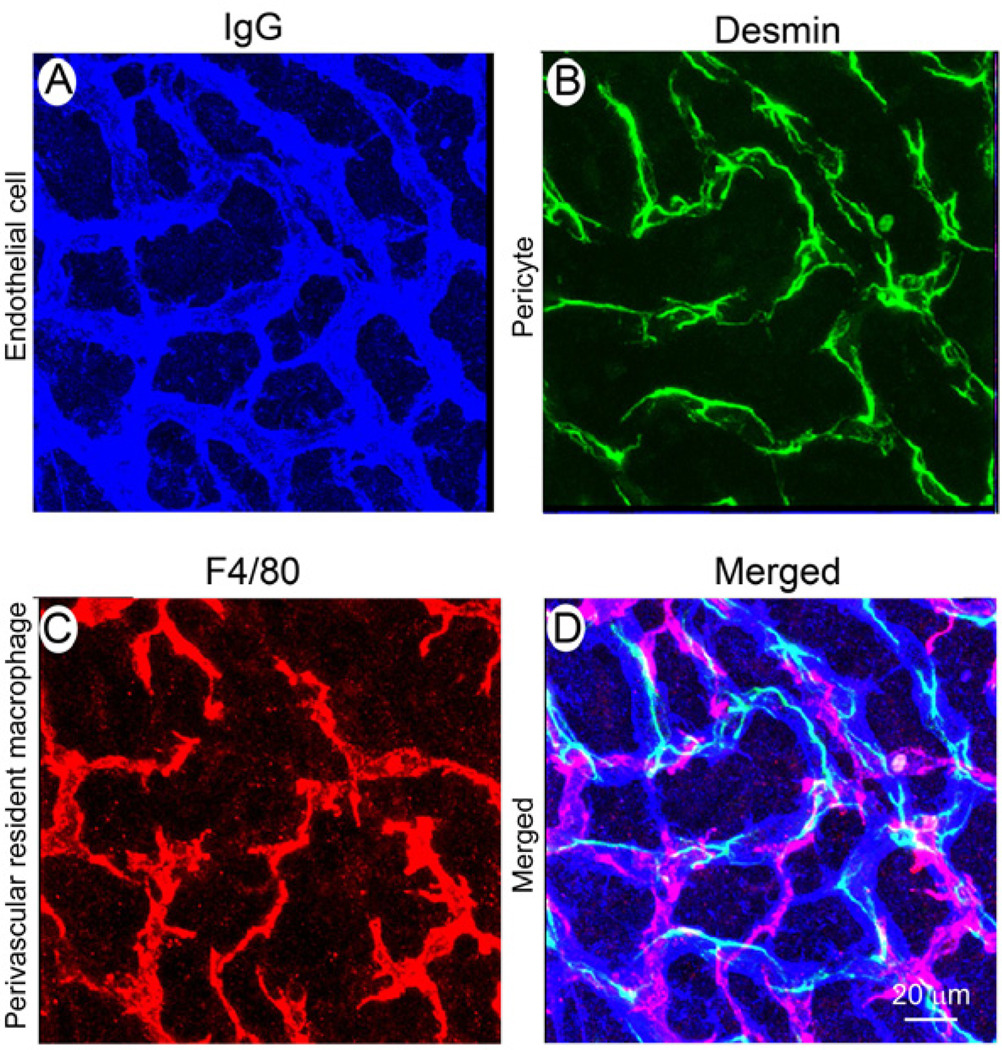
The capillary bed in the stria vascularis is essential for solute homeostasis and preventing the influx of toxic substances into the inner ear (Juhn et al., 1981; Juhn et al., 2001). In the classic view, the BLB is composed of endothelial cells and an underlying basement membrane (Sakagami et al., 1982; Sakagami et al., 1987). Endothelial cells connect to each other by tight junctions (Sakagami et al., 1982; Takeuchi et al., 2001) and form a diffusion barrier which selectively excludes most blood-borne substances from entering the ear, protecting it from systemic influences (Juhn, 1988; Juhn et al., 1981). In a recent study, the BLB was discovered to include, in addition to endothelial cells and basement membrane, a large number of pericytes (Shi, 2009; Shi et al., 2008; Takeuchi et al., 2001) and perivascular resident macrophages (Shi, 2010) (Figure 6). The perivascular resident macrophages, with foot processes strikingly rich in mitochondria and vesicles, are highly invested on the abluminal surface of capillaries. They are positive for several macrophage surface molecules, including F4/80, CD68, and CD11b (Shi, 2010). They are also similar to astrocytes in the brain and glial cells in the retina, both of which are known to have an essential role in regulating barrier integrity (Abbott, 2002; Abbott et al., 2006; Cardoso et al., 2010; Prat et al., 2001). In the absence of astrocytes and glial cells, BBB and BRB lose tight junction proteins and become leaky to large molecules (Abbott, 2002; Abbott et al., 2006; Haseloff et al., 2005; Willis, 2011). Presence of perivascular resident macrophages in the BLB may suggest an analogous regulatory mechanism in the cochlea. Pericytes in the BLB are rich in the structural protein desmin which gives mechanical strength to the capillary and enhances general integrity of the network. Blood vessels deficient of pericytes are abnormally large and leaky (Hellstrom et al., 2001). Perivascular resident macrophages and pericytes are new classes of cells in the BLB, and their function is largely uncharacterized.
Figure 6. Cellular structure of the blood-labyrinth barrier.
Endothelial cells in normal BLB are identified with an antibody for mouse endothelial IgG (A, blue), pericytes with an antibody for desmin (B, green), and macrophages with an antibody for F4/80 (C, red). The merged image (D) shows the complexity of the blood-labyrinth-barrier.
3.2. Molecular composition of blood-labyrinth barrier
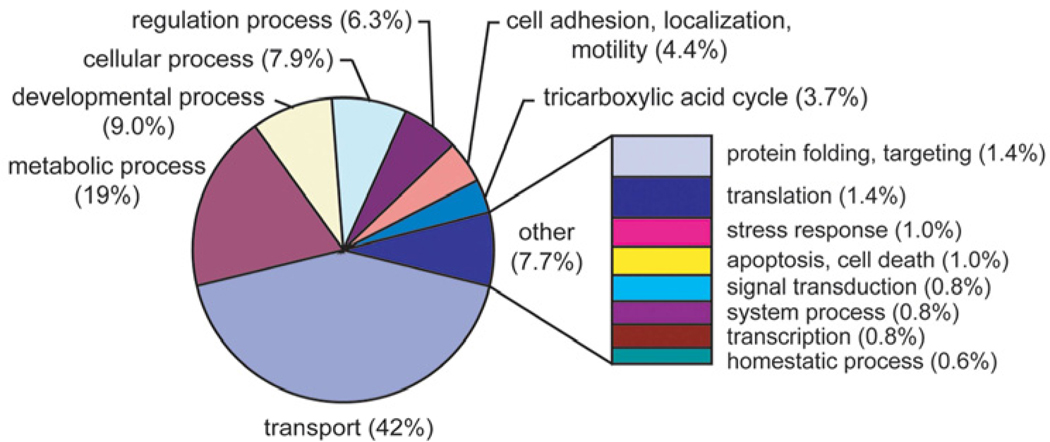
The BLB contains an array of enzymes and transporters, which together maintain the necessary extracellular environment of the cochlear system (Saito et al., 1997; Yang et al., 2011). Using a mass-spectrometry, shotgun-proteomics approach, combined with a novel “sandwich-dissociation” method of isolating capillaries from the stria vascularis, more than 600 strial capillary proteins have been identified (Yang et al., 2011) (Figure 7). Strikingly, a high number of identified proteins are involved in metabolism and transport. For example, the most abundant protein identified in the blood-labyrinth barrier is the ion transporter subunit, Na+/K+-ATPase α1. In addition, a large number of proteins are metabolic enzymes, including glutathione S-transferase (GST), prosaposin, leukotriene A4 hydrolase, and glutamate oxaloacetate transaminase. Prosaposin, synthesized and secreted by the stria vascularis, is pivotal to maintaining homeostasis in the auditory system (Terashita et al., 2007). LTB4 is a vasoconstrictor which can cause hearing loss by down-regulating cochlear blood flow (Rhee et al., 1999). The large number of transporters and metabolic enzymes in the blood-labyrinth barrier is indicative of a high level of energy and transport activity. Moreover, stria vascularis capillaries are rich in tight junction and cell adhesion proteins, which is consistent with blood-labyrinth barrier function.
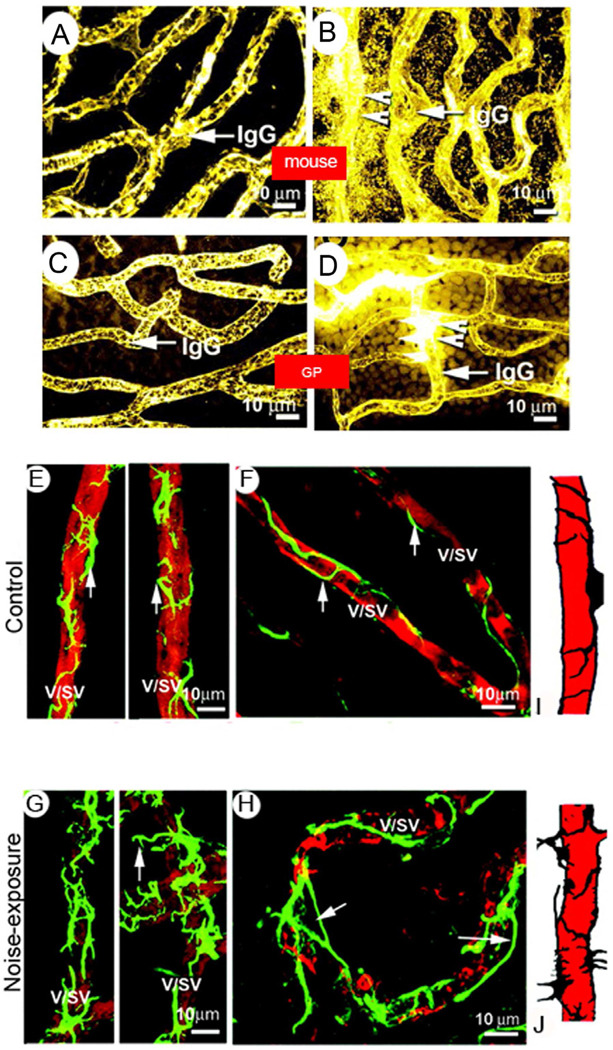
Figure 7. Noise induces breakdown of the blood-labyrinth-barrier and causes irregularities in pericyte coverage.
