Abstract
BACKGROUND AND OBJECTIVE:
Lipid levels are linked to early atherosclerosis. Risk stratification may be improved by using triglyceride to high-density lipoprotein cholesterol ratio (TG/HDL-C), which relates to arterial stiffness in adults. We tested whether TG/HDL-C was an independent predictor of arterial stiffness in youth.
METHODS:
Subjects 10 to 26 years old (mean 18.9 years, 39% male, 56% non-Caucasian, n = 893) had laboratory, anthropometric, blood pressure, and arterial stiffness data collected (brachial distensibility, augmentation index, carotid-femoral pulse-wave velocity). Subjects were stratified into tertiles of TG/HDL-C (low, n = 227; mid, n = 288; high, n = 379).
RESULTS:
There was a progressive rise in cardiovascular (CV) risk factors and arterial stiffness across TG/HDL-C ratio. The high TG/HDL-C ratio group had the stiffest vessels (all P < .03 by analysis of variance). TG/HDL-C as a continuous variable was an independent determinant of brachial distensibility in CV risk factor adjusted model and for carotid-femoral pulse-wave velocity in obese subjects, with trend for higher augmentation index.
CONCLUSIONS:
TG/HDL-C, an estimate of small, dense low-density lipoprotein cholesterol, is an independent determinant of arterial stiffness in adolescents and young adults, especially in obese youth. These data suggest that use of TG/HDL-C may be helpful in identifying young adults requiring aggressive intervention to prevent atherosclerotic CV diseases.
Keywords: arterial stiffness, dyslipidemia, pediatrics
What’s Known on This Subject:
The triglyceride to high-density lipoprotein cholesterol ratio (TG/HDL-C) estimates atherogenic small, dense low-density lipoprotein cholesterol and predicts arterial stiffness and hard cardiovascular events in adults. Whether TG/HDL-C predicts intermediate noninvasive end points (arterial stiffness) in youth is not known.
What This Study Adds:
This study is the first to document stiffer vessels in youth with higher cardiovascular risk factor–adjusted TG/HDL-C, with the effect especially strong in obese subjects. Evaluating TG/HDL-C may be helpful in identifying young subjects at risk for obesity-related atherosclerosis.
Cholesterol levels were first identified as a cardiovascular (CV) risk factor in 1959 in the Framingham Heart Study.1 Since then, lipid parameters have been linked to early atherosclerosis on autopsy,2 increased carotid intima-media thickness,3 and arterial stiffness.4 Risk stratification may be improved by testing for apolipoprotein B5 or use of lipid ratios, such as non–high-density lipoprotein (HDL)-cholesterol. Triglyceride (TG) to HDL-cholesterol ratio (TG/HDL-C) may be a better predictor of small, dense low-density lipoprotein (LDL),6 an atherogenic lipoprotein particle that strongly predicts coronary heart disease.7 In adults with low total LDL cholesterol (LDL-C), higher TG and lower HDL-cholesterol (HDL-C) were found to predict coronary heart disease.8 Data on adults show a relationship among TG, HDL-C, and arterial stiffness.9,10 TG/HDL-C does correlate to LDL particle size in youth,11 but correlations to vascular damage are lacking. Therefore, we explored TG/HDL-C as an independent predictor of arterial stiffness in children, adolescents, and young adults.
Methods
Study Population
Analyses were performed on pooled data from 2 studies from Cincinnati by using the same techniques.12,13 One compared CV structure in children, adolescents, and young adults with type 2 diabetes mellitus (T2DM) with nondiabetic controls (total n = 784, subjects included in these analyses, n = 513).12 The other was a longitudinal school-based study exploring development of insulin resistance in healthy adolescents and young adults (total n = 439, subjects included in these analyses, n = 380).13 Subjects with diabetes, pregnant female subjects, and individuals with chronic disease or taking medication affecting carbohydrate metabolism were excluded from both studies. All subjects had nondiabetic level fasting glucose (school study and lean subjects from T2DM study) or normal oral glucose tolerance (obese subjects from T2DM study) test per American Diabetes Association guidelines.14 Written informed consent was obtained from subjects ≥18 years old or the parent or guardian if <18 years old. Written assent was obtained for subjects <18 years old according to the institutional review board at Cincinnati Children’s Hospital.
Data Collection
After a 10-hour fast, participants had questionnaire, anthropometric, blood pressure (BP), laboratory, and arterial stiffness data collected. Trained personnel obtained height using a calibrated stadiometer (Veeder-Rood, Elizabethtown, NC; RoadRod, Quick Medical, North Bend, WA; or Accustat, Genentech, South San Francisco, CA). Weight was measured by using a calibrated digital scale (Health-O-Meter; Sunbeam Products, Boca Raton, FL or SECA 770; SECA, Hanover, MD). The averages of 2 height and weight measurements were used. BMI was calculated as kg/m2. The average of 3 resting measures of systolic BP, diastolic BP (DBP), and mean arterial pressure (MAP) were obtained with a validated oscillometric device (DynaPulse Pathway; PulseMetric, Inc, San Diego, CA) by using the method of pulse wave form analysis.15
The same sample processing and laboratory (Cincinnati Children’s Hospital laboratory) were used for both studies. Fasting plasma glucose was measured by using a Hitachi model 704 glucose analyzer (Roche Hitachi, Indianapolis, IN) with intra-assay and inter-assay coefficients of variation of 1.2% and 1.6%.16 Plasma insulin was measured by radioimmunoassay using an anti-insulin serum raised in guinea pigs, 125I-labeled insulin (Linco, St. Louis, MO), and a double antibody method to separate bound from free tracer with a sensitivity of 2 pmol and intra- and interassay coefficients of variation of 5% and 8%.17 High-sensitivity C-reactive protein (CRP) was measured by using a high-sensitivity enzyme-linked immunosorbent assay.18 Lipid profiles were performed at a National Heart, Lung, and Blood Institute–Centers for Disease Control and Prevention standardized laboratory. LDL-C was calculated by using the Friedewald equation.19 TG/HDL-C was calculated. Subjects were stratified by TG/HDL-C (low, n = 227; mid, n = 288; high, n = 379) based on the race-specific tertiles for lean subjects with BMI <85th percentile (whites: low = 0.83 to <1.28, mid = 1.28 to <1.92, high = > 1.92; blacks: low = 0.78 to <0.90, mid = 0.90 to <1.40, high = > 1.40).
