Abstract
A number of widespread and devastating chronic diseases, including atherosclerosis, type 2 diabetes, and Alzheimer’s disease, have a pathophysiologically important inflammatory component. In these diseases, the precise identity of the inflammatory stimulus is often unknown and, if known, is difficult to remove. Thus, there is interest in therapeutically targeting the inflammatory response. Although there has been success with anti-inflammatory therapy in chronic diseases triggered by primary inflammation dysregulation or autoimmunity, there are considerable limitations. In particular, the inflammatory response is critical for survival. As a result, redundancy, compensatory pathways, and necessity narrow the risk:benefit ratio of anti-inflammatory drugs. However, new advances in understanding inflammatory signaling and its links to resolution pathways, together with new drug development, offer promise in this area of translational biomedical research.
All living organisms depend on the ability to protect themselves from exogenous pathogens (1) and to repair tissue damage that results from infection or trauma (Figs. 1, A and B, and 2A). Nonetheless, there are settings when the inflammatory response itself damages host tissue and causes organ dysfunction. In one setting, there is an overly exuberant acute or subacute inflammatory response (cytokine storm) that occurs in response to pathogens (sepsis) or debris from damaged host cells (2). In another type of pathologic inflammation, a primary defect in the regulation of an inflammatory pathway triggers chronic disease. An example is cryopyrin-associated periodic syndromes (CAPS), which are inflammasome diseases caused by mutations in NACHT-leucine-rich repeat protein 3 (3). A third class of chronic diseases is driven by pathologic inflammation but is not triggered by acute sepsis, acute tissue injury, or a primary defect in inflammation regulation. In these diseases, the inflammatory response does not eradicate the primary stimulus, as would normally occur in most cases of infection or injury, and thus a chronic form of inflammation ensues that ultimately contributes to tissue damage. In diseases such as seropositive rheumatoid arthritis (RA), the inflammatory response is triggered by an exuberant, pathologic autoimmune cascade (4) (Fig. 1C). However, in other types of chronic inflammatory diseases, autoimmunity is not the key, primary pathogenic event (Fig. 2B) (5). Diseases in this category are often associated with aging and include atherosclerosis (Fig. 1D), obesity and insulin resistance, and certain neurodegenerative diseases such as Alzheimer’s disease.
Fig. 1.
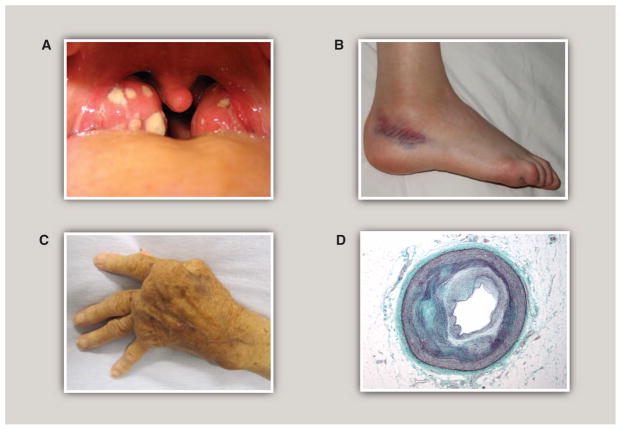
Inflammation in infection, injury, and chronic disease. (A) Acute tonsillitis. White patches represent the accumulation of neutrophils in the tonsils in response to bacterial infection. The tonsils are swollen, erythematous, and painful. Normal tissue homeostasis is usually restored over the course of several days upon eradication of the infection. [Source: Michaelbladon/Wikimedia Commons] (B) Sprained ankle. An example of a sterile tissue injury. Swelling and redness result from the inflammatory response to tissue damage and hemorrhage. Normal tissue homeostasis is usually restored over a time course of weeks after clearance of dead or damaged cells and activation of tissue repair programs. [Source: Boldie/ Wikimedia Commons] (C) Rheumatoid arthritis. An example of chronic inflammation in a disease triggered primarily by autoimmunity. Persistent inflammation, driven in part by TNFα, results in severe tissue damage, joint destruction, and loss of function. [Source: James Heilman, MD/Wikimedia Commons] (D) Cross section of a coronary artery at the location of an atherosclerotic lesion. An example of a chronic inflammatory disease that is triggered initially by a process other than infection, tissue injury, or autoimmunity. Elevated levels of apolipoprotein B–containing lipoproteins in the artery wall induce a chronic inflammatory state characterized by activated endothelial cells and recruitment and activation of macrophages and other immune cells. As lesions evolve, inflammation fails to resolve in the setting of persistent arterial-wall lipoproteins. Nonresolving inflammation leads to cell death and necrotic core formation; cycles of extracellular matrix deposition and degradation; and calcification. [Source: Nephron/Wikimedia Commons]
Fig. 2.
