Abstract
OBJECTIVES:
To determine (1) between-hospital variation in diuretic use for infants with bronchopulmonary dysplasia (BPD), including hospital-specific treatment frequency, treatment duration, and percentage of infants receiving short (≤5 consecutive days) versus longer (>5 days) courses, and to determine (2) demographic and clinical variables associated with diuretic administration.
METHODS:
A retrospective cohort study was conducted with the use of the Pediatric Health Information System to determine between-hospital variation in diuretic utilization patterns (primary outcome) and variables associated with diuretic use among <29-week-gestation infants with evolving BPD at age 28 days who were discharged between January 2007 and June 2011.
RESULTS:
During the 54-month study period, 1429 infants within 35 hospitals met the inclusion criteria for BPD at age 28 days, with 1222 (86%) receiving diuretic therapy for a median of 9 days (25th–75th percentile: 2–33 days). Short courses were administered to 1203 (83%) infants, and 570 (40%) infants received treatment for >5 consecutive days. Furosemide was the most widely prescribed diuretic (1218 infants; 85%), although chlorothiazide had the longest median duration of use (21 days; 25th–75th percentile: 8–46 days). The range of infants receiving a diuretic course of >5 days duration varied by hospital from 4% to 86%, with wide between-hospital variation even after adjustment for confounding variables.
CONCLUSIONS:
The frequency of diuretic administration to infants with BPD at US children’s hospitals, as well as the specific diuretic regimen used, varies markedly by institution. Safety and effectiveness research of long-term diuretic therapy for BPD patients is needed to develop evidence-based recommendations.
Keywords: pharmacoepidemiology, drug utilization, practice variation, prematurity, diuretics, bronchopulmonary dysplasia, comparative effectiveness, patient-centered outcomes
What’s Known on This Subject:
Diuretics are used in preterm infants to treat the symptoms of bronchopulmonary dysplasia (BPD), although there is little evidence of their effectiveness in improving long-term outcomes. Prescribing patterns and frequency of diuretic use in patients with BPD are unknown.
What This Study Adds:
The use of diuretics in infants with BPD, including the specific medications used and length of treatment, varies widely by institution. Long-term diuretic administration to patients with BPD is commonly practiced despite minimal evidence regarding effectiveness and safety.
Diuretic administration to preterm infants with bronchopulmonary dysplasia (BPD) has been practiced for >3 decades to improve respiratory outcomes.1 Short courses of diuretics and long-term diuresis have been shown to transiently improve pulmonary mechanics.2,3
However, evidence is limited regarding the impact of long-term diuretic treatment on important clinical outcomes.2–5 In addition, diuretic medications are associated with side effects in preterm infants and have the potential to cause serious harm.4,6
Several investigations have shown that diuretics are prescribed frequently to preterm infants.7,8 However, practice patterns for diuretic administration to infants with BPD, including frequency of use, average length of treatment, variables associated with administration, and interhospital variations in use, have never been fully reported. Pharmacoepidemiologic knowledge of current diuretic utilization patterns, including interinstitutional differences in diuretic treatment of BPD, will be essential in designing patient-centered trials to examine the comparative effectiveness of diuretic administration for improving BPD outcomes.
Therefore, our investigational objectives were (1) to determine between-hospital variation in diuretic use for infants with BPD, including hospital-specific treatment frequency, inpatient treatment duration, and percentage of infants receiving short (≤5 consecutive days) versus longer (>5 days) courses, and (2) to determine demographic and clinical variables associated with diuretic administration.