A & C, Serum protein IgG is confined to blood plasma (IgG/arrow) in vessels of the stria vascularis in normal mice (A) and guinea pigs (C). B and D, Serum protein IgG leaks from vessels (arrow/IgG) in noise-exposed mice (B) and guinea pigs (D). Arrowheads indicate sites of vascular leakage. GP: guinea pig. Pericytes containing desmin filaments are evenly distributed on the vessel walls of the stria vascularis in both guinea pigs (E) and mice (F). Pericytes are labeled with an antibody for desmin (green), and vessels with an antibody for isolectin IB4 (red). G and H: Confocal fluorescent images from noise-exposed guinea pigs and mice show abnormal pericyte morphology and increased pericyte coverage. Arrows point to irregular pericyte foot processes turning away from the vessel wall (G) and detached from it (H). I and J: Drawings illustrate the pattern of pericyte distribution on vessel walls in normal and noised-exposed animals. V/SV, vessel of the stria vascularis; NE, noise exposure; GP, guinea pig; MS, mouse.
3.3. Regulation of blood-labyrinth barrier permeability
The inner ear has an endothelial blood-tissue barrier in the stria vascularis that is as tight as the blood brain barrier. However, the mechanisms that control stria vascularis endothelial blood-barrier permeability remain largely unknown. Information on regulation of the BLB is sparse. Early studies showed that BLB permeability is more robust in developing rat cochlea (Suzuki et al., 1998). Recently, protein kinase C eta (PKCη) was found to regulate barrier permeability by directly interacting with Na+/K+-ATPase α1 and mediating tight junction protein status, for example, by affecting the phosphorylation status of occludin.
Highly regulated transport of ions in and out of the BLB maintains the fluid composition in the inner ear essential for auditory function. A better understanding of how the BLB is regulated would enable development of therapies for restoring the barrier in BLB related hearing disorders.
4. Hearing loss associated with impaired microvasculature
4.1. Noise-induced hearing loss
The cause of noise-induced hearing loss remains unclear, despite years of investigation. Insufficient blood supply is one mechanism which accounts for temporary or permanent noise-induced threshold shifts. For example, several histological and physiological studies have demonstrated signs of reduced circulation (vessel contraction and cochlear hypoxia) and inflammation, including leukocyte infiltration and up-regulation of adhesive molecules, in the cochlea after noise exposure (Hillerdal et al., 1987; Lamm et al., 1999; Quirk et al., 1992; Scheibe et al., 1993; Seidman et al., 1999b; Shi et al., 2007; Yamane et al., 1991). A recent study by Arpornchayanon et al. (2011) shows that noise exposure reduces red blood cell velocity compared to stable control measurements. In addition to noise-induced disruption of endothelial cells, noise also causes upregulation of vascular endothelial growth factor (VEGF), a potent inducer of vascular breakdown (Nag et al., 2011; Selivanova et al., 2007). Furthermore, noise exposure causes down-regulation of COX enzymes (Heinrich et al., 2010; Heinrich et al., 2006), which can decrease endogenous PGE2 (a vasodilator) levels in the cochlea. Down-regulation of PGE2, particularly EP2 and EP4, could be the cause of noise induced cochlear ischemia. In addition, structural and molecular changes in the cochlear endothelium are involved in the BLB breakdown from noise-exposure. We have observed that pericytes, hypothesized to provide structural support in the BLB, lose their tight association with endothelial cells following loud sound damage (Shi, 2009) (Figure 8).
Figure 8. Classification of isolated stria vascularis capillary proteins identified ATP1A1 as the most abundant protein in the blood-labyrinth barrier.
The pie graph shows a spectral count-weighted tabulation of the GO annotation by biological process. Proteins involved in transport (42%) and metabolism (19%) are highly expressed in the blood-labyrinth barrier
4.2. Endolymphatic hydrops
Endolymphatic hydrops is a condition in which too much endolymph is present (Pirodda et al., 2010; Semaan et al., 2010). Meniere's disease is characterized by fluctuating hearing loss, episodic vertigo, and tinnitus, and by endolymphatic hydrops found on examination post-mortem (Semaan et al., 2005). The cause of Meniere's disease remains unclear. Numerous factors play a role in the development of hydrops and in the pathogenesis of related cochleovestibular dysfunction. However, the evidence from research on animal models suggests the pathophysiology in Meniere's disease is closely associated with dysfunctional CoBF. For example, Miller and his co-worker demonstrated that the magnitude of evoked CoBF response was reduced by approximately one third in hydropic ears compared to normal ears (Miller et al., 1995b; Vass et al., 1995; Yazawa et al., 1998). Brechtelsbauer et al. (Brechtelsbauer et al., 1995) reported reduced autoregulation of CoBF in guinea pigs with endolymphatic hydrops. Significantly higher levels of plasma norepinephrine and vasopressin have been reported in patients with Meniere's disease (Juhn et al., 1999). However, the evidence is not consistent. Others have not found endolymphatic hydrops associated with blood flow. For example, Larsen et al. (Larsen et al., 1988) found no change in regional or total cochlear blood flow in hydropic ears. CoBF measurement in patients with Ménière's disease and control groups showed no statistically significant difference with respect to CoBF amplitudes (Selmani et al., 2001). Resolving the issue of whether microcirculation and ear hydrops are correlated needs to wait on development of better means to measure blood flow in the cochlea.
4.3. Presbycusis
Age-related hearing loss is the major form of hearing loss and the predominant neurodegenerative disease of aging (Frisina, 2009; Lang et al., 2010; Ohlemiller et al., 2008; Ohlemiller et al., 2010; Schacht et al., 2005). Insufficient CoBF and decline in the endocochlear potential (EP) (strial presbycusis) are considered responsible for hair cell damage and hearing loss in the elderly (Gacek, 1969; Harkins, 1981; Seidman et al., 1999b). For example, Prazma et al. (Prazma et al., 1990) reported that CoBF in old gerbils was less than in young animals. Gratton et al (Gratton et al., 1996b) reported that age-related decreases in endocochlear potential are associated with vascular abnormalities in the stria vascularis. Brown et al. (Brown et al., 1995) found that cochlear vascular reactivity to topical application of nitroprusside, a vasodilating agent, was less in old mice than in young mice. Suzuki et al. (Suzuki et al., 1998) demonstrated that autoregulation was significantly reduced in the aged group. Using a microsphere technique to quantify blood flow, they found diminished flow in morphologically normal-appearing basal turn capillaries. Changes in whole blood viscosity and red-cell rigidity have also been correlated with high-frequency hearing loss in elderly human subjects (Gatehouse et al., 1990). Furthermore, in a series of in vivo experiments using intravital microscopy of the cochlear microvasculature, Seidman et al. (Seidman et al., 1996) demonstrated age-dependent, statistically significant reductions in mean red blood cell velocity accompanied by increases in capillary permeability. Increased immunoglobulin and laminin deposits were observed in thickened basement membranes of aged strial capillaries (Sakaguchi et al., 1997a; Sakaguchi et al., 1997b). In humans, an age-related, gradual loss of capillaries in the spiral ligament of the scala vestibuli was observed. For example, in a human temporal bone study, presbycusis patients showed loss of hair cells and neurons and atrophy of the stria vascularis (Sprinzl et al., 2010).
However, the literature is also inconsistent. Hillerdal and co-workers (Hillerdal et al., 1987) reported no difference in CoBF in young and aged normotensive rats. The conflicting results may reflect a difference in the species studied or age at which animals were selected for investigation. The association of age-related pathological changes with disturbance of CoBF is not clear at present. A better understanding of cochlear homeostasis requires a way to measure CoBF in humans, as animal models currently provide our only means to study CoBF.
4.4. Sudden deafness
The pathogenesis of idiopathic hearing loss remains unknown, but vascular involvement is one hypothesis (Mosnier et al., 2011). Observational clinical studies have shown that patients with sudden idiopathic sensorineural hearing loss often present with systemic arterial hypertension, diabetes mellitus, dyslipidemias, alone or associated with systemic sclerosis, and thromboembolic risk (Nagaoka et al., 2010). The sudden deafness patient often presents with high precontrast signals in the inner-ear fluid space and an increased concentration of protein passing through blood vessels, indicating a breakdown of the blood-labyrinth barrier (Sone et al., 2009; Sugiura et al., 2006; Yoshida et al., 2008).
4.5. Genetic Hearing loss
The endocochlear potential (EP) is essential to hearing, because it provides approximately half of the driving force for the mechanoelectrical transduction current in auditory hair cells (Salt et al., 1987; Smith et al., 1954; Tasaki et al., 1958; Tawackoli et al., 2001; Wangemann, 2002a). The EP is produced in the stria vascularis (SV) (Ferrary et al., 1998; Marcus et al., 1983; Offner et al., 1987; Salt, 2001; Salt et al., 1987; Tran Ba Huy et al., 1986; Wangemann, 2002a; Wangemann, 2002b). Disruption of the endothelial barrier in the stria vascularis leads to loss of EP in genetic hearing loss (Cohen-Salmon et al., 2007). For example, connexin30 deficiency results in severe congenital hearing impairment with disruption of the BLB (Cohen-Salmon et al., 2007). In addition, hearing loss resulting from Nr3b2(−/−) mutation is associated with reduction of the density of the cochlear strial capillaries (Chen et al., 2007).
5. Measurement of CoBF
Direct measurement of CBF is difficult and techniques for assessing blood flow are still under development. Various techniques are used for evaluation of cochlear blood flow, including laser-doppler anemometry (LDA), magnetic resonance imaging (MRI), fluorescence intravital microscopy (FIVM), microendoscopy (FME), as well as approaches based on injection of radioactive or labeled microspheres into the boodstream. Here, I discuss two recent methods for measurement of cochlear blood flow.