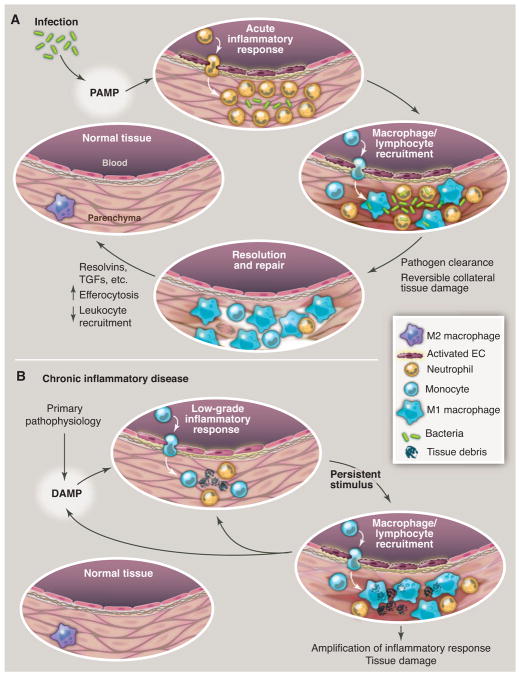
Evolution of resolving versus nonresolving inflammation at a cellular level. (A) Typical features of a normal acute inflammatory response to infection that is detected by presentation of PAMPs to pattern recognition receptors. Eradication of the pathogen eliminates the stimulus, along with causing some reversible collateral tissue damage, and sets the stage for the resolution/repair phase, leading to restoration of normal tissue homeostasis. (B) Typical features of a chronic inflammatory disease caused by a nonimmune pathophysiologic process that in one way or another triggers an initial sterile inflammatory response, often indolent and likely through production of damage-associated molecular patterns (DAMPs). This initial response then becomes amplified by cytokines and chemokines. Because this response does not eradicate the initial stimulus, persistent nonresolving inflammation occurs, ultimately resulting in tissue damage. The inflammatory response itself may positively influence the production of DAMPS, which provides an additional positive feedback loop. For example, in the case of atherosclerosis, reactive oxygen intermediates (ROI) and reactive nitrogen intermediates (RNI) may modify subendothelial lipoproteins in a manner that amplifies their ability to promote inflammation.
Improved understanding of the inflammatory response has led to important advances in the treatment of diseases with a primary defect in inflammation regulation, such as CAPS, and in autoimmune-induced inflammatory diseases, particularly seropositive RA and certain other rheumatoid diseases. Can these advances also be applied to other types of chronic diseases in which inflammation is an important driving force? In chronic autoimmune diseases, the mechanisms linking the autoimmune trigger to the maladaptive inflammatory response are often better understood than in chronic inflammatory diseases with a nonautoimmune etiology. Moreover, autoimmune diseases are usually associated with life-changing, and often painful, symptoms on a day-to-day basis, which tends to increase patient acceptance of adverse effects of treatment. Nonetheless, even with this class of diseases, treatment is not ideal. The beneficial responses are variable, particularly when anti-inflammatory therapy is started after the disease has become established; long-lasting remissions are not common; and adverse effects, particularly in the area of compromised host defense, can be substantial (6). These problems are likely to be even more pronounced with complex and often indolent chronic disease processes in which the primary trigger is thought to be something other than autoimmunity.
The challenges in targeting inflammation in any chronic inflammatory disease lie in three properties that are characteristic of processes that are critical for evolutionary survival: redundancy, compensation, and necessity. Thus, inflammation is orchestrated by many molecules, and targeting one or a few may not be enough. Inflammation is also a finely tuned process that has inherent sensors and feedback pathways, and so inhibition of a critical component of inflammation may simply trigger a compensatory proinflammatory response involving another pathway. Finally, the inflammatory response is critical for host defense, and thus when the previous two challenges are successfully overcome, the risk:benefit profile is often unacceptable. With this background, we will first review the fundamental components of the inflammatory response, with emphasis on those components that contribute to redundancy and compensation and that are targets of currently available drugs or have promise for future therapeutic intervention. We will then highlight principles of anti-inflammatory therapy in chronic autoimmune inflammatory diseases, discuss challenges and ongoing attempts to target inflammation in other chronic inflammatory diseases, and raise prospects for future therapeutic directions.