Methods
Study Design
We conducted a retrospective cohort study to evaluate diuretic use in infants with BPD. Infants were considered to have evolving BPD if they survived until at least 28 days of age and received respiratory support for the first 28 consecutive days of life via mechanical ventilation, continuous positive airway pressure (CPAP), and/or supplemental oxygen. The cohort included infants with evolving BPD born before 29 weeks of gestation with a birth weight <1500 g and admitted to NICUs in children’s hospitals at <8 days of age with discharge dates from January 2007 to June 2011, as recorded in the Children’s Hospital Association Pediatric Health Information System (PHIS) database (Shawnee Mission, KS). We chose our gestational age (<29 weeks) and birth weight (<1500 g) cutoffs to include >97% of infants with BPD.5 We excluded infants admitted after 7 days of age to minimize exposure to unmeasured diuretic treatment at outside hospitals and included only those who lived ≥28 days, the earliest age at which BPD may be assigned.9 We chose to define BPD at its 28-day onset, before severity staging at 36 weeks’ corrected age, because an infant’s respiratory condition at BPD onset was more likely to influence a clinician’s decision regarding diuretic usage throughout the remainder of the infant’s hospitalization than the 36-week outcome measurement. For reference, we also determined the frequency of mild, moderate, and severe BPD at 36 weeks. The Nationwide Children’s Hospital Institutional Review Board determined that this was not human subjects research, because it was an analysis of a preexisting, deidentified data set and involved no patient contact.
Data Source
The PHIS database contains administrative, billing, and record-review data including patient demographics, diagnoses, medications, and procedures from 43 freestanding US children’s hospitals, which account for 85% of all national freestanding children’s hospitals (Children's Hospital Association; Shawnee Mission, KS).
Only 41 hospitals contributed data on NICU admissions. Of these, 6 reported no cases of BPD as defined in this article and were excluded. The resulting study sample consisting of 1429 admissions from 35 hospitals was used for patient-level analyses. Between-hospital comparisons based on the mean proportion of hospital diuretic use were confined to the 21 hospitals with at least 15 BPD cases representing 94% (n = 1341) of the study sample to prevent bias from the overweighting of institutions with smaller BPD sample sizes.
Study Variables
Daily drug-specific diuretic administration, mechanical ventilation, CPAP, oxygen use, as well as length of stay and demographic variables were determined from each hospital’s daily charge records as included in PHIS. Thompson-Reuters Healthcare (Ann Arbor, MI), the PHIS data processing partner, maps each hospital’s daily charge codes to a common classification system, the Clinical Transaction Classification codes to ensure comparability of charge-level data between institutions. Clinical Transaction Classification codes evaluated included the following: acetazolamide (191145), bumetanide (191131), chlorothiazide (191111), ethacrynic acid (191133), furosemide (191135), hydrochlorothiazide (191113), metolazone (191121), spironolactone (191141), mechanical ventilation (521166), CPAP (521162), and oxygen delivery by cannula, tent, or mask (521171). We evaluated the use of all diuretics included in the PHIS database within our cohort population and excluded those with <1% frequency of use (metolazone, ethacrynic acid) from additional analysis. International Classification of Diseases, Ninth Revision, codes were used to determine the diagnoses of patent ductus arteriosus (PDA; 747.0), intraventricular hemorrhage (IVH; 772.1), and necrotizing enterocolitis (NEC; 777.5).
Because previous trials revealed transient efficacy for short-term diuretic therapy for BPD, but unclear evidence for longer-term diuretic use,2,3 we examined the frequency of both short- and longer-term diuretic treatment. We defined a short course of diuretics as ≤5 consecutive days’ duration and a longer course as >5 consecutive days. We chose a 5-day cutoff because 4- to 7-day treatment ranges have been frequently used in short-term BPD diuretic therapy trials.2,3
Statistical Analysis
All analyses were conducted by using Stata 12.1 (StataCorp, College Station, TX). The power of the sample to reject the null hypotheses of equal hospital-specific means of ever administering a diuretic to a patient (primary outcome) was >0.99 (β < .01) with α = .01. The same power results held for ever administering ≤5 days or ever administering >5 days of diuretics. Unadjusted odds ratios for bivariate associations between diuretic use and neonatal demographic/clinical risk factors were determined with simple logistic regression for binary outcome variables and simple ordinary least-squares regression for continuous outcomes. Multivariable mixed logistic regression modeling with a random intercept for hospitals was used to adjust odds ratios for confounding variables and to evaluate the contribution of within-hospital clustering to variation in diuretic administration. The model was created by purposeful selection and included gestational age, gender, duration of mechanical ventilation or CPAP, IVH, PDA, and NEC as variables. A second model was created with birth weight replacing gestational age to obtain adjusted odds ratios for birth weight, which was done to avoid multicollinearity between birth weight and gestational age. All statistical testing was 2-sided and an α level of .05 was considered significant.
