Abstract
OBJECTIVE:
The purpose of the study was to examine use of the Modified Checklist for Autism in Toddlers (M-CHAT) as an autism-specific screening instrument in a large, geographically diverse pediatrics-based sample.
METHODS:
The M-CHAT and the M-CHAT Follow-Up (M-CHAT/F) were used to screen 18 989 toddlers at pediatric well-child visits in 2 US geographic regions. Pediatricians directly referred children to ascertain potential missed screening cases. Screen-positive children received the M-CHAT/F; children who continued to screen positive after the M-CHAT/F received a diagnostic evaluation.
RESULTS:
Results indicated that 54% of children who screened positive on the M-CHAT and M-CHAT/F presented with an autism spectrum disorder (ASD), and 98% presented with clinically significant developmental concerns warranting intervention. An M-CHAT total score cutoff of ≥3 identifies nearly all screen-positive cases, and for ease of scoring the use of only the M-CHAT total score cutoff is recommended. An M-CHAT total score of 7 serves as an appropriate clinical cutoff, and providers can bypass the M-CHAT/F and refer immediately to evaluation and intervention if a child obtains a score of ≥7.
CONCLUSIONS:
This study provides empirical support for the utility of population screening for ASD with the use of the M-CHAT in a primary care setting. Results suggest that the M-CHAT continues to be an effective screening instrument for ASD when the 2-step screening process is used. The M-CHAT is widely used at pediatric offices, and this study provides updated results to facilitate use and scoring of the M-CHAT by clinical providers.
Keywords: autism, M-CHAT, screening, toddlers, diagnosis
What’s Known on This Subject:
Early detection for children with autism leads to better outcomes; early screening is critical. The Modified Checklist for Autism in Toddlers (M-CHAT) is a widely used instrument for early autism screening and is recommended by the American Academy of Pediatrics.
What This Study Adds:
This large study provides empirical support for population screening for autism spectrum disorders and the use of the M-CHAT in primary care settings. This study provides updated results to facilitate use and scoring of the M-CHAT by clinical providers.
Autism spectrum disorders (ASDs) are neurodevelopmental disorders characterized by impaired social interactions, communication deficits, and repetitive behaviors or unusual or limited interests.1 The current prevalence is estimated at 1 in 88 births.2 Early intervention can lead to a substantially better prognosis for children with ASD3–7; however, the median age of diagnosis is still past the third birthday8 and even later in children from disadvantaged backgrounds.9 Because intervention services before diagnosis are extremely limited, early screening and diagnosis are crucial.3 The current American Academy of Pediatrics (AAP) guidelines recommend routine ASD surveillance at every well-child visit plus standardized developmental screenings at ages 9, 18, and 30 months and ASD-specific screening at ages 18 and 24 months.10
The Modified Checklist for Autism in Toddlers (M-CHAT)11 is a 23-item parent-report autism screening tool for children 16 to 30 months of age (see Supplemental Information). The M-CHAT Follow-up (M-CHAT/F)12 gathers additional detail about at-risk responses and reduces the M-CHAT’s false-positive rate (see Supplemental Information). The aim of the study is to update findings regarding the use of the M-CHAT as an autism-specific, population-level screening instrument. A large sample of toddlers was screened in 2 US geographic regions during 18- and 24-month well-child examinations. Screen-positive children received the M-CHAT/F, and children who continued to screen positive were invited for diagnostic evaluation. Children are being rescreened between the ages of 42 and 54 months. The current project presents data from the initial screening; follow-up data will be presented in a subsequent article once data collection is complete.
Methods
This study was approved by the institutional review boards of the University of Connecticut (UConn) and Georgia State University (GSU).
Participants
Participants are children who participated in the large-scale M-CHAT screening studies conducted at UConn and GSU. The current sample includes low-risk participants who participated in previous studies13–16 and newly screened children at both sites. Children were screened with the M-CHAT between 16 and 30 months of age during a well-child visit at a participating pediatric site. Children were excluded if they were screened by an early intervention provider, screened as part of an autism sibling study, or if they were self-referred by their caregivers with autism-related concerns. Children were also excluded if they received an ASD diagnosis before being screened with the M-CHAT, if they had a severe sensory or motor disability (eg, blindness or deafness) that prevented them from completing study assessments, or if the child’s caregivers were not fluent in English or Spanish. If an excluded child presented with an “at risk” M-CHAT score, the child’s family and pediatrician were notified of the screening results and referrals were provided.