Fluorescence microendoscopy (FME)
The fluorescence microendoscope, consisting of a flexible imaging fiber, coupled to a system for detection of fluorescence, enables study of cochlear blood flow on a scale of microns. Blood flow velocity is determined by analysis of video sequences (Monfared et al., 2006). The small size of the instrument makes it versatile and suitable for relatively non-invasive imaging of regional blood flow. In 2006, Monfared et al. observed single red blood cells passing through individual capillaries in several cochlear structures, including through the round window membrane, spiral ligament, osseous spiral lamina, and basilar membrane. They determined blood flow velocity using this technique by analyzing the acquired video sequences. Fluorescence microendoscopy has several advantages: (1) The endoscope probe can be placed at the round window without disturbing the membrane; (2) The vasculature of the round window membrane itself, as well as the most proximal portion of the osseous spiral lamina and basilar membrane, can be imaged; (3) With resection of the round window membrane, the endoscope probe can easily be introduced into the scala tympani to image blood flow in the osseous spiral lamina, spiral ligament, and basilar membrane. Disadvantages include disruption of the delicate homeostatic balance in the cochlea.
Optical microangiography (OMAG)
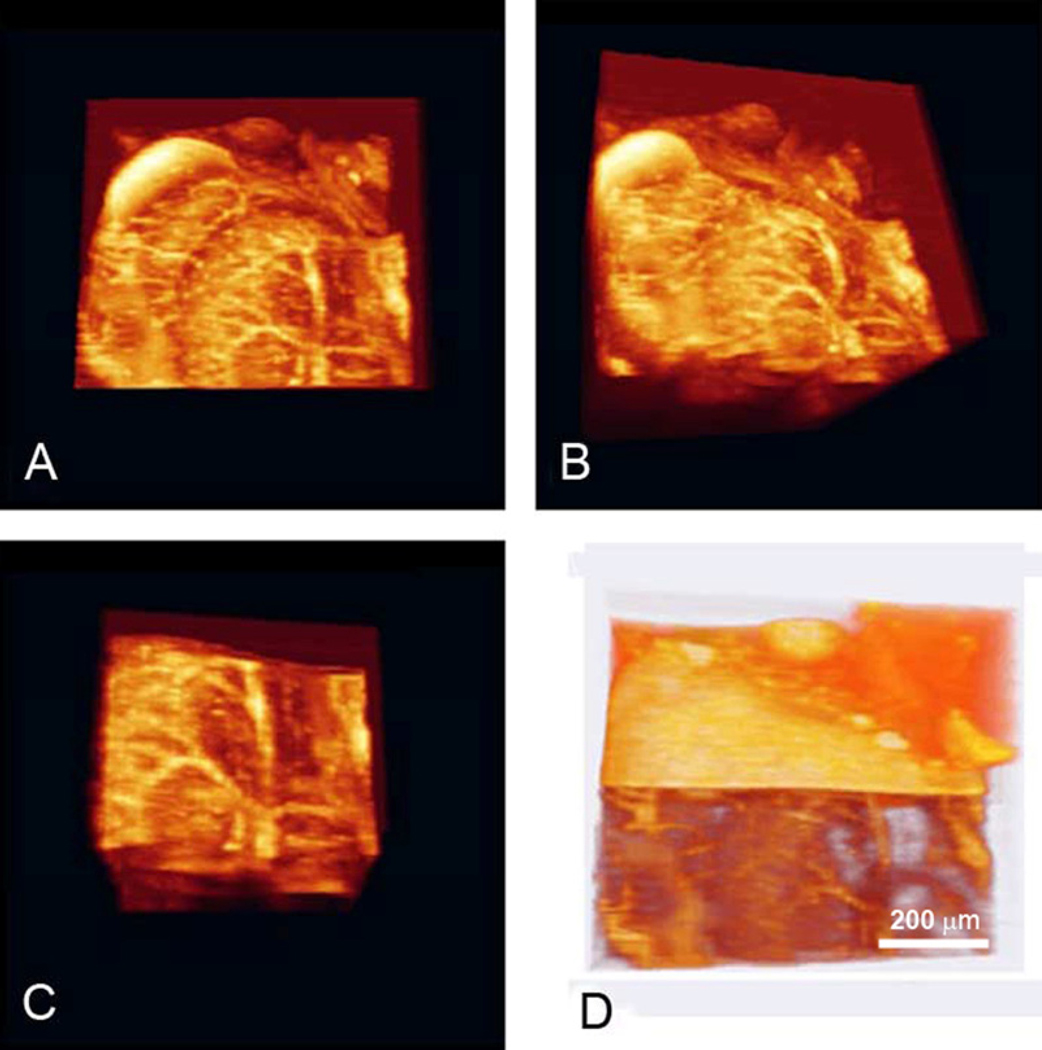
Optical microangiography (OMAG) is a recently developed technique which enables the imaging of circulation patterns at capillary level resolution in tissue beds up to 2 mm thick (Wang, 2008a; Wang, 2008b). Endogenous light scattering from moving blood cells provides the image contrast, and no exogenous contrast agents are necessary. The technique is sensitive enough to image very slow blood flow velocities, such as those found in capillary networks of the cochlea, without opening a window in the cochlear lateral wall. Volumetric reconstruction of microvascular flow in the cochlea with this technique has been demonstrated (see Figure 9). The collection to the left displays OMAG images of the otic capsule, stria vascularis of the apical (SVa) and middle (SVm) turns, and radiating arterioles that emanate from the modiolus (M) in an intact cochlea (Choudhury et al., 2009). Further improvements to the resolution of the OMAG imaging system, for example with a higher numerical aperture (NA) objective, would enable visualization of individual capillaries in the stria vascularis, as well as measurement of blood flow in the intact cochlea of living animals, without need of compromising the lateral wall.
Figure 9. The 3D volume rendering of mouse cochlea is segmented and displayed in four different orientations to provide a detailed view of the cochlear microvasculature.
A & B show a 3D volumetric perfusion image of the entire cochlea (Media3 & Media4). C is a segmented 3D volumetric microvascular perfusion at the Modiolus (Media5), and D a 3D volumetric reconstruction of the microvascular perfusion together with cochlear structures (Media6).
Conclusion
Normal blood supply to the cochlea and BLB are essential for sustaining endocochlear potential, ion transport, and endolymphatic fluid balance, and for preventing toxic substances from entering the cochlea. Many of these functions are well-documented in previous reviews (Axelsson, 1988; Axelsson et al., 1987; Kimura, 1986; Lawrence, 1980; Miller et al., 1988; Miller et al., 1995a; Nakashima et al., 2003; Nuttall, 1988; Seidman et al., 1999b; Sillman et al., 1989; Wangemann, 2002b). This review has focused on regulation of blood flow in the microvasculature, as dysfunction of cochlear blood flow and disruption of the cochlear BLB are shown to result in hearing impairment in animal models. Recent research has shown breakthroughs in explaining some of the underlying mechanisms. Progress in understanding the cellular and molecular structure of the blood-labyrinth barrier is accelerating, and new experimental methods are providing opportunities to study the physiology of the inner ear microvasculature more deeply. Future directions for cochlear microcirculation research include (i) developing novel CoBF measurement tools for diagnosis of vascular dysfunction related hearing loss; (ii) investigating the role of the blood-labyrinth-barrier in generating the endolymphatic potential; (iii) investigating the role of CoBF in cochlear homeostasis; and (iv) defining the pathological mechanisms in clinical diseases which involve flow dysregulation and barrier disruption.
> Cochlear microcirculation is essential for normal hearing function. > A better understanding of cochlear microcirculation will benefit clinic treatment. > Progress in this field is accelerating due to new methods and technologies. > This review focuses on recent discoveries on cochlear microcirculation.
Acknowledgments
Most of the data presented in this review reflects the efforts of my colleagues and students at the Oregon Hearing Research Center. In particular, the author is deeply indebted to Dr. Alfred Nuttall for stimulating discussion and advice. The author also thanks Mr. Allan Kachelmeier and Ms. Janice Moore for editorial assistance, and Dr. Min Dai for assistance with the references. I apologize to my colleagues whose work cannot be cited here due to space constraints.