Results
A total of 1429 infants met the criteria for BPD at age 28 days, of which 1222 (86%) were treated with at least 1 diuretic dose. Patients received a median of 9 days (25th–75th percentile: 2–33 days) of diuretic therapy, with 1203 (84%) receiving at least 1 short course of ≤5 days’ duration and 570 (40%) receiving at least 1 longer course of >5 days. At discharge, 171 (13%) were receiving diuretics. In the unadjusted analysis, decreasing birth weight, decreasing gestational age, IVH, NEC, PDA, and increasing duration of positive-pressure exposure via mechanical ventilation or CPAP were associated with significantly increased odds of diuretic treatment. Male gender increased the odds of longer, but not short-course, diuretic therapy. Race and study year were also examined but did not significantly affect the odds of diuretic administration (Supplemental Table 2).
When all variables were included in a multivariable logistic regression model with a random intercept to adjust for confounding and within-hospital clustering (Table 1), the odds of an infant with BPD ever receiving a ≤5-day short course were significantly associated with total days of exposure to mechanical ventilation or CPAP, PDA, and NEC. Smaller infants, except for the <500-g group, had higher odds of ever receiving a ≤5-day course of diuretics relative to 1000- to 1499-g infants. The lack of a significant association in <500-g infants could be related to small sample size (n = 40) and increased inpatient mortality risk (risk ratio: 2.93; P = .004) relative to the other infants in the cohort.
TABLE 1.
Multivariable-Adjusted Odds of ≤5-Day and >5-Day Courses of Diuretics by Demographic Categories and Clinical Risk Factors
| Variable | Ever Received ≤5-Day Course | Ever Received >5-Day Course |
|---|---|---|
| Gestation | ||
| 27–28 weeks (ref) | — | — |
| 25–26 weeks | 1.73* (1.19, 2.52) | 1.16 (0.85, 1.6) |
| ≤24 weeks | 2.38* (1.19, 4.79) | 1.24 (0.84, 1.84) |
| Birth weight | ||
| 1000–1499 g (ref) | — | — |
| 750–999 g | 1.59* (1.07, 2.37) | 1.32 (0.9, 1.92) |
| 500–749 g | 2.06* (1.21, 3.5) | 1.32 (0.87, 2.01) |
| <500 g | 2.02 (0.4, 10.18) | 1.49 (0.63, 3.51) |
| Gender | ||
| Male (ref) | — | — |
| Female | 0.86 (0.61, 1.22) | 0.70* (0.54, 0.92) |
| Days on CPAP or mechanical ventilation | ||
| ≤20 days (ref) | — | — |
| 21–35 days | 1.95* (1.26, 3.03) | 1.82* (1.1, 3) |
| 36–53 days | 4.03* (2.38, 6.82) | 4.6* (2.8, 7.55) |
| ≥54 days | 30.88* (11.37, 83.83) | 13.71* (8.11, 23.18) |
| Major comorbidities | ||
| IVH | 0.99 (0.67, 1.44) | 0.97 (0.74, 1.28) |
| PDA | 1.85* (1.3, 2.64) | 1.23 (0.93, 1.64) |
| NEC | 2.68* (1.46, 4.93) | 1.11 (0.8, 1.54) |
Data are shown as adjusted odds ratios (95% confidence interval). All regression odds ratios except for those for major comorbidities are reported relative to the reference group indicated by (—). Adjusted odds ratios were determined by using a mixed-effects logistic regression model with a random intercept for hospital, in which all variables in the table were fit in the model except for birth weight. Birth weight adjusted odds ratios were determined by a second model, which included all variables except for gestational age to avoid multicollinearity between birth weight and gestational age.
Statistically significant at α = .05. ref, reference.
In our multivariable model, the largest influence on the odds of an infant with BPD ever receiving a >5-day diuretic course was the length of positive-pressure exposure via mechanical ventilation or CPAP. In addition, male gender was associated with increased odds of ever receiving a longer course. Birth weight, gestational age, IVH, NEC, and PDA were not significantly associated with the receipt of >5-day diuretics courses.