Basic Principles of the Inflammatory Response and Inflammation Resolution
At the tissue level, acute inflammation is characterized by redness, heat, pain, and swelling, which result from local responses of immune, vascular, and parenchymal cells to infection or injury (Fig. 1, A and B). At a signaling level, infection or tissue damage is initially sensed by pattern recognition receptors (PRRs) that recognize pathogen-associated molecular patterns (PAMPs) and/or damage-associated molecular patterns (DAMPs) (Fig. 3). Several classes of such receptors are now well characterized, exemplified by the Toll-like receptors (TLRs) and NOD-like receptors (NLRs) (7). Upon binding of PAMPs or DAMPs, PRRs engage signal transduction pathways that activate signal-dependent transcription factors such as nuclear factor κB (NF-κB) and activating protein 1 (AP-1). These factors act in a combinatorial and cell-specific manner to induce the expression of genes that initiate the inflammatory response (e.g., TNFA, IL1B, and COX2), exert antimicrobial functions (e.g., inducible nitric oxide synthase), and recruit additional immune cells (e.g., chemokines), thereby setting into motion both innate and adaptive immune responses. Notably, nearly all components of this early warning system are redundant. Furthermore, there are cytokine-mediated feed-forward loops that amplify the initial inflammatory response (Fig. 3). Epigenetic regulation is also important. For example, gain of histone H3 and H4 acetylation and loss of H3K27 and H4K20 methylation occur to facilitate activation of inflammatory response genes (8–11).
Fig. 3.
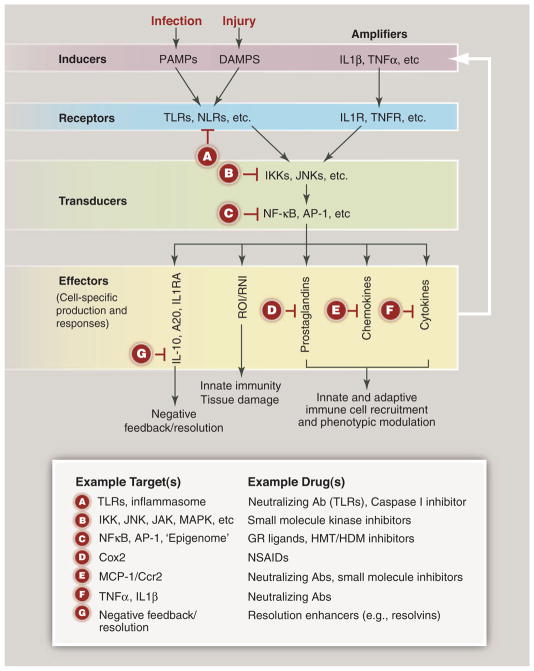
Inflammation at a signaling level and candidate therapeutic targets. Inflammation is typically initiated by pattern recognition receptors, such as TLRs and NLRs, that recognize PAMPs and/or DAMPs. These receptors typically couple to signal transduction pathways that activate latent transcription factors that include members of the NF-κB and AP-1 families. These factors in turn act in a combinatorial and cell-specific manner to induce the expression of a large number of genes that exert antimicrobial activities—e.g., generate ROI and RNI. Chemokines regulate the recruitment of additional immune cells. Production of bioactive lipids, such as prostaglandins, also regulates pro- and anti-inflammatory cell functions. Expression of inflammatory cytokines provides a feed-forward loop for amplification of the initial response. The production of anti-inflammatory/ resolution mediators, such as IL-10 and PGE2, in response to proinflammatory signals suggests that resolution programs are an inherent aspect of inflammation. Letters in red “stop signs” represent examples of points in proinflammatory signaling pathways that are existing or potential targets for therapeutic intervention. Ab, antibody; GR, glucocorticoid receptor; HDM, histone demethylase; HMT, histone methyltransferase; IKK, IκB kinase; MAPK, mitogen-activated protein kinase; MCP1, monocyte chemotactic protein–1; TNFR, tumor necrosis factor receptor Cox2, cyclooxygenase 2; NSAIDs, nonsteroidal anti-inflammatory drugs.
At a cellular level, acute inflammatory responses are characterized by marked temporal changes in quantities and characteristics of tissue immune cells. Under resting conditions, most tissues contain a population of resident macrophages that exhibit a deactivated or “resting” phenotype. There is substantial evidence that these cells play important roles in maintenance of normal tissue homeostasis. For example, lean adipose tissue contains deactivated macrophages, eosinophils, and regulatory T cells (Tregs), all of which appear to contribute to normal insulin signaling (12–14). Upon infection with a bacterial pathogen, the initial phase of the acute inflammatory response is typically characterized by rapid neutrophil invasion (Fig. 2A). This early response subsequently gives way to a secondary phase characterized by recruitment of monocyte-derived macrophages, adaptive immune cells, and stromal cells. The combination of innate and adaptive immune responses serves to eradicate the infection but also results in collateral tissue damage, in part through the production of reactive oxygen species that are cytotoxic and secretion of proteases that degrade extracellular matrix.