This work was supported by National Institutes of Health grants R03-DC008888 (X.S.), DC008888S1 (X.S.), R01-NIDCD DC010844 (X.S), P30-DC005983.DC 00105 and R01 DC00105.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. Journal of anatomy. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature reviews. 2006.;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Adams J. Immunocytochemical Traits of Type IV Fibrocytes and Their Possible Relations to Cochlear Function and Pathology. J Assoc Res Otolaryng. 2009 doi: 10.1007/s10162-009-0165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimoni C, Bianchini C, Borin M, Ciorba A, Fellin R, Martini A, Scanelli G, Volpato S. Diabetes, cardiovascular risk factors and idiopathic sudden sensorineural hearing loss: A case-control study. Audiol Neurotol. 2010;15:111–115. doi: 10.1159/000231636. [DOI] [PubMed] [Google Scholar]
- Albera R, Ferrero V, Canale A, De Siena L, Pallavicino F, Poli L. Cochlear blood flow modifications induced by anaesthetic drugs in middle ear surgery: comparison between sevoflurane and propofol. Acta oto-laryngologica. 2003;123:812–816. doi: 10.1080/00016480310002230. [DOI] [PubMed] [Google Scholar]
- Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells, tissues, organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- Ando M, Takeuchi S. Postnatal vascular development in the lateral wall of the cochlear duct of gerbils: quantitative analysis by electron microscopy and confocal laser microscopy. Hearing research. 1998;123:148–156. doi: 10.1016/s0378-5955(98)00109-9. [DOI] [PubMed] [Google Scholar]
- Arpornchayanon W, Canis M, Suckfuell M, Ihler F, Olzowy B, Strieth S. Modeling the Measurements of Cochlear Microcirculation and Hearing Function after Loud Noise. Otolaryngol Head Neck Surg. 2011 doi: 10.1177/0194599811407829. [DOI] [PubMed] [Google Scholar]
- Axelsson A. The vascular anatomy of the cochlea in the guinea pig and in man. Acta oto-laryngologica. 1968;(Suppl 243) 3+ [PubMed] [Google Scholar]
- Axelsson A. Comparative anatomy of cochlear blood vessels. American journal of otolaryngology. 1988;9:278–290. doi: 10.1016/s0196-0709(88)80036-x. [DOI] [PubMed] [Google Scholar]
- Axelsson A, Dengerink H. The effects of noise on histological measures of the cochlear vasculature and red blood cells: a review. Hearing research. 1987;31:183–191. doi: 10.1016/0378-5955(87)90125-0. [DOI] [PubMed] [Google Scholar]
- Axelsson A, Ryan A, Woolf N. The early postnatal development of the cochlear vasculature in the gerbil. Acta Otolaryngol. 1986;101:75–87. doi: 10.3109/00016488609108610. [DOI] [PubMed] [Google Scholar]
- Axelsson A, Nuttall AL, Miller JM. Observations of cochlear microcirculation using intravital microscopy. Acta oto-laryngologica. 1990;109:263–270. doi: 10.3109/00016489009107442. [DOI] [PubMed] [Google Scholar]
- Barkhuizen A, Lim L, Trune D, Rosenbaum J. Ocular, Aural & Oral Manifestations. In: Wallace D, Hahn B, editors. Dubois' Lupus Erythematosus. Baltimore: Lippincott, Williams & Wilkins; 2006. pp. 789–800. [Google Scholar]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neurooncology. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis. Exs. 2005:115–125. doi: 10.1007/3-7643-7311-3_8. [DOI] [PubMed] [Google Scholar]
- Brechtelsbauer PB, Nuttall AL, Miller JM. Basal nitric oxide production in regulation of cochlear blood flow. Hearing research. 1994;77:38–42. doi: 10.1016/0378-5955(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Brechtelsbauer PB, Ren TY, Miller JM, Nuttall AL. Autoregulation of cochlear blood flow in the hydropic guinea pig. Hearing research. 1995;89:130–136. doi: 10.1016/0378-5955(95)00130-4. [DOI] [PubMed] [Google Scholar]
- Brechtelsbauer PB, Prazma J, Garrett CG, Carrasco VN, Pillsbury HC., 3rd Student Research Award 1990. Catecholaminergic innervation of the inner ear. Otolaryngol Head Neck Surg. 1990;103:566–574. doi: 10.1177/019459989010300407. [DOI] [PubMed] [Google Scholar]
- Brown JN, Nuttall AL. Autoregulation of cochlear blood flow in guinea pigs. The American journal of physiology. 1994;266:H458–H467. doi: 10.1152/ajpheart.1994.266.2.H458. [DOI] [PubMed] [Google Scholar]
- Brown JN, Miller JM, Nuttall AL. Age-related changes in cochlear vascular conductance in mice. Hear Res. 1995;86:189–194. doi: 10.1016/0378-5955(95)00070-k. [DOI] [PubMed] [Google Scholar]
- Cadoni G, Fetoni AR, Agostino S, Santis AD, Manna R, Ottaviani F, Paludetti G. Autoimmunity in Sudden Sensorineural Hearing Loss: Possible Role of Anti-endothelial Cell Autoantibodies. Acta Oto-Laryngologica. 2002;122:30–33. doi: 10.1080/00016480260094947. [DOI] [PubMed] [Google Scholar]
- Cao C, Goo JH, Lee-Kwon W, Pallone TL. Vasa recta pericytes express a strong inward rectifier K+ conductance. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1601–R1607. doi: 10.1152/ajpregu.00877.2005. [DOI] [PubMed] [Google Scholar]
- Cardoso FL, Brites D, Brito MA. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain research reviews. 2010;64:328–363. doi: 10.1016/j.brainresrev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Carlisle L, Aberdeen J, Forge A, Burnstock G. Neural basis for regulation of cochlear blood flow: peptidergic and adrenergic innervation of the spiral modiolar artery of the guinea pig. Hear Res. 1990;43:107–113. doi: 10.1016/0378-5955(90)90219-f. [DOI] [PubMed] [Google Scholar]
- Chen J, Nathans J. Estrogen-related receptor beta/NR3B2 controls epithelial cell fate and endolymph production by the stria vascularis. Dev Cell. 2007;13:325–337. doi: 10.1016/j.devcel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Chen YS, Tseng FY, Liu TC, Lin-Shiau SY, Hsu CJ. Involvement of nitric oxide generation in noise-induced temporary threshold shift in guinea pigs. Hearing Research. 2005a;203:94–100. doi: 10.1016/j.heares.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Chen YS, Tseng FY, Liu TC, Lin-Shiau SY, Hsu CJ. Involvement of nitric oxide generation in noise-induced temporary threshold shift in guinea pigs. Hearing research. 2005b;203:94–100. doi: 10.1016/j.heares.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Choudhury N, Chen F, Shi X, Nuttall AL, Wang RK. Volumetric Imaging of Blood Flow within Cochlea in Gerbil in vivo. IEEE J Sel Top Quantum Electron. 2009:1–6. doi: 10.1109/JSTQE.2009.2032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Salmon M, Regnault B, Cayet N, Caille D, Demuth K, Hardelin JP, Janel N, Meda P, Petit C. Connexin30 deficiency causes instrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proc Natl Acad Sci U S A. 2007;104:6229–6234. doi: 10.1073/pnas.0605108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M, Shi X. Fibro-Vascular Coupling in the Control of Cochlear Blood Flow. PloS one. 2011;6:e20652. doi: 10.1371/journal.pone.0020652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M, Nuttall A, Yang Y, Shi X. Visualization and contractile activity of cochlear pericytes in the capillaries of the spiral ligament. Hearing research. 2009;254:100–107. doi: 10.1016/j.heares.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M, Yang Y, Fletcher R, Xiu RJ, Nuttall AL, Shi XR. Lactate Dilates Pre-Capillaries of the Spiral Ligament Via Type V Fibrocyte-Pericyte Coupling. ARO abstracts. 2010;33:H69–H78. [Google Scholar]
- Degoute CS, Preckel MP, Dubreuil C, Banssillon V, Duclaux R. Sympathetic nerve regulation of cochlear blood flow during increases in blood pressure in humans. European journal of applied physiology and occupational physiology. 1997;75:326–332. doi: 10.1007/s004210050168. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1966–1975. doi: 10.1161/01.ATV.0000232525.65682.a2. [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Varela H, Rancel N, Valladares F. Microvascular pericytes: a review of their morphological and functional characteristics. Histology and histopathology. 1991;6:269–286. [PubMed] [Google Scholar]
- Doherty JK, Linthicum FHJ. Spiral ligament and stria vascularis changes in cochlear otosclerosis: effect on hearing level. Otol Neurotol. 2004;25:457–464. doi: 10.1097/00129492-200407000-00010. [DOI] [PubMed] [Google Scholar]
- Donoghue L, Tyburski JG, Steffes CP, Wilson RF. Vascular endothelial growth factor modulates contractile response in microvascular lung pericytes. American journal of surgery. 2006;191:349–352. doi: 10.1016/j.amjsurg.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Fattori B, Nacci A, Casani A, Cristofani R, Sagripanti A. Hemostatic alterations in patients with acute, unilateral vestibular paresis. Otolaryngology - Head and Neck Surgery. 2001;124:401–407. doi: 10.1067/mhn.2001.114795. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor. Clinical and experimental pharmacology & physiology. 1996;23:1082–1090. doi: 10.1111/j.1440-1681.1996.tb01174.x. [DOI] [PubMed] [Google Scholar]
- Ferrary E, Sterkers O. Mechanisms of endolymph secretion. Kidney Int Suppl. 1998;65:S98–S103. [PubMed] [Google Scholar]
- Frank RN, Dutta S, Mancini MA. Pericyte coverage is greater in the retinal than in the cerebral capillaries of the rat. Investigative ophthalmology & visual science. 1987;28:1086–1091. [PubMed] [Google Scholar]
- Frisina RD. Age-related hearing loss: ear and brain mechanisms. Ann N Y Acad Sci. 2009;1170:708–717. doi: 10.1111/j.1749-6632.2009.03931.x. [DOI] [PubMed] [Google Scholar]
- Fujimura T, Suzuki H, Shimizu T, Tokui N, Kitamura T, Udaka T, Doi Y. Pathological alterations of strial capillaries in dominant white spotting W/Wv mice. Hearing research. 2005;209:53–59. doi: 10.1016/j.heares.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Gacek RR. The course and central termination of first order neurons supplying vestibular endorgans in the cat. Acta Otolaryngol Suppl. 1969;254:1–66. [PubMed] [Google Scholar]