Utilization of Specific Diuretics
We determined the patterns and frequencies of use for the 6 diuretics that were prescribed to >1% of infants within the cohort. We also determined the drug-specific median diuretic days per patient. We chose to report the median measure of central tendency because the distributions for days of use for all the diuretics were skewed to the right, indicating wide variation in treatment duration.
Within the cohort of BPD patients, acetazolamide was administered to 74 (5%) infants, bumetanide to 35 (2%), chlorothiazide to 202 (14%), hydrochlorothiazide to 61 (4%), and spironolactone to 178 (12%). Furosemide was the most widely prescribed diuretic overall (1218 infants; 85%), as well as for short-term therapy (1198 infants; 84%) and longer courses (466 infants; 33%). However, on average, chlorothiazide was the diuretic prescribed for the most days per patient (median: 21 days; 25th–75th percentile: 8–46 days). Spironolactone was prescribed for a median of 18 (7–38) days per patient, hydrochlorothiazide for 15 (3–32) days, furosemide for 10 (3–28) days, bumetanide for 5 (2–29) days, and acetazolamide for 3 (1–6) days.
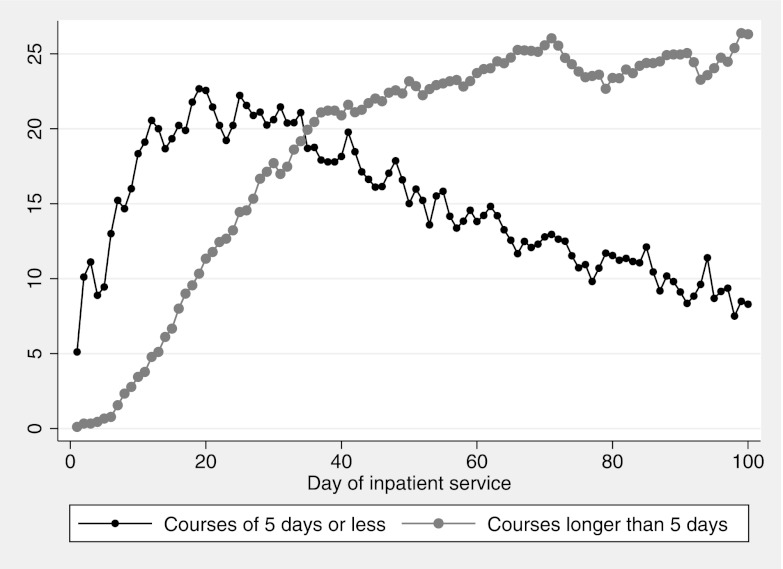
Figure 1 shows the percentage of admitted patients receiving each diuretic by day of hospitalization. Overall, diuretic courses ≤5 days in length predominated early in infants who developed BPD, whereas longer >5-day courses were more common after hospital day 34. Similar patterns were noted for furosemide, with longer courses predominating, on average, after 42 inpatient days, and for chlorothiazide, with more longer courses after day 55 (Supplemental Figure 5). Short courses of hydrochlorothiazide and spironolactone were rarely given (<1%). Average daily bumetanide and acetazolamide use was <1% with no clear course length trend over time, and they were excluded from Supplemental Figure 5.
FIGURE 1.
Percentage of infants with BPD receiving a ≤5-consecutive-day short course or >5-day course of diuretics by day of hospitalization.
When we examined the correlations between the percentage of patient days on specific diuretics to determine if the use of 1 diuretic was associated with the use of another, we found strong positive correlations between chlorothiazide and spironolactone (R = 0.85, P < .0001) and hydrochlorothiazide and spironolactone (R = 0.89, P < .0001).
Between-Hospital Variation in Diuretic Treatment of Infants With BPD
Diuretic use for infants with BPD varied between children’s hospitals. Figure 2 shows between-hospital variation in percentage of patient days receiving diuretics, and Fig 3 shows between-hospital variation in the percentage of patients who ever received a ≤5-day short course, as well as the percentage ever receiving courses >5 days. There was only moderate correlation (R = 0.46, P = .005) between the percentage of short courses and long courses administered by the hospital, and no significant correlation between patient volume and the percentage of short or longer courses administered.