Arterial Stiffness Measurements
The averages of 3 recordings of vascular function collected after 5 minutes of rest were used. Brachial artery distensibility (BrachD) was measured by a DynaPulse Pathway instrument (Pulse Metric, Inc., San Diego, CA).20 This device derives brachial artery pressure curves from arterial pressure signals obtained from a standard cuff sphygmomanometer assuming a straight tube brachial artery and T-tube aortic system.20 Our coefficients of variability were <9%.12
A SphygmoCor SCOR-PVx System (Atcor Medical, Sydney, Australia) was used for heart rate (HR), carotid-femoral pulse wave velocity (PWV), and augmentation index (AIx), an arterial stiffness measure that measures wave reflections.21 Electrocardiogram-gated pressure wave data were obtained with a tonometer placed on the artery. PWV is the difference in carotid-to-femoral path length (measured directly) divided by the difference in the electrocardiogram R-wave to foot of the pressure wave. For AIx, the pressure waves were calibrated by using MAP and DBP obtained in the same arm. A validated generalized transfer function was used to calculate central aortic pressure and AIx.22 AIx was adjusted to an HR of 75 beats per minute. Coefficients of variability are <7% for PWV and intraclass correlation coefficients are 0.7 to 0.9 for AIx.12
Statistical Analysis
Analyses were performed with Statistical Analyses Software (SAS, version 9.2, SAS, Inc., Cary, NC). Average values for demographic, anthropometric, and laboratory data were obtained by TG/HDL-C. One-way analysis of variance was performed (χ2 analyses for categorical variables) to determine if differences in mean levels of variables existed by TG/HDL-C tertiles. Least square means were calculated to adjust for differences in sample size among tertiles to allow for category-by-category comparisons in mean levels. Variance stabilizing measures were performed as needed. General linear models were constructed by using significant covariates from correlation analyses to determine if TG/HDL-C was an independent determinate of arterial stiffness. The full model contained TG/HDL-C, age, demographics, anthropometrics, MAP, HR (except for AIx), LDL-C, CRP, glucose, and insulin. Height was added for AIx to control for distance of wave reflection sites from the heart related to height. Significance of each covariate was assessed. Nonsignificant terms were removed until remaining covariates or interactions (effect modifiers) were significant (P < .05).
Results
The population consisted of 893 subjects aged 10 to 26 years (mean 18.9 years, 39% male, 56% non-Caucasian, mostly African American). TG/HDL-C tertile groups did not differ by age but there were fewer non-Caucasian subjects in the low tertile and more male subjects in the high tertile (Table 1). There was a progressive increase in BMI and BMI z-score (data not shown) across TG/HDL-C tertiles, with similar patterns for BP, LDL-C, glucose, insulin, and CRP. The prevalence of obesity was high (37.6% with BMI ≥95th% for age and sex). Prevalence of hypertension was 5.3% for systolic BP and 3.0% for DBP. Elevated LDL-C (≥160 mg/dL) was present in 1.6% of subjects, 42.0% had elevated TG/HDL-C, and 16.5% had strong family history of CV disease (data not shown). Elevated CRP (≥1 mg/L) was found in 4.6% of lean (BMI <85th%) and 30% of obese subjects (BMI ≥95th%). Impaired fasting glucose (≥100 mg/dL) was found in 1.8% of lean and 5.6% of obese subjects. Defining abnormal arterial stiffness as >90th% for AIx and PWV or <10th% for BrachD for our healthy lean subjects, 33% had an abnormality in 1 measure (11% high Aix; 32% low BrachD; 24% high PWV), 13% had abnormality in 2 measures, and 3% had abnormality in all 3 measures. The prevalence of abnormalities in BrachD and PWV were significantly higher in the high TG/HDL-C tertile (P for χ2 <.0001 for both).
TABLE 1.
CV Risk Factors and Arterial Stiffness Parameters Stratified by Tertiles of TG/HDL-C
| Variable | Low (n = 227) | Mid (n = 288) | High (n = 378) | ANOVAa |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age, y | 18.7 (3.5) | 19.0 (3.1) | 19.0 (3.2) | NS |
| Race, n (% non-Caucasian) | 107 (47.1) | 169 (58.7) | 220 (58.2) | L<M & H |
| Gender, n (% male) | 78 (34.4) | 102 (35.4) | 165 (43.7) | L & M>H |
| Height, cm | 167.1 (10.5) | 167.8 (10.2) | 168.8 (10.3) | NS |
| Weight, kg | 65.1 (17.7) | 78.7 (26.0) | 90.9 (26.9) | L<M<H |
| BMI, kg/m2 | 23.2 (5.6) | 27.8 (8.6) | 31.9 (9.2) | L<M<H |
| Systolic BP, mm Hg | 110.1 (12.4) | 112.3 (11.6) | 116.2 (12.4) | L<M<H |
| DBP, mm Hg | 63.9 (11.6) | 65.4 (11.6) | 67.7 (11.0) | L & M<H |
| MAP, mm Hg | 79.1 (7.6) | 81.4 (7.9) | 83.7 (8.8) | L<M<H |
| HR, beats/min | 62.5 (9.6) | 64.1 (10.1) | 64.4 (10.0) | L<H |
| Total cholesterol, mg/dL | 156.0 (30.0) | 162.7 (30.2) | 171.8 (31.9) | L<M<H |
| LDL-C, mg/dL | 82.4 (23.1) | 94.4 (25.0) | 104.0 (28.0) | L<M<H |
| HDL-C, mg/dL | 63.6 (13.9) | 54.1 (10.3) | 44.9 (8.5) | L>M>H |
| TG | 50.4 (14.1) | 70.7 (18.7) | 115.8 (50.7) | L<M<H |
| TG/HDL-C | 0.8 (0.2) | 1.3 (0.3) | 2.7 (1.6) | L<M<H |
| Glucose, mg/dL | 87.4 (6.1) | 89.5 (7.0) | 90.9 (7.3) | L<M<H |
| Insulin, µU/mL | 12.0(6.1) | 15.4 (8.6) | 19.7 (12.1) | L<M<H |
| High-sensitivity CRP, mg/L | 1.02 (1.35) | 1.87 (2.13) | 2.57 (2.60) | L<M<H |
| AIx, % | −1.16 (12.11) | 0.60 (11.04) | 1.72 (11.11) | L<H |
| BrachD, %/mm Hg | 6.76 (1.23) | 6.37 (1.26) | 5.89 (1.23) | L>M>H |
| PWV, m/s | 5.60 (0.90) | 5.84 (0.87) | 6.13 (1.09) | L<M<H |
ANOVA, analysis of variance.