A total of 18 989 children were screened with the M-CHAT: 9088 at GSU and 9901 at UConn (see Table 1). The sample was evenly divided between boys and girls; there was no difference in the male to female ratio between sites (χ2 [1, N = 18 741] = 0.260; P = .610). Parents reported race and ethnicity for a subset of toddlers (UConn, n = 3574; GSU, n = 5469). Due to the relatively smaller numbers of nonwhite children, the samples were divided into white (not Hispanic/Latino) and all other races and ethnicities for comparison across sites. There was a statistically significant difference in the racial/ethnic composition between the 2 sites (GSU = 67.6% white; UConn = 69.6% white) (χ2 [1, N = 9043] = 4.13; P = .042), although the effect size was small (Φ = 0.02). The comparison of mean age at screening (GSU = 20.7 months; UConn = 20.1 months) was significant due to the large sample size (t [18 989] = 13.377; P < .001); however, the effect size was small (d = 0.19), and the difference (0.06 month) was not clinically meaningful.
TABLE 1.
Characteristics of Screening and Evaluation Samples
| GSU Screening Sample | UConn Screening Sample | P | d | Total Screening Sample | Total Evaluation Sample | |
|---|---|---|---|---|---|---|
| Sample size | 9088 | 9901 | 18 989 | 207 | ||
| Male, n (%) | 4561 (50.2) | 5040 (50.9) | .610 | — | 9601 (50.6) | 159 (76.8) |
| White (non-Hispanic), n (%)a | 3696 (67.6) | 2488 (69.6) | .042 | — | 6184 (68.4) | 128 (61.8) |
| Nonwhite, n (%)a | 1773 (32.4) | 1086(30.4) | .042 | — | 1186 (13.1) | 28 (13.5) |
| Age at screening, mo (SD) | 20.67 (3.12) | 20.07 (3.06) | <.001 | 0.19 | 20.4 (3.1) | — |
| Age at evaluation, mo (SD) | 25.35 (5.0) | 26.01 (4.17) | .304 | — | — | 25.75 (4. 51) |
Ethnicity percentages were calculated on the basis of cases with reported ethnicity data (n = 9043).
Screening Instrument
The M-CHAT was administered and scored by using previously published cutoffs. A positive screen was indicated by screening positive on 2 of 6 critical items or on 3 of 23 items overall on both the M-CHAT and M-CHAT/F.14 The M-CHAT and M-CHAT/F are available at www.mchatscreen.com.
Procedure
Screening Procedure
Pediatric offices were recruited by mailings to members of state AAP organizations. Participating offices agreed to offer the informed consent and M-CHAT in English or Spanish to all eligible families presenting for 18- and 24-month well-child visits. Office staff collected and mailed completed forms to the research office for scoring. Although not required by the study, many pediatric offices scored the M-CHAT independently and kept a copy in the child’s file.
Caregivers of screen-positive cases completed the M-CHAT/F, primarily over the telephone, with research assistants; cases that screened positive on the M-CHAT/F were offered free diagnostic evaluations. Families received compensation for time and travel.
Evaluation Procedure
Diagnostic evaluations were conducted by a licensed clinical psychologist or developmental pediatrician and a psychology doctoral student, in English or Spanish depending on the family’s preference. Evaluations included the Autism Diagnostic Observation Schedule,17 the Autism Diagnostic Interview-Revised or Toddler Edition,18 the Mullen Scales of Early Learning,19 the Vineland Adaptive Behavior Scales,20–21 and the Childhood Autism Rating Scale.22 If the diagnostic instruments disagreed, the discrepancy was resolved by clinical judgment.23 Diagnosis was made by clinical judgment of the licensed clinician. Evaluated children were given all appropriate Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), diagnoses; others were classified as having “developmental concerns” or as typically developing. The label of “developmental concerns” was given to children who did not meet criteria for any DSM-IV diagnosis but who presented with subthreshold characteristics of a diagnostic condition (eg, clinically significant social difficulties in the absence of an ASD diagnosis or clinically significant speech and language delays not meeting criteria for a DSM-IV language disorder). Families received oral and written feedback that included recommendations for intervention.