- Gatehouse S, Lowe GD. Whole blood viscosity and red cell filterability as factors in sensorineural hearing impairment in the elderly. Acta Otolaryngol Suppl. 1990;476:37–43. doi: 10.3109/00016489109127254. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell and tissue research. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Mulligan SJ, MacVicar BA. Astrocyte control of the cerebrovasculature. Glia. 2007;55:1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton MA, Schmiedt RA, Schulte BA. Age-related decreases in endocochlear potential are associated with vascular abnormalities in the stria vascularis. Hear Res. 1996a;102:181–190. doi: 10.1016/s0378-5955(96)90017-9. [corrected and republished article originallly printed in Hear Res 1996 May;94(1– 2):116-24] [DOI] [PubMed] [Google Scholar]
- Gratton MA, Schmiedt RA, Schulte BA. Age-related decreases in endocochlear potential are associated with vascular abnormalities in the stria vascularis. Hearing research. 1996b;102:181–190. doi: 10.1016/s0378-5955(96)90017-9. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Schulte BA, Smythe NM. Quantification of the stria vascularis and strial capillary areas in quiet-reared young and aged gerbils. Hear Res. 1997;114:1–9. doi: 10.1016/s0378-5955(97)00025-7. [DOI] [PubMed] [Google Scholar]
- Gruber DD, Dang H, Shimozono M, Scofield MA, Wangemann P. Alpha1A-adrenergic receptors mediate vasoconstriction of the isolated spiral modiolar artery in vitro. Hear Res. 1998;119:113–124. doi: 10.1016/s0378-5955(98)00036-7. [DOI] [PubMed] [Google Scholar]
- Haefliger IO, Zschauer A, Anderson DR. Relaxation of retinal pericyte contractile tone through the nitric oxide-cyclic guanosine monophosphate pathway. Investigative ophthalmology & visual science. 1994;35:991–997. [PubMed] [Google Scholar]
- Harkins SW. Effects of age and interstimulus interval on the brainstem auditory evoked potential. Int J Neurosci. 1981;15:107–118. doi: 10.3109/00207458108985851. [DOI] [PubMed] [Google Scholar]
- Haseloff RF, Blasig IE, Bauer HC, Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cellular and molecular neurobiology. 2005;25:25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JE., Jr The role of vasoconstriction in noise-induced hearing loss. Ann Otol Rhinol Laryngol. 1971;80:903–913. doi: 10.1177/000348947108000617. [DOI] [PubMed] [Google Scholar]
- Heinrich UR, Selivanova O, Schmidtmann I, Feltens R, Brieger J, Mann WJ. Noise exposure alters cyclooxygenase 1 (COX-1) and 5-lipoxygenase (5-LO) expression in the guinea pig cochlea. Acta oto-laryngologica. 2010;130:358–365. doi: 10.1080/00016480903168066. [DOI] [PubMed] [Google Scholar]
- Heinrich UR, Brieger J, Selivanova O, Feltens R, Eimermacher A, Schafer D, Mann WJ. COX-2 expression in the guinea pig cochlea is partly altered by moderate sound exposure. Neuroscience letters. 2006;394:121–126. doi: 10.1016/j.neulet.2005.10.039. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. The Journal of cell biology. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog M, Scherer EQ, Albrecht B, Rorabaugh B, Scofield MA, Wangemann P. CGRP receptors in the gerbil spiral modiolar artery mediate a sustained vasodilation via a transient cAMP-mediated Ca2+-decrease. J Membr Biol. 2002;189:225–236. doi: 10.1007/s00232-002-1017-5. [DOI] [PubMed] [Google Scholar]
- Hillerdal M, Jansson B, Engstrom B, Hultcrantz E, Borg E. Cochlear blood flow in noise-damaged ears. Acta oto-laryngologica. 1987;104:270–278. doi: 10.3109/00016488709107328. [DOI] [PubMed] [Google Scholar]
- Hirose K, Liberman MC. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. Jaro. 2003;4:339–352. doi: 10.1007/s10162-002-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, D'Amore PA. Pericytes in the microvasculature. Cardiovascular research. 1996;32:687–698. [PubMed] [Google Scholar]
- Iwagaki T, Suzuki T, Nakashima T. Development and regression of cochlear blood vessels in fetal and newborn mice. Hearing research. 2000;145:75–81. doi: 10.1016/s0378-5955(00)00075-7. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Shi X, Zhao H, Si JQ, Nuttall AL. Basal nitric oxide production contributes to membrane potential and vasotone regulation of guinea pig in vitro spiral modiolar artery. Hearing research. 2004;189:92–100. doi: 10.1016/S0378-5955(03)00398-8. [DOI] [PubMed] [Google Scholar]
- Juhn SK. Barrier systems in the inner ear. Acta Otolaryngol Suppl. 1988;458:79–83. doi: 10.3109/00016488809125107. [DOI] [PubMed] [Google Scholar]
- Juhn SK, Rybak LP. Labyrinthine barriers and cochlear homeostasis. Acta oto-laryngologica. 1981;91:529–534. doi: 10.3109/00016488109138538. [DOI] [PubMed] [Google Scholar]
- Juhn SK, Hunter BA, Odland RM. Blood-labyrinth barrier and fluid dynamics of the inner ear. Int Tinnitus J. 2001;7:72–83. [PubMed] [Google Scholar]
- Juhn SK, Li W, Kim JY, Javel E, Levine S, Odland RM. Effect of stress-related hormones on inner ear fluid homeostasis and function. The American journal of otology. 1999;20:800–806. [PubMed] [Google Scholar]
- Kellerhals B. Acoustic trauma and cochlear microcirculation. An experimental and clinical study on pathogenesis and treatment of inner ear lesions after acute noise exposure. Adv Otorhinolaryngol. 1972;18:91–168. [PubMed] [Google Scholar]
- Kim JA, Tran ND, Li Z, Yang F, Zhou W, Fisher MJ. Brain endothelial hemostasis regulation by pericytes. J Cereb Blood Flow Metab. 2006;26:209–217. doi: 10.1038/sj.jcbfm.9600181. [DOI] [PubMed] [Google Scholar]
- Kimura RS. Animal models of inner ear vascular disturbances. Am J Otolaryngol. 1986;7:130–139. doi: 10.1016/s0196-0709(86)80042-4. [DOI] [PubMed] [Google Scholar]
- Klijin E, Den Uil CA, Bakker J, Ince C. The heterogeneity of the microcirculation in critical illness. Clinic in Chest Medicine. 2008;29:643–654. doi: 10.1016/j.ccm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Konishi K, Yamane H, Iguchi H, Takayama M, Nakagawa T, Sunami K, Nakai Y. Local substances regulating cochlear blood flow. Acta Otolaryngol Suppl. 1998;538:40–46. [PubMed] [Google Scholar]
- Lamm K, Arnold W. The effect of prednisolone and non-steroidal anti-inflammatory agents on the normal and noise-damaged guinea pig inner ear. Hear Res. 1998;115:149–161. doi: 10.1016/s0378-5955(97)00186-x. [DOI] [PubMed] [Google Scholar]
- Lamm K, Arnold W. Successful treatment of noise-induced cochlear ischemia, hypoxia, and hearing loss. Annals of the New York Academy of Sciences. 1999;884:233–248. doi: 10.1111/j.1749-6632.1999.tb08645.x. [DOI] [PubMed] [Google Scholar]
- Lang H, Jyothi V, Smythe NM, Dubno JR, Schulte BA, Schmiedt RA. Chronic reduction of endocochlear potential reduces auditory nerve activity: further confirmation of an animal model of metabolic presbyacusis. J Assoc Res Otolaryngol. 2010;11:419–434. doi: 10.1007/s10162-010-0214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen HC, Albers F, Jansson B, Angelborg C, Veldman J. Cochlear blood flow in endolymphatic hydrops. Acta oto-laryngologica. 1988;106:404–408. doi: 10.3109/00016488809122263. [DOI] [PubMed] [Google Scholar]
- Laurikainen EA, Kim D, Didier A, Ren T, Miller JM, Quirk WS, Nuttall AL. Stellate ganglion drives sympathetic regulation of cochlear blood flow. Hearing research. 1993;64:199–204. doi: 10.1016/0378-5955(93)90006-m. [DOI] [PubMed] [Google Scholar]
- Laurikainen EA, Costa O, Miller JM, Nuttall AL, Ren TY, Masta R, Quirk WS, Robinson PJ. Neuronal regulation of cochlear blood flow in the guinea-pig. J Physiol. 1994;480(Pt 3):563–573. doi: 10.1113/jphysiol.1994.sp020384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M. Control mechanisms of inner ear microcirculation. Am J Otolaryngol. 1980;1:324–333. doi: 10.1016/s0196-0709(80)80035-4. [DOI] [PubMed] [Google Scholar]
- Legler DF, Bruckner M, Uetz-von Allmen E, Krause P. Prostaglandin E2 at new glance: novel insights in functional diversity offer therapeutic chances. The international journal of biochemistry & cell biology. 2010;42:198–201. doi: 10.1016/j.biocel.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Lin DW, Trune DR. Breakdown of stria vascularis blood-labyrinth barrier in C3H/lpr autoimmune disease mice. Otolaryngol Head Neck Surg. 1997;117:530–534. doi: 10.1016/S0194-59989770026-3. [DOI] [PubMed] [Google Scholar]
- Lockhart CJ, Hamilton PK, Quinn CE, McVeigh GE. End-organ dysfunction and cardiovascular outcomes: the role of the microcirculation. Clinical Science. 2009;116:175–190. doi: 10.1042/CS20080069. [DOI] [PubMed] [Google Scholar]
- Lombard JH. A novel mechanism for regulation of retinal blood flow by lactate: gap junctions, hypoxia, and pericytes. Am J Physiol Heart Circ Physiol. 2006;290:H921–H922. doi: 10.1152/ajpheart.01268.2005. [DOI] [PubMed] [Google Scholar]
- Marcus DC, Rokugo M, Ge XX, Thalmann R. Response of cochlear potentials to presumed alterations of ionic conductance: endolymphatic perfusion of barium, valinomycin and nystatin. Hear Res. 1983;12:17–30. doi: 10.1016/0378-5955(83)90116-8. [DOI] [PubMed] [Google Scholar]
- Marcus DC, Wu T, Wangemann P, Kofuji P. KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. American journal of physiology. 2002;282:C403–C407. doi: 10.1152/ajpcell.00312.2001. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Puro DG. Topographical heterogeneity of K(IR) currents in pericyte-containing microvessels of the rat retina: effect of diabetes. The Journal of physiology. 2006;573:483–495. doi: 10.1113/jphysiol.2006.107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek B, Haupt H, Georgiewa P, Klapp BF, Reisshauer A. A model of peripherally developing hearing loss and tinnitus based on the role of hypoxia and ischemia. Med. Hypotheses. 2006 doi: 10.1016/j.mehy.2006.03.040. article in press. [DOI] [PubMed] [Google Scholar]