At this stage, the inflammatory response transitions to an active resolution phase (Fig. 2A) (1, 15). Cellular processes involved in inflammation resolution include clearance of pathogens, pro-inflammatory debris, and cytokines; apoptosis of neutrophils and their non–inflammation-inducing removal by phagocytes (efferocytosis); egress of inflammatory macrophages and recruitment or phenotypic switching of macrophages to a pro-resolving phenotype; recruitment of Tregs; activation of anti-inflammatory nuclear receptors; and overall repair and normalization of tissue architecture and function, including reestablishment of the vasculature. Many of these cellular processes are activated by and/or generate proresolving soluble factors produced by the inflammatory cells themselves or by cells recruited during the initial resolution phase. For example, prostaglandin E2 (PGE2), omega-3 fatty acids, and neutrophil-derived microparticles in edema fluid can trigger resolution responses (16–18). Factors that mediate resolution include interleukin-10 (IL-10), transforming growth factor–β (TGFβ), proresolving proteins such as annexin A1, and small lipid mediators called lipoxins, resolvins, protectins, and maresins that are derived from arachidonic acid and omega-3 fatty acids through 5- or 15-lypoxygenase pathways (1, 15).
Principles of Anti-Inflammatory Therapy in Chronic Autoimmune Inflammatory Diseases
The understanding that the pathology of chronic autoimmune diseases is driven by maladaptive, nonresolving inflammation led to trials and then successful use of anti-inflammatory therapy, including nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and disease-modifying agents of rheumatoid diseases (DMARDs) (Fig. 3) (19). The first two classes are used mostly to relieve symptoms, whereas DMARDs are distinguished by their ability to reduce or prevent tissue damage caused by the inflammatory attack, especially when used early in the course of the disease. Nonbiologic DMARDs, such as low-dose methotrexate (LD-MTX), suppress various inflammatory processes in multiple types of immune cells. Biologic DMARDs are genetically engineered recombinant proteins (e.g., humanized antibodies) that target specific inflammatory molecules or their receptors or signaling pathways—for example, tumor necrosis factor–α (TNFα), IL-1β, and the IL-1 receptor (Fig. 3). Combination therapy with a non-biological plus a biological DMARD is commonly used and very effective, but, apropos of the “necessity” challenge, combinations of different classes of biologic DMARDs are not used due to high risk of infection.
An example of how the development of a biologic DMARD met some of the challenges of anti-inflammatory therapy can be appreciated by briefly reviewing the history of antibody to TNFα (anti-TNFα) as therapy for antibody to citrinullated protein (ACPA)–positive RA (6). The challenge of redundancy and compensation, with many inflammatory cytokines and other mediators present in the arthritic joints, was daunting and an early deterrent to drug development. Cleverly conducted ex vivo rheumatoid synovium experiments revealed, however, that many of the inflammatory mediators were in the same pathway and that TNFα was a proximal trigger. Thus, anti-TNFα blocked inflammatory cell production of many other cytokines in addition to TNFα and, importantly, chemokines. The decrease in chemokines then amplified the beneficial effect by reducing trafficking of the inflammatory cells to the joints. Subsequent studies in mouse models confirmed this hierarchy. In striking contrast, therapeutic targeting of another inflammatory mediator in ACPA-positive RA, the mitogen-activated protein kinase p38, was found to be too transient to be useful, suggesting that the challenges of redundancy and/or compensation were not overcome in the targeting of this particular pathway (20). Moreover, anti-TNFα is not effective in certain other inflammatory conditions, such as septic shock, and even appears to be harmful in multiple sclerosis, perhaps because inhibiting this cytokine compromises the transition to resolution in these settings (21, 22).