Ascertaining Missed Screen-Positive Cases
Potential missed cases were ascertained by (1) asking pediatricians to “red flag” children about whom they had autism-related concerns and (2) concurrently screening with a second instrument. Regardless of M-CHAT score, children were offered evaluations on the basis of a pediatrician’s red flag, a positive screen on the Yale Screener (previously under development and administered to 3570 UConn cases), or a positive screen on the Screening Tool for Autism in Two-Year-Olds or STAT24 (administered to 3 randomly selected GSU cases as pilot data for a new study).
Results
Screening Results
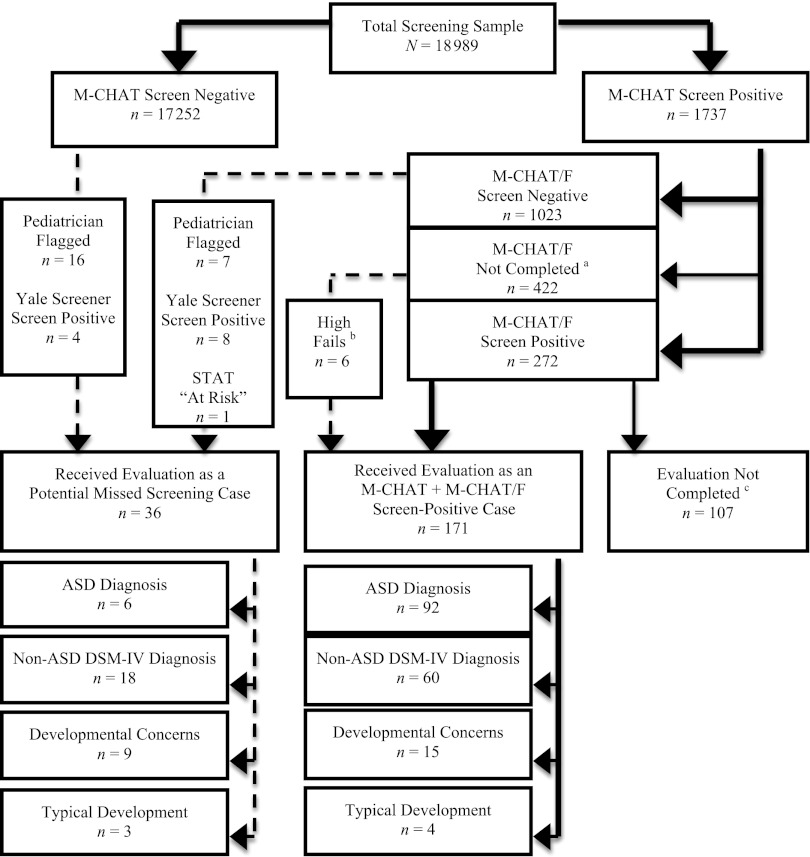
Of the 18 989 children screened with the M-CHAT, 9.1% screened positive and required the M-CHAT/F. Of the 1737 screen-positive cases, 74.6% completed the M-CHAT/F, 1023 children (79%) screened negative and required no additional follow-up, and 272 children (21%) continued to screen positive after the M-CHAT/F and were offered an evaluation. Of the eligible children, 60.7% attended the evaluation (see Fig 1).
FIGURE 1.
Study participation flowchart. The bold lines indicate the standard path of participation in the study; the dashed lines indicate ways in which children received evaluations as potential missed screening cases. aOf the 422 M-CHAT/F noncompleters, 271 were unable to be contacted for follow-up, 38 declined additional participation, 5 were excluded due to a language barrier, 6 received an evaluation without completing the M-CHAT/F due to failing a high number of items on the M-CHAT (indicating a high risk of ASD), and 122 cases had missing data. b“High Fails” refers to children who screened positive on ≥8 items on the M-CHAT screening questionnaire (indicating a high risk of ASD) and who were referred directly to evaluation, bypassing the M-CHAT/F. cOf the 107 children who did not complete an evaluation, 65 declined an evaluation or failed to show up for multiple appointments; 22 could not be contacted to schedule the evaluation; 7 were excluded due to a previous medical diagnosis or the presence of a severe neurologic, physical, visual, or hearing deficit that precluded the child’s ability to complete the standardized evaluation measures; and 13 cases had missing data. STAT, Screening Tool for Autism in Two-Year-Olds.