- McLaren GM, Quirk WS, Laurikainen E, Coleman JK, Seidman MD, Dengerink HA, Nuttall AL, Miller JM, Wright JW. Substance P increases cochlear blood flow without changing cochlear electrophysiology in rats. Hear Res. 1993;71:183–189. doi: 10.1016/0378-5955(93)90033-w. [DOI] [PubMed] [Google Scholar]
- Mendrinos E, Petropoulos IK, Mangioris G, Papadopoulou DN, Stangos AN, Pournaras CJ. Lactate-induced retinal arteriolar vasodilation implicates neuronal nitric oxide synthesis in minipigs. Investigative ophthalmology & visual science. 2008;49:5060–5066. doi: 10.1167/iovs.08-2087. [DOI] [PubMed] [Google Scholar]
- Miller JM, Dengerink H. Control of inner ear blood flow. American journal of otolaryngology. 1988;9:302–316. doi: 10.1016/s0196-0709(88)80038-3. [DOI] [PubMed] [Google Scholar]
- Miller JM, Ren TY, Nuttall AL. Studies of inner ear blood flow in animals and human beings. Otolaryngol Head Neck Surg. 1995a;112:101–113. doi: 10.1016/S0194-59989570308-X. [DOI] [PubMed] [Google Scholar]
- Miller JM, Brown JN, Schacht J. 8-iso-prostaglandin F-2a, a product of noise exposure, reduces inner ear blood flow. Audiol. Neuro-Otol. 2003;8:207–221. doi: 10.1159/000071061. [DOI] [PubMed] [Google Scholar]
- Miller JM, Ren TY, Laurikainen E, Golding-Wood D, Nuttall AL. Hydrops-induced changes in cochlear blood flow. The Annals of otology, rhinology, and laryngology. 1995b;104:476–483. doi: 10.1177/000348949510400611. [DOI] [PubMed] [Google Scholar]
- Mistrik P, Ashmore J. The role of potassium recirculation in cochlear amplification. Current opinion in otolaryngology & head and neck surgery. 2009;17:394–399. doi: 10.1097/MOO.0b013e328330366f. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Gopinath B, McMahon CM, Rochtchina E, Wang JJ, Boyages SC, Leeder SR. Relationship of Type 2 diabetes to the prevalence, incidence and progression of age-related hearing loss. Diabetic Medicine. 2009;26:483–488. doi: 10.1111/j.1464-5491.2009.02710.x. [DOI] [PubMed] [Google Scholar]
- Monfared A, Blevins NH, Cheung EL, Jung JC, Popelka G, Schnitzer MJ. In vivo imaging of mammalian cochlear blood flow using fluorescence microendoscopy. Otol Neurotol. 2006;27:144–152. doi: 10.1097/01.mao.0000190708.44067.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SK, Park R, Lee HY, Nam GJ, Cha K, Andalibi A, Lim DJ. Spiral ligament fibrocytes release chemokines in response to otitis media pathogens. Acta Otolaryngol. 2006;126:564–569. doi: 10.1080/00016480500452525. [DOI] [PubMed] [Google Scholar]
- Mosnier I, Stepanian A, Baron G, Bodenez C, Robier A, Meyer B, Fraysse B, Bertholon P, Defay F, Ameziane N, Ferrary E, Sterkers O, de Prost D. Cardiovascular and thromboembolic risk factors in idiopathic sudden sensorineural hearing loss: a case-control study. Audiology & neuro-otology. 2011;16:55–66. doi: 10.1159/000312640. [DOI] [PubMed] [Google Scholar]
- Mouadeb DA, Ruckenstein MJ. Antiphospholipid inner ear syndrome. The Laryngoscope. 2005;115:879–883. doi: 10.1097/01.MLG.0000158666.15447.37. [DOI] [PubMed] [Google Scholar]
- Mudry A, Tange RA. The vascularization of the human cochlea: its historical background. Acta Otolaryngol Suppl. 2009:3–16. doi: 10.1080/00016480902924469. [DOI] [PubMed] [Google Scholar]
- Munoz DJ, McFie C, Thorne PR. Modulation of cochlear blood flow by extracellular purines. Hearing research. 1999;127:55–61. doi: 10.1016/s0378-5955(98)00161-0. [DOI] [PubMed] [Google Scholar]
- Naarendorp M, Spiera H. Sudden sensorineural hearing loss in patients with systemic lupus erythematosus or lupus-like syndromes and antiphospholipid antibodies. The Journal of rheumatology. 1998;25:589–592. [PubMed] [Google Scholar]
- Nag S, Kapadia A, Stewart DJ. Review: molecular pathogenesis of blood-brain barrier breakdown in acute brain injury. Neuropathology and applied neurobiology. 2011;37:3–23. doi: 10.1111/j.1365-2990.2010.01138.x. [DOI] [PubMed] [Google Scholar]
- Nagahara K, Aoyama T, Fukuse S, Noi O. Effects of prostaglandins on perilymphatic oxygenation. Enhancement of cochlear autoregulation by prostacyclin. Acta Otolaryngol Suppl. 1988;456:143–150. doi: 10.3109/00016488809125092. [DOI] [PubMed] [Google Scholar]
- Nagaoka J, Anjos MF, Takata TT, Chaim RM, Barros F, Penido Nde O. Idiopathic sudden sensorineural hearing loss: evolution in the presence of hypertension, diabetes mellitus and dyslipidemias. Brazilian journal of otorhinolaryngology. 2010;76:363–369. doi: 10.1590/S1808-86942010000300015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T. Roles of prostaglandin E2 in the cochlea. Hearing research. 2011;276:27–33. doi: 10.1016/j.heares.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Masutani H, Cho H. Scanning electron microscopy of the microvascular system in the inner ear. Scanning electron microscopy. 1986:543–548. [PubMed] [Google Scholar]
- Nakashima T. Autoregulation of cochlear blood flow. Nagoya journal of medical science. 1999;62:1–9. [PubMed] [Google Scholar]
- Nakashima T, Naganawa S, Sone M, Tominaga M, Hayashi H, Yamamoto H, Liu X, Nuttall AL. Disorders of cochlear blood flow. Brain Res Brain Res Rev. 2003;43:17–28. doi: 10.1016/s0165-0173(03)00189-9. [DOI] [PubMed] [Google Scholar]
- Nehls V, Drenckhahn D. The versatility of microvascular pericytes: from mesenchyme to smooth muscle? Histochemistry. 1993;99:1–12. doi: 10.1007/BF00268014. [DOI] [PubMed] [Google Scholar]
- Nelson EG, Hinojosa R. Presbycusis: A human temporal bone study of individuals with downward sloping audiometric patterns of hearing loss and review of the literature. The Laryngoscope. 2006;116:1–12. doi: 10.1097/01.mlg.0000236089.44566.62. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. The American journal of physiology. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Nie L, Gratton MA, Mu KJ, Dinglasan JN, Feng W, Yamoah EN. Expression and functional phenotype of mouse ERG K+ channels in the inner ear: potential role in K+ regulation in the inner ear. J Neurosci. 2005;25:8671–8679. doi: 10.1523/JNEUROSCI.1422-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Nario K, Hosoi H. Effects of intravenous administration of prostaglandin E(1) and lipo-prostaglandin E(1) on cochlear blood flow in guinea pigs. Eur Arch Otorhinolaryngol. 2002;259:253–256. doi: 10.1007/s00405-002-0453-2. [DOI] [PubMed] [Google Scholar]
- Nuttall AL. Techniques for the observation and measurement of red blood cell velocity in vessels of the guinea pig cochlea. Hearing research. 1987;27:111–119. doi: 10.1016/0378-5955(87)90012-8. [DOI] [PubMed] [Google Scholar]
- Nuttall AL. Cochlear blood flow: measurement techniques. Am J Otolaryngol. 1988;9:291–301. doi: 10.1016/s0196-0709(88)80037-1. [DOI] [PubMed] [Google Scholar]
- Nuttall AL. Sound-induced cochlear ischemia/hypoxia as a mechanism of hearing loss. Noise Health. 1999a;2:17–31. [PubMed] [Google Scholar]
- Nuttall AL. Sound-Induced Cochlear Ischemia/Hypoxia as a Mechanism of Hearing Loss. Noise & health. 1999b;2:17–32. [PubMed] [Google Scholar]
- Offner FF, Dallos P, Cheatham MA. Positive endocochlear potential: mechanism of production by marginal cells of stria vascularis. Hear Res. 1987;29:117–124. doi: 10.1016/0378-5955(87)90160-2. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Mechanisms and genes in human strial presbycusis from animal models. Brain research. 2009 doi: 10.1016/j.brainres.2009.02.079. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Rice ME, Gagnon PM. Strial microvascular pathology and age-associated endocochlear potential decline in NOD congenic mice. Hear Res. 2008;244:85–97. doi: 10.1016/j.heares.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Dahl AR, Gagnon PM. Divergent aging characteristics in CBA/J and CBA/CaJ mouse cochleae. J Assoc Res Otolaryngol. 2010;11:605–623. doi: 10.1007/s10162-010-0228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani F, Cadoni G, Marinelli L, Fetoni AR, De Santis A, Romito A, Vulpiani P, Manna R. Anti-endothelial autoantibodies in patients with sudden hearing loss. Laryngoscope. 1999;109:1084–1087. doi: 10.1097/00005537-199907000-00014. [DOI] [PubMed] [Google Scholar]
- Pallone TL, Silldorff EP. Pericyte regulation of renal medullary blood flow. Experimental nephrology. 2001;9:165–170. doi: 10.1159/000052608. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirodda A, Brandolini C, Raimondi MC, Ferri GG, Modugno GC, Borghi C. Meniere's disease: update of etiopathogenetic theories and proposal of a possible model of explanation. Acta Clin Belg. 2010;Vol. 65:170–175. doi: 10.1179/acb.2010.036. [DOI] [PubMed] [Google Scholar]
- Prat A, Biernacki K, Wosik K, Antel JP. Glial cell influence on the human blood-brain barrier. Glia. 2001;36:145–155. doi: 10.1002/glia.1104. [DOI] [PubMed] [Google Scholar]
- Prazma J, Carrasco VN, Butler B, Waters G, Anderson T, Pillsbury HC. Cochlear microcirculation in young and old gerbils. Archives of otolaryngology--head & neck surgery. 1990;116:932–936. doi: 10.1001/archotol.1990.01870080054015. [DOI] [PubMed] [Google Scholar]
- Qiu J, Steyger PS, Trune DR, Nuttall AL. Co-existence of tyrosine hydroxylase and calcitonin gene-related peptide in cochlear spiral modiolar artery of guinea pigs. Hear Res. 2001;155:152–160. doi: 10.1016/s0378-5955(01)00231-3. [DOI] [PubMed] [Google Scholar]