Even in the case of successful anti-TNFα therapy, the response is variable, so we can learn lessons from its limitations (6). First, patient benefit of anti-TNFα is better in early disease than in late disease, where irreversible tissue damage has already occurred. This property of anti-inflammatory therapy is inherent to all chronic inflammatory diseases. Therefore, methods to identify early disease, such as biomarkers and imaging techniques, and finding drugs that can be tolerated for very long periods of time are important in the overall design of anti-inflammatory strategies for chronic diseases. Second, despite the fact that anti-TNFα has been relatively successful in the face of redundancy and compensatory pathways, these challenges still exist. Finally, basic principles of necessity dictate that the more potent the anti-inflammatory therapy, the greater chance for adverse effects related to host defense. Thus, it is not surprising that patients taking anti-TNFα therapy are at increased risk for infections. In the end, the devastating nature of RA creates a risk:benefit ratio that more often than not favors drug treatment.
Targeting Inflammation in Chronic Diseases with an Inflammatory Component Not Triggered by Autoimmunity
Chronic diseases associated with an inflammatory component not directly induced by an auto-immune process are the most common diseases of aging and represent our greatest health threats (3). These include most forms of cardiovascular disease, type 2 diabetes, and virtually all neurodegenerative diseases. In each case, a nonautoimmune primary pathological process—for example, excess subendothelial apolipoprotein B–containing lipoproteins, saturated fatty acids, or formation of protein aggregates, respectively—results in the generation of DAMPs that are detected by PRRs (Fig. 2B). Moreover, the inflammatory response itself may amplify the production of disease-specific DAMPs, resulting in positive-feedback loops that accelerate the underlying disease process. For example, inflammation promotes formation of oxidized phospholipids that may serve as important DAMPs in atherosclerosis (23) and may enhance the formation of β-amyloid and tau aggregates in Alzheimer’s disease (24). As with autoimmune diseases, inhibition of inflammation could reduce the rate of disease progression to the point of substantial clinical benefit despite not altering the underlying pathogenic process. In contrast with primary inflammatory or autoimmune diseases, however, there is little evidence as yet for efficacy of this approach in humans.
Each of the diseases in this category has unique therapeutic opportunities and challenges. For example, the evaluation of anti-inflammatory drugs for type 2 diabetes, as compared with atherosclerosis and, especially, Alzheimer’s disease, is more feasible in terms of end-point analysis (fasting blood sugar, hemoglobin A1c, and plasma insulin levels) and may not be as demanding in terms of the necessity for early-stage treatment (25). For a discussion of targeting inflammation in metabolic and neurodegenerative diseases, see the Reviews in this issue by Odegaard and Chawla (26) and Aguzzi et al. (27), respectively. The focus here will be on atherosclerosis, which is an important and instructive example of the challenges in this area (28).
Subendothelial retention of apolipoprotein B–containing lipoproteins triggers a maladaptive, nonresolving inflammatory response that drives atherogenesis [see the Review in this issue by Swirski and Nahrendorf (29)]. Here, we emphasize a few points that are relevant to the discussion that follows. First, the statin class of drugs are used in almost all subjects at high risk for atherosclerotic disease. Thus, anti-inflammatory therapy would be used mostly in combination with statins and would have to show effectiveness greater than that with statins alone, which themselves may have some anti-inflammatory effects (30). Second, although early use of anti-inflammatory therapy might be most effective in combating atherosclerosis before certain aspects of the pathology become irreversible, this would require a very long treatment period and may therefore have an unacceptable risk:benefit ratio. Conversely, although the risk:benefit ratio might be most acceptable for shorter-term treatment of advanced atherosclerosis, it is precisely in this situation that anti-inflammatory therapy would be least effective. In this setting, the value may lie in preventing the progression of earlier lesions that are known to coexist with advanced lesions. Third, it is important to consider the possibility of unique adverse effects of targeting inflammation in atherosclerosis. For example, recurrence of a myocardial infarction (MI, or heart attack) is particular prominent in the year following an initial MI. This heightened risk may be driven in part by a post-MI monocytosis, but inhibiting monocytosis through anti-inflammatory therapy may delay tissue repair of the infarcted myocardium itself (31). Furthermore, inhibition of a master regulator of inflammation, NF-κB, or deletion in macrophages of tumor necrosis factor receptor–associated factor 6 (TRAF6), which is an adaptor of IL-1R and TLRs, actually increases atherosclerosis in mouse models by mechanisms that appear to involve compensatory signaling (32, 33). On the other hand, a possible additional benefit of targeting systemic inflammation in atherosclerosis may be its effects on obesity and insulin resistance (34), which itself may be lessened by anti-inflammatory therapy (26).