FIGURE 2.
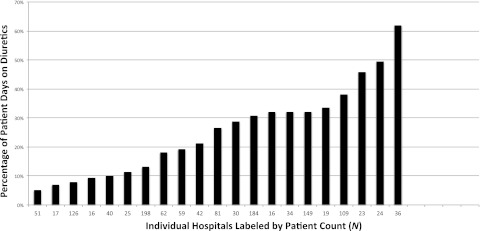
Mean proportion of days that infants received diuretics during their NICU stay, by hospital. Range: 5.1% to 61.9%. Patient days = (mean patient days on any diuretic/mean length of stay). Hospitals were excluded if <15 patients developed BPD during the study period.
FIGURE 3.
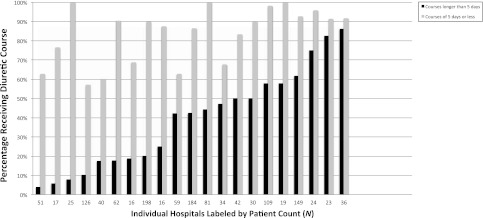
Percentage of patients by hospital who ever received a ≤5-day short course of diuretics or a course >5 days. Ranges: longer courses, 3.9% to 86% (median: 42.4%); short courses, 62.7% to 91.7% (median: 89.9%). Hospitals are listed in order of increasing >5-day courses.
The variation in diuretic course length between hospitals persisted even after controlling for length of exposure to positive pressure via mechanical ventilation or CPAP, birth weight, gestational age, IVH, PDA, and NEC in our multivariable logistic regression models with random intercepts (Table 1). The intraclass correlation coefficient (ICC), a measure of the proportion of total variance in diuretic use due to variation between hospitals, indicated that clustering by hospital was a significant component of the overall variation in the frequency of both short and longer courses prescribed (ICC for ≤5-day courses = 0.33; 95% CI: 0.19, 0.52) (ICC for >5-day courses =0.35; 95% CI: 0.23, 0.51).
In addition, the specific diuretic or diuretics chosen by clinicians varied widely among hospitals. Figure 4 shows the range of infants receiving chlorothiazide (0–72%), furosemide (0–87%), hydrochlorothiazide (0–30%), and spironolactone (0–67%) by institution.
FIGURE 4.
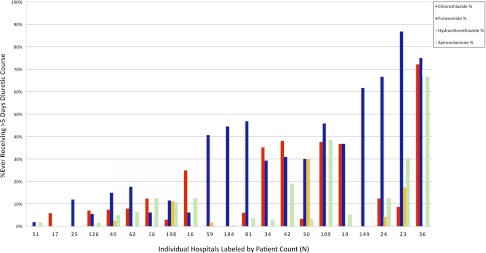
Percentage of infants by hospital who ever received a >5-consecutive-day course of chlorothiazide, furosemide, hydrochlorothiazide, or spironolactone. Hospitals are listed in order of increasing >5-day courses.
Cohort at 36 Weeks’ Postmenstrual Age
At 36 weeks’ postmenstrual age, 78% (n = 1116) of the cohort remained hospitalized after 53 deaths and 313 discharges. Oxygen requirement (moderate BPD as defined by Ehrenkranz et al10) was noted in 39% (n = 431) and 28% (n = 314) required CPAP or mechanical ventilation (severe BPD10). Of these infants, 122 (33%) with mild BPD (n = 371), 143 (33%) with moderate BPD, and 159 (51%) with severe BPD received at least 1 >5-day diuretic course during their stay. Death occurred in 24 hospitalized infants after 36 weeks.
Discussion
Our investigation indicates that diuretic use in infants with BPD at US children’s hospitals is common, but patterns of utilization vary markedly between institutions. Overall, 86% of the cohort received a diuretic, with 84% receiving at least 1 ≤5-day course and 40% receiving courses of >5 consecutive days. We found that duration of mechanical ventilation or CPAP exposure, a marker of respiratory disease severity, was the greatest predictor of diuretic exposure. On average, among infants that develop BPD, courses of ≤5 days predominate in the first month of life with >5 days of treatment more common thereafter.