Evaluation Results
Children were evaluated on the basis of positive screens on the M-CHAT and M-CHAT/F (n = 171; of note, 6 of these children screened positive on a high number of items on the M-CHAT and were offered an evaluation without completing M-CHAT/F) or if they screened negative on the M-CHAT or M-CHAT/F but were identified as a potential missed case (n = 36). The sample was 76.8% male; the mean evaluation age was 25.75 months (SD = 4.51; see Table 1).
Of the 171 screen-positive cases, 92 (53.8%) were diagnosed with an ASD and all but 4 (97.7%) were identified as having either a DSM-IV diagnosis or “developmental concerns” and were referred for intervention (see Table 2). Of the 36 potential missed cases, 6 were diagnosed with ASD: 3 were missed by the M-CHAT and 3 were missed by the M-CHAT/F. Of the 36 potential missed cases, 23 were flagged by a pediatrician (5 ASD), 12 screened positive on the Yale Screener (0 ASD), and 1 child screened positive on the Screening Tool for Autism in Two-Year-Olds (1 ASD).
TABLE 2.
Diagnostic Breakdown of Evaluation Sample
| Diagnosis | Positive Screens on M-CHAT+M-CHAT/F (n = 171),a n (%) | Potential Missed Screening Cases (n = 36), n (%) | |
|---|---|---|---|
| All ASDs | |||
| Autistic disorder | 44 (25.7) | 1 (2.8) | |
| PDD-NOS | 48 (28.1) | 5 (13.9) | |
| All non-ASD disorders | |||
| Developmental delay | 37 (21.6) | 11 (30.6) | |
| Developmental language disorder | 18 (10.5) | 6 (16.7) | |
| Other DSM-IV diagnoses | 5 (2.9) | 1 (2.8) | |
| Developmental concerns | 15 (8.8) | 9 (25) | |
| Typical development | 4 (2.3) | 3 (8.3) | |
The 6 children who screened positive on a high number of items on the M-CHAT and were offered an evaluation without completing the M-CHAT/F are included in the positive screen sample.
M-CHAT Total and Critical Cutoff Scores
The M-CHAT has 2 cutoff scores that can be used to identify screen-positive cases; the total score cutoff requires a child to screen positive on ≥3 items, and the critical score cutoff requires a child to screen positive on ≥2 of 6 critical items determined to be the best discriminators of ASD and non-ASD on the basis of the first 600 children screened.13 The utility of these cutoffs was assessed in the sample of 92 children with ASD who screened positive on the M-CHAT and M-CHAT/F. Of the 92 children, 91 children (98.9%) exceeded the M-CHAT total score cutoff, whereas only 78 (84.8%) exceeded the critical score cutoff. Only 1 child screened positive on the critical score without also screening positive on the total score.
M-CHAT Cutoff Score for Clinical Referral
By using the subsample who completed the M-CHAT/F (n = 1295), M-CHAT total scores were cross-tabulated with the M-CHAT/F results to identify an M-CHAT cutoff for bypassing the M-CHAT/F for immediate referral for evaluation and intervention (see Table 3). By using a cutoff of M-CHAT total score ≥7, 82.2% continued to screen positive after the M-CHAT/F, whereas 87.4% of children with a score of 3 to 6 reverted to screen negative after follow-up.
TABLE 3.