- Qu C, Liang F, Smythe NM, Schulte BA. Identification of ClC-2 and CIC-K2 chloride channels in cultured rat type IV spiral ligament fibrocytes. J Assoc Res Otolaryng. 2007;8:205–219. doi: 10.1007/s10162-007-0072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quignard JF, Harley EA, Duhault J, Vanhoutte PM, Feletou M. K+ channels in cultured bovine retinal pericytes: effects of beta-adrenergic stimulation. Journal of cardiovascular pharmacology. 2003;42:379–388. doi: 10.1097/00005344-200309000-00009. [DOI] [PubMed] [Google Scholar]
- Quirk WS, Avinash G, Nuttall AL, Miller JM. The influence of loud sound on red blood cell velocity and blood vessel diameter in the cochlea. Hearing research. 1992;63:102–107. doi: 10.1016/0378-5955(92)90079-3. [DOI] [PubMed] [Google Scholar]
- Rauchegger H, Spoendlin H. Damage of the basilar membrane by acoustic stimulation. Arch Otorhinolaryngol. 1981;232:117–122. doi: 10.1007/BF00505030. [DOI] [PubMed] [Google Scholar]
- Ren T, Nuttall AL, Miller JM. ATP-induced cochlear blood flow changes involve the nitric oxide pathway. Hear Res. 1997;112:87–94. doi: 10.1016/s0378-5955(97)00109-3. [DOI] [PubMed] [Google Scholar]
- Ren TY, Laurikainen E, Quirk WS, Miller JM, Nuttall AL. Effects of electrical stimulation of the superior cervical ganglion on cochlear blood flow in guinea pig. Acta oto-laryngologica. 1993;113:146–151. doi: 10.3109/00016489309135783. [DOI] [PubMed] [Google Scholar]
- Rhee CK, Park YS, Jung TT, Park CI. Effects of leukotrienes and prostaglandins on cochlear blood flow in the chinchilla. Eur Arch Otorhinolaryngol. 1999;256:479–483. doi: 10.1007/s004050050195. [DOI] [PubMed] [Google Scholar]
- Rickheit G, Maier H, Strenzke N, Andreescu CE, De Zeeuw CI, Muenscher A, Zdebik AA, Jentsch TJ. Endocochlear potential depends on Cl- channels: mechanism underlying deafness in Bartter syndrome IV. The EMBO journal. 2008;27:2907–2917. doi: 10.1038/emboj.2008.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan RS. Possible roles of nitric oxide in the physiology and pathophysiology of the mammalian cochlea. Annals of the New York Academy of Sciences. 2002;962:260–274. doi: 10.1111/j.1749-6632.2002.tb04073.x. [DOI] [PubMed] [Google Scholar]
- Ruckenstein MJ, Milburn M, Hu L. Strial dysfunction in the MRL-Faslpr mouse. Otolaryngology - Head and Neck Surgery. 1999;121:452–456. doi: 10.1016/S0194-5998(99)70236-6. [DOI] [PubMed] [Google Scholar]
- Sadanaga M, Liu J, Wangemann P. Endothelin-A receptors mediate vasoconstriction of capillaries in the spiral ligament. Hear Res. 1997;112:106–114. doi: 10.1016/s0378-5955(97)00121-4. [DOI] [PubMed] [Google Scholar]
- Saito T, Zhang ZJ, Tsuzuki H, Ohtsubo T, Yamada T, Yamamoto T, Saito H. Expression of P-glycoprotein in inner ear capillary endothelial cells of the guinea pig with special reference to blood-inner ear barrier. Brain Res. 1997;767:388–392. doi: 10.1016/s0006-8993(97)00821-4. [DOI] [PubMed] [Google Scholar]
- Sakagami K, Kawamura H, Wu DM, Puro DG. Nitric oxide/cGMP-induced inhibition of calcium and chloride currents in retinal pericytes. Microvascular research. 2001;62:196–203. doi: 10.1006/mvre.2001.2343. [DOI] [PubMed] [Google Scholar]
- Sakagami M, Matsunaga T, Hashimoto PH. Fine structure and permeability of capillaries in the stria vascularis and spiral ligament of the inner ear of the guinea pig. Cell and tissue research. 1982;226:511–522. doi: 10.1007/BF00214780. [DOI] [PubMed] [Google Scholar]
- Sakagami M, Harada T, Sano M, Sakai S, Matsunaga T. Quantitative evaluation of pinocytosis of capillaries of the stria vascularis under normal and experimental conditions. Acta oto-laryngologica. 1987;103:189–197. [PubMed] [Google Scholar]
- Sakaguchi N, Spicer SS, Thomopoulos GN, Schulte BA. Immunoglobulin deposition in thickened basement membranes of aging strial capillaries. Hearing research. 1997a;109:83–91. doi: 10.1016/s0378-5955(97)00048-8. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N, Spicer SS, Thomopoulos GN, Schulte BA. Increased laminin deposition in capillaries of the stria vascularis of quiet-aged gerbils. Hearing research. 1997b;105:44–56. doi: 10.1016/s0378-5955(96)00180-3. [DOI] [PubMed] [Google Scholar]
- Salt AN. Regulation of endolymphatic fluid volume. Ann N Y Acad Sci. 2001;942:306–312. doi: 10.1111/j.1749-6632.2001.tb03755.x. [DOI] [PubMed] [Google Scholar]
- Salt AN, Melichar I, Thalmann R. Mechanisms of endocochlear potential generation by stria vascularis. Laryngoscope. 1987;97:984–991. [PubMed] [Google Scholar]
- Schacht J, Hawkins JE. Sketches of otohistory. Part 9: presby[a]cusis. Audiol Neurootol. 2005;10:243–247. doi: 10.1159/000086524. [DOI] [PubMed] [Google Scholar]
- Scheibe F, Haupt H, Hache U. Comparative studies of lactate concentration in the perilymph, blood and cerebrospinal fluid of normal and sound exposed guinea pigs (author's transl) Archives of oto-rhino-laryngology. 1976;214:19–25. doi: 10.1007/BF00455106. [DOI] [PubMed] [Google Scholar]
- Scheibe F, Haupt H, Ludwig C. Intensity-related changes in cochlear blood flow in the guinea pig during and following acoustic exposure. Eur Arch Otorhinolaryngol. 1993;250:281–285. doi: 10.1007/BF00186226. [DOI] [PubMed] [Google Scholar]
- Scherer EQ, Arnold W, Wangemann P. Pharmacological reversal of endothelin-1 mediated constriction of the spiral modiolar artery: a potential new treatment for sudden sensorineural hearing loss. BMC Ear Nose Throat Disord. 2005;5:10. doi: 10.1186/1472-6815-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman MD, Quirk WS, Shirwany NA. Mechanisms of alterations in the microcirculation of the cochlea. Ann NY Acad Sci. 1999a;249(6):332–335. doi: 10.1111/j.1749-6632.1999.tb08644.x. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Quirk WS, Shirwany NA. Mechanisms of alterations in the microcirculation of the cochlea. Annals of the New York Academy of Sciences. 1999b;884:226–232. doi: 10.1111/j.1749-6632.1999.tb08644.x. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Khan MJ, Dolan DF, Quirk WS. Age-related differences in cochlear microcirculation and auditory brain stem response. Archives of otolaryngology--head & neck surgery. 1996;122:1221–1226. doi: 10.1001/archotol.1996.01890230067013. [DOI] [PubMed] [Google Scholar]
- Selivanova O, Heinrich UR, Brieger J, Feltens R, Mann W. Fast alterations of vascular endothelial growth factor (VEGF) expression and that of its receptors (Flt-1, Flk-1 and Neuropilin) in the cochlea of guinea pigs after moderate noise exposure. Eur Arch Otorhinolaryngol. 2007;264:121–128. doi: 10.1007/s00405-006-0154-3. [DOI] [PubMed] [Google Scholar]
- Selmani Z, Pyykko I, Ishizaki H, Marttila TI. Cochlear blood flow measurement in patients with Meniere's disease and other inner ear disorders. Acta Otolaryngol. 2001;(Suppl 545):10–13. [PubMed] [Google Scholar]
- Semaan MT, Megerian CA. Contemporary perspectives on the pathophysiology of Meniere's disease: implications for treatment. Current opinion in otolaryngology & head and neck surgery. 2010;18:392–398. doi: 10.1097/MOO.0b013e32833d3164. [DOI] [PubMed] [Google Scholar]
- Semaan MT, Alagramam KN, Megerian CA. The basic science of Meniere's disease and endolymphatic hydrops. Current opinion in otolaryngology & head and neck surgery. 2005;13:301–307. doi: 10.1097/01.moo.0000186335.44206.1c. [DOI] [PubMed] [Google Scholar]
- Shepro D, Morel NM. Pericyte physiology. Faseb J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- Shi X. Cochlear pericyte responses to acoustic trauma and the involvement of hypoxia-inducible factor-1alpha and vascular endothelial growth factor. The American journal of pathology. 2009;174:1692–1704. doi: 10.2353/ajpath.2009.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X. Resident macrophages in the cochlear blood-labyrinth barrier and their renewal via migration of bone-marrow-derived cells. Cell and tissue research. 2010;342:21–30. doi: 10.1007/s00441-010-1040-2. [DOI] [PubMed] [Google Scholar]
- Shi X, Nuttall AL. The demonstration of nitric oxide in cochlear blood vessels in vivo and in vitro: the role of endothelial nitric oxide in venular permeability. Hearing research. 2002;172:73–80. doi: 10.1016/s0378-5955(02)00513-0. [DOI] [PubMed] [Google Scholar]
- Shi X, Nuttall AL. Dependence of loud sound induced cochlear stria vascularis injury on poly (ADP-Ribose) polymerase. Association for Research in Otolaryngology Midwinter Meeting Daytona Beach Florida. 2003 [Google Scholar]
- Shi X, Nuttall AL. Expression of adhesion molecular proteins in the cochlear lateral wall of normal and PARP-1 mutant mice. Hearing research. 2007;224:1–14. doi: 10.1016/j.heares.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Shi X, Ren T, Nuttall AL. Nitric oxide distribution and production in the guinea pig cochlea. Hearing research. 2001;153:23–31. doi: 10.1016/s0378-5955(00)00254-9. [DOI] [PubMed] [Google Scholar]
- Shi X, Han W, Yamamoto H, Tang W, Lin X, Xiu R, Trune DR, Nuttall AL. The cochlear pericytes. Microcirculation. 2008;15:515–529. doi: 10.1080/10739680802047445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si JQ, Zhao H, Yang Y, Jiang ZG, Nuttall AL. Nitric oxide induces hyperpolarization by opening ATP-sensitive K (+) channels in guinea pig spiral modiolar artery. Hearing research. 2002;171:167–176. doi: 10.1016/s0378-5955(02)00497-5. [DOI] [PubMed] [Google Scholar]
- Sillman JS, LaRouere MJ, Masta RI, Miller JM, Nuttall AL. Electrically stimulated increases in cochlear blood flow: I. Frequency and intensity effects. Otolaryngol Head Neck Surg. 1989;100:308–316. doi: 10.1177/019459988910000411. [DOI] [PubMed] [Google Scholar]
- Sims DE. Diversity within pericytes. Clinical and experimental pharmacology & physiology. 2000;27:842–846. doi: 10.1046/j.1440-1681.2000.03343.x. [DOI] [PubMed] [Google Scholar]