The percentage of BPD patients receiving diuretics as well as the frequency of shorter- and longer-term courses varied widely among hospitals. Among hospitals with ≥15 BPD patients during the study period, the percentage receiving a diuretic course of >5 days ranged from 4% to 86%. Variation among centers in the percentage of infants receiving short and longer courses persisted even after controlling for confounding variables associated with diuretic use. However, the range of variation was narrower for short courses relative to long courses, perhaps due to greater evidence for short-term diuretic effects.2,3
When we examined diuretic utilization by hospital for >5-day courses, we found that the most frequently administered class of diuretic varied between institutions. Clinical studies have focused primarily on 2 classes of diuretics for the treatment of BPD: loop diuretics (eg, furosemide) and diuretics acting on the distal tubule (eg, thiazides or spironolactone).
Furosemide, the most widely studied loop diuretic in infants, can improve lung mechanics through both diuretic-dependent and diuretic-independent responses.6 Furosemide increases local prostaglandin production leading to pulmonary vasodilation,11,12 enhances lung fluid absorption,13,14 inhibits bronchial smooth muscle contraction resulting in bronchodilation,15 and decreases inflammatory mediator release.16 These potential benefits must be balanced against important systemic side effects including electrolyte disturbances, ototoxicity, and renal calcium excretion potentially leading to nephrocalcinosis and osteopenia.4,6 Furosemide also vasodilates the ductus arteriosus17 and has been associated with increased PDA when administered to preterm infants with respiratory distress syndrome.18,19 A Cochrane systematic review of 6 trials found that although furosemide improves pulmonary compliance, minute ventilation, and oxygen requirement in infants aged >3 weeks with BPD, there was no beneficial effect in duration of oxygen requirement or mechanical ventilation.2 Although none of the included trials evaluated long-term furosemide use or clinically important outcomes such as mortality, length of stay, BPD at 36 weeks, or bone demineralization, we found that multiple centers appear to use furosemide almost exclusively for long-term diuresis of BPD patients.
Diuretics acting primarily at the distal tubules, including thiazides and spironolactone, are less potent than loop diuretics but potentially cause fewer electrolyte abnormalities. Thiazides do not increase renal calcium excretion nor does spironolactone when used individually.6 However, spironolactone is associated with hypercalciuria in combination with thiazides.20 We found that spironolactone administration was correlated with both chlorothiazide and hydrochlorothiazide, likely indicating its synergistic use with these medications as a potassium-sparing diuretic. However, thus far, combination therapy with spironolactone has not been proven beneficial in neonates. When Hoffman et al21 randomly assigned a total of 33 infants to 2-week courses of a thiazide with spironolactone versus thiazide alone they found no between-group differences in serum electrolytes, the need for potassium chloride or sodium chloride supplementation, pulmonary mechanics, or fraction of inspired oxygen.
Only 2 single-center randomized trials, the first which occurred before surfactant use and both which occurred before the era of routine antenatal steroid administration, have examined long-term use of distal tubule diuretics for infants with BPD.1,22 Albersheim et al22 reported reduced mortality for ≥30-day-old, ventilated preterm infants randomly assigned to hydrochlorothiazide/spironolactone. However, placebo-group mortality was 53%,22 higher than that in contemporary investigations in chronically ventilated BPD patients.23 A Cochrane systematic review that included both trials determined thiazide and spironolactone improved lung compliance and decreased intermittent furosemide use in >3-week-old preterm infants with chronic lung disease.3 The authors found little evidence that distal diuretic administration reduces duration of ventilation or length of stay. 3
Despite this lack of evidence to support long-term diuretic use and minimal data on long-term side effects, we found that such use is a routine occurrence in NICUs. Surprisingly, a recent survey of 400 US neonatologists by Hagadorn et al24 to determine factors that influenced a clinician’s decision to use diuretics revealed that 66% of respondents expected decreased ventilator days and 59% decreased length of stay.