Children with a Screen-Positive M-CHAT Score Who Continued to Screen Positive After M-CHAT/F
| M-CHAT Total Score | Continued to Screen Positive After M-CHAT/F (Evaluation Needed), n | Total, n | Percentage of Children Who Continued to Screen Positive After M-CHAT/F | |
|---|---|---|---|---|
| No | Yes | |||
| 2a | 12 | 4 | 16 | 25 |
| 3 | 609 | 38 | 647 | 5.9 |
| 4 | 241 | 38 | 279 | 13.6 |
| 5 | 92 | 26 | 118 | 22 |
| 6 | 41 | 37 | 78 | 47.4 |
| 7 | 9 | 23 | 32 | 71.9 |
| 8 | 10 | 22 | 32 | 68.8 |
| 9 | 2 | 18 | 20 | 90 |
| 10 | 2 | 14 | 16 | 87.5 |
| 11 | 3 | 14 | 17 | 82.4 |
| 12 | 1 | 9 | 10 | 90 |
| 13 | 0 | 10 | 10 | 100 |
| 14 | 0 | 6 | 6 | 100 |
| 15 | 0 | 6 | 6 | 100 |
| 16 | 0 | 1 | 1 | 100 |
| 17 | 1 | 2 | 3 | 66.7 |
| 18 | 0 | 3 | 3 | 100 |
| 19 | 0 | 1 | 1 | 100 |
| Total | 1023 | 272 | 1295 | |
Children in this sample who failed 2 M-CHAT items failed 2 of the 6 critical items. This result may be why the percentage of children who continued to screen positive after the M-CHAT/F (25%) is higher for the M-CHAT total score of 2.
Positive Predictive Value
The positive predictive value (PPV; true positives/all screen positives) of the M-CHAT was calculated on the basis of the diagnostic classification of children who completed evaluations, conservatively presuming non-ASD for those children who screened positive on the M-CHAT but then screened negative on the M-CHAT/F. The 6 “high fail” cases who bypassed the M-CHAT/F were combined with the M-CHAT and M-CHAT/F screen-positive cases in the calculation of the PPV.
Ninety-five children diagnosed with ASD screened positive on the M-CHAT, out of 1737 screen-positive cases, yielding a PPV of 0.06 (see Table 3). Completing the M-CHAT/F is essential for PPV (Robins, 200816); 92 children diagnosed with ASD screened positive on the M-CHAT and M-CHAT/F out of 171 screen-positive cases, yielding a PPV for identifying ASD of 0.54. Only 4 evaluated children were typically developing, yielding a PPV for identifying any developmental concerns of 0.98; results were similar across sites (see Table 4).
TABLE 4.
Positive Predictive Value of M-CHAT and M-CHAT/F
| ASD Diagnosis | Any DSM-IV Diagnosis | Any Diagnosis + Developmental Concerns | |
|---|---|---|---|
| M-CHAT only | |||
| GSU | 0.05 | 0.08 | 0.09 |
| UConn | 0.06 | 0.11 | 0.12 |
| Combined | 0.06 | 0.09 | 0.11 |
| M-CHAT + M-CHAT/F | |||
| GSU | 0.51 | 0.83 | 0.96 |
| UConn | 0.56 | 0.93 | 0.99 |
| Combined | 0.54 | 0.89 | 0.98 |
Discussion
The current study is one of the largest pediatrics-based autism screening studies to date. The 18 989 participating children were screened across 2 geographically diverse US regions, and the screening results and PPV rates were consistent across the 2 sites, establishing cross-validation.
Results indicate that ∼54% of children who screened positive on the M-CHAT and MCHAT/F presented with an ASD, and 98% presented with clinically significant developmental concerns warranting intervention; therefore, confidence that a positive screen on the M-CHAT and M-CHAT/F warrants immediate referrals for evaluation and intervention is very high. Although the M-CHAT does not detect ASD at the current 1 in 88 prevalence rate,2 it was expected that the prevalence of ASD in the current sample would be lower, given the young age of the sample, which largely precluded diagnoses of Asperger syndrome, and the fact that children already receiving early intervention and siblings of children with ASD (children who present with a higher likelihood of being diagnosed with an ASD) were excluded from the study. In addition, we anticipate that many of the ASD cases not evaluated in this study were identified by the M-CHAT screen but failed to complete full participation in the study, because not all families whose children initially screened positive participated in the clinical follow-up. Failure to follow up on screening results and complete a clinical evaluation remains a significant barrier to population screening. Despite this difficulty, the current study evaluated 60.6% of cases who screened positive on the M-CHAT and M-CHAT/F, which represents a higher evaluation participation rate than with other screening studies.25,26
This study provides several updates to simplify the clinical use of the M-CHAT. Assessment of M-CHAT cutoffs indicates that the M-CHAT total score cutoff (≥3) identifies the vast majority (98.9%) of screen-positive cases and suggests that the critical score does not improve the performance of the tool. For ease of scoring, it is recommended that only the M-CHAT total score cutoff be used to identify screen-positive cases.