All model P for χ2 or ANOVA ≤.0001; all P for comparison of least square means ≤.03; L = low, M = mid, H = high TG/HDL-C tertile.
When stratified by tertiles (Table 1), arterial stiffness increased across TG/HDL-C tertile groups with significantly higher PWV and lower BrachD. For AIx, only the high and low tertiles differed. There are no published normal values for TG/HDL-C; however, using lipid tables from the National Institutes of Health Pediatric CV Risk Reduction Initiative23 to calculate TG/HDL-C for youth 10 to 19 years old produces low, mid, and high levels of ≤2, >2 to <3.25, and ≥3.25. Data from the Healthy study, a slightly younger cohort (n = 6365, age 11.8 years), would estimate high TG/HDL-C as 3.14.24 Most of our subjects had TG/HDL-C below the high-tertile cut point from these studies, suggesting that vascular change may be occurring at much lower levels than expected based on the normative distribution for TG/HDL-C in American youth.
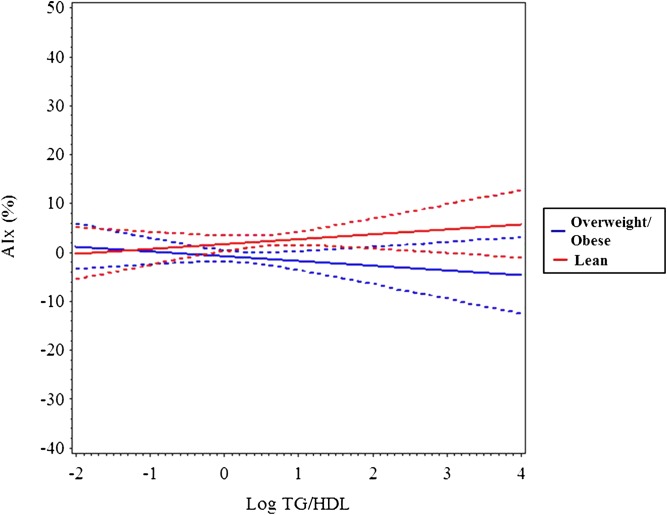
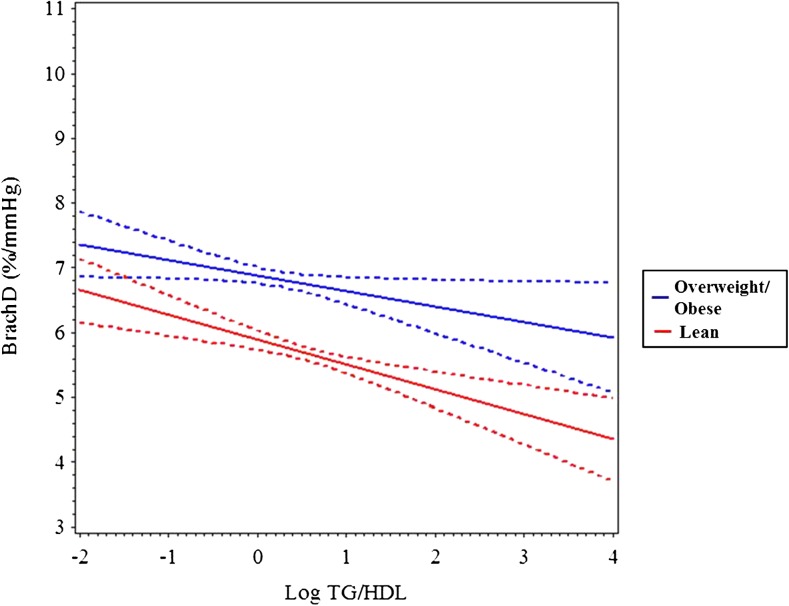
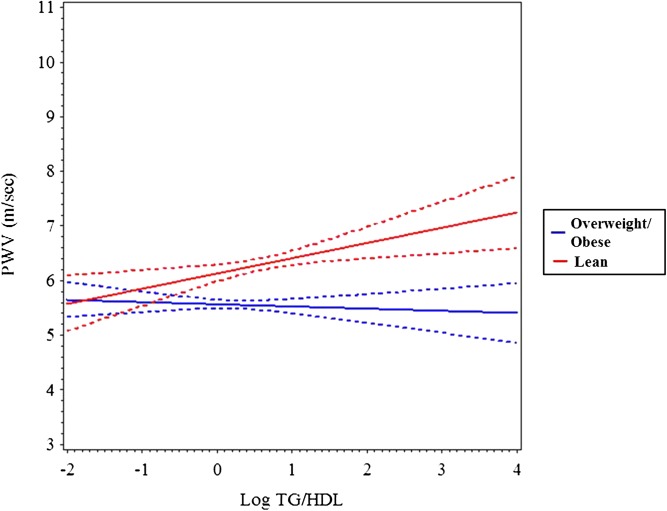
In multivariable analyses, TG/HDL-C as a continuous variable was not an independent predictor of AIx (Table 2), but using TG/HDL-C tertile as a categorical variable produced a trend for higher AIx in the high versus low tertiles (P ≤ .059, data not shown). AIx was influenced by age, female gender, non-white race, MAP, and fasting glucose (R2 = 0.23, P ≤ .0001). TG/HDL-C was a significant independent determinant of BrachD (R2 = 0.36, P ≤ .001) and PWV (R2 = 0.48, P ≤ .001) even after adjustment for demographics, MAP, and HR. This was strongest in overweight and obese subjects (TG/HDL-C by BMI z-score interaction was significant). To better visualize the interaction, we stratified by BMI z-score (lean ≤1 or overweight/obese >1). Plotting the regression (Figs 1, 2, and 3) showed no relationship between AIx and TG/HDL-C but a decline in BrachD for all subjects with the increase in PWV evident predominantly in the nonlean subjects.