Due to the retrospective and observational nature of our investigation, we were unable to study the causal effects of diuretics on important clinical outcomes such as duration of ventilation, duration of oxygen use, severity of BPD at 36 weeks, and length of stay. All of these outcomes were associated with increased diuretic use in our cohort, but our ability to control for selection bias due to severity of respiratory illness was limited to observed covariates. Our diagnoses, including BPD, were based on hospital records, and potential recording errors might reduce the accuracy of our diagnoses. Although we used hospital charge data for specific date of service to determine days receiving oxygen, days on mechanical ventilation and CPAP, and dates of diuretic administration, we had to rely on less specific International Classification of Diseases, Ninth Revision, codes for the diagnoses of IVH, PDA, and NEC. Even though PHIS data are rigorously screened for errors and rejected if quality thresholds for inclusion are not met, they were initially collected for hospital administrative purposes instead of specifically for research.
Despite these limitations, our cohort study has multiple strengths. It benefitted from the large sample size and nationally representative sample of children’s hospitals included in the PHIS database, as well as measures taken by the Children’s Hospital Association to ensure data quality. Our findings are likely generalizable to most NICUs within large US children’s hospitals.
These baseline findings will serve to inform the design of prospective, comparative effectiveness investigations to determine whether long-term use of diuretics in BPD patients is beneficial. There is a critical need for investigation of harmful side effects in diuretic-exposed patients, including decreased bone density with increased fractures and the longitudinal effect of chronic electrolyte depletion requiring supplementation. If long-term diuretic administration is revealed to be beneficial for BPD patients, investigations will be needed to determine which diuretic or diuretics provide the most benefit and the lowest long-term risks.
Conclusions
Diuretic therapy is commonplace for infants with BPD at US children’s hospitals. The frequency of diuretic administration, as well as the specific diuretic regimens used, varies markedly by institution even after adjustment for confounding variables. Research is needed to determine the effectiveness and safety of long-term diuretic therapy for BPD patients to develop evidence-based recommendations.
Supplementary Material
Acknowledgment
We thank Mark Klebanoff, MD, MPH, for his critical review of the manuscript draft.
Glossary
- BPD
bronchopulmonary dysplasia
- CI
confidence interval
- CPAP
continuous positive airway pressure
- ICC
intraclass correlation coefficient
- IVH
intraventricular hemorrhage
- NEC
necrotizing enterocolitis
- PDA
patent ductus arteriosus
- PHIS
Pediatric Health Information System
Footnotes
Dr Slaughter developed the idea for the study; Drs Slaughter and Stenger wrote the original draft of the manuscript; Drs Reagan and Slaughter performed statistical analysis; and Drs Slaughter, Reagan, and Stenger interpreted the data, revised the manuscript for important intellectual content, and approved the final draft for submission.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Agency for Healthcare Research and Quality.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by KL2RR025754 (Dr Slaughter, principal investigator) from the National Center For Research Resources, which is now at the National Center for Advancing Translational Sciences, grant 8KL2TR000112-05, and R21 HS19524-01 from the Agency for Healthcare Research and Quality (Dr Reagan). Funded by the National Institutes of Health (NIH).