Results indicate that children with an M-CHAT score of ≥7 can bypass the M-CHAT/F and be referred directly for evaluation and intervention, because 82.2% of children with a score of ≥7 continue to screen positive after the M-CHAT/F.
M-CHAT total scores of 3 to 6 require follow-up with the M-CHAT/F. The results of the current study support Robins’16 framing of the M-CHAT as a “two-step” screening instrument consisting of an initial screening questionnaire (step 1) and a follow-up of positive screens with the M-CHAT/F (step 2) for scores within this range. Currently, the use of the M-CHAT without the M-CHAT/F for scores in this range is not recommended in low-risk samples. Of note, <10% of a low-risk sample will require the second step of the screening, particularly if children with high scores (≥7) are referred immediately to evaluation and intervention. The M-CHAT/F is brief and is easily administered in a conversational format, which, in addition to reducing the false-positive rate of the M-CHAT, can serve to facilitate a clinical discussion about behaviors indicating possible ASD between parents and clinical providers.
Data from this study negate concerns about the potential negative impact of false-positive cases. Results indicate that 98% of M-CHAT and M-CHAT/F screen-positive cases presented with a diagnosable disorder or developmental concerns requiring a referral to early intervention, which indicates that following up with screen-positive cases is not an inefficient use of time or resources and will not alarm families unnecessarily. When discussing universal screening for ASD, it is important to acknowledge that it may not be feasible to develop a screening instrument with a high sensitivity for ASD that does not also identify children with other developmental delays due to symptom overlap between diagnoses and the heterogeneity of symptom presentation in ASD. Therefore, it is essential to examine the PPV for ASD and for all developmental delays or concerns.26
The best age for autism screening is an ongoing debate, and the AAP currently recommends autism-specific screening at both 18 and 24 months of age. This topic is beyond the scope of the current article; however, our research group is currently exploring this question in a separate project.
The largest limitation of the screening study is the possibility of missed screening cases. Due to resource limitations, the study was unable follow up with all screen-negative cases and as a result there was no way to detect all cases of ASD. In addition, it is important to note that screening negative on the M-CHAT does not rule out the possibility of other delays. Future research includes validation of an electronic version of the M-CHAT with automatic computerized scoring to simplify scoring for clinical providers.
Conclusions
The current study provides empirical support for the utility of population-level screening for ASD by using the M-CHAT in the primary care setting. Results suggest that the M-CHAT continues to be an effective screening instrument for ASD, particularly when the 2-step screening process is used. Screening with the M-CHAT has the potential to greatly reduce the age at diagnosis, facilitate early intervention, and optimize long-term prognosis; children screened with the M-CHAT in the current study were diagnosed >1 year earlier (mean age at diagnosis of 25 months) than the current median age at diagnosis in the United States.8 The M-CHAT is a widely used screening instrument in pediatric offices, and this update, which provides psychometrics and proper procedures for using the instrument, can improve screening at well-child visits at ages 18 and 24 months, which is consistent with the autism screening guidelines outlined by the AAP.
Supplementary Material
Acknowledgments
We thank all of the children and families who participated in our study. This study would not have been possible without the assistance of participating pediatricians and the staff in their offices, and we thank them for their participation and support. We thank Jillian Wood, Executive Director of the Hezekiah Beardsley Chapter of the AAP, and the Georgia chapter of the AAP for assistance in recruiting participating physicians’ offices and in promoting the study statewide. We gratefully acknowledge Ann Hazzard, PhD, for her work as liaison for Children’s HealthCare of Atlanta at Hughes Spalding and Thyde Dumont-Mathieu, MD, for her work as liaison for pediatric providers in the state of Connecticut. We also thank Dr Dumont-Mathieu for her substantial contributions to the day-to-day operation of the study and for her clinical and medical wisdom. We thank James Green, PhD, for sharing his statistical expertise with the project. We gratefully acknowledge the clinicians who have completed clinical evaluations for the project including Ann Hazzard, PhD, and Oscar Tanaka, PhD, at GSU and Thyde Dumont-Mathieu, MD, Sarah Hodgson, PhD, and Jamie Kleinman, PhD, at UConn. We sincerely thank all of the undergraduate and postbaccalaureate research assistants at UConn and GSU who have assisted with data collection and data entry over the years. This research would not have been possible without the significant contributions from the many graduate students and research coordinators who have worked on the M-CHAT project over the years and who have been invaluable in conducting all aspects of the study (see Appendix).