TABLE 2.
Multivariable Models for Independent Determinants of Arterial Stiffness (Beta-Coefficients [SE] and Total Variance Explained)
| Variable | AIx | BrachD | PWV |
|---|---|---|---|
| (higher = stiffer) | (lower = stiffer) | (higher = stiffer) | |
| Intercept | −43.5 (22.3) | 2.16 (0.68) | −6.38 (0.30) |
| log TG/HDL-C | −0.018 (0.014)* | −0.46 (0.060)* | |
| Age | 0.39 (0.12) | 0.12 (0.0082) | |
| Female | 4.25 (0.89) | 0.087 (0.012) | |
| Non-white race | 1.39 (0.72) | 0.29 (0.053) | |
| Height | −0.32 (0.044) | ||
| BMI z-score | −0.070 (0.0065) | 0.16 (0.028) | |
| Log TG/HDL-C * BMI z-score | −0.018 (0.0086) | 0.1 (0.037) | |
| MAP | 0.045 (0.045) | 0.0035 (0.0079) | 0.034 (0.0036) |
| HR | −0.018 (0.00063) | 0.01 (0.0026) | |
| Fasting glucose | 11.73 (4.64) | ||
| R2 | 0.23 | 0.36 | 0.48 |
BrachD and PWV transformed to normal distribution. All models have P ≤ .0001 and unless indicated with asterisk, all parameters have P ≤ .05.
FIGURE 1.
Mean AIx (solid line) and 95% confidence interval (dashed lines) by log TG/HDL-C stratified by BMI z-score group (lean = black, overweight/obese = gray). There is no significant increase across TG/HDL-C ratio regardless of BMI.
FIGURE 2.
Mean BrachD (solid line) and 95% confidence interval (dashed lines) by log TG/HDL-C stratified by BMI z-score group (lean = black, overweight/obese = gray). The decline in BrachD by TG/HDL-C is steeper in the obese group (P ≤ .04).
FIGURE 3.
Mean PWV (solid line) and 95% confidence interval (dashed lines) by log TG/HDL-C stratified by BMI z-score group (lean = black, overweight/obese = gray). The increase in PWV by TG/HDL-C is higher in the obese group (P ≤ .006).
Analyses were repeated by using TG or HDL-C alone (data not shown). There were no differences in the results for AIx, as no lipid variables were independent determinants. Use of TG alone failed to reveal a difference between the mid- and high-tertile groups for BrachD and PWV by analysis of variance, as was seen using TG/HDL-C (Table 1). HDL alone failed to delineate between mid- and high-tertile group for PWV but results for BrachD were similar for TG/HDL-C (low > mid > high). Multivariable analyses with HDL-C alone explained slightly less of the variance in BrachD (R2 0.363 vs 0.364), suggesting TG/HDL-C may be a more robust lipid variable for explaining differences in arterial stiffness.
Discussion
We show that TG/HDL-C is a significant determinant of arterial distensibility (BrachD) and pulse propagation (PWV) in apparently healthy adolescents and young adults. There is a trend for influence on measures incorporating wave reflections (AIx). TG/HDL-C may be especially helpful in predicting increased arterial stiffness in young individuals who have developed obesity. These data suggest that using the TG/HDL-C may be helpful for identifying young adults requiring aggressive intervention to prevent atherosclerotic CV diseases.
In adults, small, dense LDL25 and TG/HDL-C26 predicted burden of coronary artery disease found on cardiac catheterization. Furthermore, higher TG/HDL-C was associated with abnormal HR recovery in a large cohort of 4963 healthy adults and presence of both of these abnormalities predicted increased mortality.27 Small, dense LDL also predicted preclinical change as measured by carotid intima-media thickness independently of CV risk factors,28,29 whereas TG/HDL-C independently predicted carotid intima-media thickness progression.30 No other studies specifically examined the relationship between TG/HDL-C and arterial stiffness; however, some publications examined related lipid measures. Non–HDL-C was also found to be correlated with leg arterial compliance but only in healthy women, not men.31 In addition, TG, total cholesterol, and LDL-C were found to be correlated with PWV, but none were independent predictors in a study of hypertensive adults.10 Furthermore, a small case-control study found higher PWV in subjects with metabolic syndrome, which is defined by a high TG and low HDL-C phenotype,32 and HDL-C remained an independent predictor of PWV after correction for other CV risk factors.9 For AIx, 1 investigator found a relationship between higher TG and AIx, but this relationship was independent only in men.33 Another small study found no relationship between low HDL-C and AIx in healthy adults,34 but a larger cohort demonstrated that low HDL-C in youth correlated with AIx measured 18 years later, albeit in a high-risk population with type 1 diabetes.35 Clearly, more study is needed to determine the importance of TG/HDL-C as a reflection of small, dense LDL in determining arterial health, as the atherogenicity of TG (TG was associated with coronary heart disease)36and the cardioprotective effects of HDL-C (low HDL-C predicted incident heart disease)8 may also play a role in the utility of using the ratio to predict vascular health.