With these considerations in mind, how might the inflammatory nature of atherosclerosis lead to new therapeutic advances? First and foremost, atherosclerosis has a property that is lacking in most chronic inflammatory diseases, namely, the potential for removing the inflammatory stimulus. As with gout, where lowering of uric acid levels can help prevent the formation of uric acid crystals in joints and thereby reduce inflammation, lowering of plasma low-density lipoprotein (LDL) can prevent subendothelial retention of lipoproteins and thereby decrease inflammatory atherosclerotic disease (35). However, the goal of therapeutically decreasing plasma LDL to low enough levels and early enough in the disease to eliminate atherosclerosis has not yet been achieved, so other treatment options are being sought.
In this context, investigators are beginning to ask whether drugs used for chronic primary inflammatory diseases might be useful to prevent atherosclerotic disease, and they have recently turned their attention to DMARDs. The genetic and pharmacologic targeting of many individual inflammatory processes and molecules in mouse models of atherosclerosis leads to decreases in aortic atherosclerosis. However, it has been argued that although testing new antiatherosclerosis drugs in vitro and in preclinical models in vivo is a necessary first step, it is often a poor way to predict success in vascular disease in humans. Indeed, clinical trials may be the best way to investigate proof-of-concept usefulness (28). In that spirit, two human DMARD coronary artery disease (CAD) trials are now being initiated. One will investigate the use of LD-MTX, which was shown in post hoc analyses of several RA and psoriatic arthritis trials to have potentially beneficial effects on cardiovascular disease (36). The other trial will test a humanized monoclonal antibody against IL-1β (canakinumab), which is being used or evaluated for two types of inflammasome/IL-1β–mediated diseases, CAPS and gout, respectively (37). Part of the rationale for IL-1–targeted therapy is evidence for inflammasome activation in murine atherosclerosis, perhaps triggered by cholesterol crystals (38), and the success of targeting IL-1β in these mouse models.
It is instructive to view these two proposed trials in the context of the successful development of anti-TNFα and LD-MTX for ACPA-positive RA and the challenges of redundancy, compensation, and necessity. Atherosclerotic lesions, like the RA synovium, express many cytokines and chemokines that have been implicated in the pathogenesis of atherosclerosis. As noted above, TNFα has a distinct role in synovial inflammation in that it controls the production of many other cytokines and, more generally, leukocyte trafficking. Whether IL-1β has a similar upstream role in human atherosclerosis is unknown, and the successful use of canakinumab in CAPS and gout may be because these diseases are primary inflammasome/IL-1β diseases. If the role of IL-1β is not particularly dominant in atherosclerotic plaque progression in humans, the benefit above that seen with statins alone may be difficult to detect. Moreover, although neutralization of IL-1β diminishes atherosclerotic lesion size in mice, deletion of IL-1α/β receptors was found to actually worsen plaque stability in a mouse model of advanced atherosclerosis through effects on the extracellular matrix (39). Finally, the potential benefit of long-term use of canakinumab in humans at high risk for atherosclerotic vascular disease must be substantial enough to counter the increased risk of infection, which was 67% in a recently conducted 2-year CAPS trial vs. 25% for placebo.
Despite the aforementioned favorable signal from post hoc analyses of previous clinical trials, there is more uncertainty with LD-MTX because the exact mechanisms of action are not completely understood. To the extent that the benefit of LD-MTX may rely, at least in part, on adenosine-mediated anti-inflammatory signaling, mouse atherosclerosis studies would predict promise (40, 41). Perhaps a greater challenge will be related to adverse effects. Although LD-MTX is considered to be a relatively safe and well-tolerated drug, it has several mild and usually self-limited nuisance-type side effects, including nausea, stomatitis, and fatigue, and long-term use has rarely been associated with more important adverse effects, such as hepatotoxicity, pulmonary disease, and infection (42). If LD-MTX is effective in atherosclerosis but requires long-term use, these side effects might become more important. Moreover, the risk of liver injury and infection is increased in subjects with type 2 diabetes (42, 43), which represents a sizable percentage of the high-risk CAD population that would be considered for this drug. In the end, it will be important to consider these principles both for the monitoring and evaluation of the canakinumab and LD-MTX trials and in the design of and decision to move forward with new trials in this arena.
Future Directions
Ongoing efforts to widen the benefit-to-risk window of anti-inflammatory therapy in chronic diseases will require efforts on a number of complementary fronts. To the extent that there is the potential to remove the inflammatory stimulus in these diseases, as there is in atherosclerosis (atherogenic lipoproteins) and obesity (nutrient excess), ongoing efforts in this area are crucial. For example, in atherosclerosis, there may be innovative therapeutic approaches to prevent the retention of atherogenic lipoproteins in addition to lowering plasma LDL (44). In general, however, these goals have been difficult to attain even where they are theoretically possible, and it is not yet feasible in other chronic diseases, such as neurodegenerative disease.