Glossary
- AAP
American Academy of Pediatrics
- ASD
autism spectrum disorder
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- GSU
Georgia State University
- M-CHAT
Modified Checklist for Autism in Toddlers
- M-CHAT/F
Modified Checklist for Autism in Toddlers Follow-Up
- PPV
positive predictive value
- UConn
University of Connecticut
Appendix
GSU graduate students: Sharlet Anderson, Nicolle Angeli, Bianca Brooks, Lama Farran, Julia Juechter, Meena Khowaja, Kayla Loggins, Natasha Ludwig, Kimberly Oliver, Vivian Piazza, and Lisa Wiggins.
GSU research coordinators: Marisa Arroyo, Karis Casagrande, AnnMarie Newman, and Jamie Zaj
UConn graduate students: Leandra Berry, Hilary C. Boorstein, Laura Brennan, Katelin Carr, Krista Dalbec-Mraz, Emma L. Esser, Lauren Haisley, Molly Helt, Lauren Herlihy, Alex Hinnebusch, Dasal Jashar, Jamie M. Kleinman, Kelley Knoch, Juhi Pandey, Michael A. Rosenthal, Joyce Suh, Saasha Sutera, Eva Troyb, Pamela E. Ventola, and Alyssa D. Verbalis.
UConn research coordinators: Katie DeYoe, Sarah Hardy, Ashley Maltempo, Courtney Manning, and Gail Marsha.
Footnotes
Dr Chlebowski participated in the collection of data, exported data, performed data validation and statistical analysis, participated in the interpretation of data, and prepared the manuscript; Dr Robins contributed to the conception and design of the study, collected a large subset of the data, participated in the interpretation of data, and contributed to the drafting and revision of the article; Dr Barton contributed to the conception and design of the study, participated in the interpretation of data, and contributed to the drafting and revision of the article; and Dr Fein, as principal investigator, obtained funding and supervised all stages of the study, contributed to the conception and design of the study, participated in the interpretation of data, and contributed to the drafting and revision of the article. All of the authors provided final approval of the submitted version of the manuscript.
FINANCIAL DISCLOSURE: Drs Robins, Fein, and Barton are co-owners of M-CHAT, LLC, which licenses use of the M-CHAT to companies/individuals that create and distribute electronic versions of the M-CHAT. Dr Robins receives royalties from the electronic M-CHAT, Dr Fein’s royalties are donated to the University of Connecticut Foundation, and Dr Barton’s royalties are donated to the University of Connecticut Psychological Services Clinic. However, all of the data reported in the current study were derived from the paper-and-pencil version of the M-CHAT, which is available free of charge for research, teaching, and clinical use. Dr Chlebowski has indicated she has no financial relationships relevant to this article to disclose.
FUNDING: Supported by National Institutes of Health (NIH) grant R01 HD039961, National Institute of Mental Health grant F31 MH12550-1-2, Maternal and Child Health Bureau grant R40 MC00270, a US Department of Education student-initiated research grant, a Centers for Disease Control and Prevention–Georgia State University seed grant in the social and behavioral sciences, and a grant from the National Association for Autism Research. Funded by the National Institutes of Health (NIH).