Similar to the gaps in knowledge found in the literature regarding adults, few data are available relating TG/HDL-C to vascular measures in youth. The Pathobiological Determinants of Atherosclerosis in Youth study demonstrated a relationship between low HDL-C and extent of fatty streaks and raised lesions in the thoracic and abdominal aorta and the right coronary artery.2 Low HDL-C measured in youth also predicted thicker carotid intima-media thickness in adulthood in a combined longitudinal cohort of children from the Young Finns study, Bogalusa Heart Study, and Childhood Determinants study from Australia.37 The Bogalusa Heart Study also found that elevated non–HDL-C and total/HDL-C cholesterol ratio measured at age 5 to 17 years predicted thicker carotid intima-media thickness 16 to 19 years later with odds ratios of 2.60 and 1.78 respectively.38 Recent data from the Young Finns study found apolipoprotein B and non–HDL-C in young adulthood predicted PWV 6 years later.39 Similarly, 2 small studies of obese youth found that TG was an independent predictor of carotid thickness,40,41 and a larger community-based study of healthy youth found low HDL-C was an independent predictor,42 especially if the metabolic syndrome was present.43 A few studies have examined the relationship of PWV to TG and HDL-C but the only study examining the ratio included only cardiac (left ventricular mass)44 and not vascular outcomes. Interestingly, a relationship between TG/HDL-C and left ventricular mass was found to be independent of other CV risk factors.44 None of the pediatric studies examining vascular measures performed multivariable analysis to determine the independent contribution of these lipid parameters to arterial stiffness. Three found higher PWV in subjects with higher TG and lower HDL-C levels.45–47 One found higher PWV only with higher TG but not lower HDL-C,48 and 1 found no relation between PWV and TG.49 Other studies examined either carotid PWV,50 or brachial-ankle PWV,51,52 parameters that differ substantially from carotid-femoral PWV measured in this study. One study also found high TG and low HDL-C correlated with AIx,46 whereas another found low HDL-C but not high TG correlated with AIx.48 The only study in youth that examined BrachD found no relationship with TG or HDL-C; however, the authors used a wall-tracker technique that differs from the device used in this study.53 Our data are the first to systematically study TG/HDL-C in relation to 3 distinct measures of arterial stiffness in a healthy adolescent and young adult population. Our data also suggest that measures of distensibility (BrachD) and pulse propagation (PWV) may be more effective in identifying target organ damage related to dyslipidemia than measures of wave reflection (AIx).
The usefulness of a marker of CV risk can be demonstrated by reduction in risk with modification of the risk factor. Although no studies have directly linked reduction in small, dense LDL to improvement in arterial stiffness, inferences from the existing literature can be made. For instance, LDL particle size was improved with treatment with statins.54 Although LDL particle size was not measured, a meta-analysis of the effect of statin therapy on PWV found improvement in arterial stiffness in most studies.55 Conversely, 1 study used a treatment that was effective in lowering TG level but did not change LDL particle size and no change in distensibility was found.56 Data in children are even more sparse, but in 1 study, diet and exercise were shown to drop TG levels in children followed over 2 years,57 although no assessment of vascular function was performed. When a statin was used to lower TG and raise HDL-C levels in children with familial hypercholesterolemia, an increase in brachial flow-mediated dilation, a measure of endothelial function, was seen,58 whereas a study of the use of plant stanols, which did not change TG or HDL-C levels, failed to show improved flow-mediated dilation in children with familial hypercholesterolemia.59 To date, no investigations have studied diet or drug therapy for TG and HDL-C by using arterial stiffness as an end point in children.
Our data linking TG/HDL-C to vascular target organ damage in children are limited by our cross-sectional design. A longitudinal study design could provide a more robust argument that worsening of arterial stiffness over time is related to small, dense LDL as estimated by TG/HDL. Furthermore, an interventional study demonstrating improvement in arterial stiffness with treatment of elevated TG/HDL-C in both adults and children would provide evidence for the effect of this lipid pattern on arterial stiffness. Pooling data from 2 different studies was performed to increase the power to detect differences in this young cohort with a limited lifetime exposure to dyslipidemia. This approach may lead to selection bias, however, as 1 study recruited students from a school-based population and 1 included targeted diabetic youth and matched them to obese and lean controls. However, obesity, insulin resistance, and diabetes are increasing in prevalence in the United States, and these are the populations most likely to demonstrate the abnormal TG/HDL-C pattern60 and, conversely, youth with high TG/HDL-C are more likely to be insulin resistant.61,62 Therefore, we do believe our analyses are relevant. Another limitation is that these data may have limited clinical applicability, as many pediatric practitioners do not have access to the types of devices used in our study to measure arterial stiffness; however, increasingly, large pediatric hospitals are acquiring expertise with these measures and more normative data are being published. Our cohort was predominantly female and African American because of the race and gender breakdown of the parent studies from which subjects were recruited. This may limit generalizability to other populations, although we did correct for race and gender in our multivariable models. Also of note is that the relationship between TG/HDL-C and insulin resistance, another risk factor for increased arterial stiffness in youth,13 may differ by race in obese youth.61 Another limitation is that we were not able to measure other lipoprotein parameters that could contribute to increased vascular stiffness or for which TG/HDL-C might be a marker, such as small, dense LDL, Lipoprotein(a), oxidized LDL, lipid peroxidation products, and lipoprotein-associated phospholipase-A2. Furthermore, although TG/HDL-C is related to small, dense LDL,6 there is controversy as to whether increased arterial stiffness is related directly to the level of small, dense LDL, or if it is more closely associated with elevated levels of TG-rich lipoprotein remnants, elevated LDL particle numbers,63 or the obesity-related inflammation seen in the metabolic syndrome.
Conclusions
The report of the National Institutes of Health–appointed expert panel on Integrated Guidelines for CV Health and Risk Reduction in Children and Adolescents has confirmed the importance of screening for dyslipidemias in youth, including hypertriglyceridemia.23 However, this comprehensive review of the evidence identified many gaps in the knowledge base, especially with regard to the utility of targeting specific lipid abnormalities in an effort to improve intermediate markers of future CV disease. Whether TG/HDL-C provides incremental data compared with plasma TG alone is still controversial,64 but it is easier to perform than advanced lipid testing and may prove useful in identifying subjects at high risk for accelerated vascular aging who need more aggressive, early therapy to improve long-term CV outcome. Future research should also focus on the usefulness of noninvasive measures of arterial stiffness in stratifying CV risk in young patients.