- Smith CA, Lowry OH, Wu ML. The electrolytes of the labyrinthine fluids. The Laryngoscope. 1954;64:141–153. [Google Scholar]
- Sone M, Mizuno T, Naganawa S, Nakashima T. Imaging analysis in cases with inflammation-induced sensorineural hearing loss. Acta Otolaryngol. 2009;129:239–243. doi: 10.1080/00016480802226163. [DOI] [PubMed] [Google Scholar]
- Sone M, Hayashi H, Yamamoto H, Tominaga M, Nakashima T. A comparative study of intratympanic steroid and NO synthase inhibitor for treatment of cochlear lateral wall damage due to acute otitis media. European journal of pharmacology. 2003;482:313–318. doi: 10.1016/j.ejphar.2003.09.051. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. Differentiation of inner ear fibrocytes according to their ion transport related activity. Hear Res. 1991;56:53–64. doi: 10.1016/0378-5955(91)90153-z. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. Spiral ligament pathology in quiet-aged gerbils. Hearing research. 2002a;172:172–185. doi: 10.1016/s0378-5955(02)00581-6. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. Spiral ligament pathology in quiet-aged gerbils. Hearing Research. 2002b;172:172–185. doi: 10.1016/s0378-5955(02)00581-6. [DOI] [PubMed] [Google Scholar]
- Sprinzl GM, Riechelmann H. Current trends in treating hearing loss in elderly people: a review of the technology and treatment options - a mini-review. Gerontology. 2010;56:351–358. doi: 10.1159/000275062. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Naganawa S, Sato E, Nakashima T. Visualization of a high protein concentration in the cochlea of a patient with a large endolymphatic duct and sac, using three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging. J Laryngol Otol. 2006;120:1084–1086. doi: 10.1017/S0022215106003331. [DOI] [PubMed] [Google Scholar]
- Suko T, Ichimiya I, Yoshida K, Suzuki M, Mogi G. Classification and culture of spiral ligament fibrocytes from mice. Hear Res. 2000;140:137–144. doi: 10.1016/s0378-5955(99)00191-4. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Yamasoba T, Kaga K. Development of the blood-labyrinth barrier in the rat. Hearing research. 1998;116:107–112. doi: 10.1016/s0378-5955(97)00208-6. [DOI] [PubMed] [Google Scholar]
- Takago H, Yokoyama K, Kitamura K. A vasoactive agent enhances the effect of ATP on cochlear blood flow. Acta Otolaryngol. 2001;121:130–134. doi: 10.1080/000164801300043181. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Ando M, Sato T, Kakigi A. Three-dimensional and ultrastructural relationships between intermediate cells and capillaries in the gerbil stria vascularis. Hearing research. 2001;155:103–112. doi: 10.1016/s0378-5955(01)00252-0. [DOI] [PubMed] [Google Scholar]
- Tasaki I, Spyropoulos CS. Nonuniform response in the squid axon membrana under voltage-clamp. The American journal of physiology. 1958;193:309–317. doi: 10.1152/ajplegacy.1958.193.2.309. [DOI] [PubMed] [Google Scholar]
- Tawackoli W, Chen GD, Fechter LD. Disruption of cochlear potentials by chemical asphyxiants. Cyanide and carbon monoxide. Neurotoxicology and teratology. 2001;23:157–165. doi: 10.1016/s0892-0362(01)00135-0. [DOI] [PubMed] [Google Scholar]
- Terashita T, Saito S, Miyawaki K, Hyodo M, Kobayashi N, Shimokawa T, Saito K, Matsuda S, Gyo K. Localization of prosaposin in rat cochlea. Neuroscience research. 2007;57:372–378. doi: 10.1016/j.neures.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Thomas WE. Brain macrophages: on the role of pericytes and perivascular cells. Brain research. 1999;31:42–57. doi: 10.1016/s0165-0173(99)00024-7. [DOI] [PubMed] [Google Scholar]
- Tian F, Fessenden JD, Schacht J. Cyclic GMP-dependent protein kinase-I in the guinea pig cochlea. Hearing research. 1999;131:63–70. doi: 10.1016/s0378-5955(99)00015-5. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Yamamoto H, Sone M, Teranishi MA, Nakashima T. Response of cochlear blood flow to prostaglandin E1 applied topically to the round window. Acta oto-laryngologica. 2006;126:232–236. doi: 10.1080/00016480500316803. [DOI] [PubMed] [Google Scholar]
- Tono T, Ueki Y, Nagata N, Haruta A, Komune S. Effects of trimetaphan-induced deliberate hypotension on human cochlear blood flow. Acta Otolaryngol. 1998;(Suppl 539):40–43. [PubMed] [Google Scholar]
- Tran Ba Huy P, Bernard P, Schacht J. Kinetics of gentamicin uptake and release in the rat. Comparison of inner ear tissues and fluids with other organs. J Clin Invest. 1986;77:1492–1500. doi: 10.1172/JCI112463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowe MO, Maier H, Schweizer M, Kispert A. Deafness in mice lacking the T-box transcription factor Tbx18 in otic fibrocytes. Development. 2008;135:1725–1734. doi: 10.1242/dev.014043. [DOI] [PubMed] [Google Scholar]
- Umemura K, Nakashima M. Effect of prostaglandin E1 on the rat inner ear microvascular thrombosis. General pharmacology. 1997;28:221–224. doi: 10.1016/s0306-3623(96)00192-9. [DOI] [PubMed] [Google Scholar]
- Vass Z, Brechtelsbauer B, Nuttall AL, Miller JM. Effect of endolymphatic hydrops on capsaicin-evoked increase in cochlear blood flow. Acta oto-laryngologica. 1995;115:754–758. doi: 10.3109/00016489509139398. [DOI] [PubMed] [Google Scholar]
- von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Experimental cell research. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Wagenaar M, Schuknecht H, Nadol J, Jr, Benraad-van Rens M, Pieke-Dahl S, Kimberling W, Cremers C. Histopathologic Features of the Temporal Bone in Usher Syndrome Type I. Arch Otolaryngol Head Neck Surg. 2000;126:1018–1023. doi: 10.1001/archotol.126.8.1018. [DOI] [PubMed] [Google Scholar]
- Wang RK. In vivo volumetric blood flow imaging using optical microangiography at capillary level resolution. Conf Proc IEEE Eng Med Biol Soc. 2008a;2008:804. doi: 10.1109/IEMBS.2008.4649275. [DOI] [PubMed] [Google Scholar]
- Wang RK. Directional blood flow imaging in volumetric optical microangiography achieved by digital frequency modulation. Opt Lett. 2008b;33:1878–1880. doi: 10.1364/ol.33.001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P. K+ cycling and the endocochlear potential. Hear Res. 2002a;165:1–9. doi: 10.1016/s0378-5955(02)00279-4. [DOI] [PubMed] [Google Scholar]
- Wangemann P. Cochlear blood flow regulation. Adv Otorhinolaryngol. 2002b;59:51–57. doi: 10.1159/000059241. [DOI] [PubMed] [Google Scholar]
- Wangemann P. K+ cycling and the endocochlear potential. Hearing Research. 2002c;165:1–9. doi: 10.1016/s0378-5955(02)00279-4. [DOI] [PubMed] [Google Scholar]
- Wangemann P, Liu J. Osmotic water permeability of capillaries from the isolated spiral ligament: new in-vitro techniques for the study of vascular permeability and diameter. Hear Res. 1996;95:49–56. doi: 10.1016/0378-5955(96)00007-x. [DOI] [PubMed] [Google Scholar]
- Wangemann P, Cohn ES, Gruber DD, Gratton MA. Ca2+-dependence and nifedipine-sensitivity of vascular tone and contractility in the isolated superfused spiral modiolar artery in vitro. Hear Res. 1998;118:90–100. doi: 10.1016/s0378-5955(98)00017-3. [DOI] [PubMed] [Google Scholar]
- Willis CL. Glia-induced reversible disruption of blood-brain barrier integrity and neuropathological response of the neurovascular unit. Toxicologic pathology. 2011;39:172–185. doi: 10.1177/0192623310385830. [DOI] [PubMed] [Google Scholar]
- Wonneberger K, Scofield MA, Wangemann P. Evidence for a calcium-sensing receptor in the vascular smooth muscle cells of the spiral modiolar artery. J Membr Biol. 2000;175:203–212. doi: 10.1007/s00232001068. [DOI] [PubMed] [Google Scholar]
- Wu T, Marcus D. Age-Related Changes in Cochlear Endolymphatic Potassium and Potential in CD-1 and CBA/CaJ Mice. JARO - Journal of the Association for Research in Otolaryngology. 2003;4:353–362. doi: 10.1007/s10162-002-3026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Dai M, Shi XR, Jiang ZG, Nuttall AL. Functional expression of P2X4 receptor in capillary endothelial cells of the cochlear spiral ligament and its role in regulating the capillary diameter. Am J Physiol Heart Circ Physiol. 2010;301:H69–H78. doi: 10.1152/ajpheart.01035.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S, Imaizumi T. Pericyte biology and diseases. International journal of tissue reactions. 2005;27:125–135. [PubMed] [Google Scholar]
- Yamane H, Nakai Y, Konishi K, Sakamoto H, Matsuda Y, Iguchi H. Strial circulation impairment due to acoustic trauma. Acta oto-laryngologica. 1991;111:85–93. doi: 10.3109/00016489109137358. [DOI] [PubMed] [Google Scholar]
- Yang T. Microsomal prostaglandin E synthase-1 and blood pressure regulation. Kidney international. 2007;72:274–278. doi: 10.1038/sj.ki.5002326. [DOI] [PubMed] [Google Scholar]
- Yang Y, Dai M, Wilson TM, Omelchenko I, Klimek JE, Wilmarth PA, David LL, Nuttall AL, Gillespie PG, Shi X. Na+/K+-ATPase alpha1 identified as an abundant protein in the blood-labyrinth barrier that plays an essential role in the barrier integrity. PloS one. 2011;6:e16547. doi: 10.1371/journal.pone.0016547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa Y, Kitano H, Suzuki M, Tanaka H, Kitajima K. Studies of cochlear blood flow in guinea pigs with endolymphatic hydrops. ORL; journal for oto-rhino-laryngology and its related specialties. 1998;60:4–11. doi: 10.1159/000027554. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Sugiura M, Naganawa S, Teranishi M, Nakata S, Nakashima T. Three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging findings and prognosis in sudden sensorineural hearing loss. Laryngoscope. 2008;118:1433–1437. doi: 10.1097/MLG.0b013e318172ef85. [DOI] [PubMed] [Google Scholar]