References
- 1.Kao LC, Warburton D, Sargent CW, Platzker ACG, Keens TG. Furosemide acutely decreases airways resistance in chronic bronchopulmonary dysplasia. J Pediatr. 1983;103(4):624–629 [DOI] [PubMed] [Google Scholar]
- 2.Stewart A, Brion LP. Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst Rev. 2011;(9):CD001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart A, Brion LP, Ambrosio-Perez I. Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst Rev. 2011;(9):CD001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart AL, Brion LP. Routine use of diuretics in very-low birth-weight infants in the absence of supporting evidence. J Perinatol. 2011;31(10):633–634 [DOI] [PubMed] [Google Scholar]
- 5.Walsh MC, Szefler S, Davis J, et al. Summary proceedings from the bronchopulmonary dysplasia group. Pediatrics. 2006;117(3 pt 2 suppl 1):S52–S56 [DOI] [PubMed] [Google Scholar]
- 6.Segar JL. Neonatal diuretic therapy: furosemide, thiazides, and spironolactone. Clin Perinatol. 2012;39(1):209–220 [DOI] [PubMed] [Google Scholar]
- 7.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117(6):1979–1987 [DOI] [PubMed] [Google Scholar]
- 8.Lindner U, Hilgendorff A, Frey G, Gortner L. Drug utilisation in very preterm infants: any changes during the past decade? Klin Padiatr. 2008;220(4):238–242 [DOI] [PubMed] [Google Scholar]
- 9.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729 [DOI] [PubMed] [Google Scholar]
- 10.Ehrenkranz RA, Walsh MC, Vohr BR, et al. National Institutes of Child Health and Human Development Neonatal Research Network . Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–1360 [DOI] [PubMed] [Google Scholar]
- 11.Bland RD, McMillan DD, Bressack MA. Decreased pulmonary transvascular fluid filtration in awake newborn lambs after intravenous furosemide. J Clin Invest. 1978;62(3):601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demling RH, Will JA. The effect of furosemide on the pulmonary transvascular fluid filtration rate. Crit Care Med. 1978;6(5):317–319 [DOI] [PubMed] [Google Scholar]
- 13.Dikshit K, Vyden JK, Forrester JS, Chatterjee K, Prakash R, Swan HJ. Renal and extrarenal hemodynamic effects of furosemide in congestive heart failure after acute myocardial infarction. N Engl J Med. 1973;288(21):1087–1090 [DOI] [PubMed] [Google Scholar]
- 14.Lundergan CF, Fitzpatrick TM, Rose JC, Ramwell PW, Kot PA. Effect of cyclooxygenase inhibition on the pulmonary vasodilator response to furosemide. J Pharmacol Exp Ther. 1988;246(1):102–106 [PubMed] [Google Scholar]
- 15.Almirall JJ, Dolman CS, Eidelman DH. Furosemide-induced bronchodilation in the rat bronchus: evidence of a role for prostaglandins. Lung. 1997;175(3):155–163 [DOI] [PubMed] [Google Scholar]
- 16.Anderson SD, He W, Temple DM. Inhibition by furosemide of inflammatory mediators from lung fragments. N Engl J Med. 1991;324(2):131. [DOI] [PubMed] [Google Scholar]
- 17.Toyoshima K, Momma K, Nakanishi T. In vivo dilatation of the ductus arteriosus induced by furosemide in the rat. Pediatr Res. 2010;67(2):173–176 [DOI] [PubMed] [Google Scholar]
- 18.Green TP, Thompson TR, Johnson D, Lock JE. Furosemide use in premature infants and appearance of patent ductus arteriosus. Am J Dis Child. 1981;135(3):239–243 [DOI] [PubMed] [Google Scholar]
- 19.Green TP, Thompson TR, Johnson DE, Lock JE. Furosemide promotes patent ductus arteriosus in premature infants with the respiratory-distress syndrome. N Engl J Med. 1983;308(13):743–748 [DOI] [PubMed] [Google Scholar]
- 20.Atkinson SA, Shah JK, McGee C, Steele BT. Mineral excretion in premature infants receiving various diuretic therapies. J Pediatr. 1988;113(3):540–545 [DOI] [PubMed] [Google Scholar]
- 21.Hoffman DJ, Gerdes JS, Abbasi S. Pulmonary function and electrolyte balance following spironolactone treatment in preterm infants with chronic lung disease: a double-blind, placebo-controlled, randomized trial. J Perinatol. 2000;20(1):41–45 [DOI] [PubMed] [Google Scholar]
- 22.Albersheim SG, Solimano AJ, Sharma AK, et al. Randomized, double-blind, controlled trial of long-term diuretic therapy for bronchopulmonary dysplasia. J Pediatr. 1989;115(4):615–620 [DOI] [PubMed] [Google Scholar]
- 23.Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB, DART Study Investigators . Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: a multicenter, international, randomized, controlled trial. Pediatrics. 2006;117(1):75–83 [DOI] [PubMed] [Google Scholar]
- 24.Hagadorn JI, Sanders MR, Staves C, Herson VC, Daigle K. Diuretics for very low birth weight infants in the first 28 days: a survey of the U.S. neonatologists. J Perinatol. 2011;31(10):677–681 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.