Acknowledgments
We acknowledge the work of the entire CV Aging Study and Princeton Sub-Study teams. We also thank the participants of these studies and their families.
Glossary
- AIx
augmentation index
- BP
blood pressure
- BrachD
brachial artery distensibility
- CRP
high-sensitivity C-reactive protein
- CV
cardiovascular
- DBP
diastolic BP
- HDL-C
high-density lipoprotein cholesterol
- HR
heart rate
- LDL-C
low-density lipoprotein cholesterol
- MAP
mean arterial pressure
- PWV
carotid-femoral pulse wave velocity
- T2DM
type 2 diabetes mellitus
- TG
triglycerides
- TG/HDL-C
triglyceride to HDL-cholesterol ratio
Footnotes
Dr Urbina contributed to the design of the study, acquisition of data, analyses, interpretation, and drafting the article; Mr Khoury contributed to the design of the study, analyses, and drafting the article; Ms McCoy contributed to the acquisition of data, and drafting the article; Drs Dolan, Daniels, and Kimball contributed to the design of the study, interpretation of data, and drafting the article; and all authors approved the final version of the manuscript.
FINANCIAL DISCLOSURE: Dr Daniels has a consultancy with Merck, a company that manufactures lipid-lowering agents; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by National Institutes of Health (NIH) (National Heart, Lung, and Blood Institute) grant R01 HL076269 (Cardiovascular Disease in Adolescents with Type 2 Diabetes) and by NIH grants K23 HL080447-01A1 (Mechanisms of Vascular Dysfunction in Obesity and the Metabolic Syndrome) and in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through grant 8 UL1 TR000077-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. Funded by the National Institutes of Health (NIH).
References
- 1.Dawber TRKW, Kannel WB, Revotskie N, Stokes J, III, Kagan A, Gordon T. Some factors associated with the development of coronary heart disease: six years’ follow-up experience in the Framingham study. Am J Public Health Nations Health. 1959;49:1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rainwater DL, McMahan CA, Malcom GT, et al. The PDAY Research Group . Lipid and apolipoprotein predictors of atherosclerosis in youth: apolipoprotein concentrations do not materially improve prediction of arterial lesions in PDAY subjects. Arterioscler Thromb Vasc Biol. 1999;19(3):753–761 [DOI] [PubMed] [Google Scholar]
- 3.Heiss G, Sharrett AR, Barnes R, Chambless LE, Szklo M, Alzola C. Carotid atherosclerosis measured by B-mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC study. Am J Epidemiol. 1991;134(3):250–256 [DOI] [PubMed] [Google Scholar]
- 4.Brouwers MC, Reesink KD, van Greevenbroek MM, et al. Increased arterial stiffness in familial combined hyperlipidemia. J Hypertens. 2009;27(5):1009–1016 [DOI] [PubMed] [Google Scholar]
- 5.Ramjee V, Sperling LS, Jacobson TA. Non-high-density lipoprotein cholesterol versus apolipoprotein B in cardiovascular risk stratification: do the math. J Am Coll Cardiol. 2011;58(5):457–463 [DOI] [PubMed] [Google Scholar]
- 6.Tsimihodimos V, Gazi I, Kostara C, Tselepis AD, Elisaf M. Plasma lipoproteins and triacylglycerol are predictors of small, dense LDL particles. Lipids. 2007;42(5):403–409 [DOI] [PubMed] [Google Scholar]
- 7.Onat A, Can G, Kaya H, Hergenç G. “Atherogenic index of plasma” (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol. 2010;4(2):89–98 [DOI] [PubMed] [Google Scholar]
- 8.Blankstein R, Budoff MJ, Shaw LJ, et al. Predictors of coronary heart disease events among asymptomatic persons with low low-density lipoprotein cholesterol MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2011;58(4):364–374 [DOI] [PubMed] [Google Scholar]
- 9.Roes SD, Alizadeh Dehnavi R, Westenberg JJM, et al. Assessment of aortic pulse wave velocity and cardiac diastolic function in subjects with and without the metabolic syndrome: HDL cholesterol is independently associated with cardiovascular function. Diabetes Care. 2008;31(7):1442–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legedz L, Bricca G, Lantelme P, et al. Insulin resistance and plasma triglyceride level are differently related to cardiac hypertrophy and arterial stiffening in hypertensive subjects. Vasc Health Risk Manag. 2006;2(4):485–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stan S, Levy E, Delvin EE, et al. Distribution of LDL particle size in a population-based sample of children and adolescents and relationship with other cardiovascular risk factors. Clin Chem. 2005;51(7):1192–1200 [DOI] [PubMed] [Google Scholar]
- 12.Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens. 2010;28(8):1692–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urbina E, Gao Z, Khoury P, Martin L, Dolan L. Insulin resistance and arterial stiffness in healthy adolescents and young adults. Diabetologia. 2012;55(3):625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes Association . Type 2 diabetes in children and adolescents. Diabetes Care. 2000;23(3):381–389 [DOI] [PubMed] [Google Scholar]
- 15.Brinton TJ, Cotter B, Kailasam MT, et al. Development and validation of a noninvasive method to determine arterial pressure and vascular compliance. Am J Cardiol. 1997;80(3):323–330 [DOI] [PubMed] [Google Scholar]
- 16.Goodman E, Daniels SR, Morrison JA, Huang B, Dolan LM. Contrasting prevalence of and demographic disparities in the World Health Organization and National Cholesterol Education Program Adult Treatment Panel III definitions of metabolic syndrome among adolescents. J Pediatr. 2004;145(4):445–451 [DOI] [PubMed] [Google Scholar]