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000
- 2.Centers for Disease Control and Prevention Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61:1–19 [PubMed] [Google Scholar]
- 3.Filipek PA, Accardo PJ, Baranek GT, et al. The screening and diagnosis of autistic spectrum disorders. J Autism Dev Disord. 1999;29(6):439–484 [DOI] [PubMed] [Google Scholar]
- 4.Harris SL, Handleman JS. Age and IQ at intake as predictors of placement for young children with autism: a four- to six-year follow-up. J Autism Dev Disord. 2000;30(2):137–142 [DOI] [PubMed] [Google Scholar]
- 5.Howard JS, Sparkman CR, Cohen HG, Green G, Stanislaw H. A comparison of intensive behavior analytic and eclectic treatments for young children with autism. Res Dev Disabil. 2005;26(4):359–383 [DOI] [PubMed] [Google Scholar]
- 6.Myers SM, Johnson CP, American Academy of Pediatrics Council on Children With Disabilities . Management of children with autism spectrum disorders. Pediatrics. 2007;120(5):1162–1182 [DOI] [PubMed] [Google Scholar]
- 7.Sallows GO, Graupner TD. Intensive behavioral treatment for children with autism: four-year outcome and predictors. Am J Ment Retard. 2005;110(6):417–438 [DOI] [PubMed] [Google Scholar]
- 8.Fountain C, King MD, Bearman PS. Age of diagnosis for autism: individual and community factors across 10 birth cohorts. J Epidemiol Community Health. 2011;65(6):503–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandell DS, Morales KH, Xie M, Lawer LJ, Stahmer AC, Marcus SC. Age of diagnosis among Medicaid-enrolled children with autism, 2001-2004. Psychiatr Serv. 2010;61(8):822–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson CP, Myers SM, American Academy of Pediatrics Council on Children With Disabilities . Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120(5):1183–1215 [DOI] [PubMed] [Google Scholar]
- 11.Robins DL, Fein D, Barton M. The Modified Checklist for Autism in Toddlers (M-CHAT). Self-published; 1999 [Google Scholar]
- 12.Robins DL, Fein D, Barton M. Modified Checklist for Autism in Toddlers Follow Up (M-CHAT/F). Self-published; 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robins DL, Fein D, Barton ML, Green JA. The Modified Checklist for Autism in Toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. J Autism Dev Disord. 2001;31(2):131–144 [DOI] [PubMed] [Google Scholar]
- 14.Kleinman JM, Robins DL, Ventola PE, et al. The modified checklist for autism in toddlers: a follow-up study investigating the early detection of autism spectrum disorders. J Autism Dev Disord. 2008;38(5):827–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey J, Verbalis A, Robins DL, et al. Screening for autism in older and younger toddlers with the Modified Checklist for Autism in Toddlers. Autism. 2008;12(5):513–535 [DOI] [PubMed] [Google Scholar]
- 16.Robins DL. Screening for autism spectrum disorders in primary care settings. Autism. 2008;12(5):537–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223 [PubMed] [Google Scholar]
- 18.Lord C, Pickles A, McLennan J, et al. Diagnosing autism: analyses of data from the Autism Diagnostic Interview. J Autism Dev Disord. 1997;27(5):501–517 [DOI] [PubMed] [Google Scholar]
- 19.Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service Inc; 1995 [Google Scholar]
- 20.Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales: Survey Interview Form/Caregiver Rating Form. Circle Pines, MN: American Guidance Service Inc; 1984 [Google Scholar]
- 21.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2nd ed. (Vineland II), Survey Interview Form/Caregiver Rating Form. Livonia, MN: Pearson Assessments; 2005. [Google Scholar]
- 22.Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). J Autism Dev Disord. 1980;10(1):91–103 [DOI] [PubMed] [Google Scholar]
- 23.Filipek PA, Accardo PJ, Ashwal S, et al. Practice parameter: screening and diagnosis of autism: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology. 2000;55(4):468–479 [DOI] [PubMed] [Google Scholar]
- 24.Stone WL, Coonrod EE, Ousley OY. Brief report: screening tool for autism in two-year-olds (STAT): development and preliminary data. J Autism Dev Disord. 2000;30(6):607–612 [DOI] [PubMed] [Google Scholar]
- 25.Dietz C, Swinkels S, van Daalen E, van Engeland H, Buitelaar JK. Screening for autistic spectrum disorder in children aged 14-15 months. II: population screening with the Early Screening of Autistic Traits Questionnaire (ESAT). Design and general findings. J Autism Dev Disord. 2006;36(6):713–722 [DOI] [PubMed] [Google Scholar]
- 26.Pierce K, Carter C, Gallagher N, et al. Detecting, studying, and treating autism early: the one-year well-baby check-up approach. J Pediatr. 2011;159(3):458–465e6 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.