With regard to DMARDs, a number of future developments may increase their promise in a wider array of chronic inflammatory diseases. Data from human genetic studies that link specific inflammatory genes to CAD may be helpful in identifying useful drug targets (45). In addition, detailed, disease-specific mechanistic studies will help identify targets that are upstream in signaling cascades, which will help guard against redundancy, and whose inhibition is less likely to trigger compensatory responses, compromise host defense, and suppress the transition to resolution. Some of these goals may be achievable through the use of new formulations that target DARMDs to the site of inflammation, thus minimizing systemic adverse effects.
Translating these advances into therapy will benefit from simultaneous progress in developing new classes of drugs that target inflammation. Recent examples include an inhibitor of inflammatory Janus kinase (JAK) signaling (tofacitinib), which has recently been shown to be effective in RA and ulcerative colitis (46); a potent and site-selective adenosine A(2A) receptor activator that requires ectonuclease-mediated activation at the site of inflammation (47); and drugs that target inflammatory fibroblasts, which have shown promise in preclinical models of RA and may be useful in other inflammatory diseases depending on the roles of these cells (48). There is also substantial evidence supporting the feasibility of using antisense oligonucleotides (ASOs) to target specific RNAs in humans (49). The primary attraction of ASO technology is that, in principle, it enables knockdown of any RNA-encoded target, ranging from proteins that are very difficult to target with drugs to noncoding RNAs. In addition, the sequence precision of the ASO targeting mechanism enables highly related members of druggable families to be specifically targeted in a manner that could be difficult to achieve with small molecules. For example, an ASO specific for MKK7 reduced phosphorylation of c-Jun N-terminal kinase (JNK) in synoviocytes and reduced inflammation and disease severity in a mouse model of arthritis (50). Another strategy would be to use recent advances in histone modifications. Examples include inhibitors of the bromodomain and extraterminal domain family of proteins (I-BET), which block the binding of transcription initiation mediators called BET proteins to acetylated histones and thereby inhibit transcription. The inhibitors exert remarkably selective effects on gene expression in vitro and in vivo, acting to suppress a subset of inflammatory response genes in activated macrophages and conferring protection against endotoxin-induced shock (51). Another example of epigenetic-based therapy is a druglike compound that suppresses lipopolysaccharide-induced cytokine production in macrophages by inhibiting a specific subfamily of histone demethylases (52).
An alternative strategy to inhibiting inflammation would be to commandeer nature’s own anti-inflammatory mechanisms to induce a “dominant” program of resolution. Examples include activators of liver-x receptors (LXRs), which regulate inflammation and lipid metabolism and have shown benefit in mouse models of atherosclerosis and Alzheimer’s disease (53–56), and activators of peroxisome proliferator–activated receptor (PPAR)γ, which may be able to reprogram white adipose tissue macrophages and promote Treg development to improve insulin resistance in obese adipose tissue (12). Recent studies suggest new strategies to enhance therapeutic actions of these receptors and reduce side effects (57, 58). Future therapies may be able to take advantage of two key suppressors of inflammation, IL-10 and Tregs (59). Using atherosclerosis as an example, IL-10 and Tregs have marked antiatherosclerotic effects in mouse models (60, 61). Long-duration systemic IL-10 therapy would likely have substantial adverse effects, but nanoparticle-targeted delivery of IL-10 to atherosclerosis lesions may be both feasible and effective (62). Antiatherogenic Tregs might be inducible through the use of athero-specific dendritic cell vaccine strategies (63), perhaps in combination with agents that activate endogenous cell biological processes involved in the stability and function of Tregs and/or in the feedback suppression of inflammatory T effector cells (64, 65).
Another line of attack may be therapeutic administration of lipid mediators of inflammation resolution, particularly in view of evidence that chronic inflammatory diseases may lower the levels or actions of these molecules (66). In this regard, resolvin E1 (RvE1) has shown benefit in preclinical models or clinical trials of a number of chronic inflammatory diseases, including asthma, rheumatoid arthritis, atherosclerosis, and obesity-related adipose inflammation (www.resolvyx.com) (67), and neuroprotectin D1 protected human neurons from beta-amyloid–induced inflammation and cell death in vitro (68). A key principle in the therapeutic use of inflammation resolution mediators is that they are predicted not to compromise host defense. In fact, recent work in mice has shown that RvD2 actually promotes host defense during sepsis (69).