- 17.Martin LJ, Woo JG, Daniels SR, Goodman E, Dolan LM. The relationships of adiponectin with insulin and lipids are strengthened with increasing adiposity. J Clin Endocrinol Metab. 2005;90(7):4255–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimberly MM, Vesper HW, Caudill SP, et al. Standardization of immunoassays for measurement of high-sensitivity C-reactive protein. Phase I: evaluation of secondary reference materials. Clin Chem. 2003;49(4):611–616 [DOI] [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502 [PubMed] [Google Scholar]
- 20.Urbina EM, Brinton TJ, Elkasabany A, Berenson GS. Brachial artery distensibility and relation to cardiovascular risk factors in healthy young adults (The Bogalusa Heart Study). Am J Cardiol. 2002;89(8):946–951 [DOI] [PubMed] [Google Scholar]
- 21.Laurent S, Cockcroft J, Van Bortel L, et al. European Network for Non-invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605 [DOI] [PubMed] [Google Scholar]
- 22.O'Rourke MF, Gallagher DE. Pulse wave analysis. J Hypertens. 1996;14(5):S147–S157 [PubMed] [Google Scholar]
- 23.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents Summary Report. Pediatrics. 2011;128 (Suppl 5):S213–S256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcus MD, Baranowski T, DeBar LL, et al. Severe obesity and selected risk factors in a sixth grade multiracial cohort: the HEALTHY study. J Adolesc Health. 2010;47:604–607 [DOI] [PMC free article] [PubMed]
- 25.März W, Scharnagl H, Winkler K, et al. Low-density lipoprotein triglycerides associated with low-grade systemic inflammation, adhesion molecules, and angiographic coronary artery disease: the Ludwigshafen Risk and Cardiovascular Health study. Circulation. 2004;110(19):3068–3074 [DOI] [PubMed] [Google Scholar]
- 26.Ostfeld R, Mookherjee D, Spinelli M, et al. A triglyceride/high-density lipoprotein ratio > or = 3.5 is associated with an increased burden of coronary artery disease on cardiac catheterization. J Cardiometab Syndr. 2006;1(1):13–15 [DOI] [PubMed] [Google Scholar]
- 27.Shishehbor MH, Hoogwerf BJ, Lauer MS. Association of triglyceride-to-HDL cholesterol ratio with heart rate recovery. Diabetes Care. 2004;27(4):936–941 [DOI] [PubMed] [Google Scholar]
- 28.Norata GD, Raselli S, Grigore L, et al. Small dense LDL and VLDL predict common carotid artery IMT and elicit an inflammatory response in peripheral blood mononuclear and endothelial cells. Atherosclerosis. 2009;206(2):556–562 [DOI] [PubMed] [Google Scholar]
- 29.Mora S, Szklo M, Otvos JD, et al. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2007;192(1):211–217 [DOI] [PubMed] [Google Scholar]
- 30.Maki KC, Davidson MH, Dicklin MR, Bell M, Witchger M, Feinstein SB. Predictors of anterior and posterior wall carotid intima media thickness progression in men and women at moderate risk of coronary heart disease. J Clin Lipidol. 2011;5(3):141–151 [DOI] [PubMed] [Google Scholar]
- 31.Corti R, Fuster V, Fayad ZA, et al. Effects of aggressive versus conventional lipid-lowering therapy by simvastatin on human atherosclerotic lesions: a prospective, randomized, double-blind trial with high-resolution magnetic resonance imaging. J Am Coll Cardiol. 2005;46(1):106–112 [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, American Heart Association. National Heart, Lung, and Blood Institute . Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438 [DOI] [PubMed] [Google Scholar]
- 33.Aznaouridis K, Vlachopoulos C, Dima I, Ioakeimidis N, Stefanadis C. Triglyceride level is associated with wave reflections and arterial stiffness in apparently healthy middle-aged men. Heart. 2007;93(5):613–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alagona C, Soro A, Ylitalo K, Salonen R, Salonen JT, Taskinen M-R. A low high density lipoprotein (HDL) level is associated with carotid artery intima-media thickness in asymptomatic members of low HDL families. Atherosclerosis. 2002;165(2):309–316 [DOI] [PubMed] [Google Scholar]
- 35.Prince CT, Secrest AM, Mackey RH, Arena VC, Kingsley LA, Orchard TJ. Cardiovascular autonomic neuropathy, HDL cholesterol, and smoking correlate with arterial stiffness markers determined 18 years later in type 1 diabetes. Diabetes Care. 2010;33(3):652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frohlich J, Dobiásová M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin Chem. 2003;49(11):1873–1880 [DOI] [PubMed] [Google Scholar]
- 37.Magnussen CG, Venn A, Thomson R, et al. The association of pediatric low- and high-density lipoprotein cholesterol dyslipidemia classifications and change in dyslipidemia status with carotid intima-media thickness in adulthood evidence from the cardiovascular risk in Young Finns study, the Bogalusa Heart study, and the CDAH (Childhood Determinants of Adult Health) study. J Am Coll Cardiol. 2009;53(10):860–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frontini MG, Srinivasan SR, Xu J, Tang R, Bond MG, Berenson GS. Usefulness of childhood non-high density lipoprotein cholesterol levels versus other lipoprotein measures in predicting adult subclinical atherosclerosis: the Bogalusa Heart Study. Pediatrics. 2008;121(5):924–929 [DOI] [PubMed] [Google Scholar]
- 39.Koivistoinen T, Hutri-Kähönen N, Juonala M, et al. Apolipoprotein B is related to arterial pulse wave velocity in young adults: the Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2011;214(1):220–224 [DOI] [PubMed] [Google Scholar]
- 40.Wunsch R, de Sousa G, Reinehr T. Intima-media thickness in obesity: relation to hypertension and dyslipidaemia. Arch Dis Child. 2005;90(10):1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang J, Zhang JP, Luo CX, Yu XM, Lv LQ. Carotid intima-media thickness in childhood and adolescent obesity relations to abdominal obesity, high triglyceride level and insulin resistance. Int J Med Sci. 2010;7(5):278–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayer JG, Harmer JA, Nakhla S, et al. HDL-cholesterol, blood pressure, and asymmetric dimethylarginine are significantly associated with arterial wall thickness in children. Arterioscler Thromb Vasc Biol. 2009;29(6):943–949 [DOI] [PubMed] [Google Scholar]
- 43.Juonala M, Viikari JS, Rönnemaa T, et al. Associations of dyslipidemias from childhood to adulthood with carotid intima-media thickness, elasticity, and brachial flow-mediated dilatation in adulthood: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2008;28(5):1012–1017 [DOI] [PubMed] [Google Scholar]
- 44.Di Bonito P, Moio N, Scilla C, et al. Usefulness of the high triglyceride-to-HDL cholesterol ratio to identify cardiometabolic risk factors and preclinical signs of organ damage in outpatient children. Diabetes Care. 2012;35(1):158–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gungor N, Thompson T, Sutton-Tyrrell K, Janosky J, Arslanian S. Early signs of cardiovascular disease in youth with obesity and type 2 diabetes. Diabetes Care. 2005;28(5):1219–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riggio S, Mandraffino G, Sardo MA, et al. Pulse wave velocity and augmentation index, but not intima-media thickness, are early indicators of vascular damage in hypercholesterolemic children. Eur J Clin Invest. 2010;40(3):250–257 [DOI] [PubMed] [Google Scholar]
- 47.Sakuragi S, Abhayaratna K, Gravenmaker KJ, et al. Influence of adiposity and physical activity on arterial stiffness in healthy children: the lifestyle of our kids study. Hypertension. 2009;53(4):611–616 [DOI] [PubMed] [Google Scholar]
- 48.Arnberg K, Larnkjær A, Michaelsen KF, Mølgaard C. Central adiposity and protein intake are associated with arterial stiffness in overweight children. J Nutr. 2012;142(5):878–885 [DOI] [PubMed] [Google Scholar]
- 49.Koudsi A, Oldroyd J, McElduff P, Banerjee M, Vyas A, Cruickshank JK. Maternal and neonatal influences on, and reproducibility of, neonatal aortic pulse wave velocity. Hypertension. 2007;49(1):225–231 [DOI] [PubMed] [Google Scholar]
- 50.Pandit D, Kinare A, Chiplonkar S, Khadilkar A, Khadilkar V. Carotid arterial stiffness in overweight and obese Indian children. J Pediatr Endocrinol Metab. 2011;24(1-2):97–102 [DOI] [PubMed] [Google Scholar]
- 51.Lee J-W, Lee D-C, Im J-A, Shim J-Y, Kim S-M, Lee H-R. Insulin resistance is associated with arterial stiffness independent of obesity in male adolescents. Hypertens Res. 2007;30(1):5–11 [DOI] [PubMed] [Google Scholar]
- 52.Miyai N, Arita M, Miyashita K, Morioka I, Takeda S. The influence of obesity and metabolic risk variables on brachial-ankle pulse wave velocity in healthy adolescents. J Hum Hypertens. 2009;23(7):444–450 [DOI] [PubMed] [Google Scholar]
- 53.Whincup PH, Gilg JA, Donald AE, et al. Arterial distensibility in adolescents: the influence of adiposity, the metabolic syndrome, and classic risk factors. Circulation. 2005;112(12):1789–1797 [DOI] [PubMed] [Google Scholar]
- 54.Karalis DG, Ishisaka DY, Luo D, Ntanios F, Wun C-C. Effects of increasing doses of atorvastatin on the atherogenic lipid subclasses commonly associated with hypertriglyceridemia. Am J Cardiol. 2007;100(3):445–449 [DOI] [PubMed] [Google Scholar]
- 55.Rizos EC, Agouridis AP, Elisaf MS. The effect of statin therapy on arterial stiffness by measuring pulse wave velocity: a systematic review. Curr Vasc Pharmacol. 2010;8(5):638–644 [DOI] [PubMed] [Google Scholar]
- 56.Ambring A, Friberg P, Axelsen M, et al. Effects of a Mediterranean-inspired diet on blood lipids, vascular function and oxidative stress in healthy subjects. Clin Sci (Lond). 2004;106(5):519–525 [DOI] [PubMed] [Google Scholar]
- 57.de Ferranti SD, Crean S, Cotter J, Boyd D, Osganian SK. Hypertriglyceridemia in a pediatric referral practice: experience with 300 patients. Clin Pediatr (Phila). 2011;50(4):297–307 [DOI] [PubMed]
- 58.de Jongh S, Lilien MR, op’t Roodt J, Stroes ES, Bakker HD, Kastelein JJ. Early statin therapy restores endothelial function in children with familial hypercholesterolemia. J Am Coll Cardiol. 2002;40(12):2117–2121 [DOI] [PubMed] [Google Scholar]
- 59.Jakulj L, Vissers MN, Rodenburg J, Wiegman A, Trip MD, Kastelein JJ. Plant stanols do not restore endothelial function in pre-pubertal children with familial hypercholesterolemia despite reduction of low-density lipoprotein cholesterol levels. J Pediatr. 2006;148(4):495–500 [DOI] [PubMed] [Google Scholar]
- 60.Musso C, Graffigna M, Soutelo J, et al. Cardiometabolic risk factors as apolipoprotein B, triglyceride/HDL-cholesterol ratio and C-reactive protein, in adolescents with and without obesity: cross-sectional study in middle class suburban children. Pediatr Diabetes. 2011;12(3 pt 2):229–234 [DOI] [PubMed] [Google Scholar]
- 61.Giannini C, Santoro N, Caprio S, et al. The triglyceride-to-HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care. 2011;34(8):1869–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quijada Z, Paoli M, Zerpa Y, et al. The triglyceride/HDL-cholesterol ratio as a marker of cardiovascular risk in obese children; association with traditional and emergent risk factors. Pediatr Diabetes. 2008;9(5):464–471 [DOI] [PubMed] [Google Scholar]
- 63.Davidson MH, Ballantyne CM, Jacobson TA, et al. Clinical utility of inflammatory markers and advanced lipoprotein testing: advice from an expert panel of lipid specialists. J Clin Lipidol. 2011;5(5):338–367 [DOI] [PubMed] [Google Scholar]
- 64.St-Pierre AC, Cantin B, Dagenais GR, Mauriége P, Després JP, Lamarche B. The triglyceride/high-density lipoprotein cholesterol ratio, the small dense low-density lipoprotein phenotype, and ischemic heart disease risk. Metab Syndr Relat Disord. 2004;2(1):57–64 [DOI] [PubMed] [Google Scholar]