Finally, it is important to consider how one could noninvasively monitor the anti-inflammatory or proresolving actions of drugs that target the inflammatory component of chronic diseases. This is particularly important in diseases like atherosclerosis where the actual clinical end points themselves are delayed, sporadic, and often devastating. To the extent that the drugs affect systemic inflammation, measurement of plasma cytokines and acute-phase reactants, including TNFα, IL-6, C-reactive protein, and fibrinogen, can be monitored. Monitoring inflammation at the site of disease would be particularly useful, and thus there may be a niche in this arena for techniques such as 18F-fluorodeoxyglucose positron emission tomography and fluorine-19 magnetic resonance imaging (47, 70). However, all these methods lack specificity and may also lack sensitivity when it comes to specific therapeutic areas, such as those involved in enhancing inflammation resolution. Thus, efforts to target the inflammatory and nonresolving components of chronic diseases, particularly those with delayed signs or symptoms, must be accompanied by the development of noninvasive methods to monitor the effectiveness of these new strategies.
In conclusion, the past two decades have provided a wealth of information on how maladaptive, nonresolving inflammation drives a number of widespread chronic diseases in which infection, primary defects in inflammation regulation, or autoimmunity are not the primary pathophysiologic process. Although this knowledge has the potential to open up vast opportunities for new therapeutic advances, the nature of the inflammatory response as a complex system that is critical for normal physiology renders this promise challenging. In particular, the challenges of redundancy, compensation, and necessity often create a very narrow risk:benefit window. Nonetheless, new knowledge about inflammatory signaling, particularly in the areas of endogenous homeostatic pathways and inflammation resolution, provide the promise for new therapeutic options that can successfully meet these challenges.
Acknowledgments
We thank C. Serhan, J. Bathon, R. Winchester, and G. Fredman for helpful discussions. This work was supported by National Institutes of Health grants P01-HL054591, R01-HL075662, and R01-HL057560 (to I.T.) and DK074868, DK091183, and HC088093 (to C.K.G.).
References and Notes
- 1.Nathan C, Ding A. Cell. 2010;140:871. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Castellheim A, Brekke OL, Espevik T, Harboe M, Mollnes TE. Scand J Immunol. 2009;69:479. doi: 10.1111/j.1365-3083.2009.02255.x. [DOI] [PubMed] [Google Scholar]
- 3.Kubota T, Koike R. Mod Rheumatol. 2010;20:213. doi: 10.1007/s10165-009-0271-0. [DOI] [PubMed] [Google Scholar]
- 4.McInnes IB, Schett G. N Engl J Med. 2011;365:2205. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 5.Scrivo R, Vasile M, Bartosiewicz I, Valesini G. Autoimmun Rev. 2011;10:369. doi: 10.1016/j.autrev.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Feldmann M, Williams RO, Paleolog E. Ann Rheum Dis. 2010;69(suppl 1):i97. doi: 10.1136/ard.2009.117143. [DOI] [PubMed] [Google Scholar]
- 7.Kumar H, Kawai T, Akira S. Int Rev Immunol. 2011;30:16. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 8.De Santa F, et al. EMBO J. 2009;28:3341. doi: 10.1038/emboj.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escoubet-Lozach L, et al. PLoS Genet. 2011;7:e1002401. doi: 10.1371/journal.pgen.1002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hargreaves DC, Horng T, Medzhitov R. Cell. 2009;138:129. doi: 10.1016/j.cell.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stender JD, et al. Mol Cell. 2012;48:28. doi: 10.1016/j.molcel.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cipolletta D, et al. Nature. 2012;486:549. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odegaard JI, et al. Nature. 2007;447:1116. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu D, et al. Science. 2011;332:243. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serhan CN, et al. FASEB J. 2007;21:325. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Nat Immunol. 2001;2:612. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 17.Kasuga K, et al. J Immunol. 2008;181:8677. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalli J, Serhan CN. Blood. 2012;120:e60. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh JA, et al. Arthritis Care Res. 2012;64:625. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Genovese MC. Arthritis Rheum. 2009;60:317. doi: 10.1002/art.24264. [DOI] [PubMed] [Google Scholar]
- 21.Kox WJ, Volk T, Kox SN, Volk HD. Intensive Care Med. 2000;26(suppl 1):S124. doi: 10.1007/s001340051129. [DOI] [PubMed] [Google Scholar]
- 22.Arnason B. Int MS J. 2011;17:63. [PubMed] [Google Scholar]
- 23.Miller YI, et al. Circ Res. 2011;108:235. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glass CK, Saijo K. Nat Rev Immunol. 2010;10:365. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- 25.Romeo GR, Lee J, Shoelson SE. Arterioscler Thromb Vasc Biol. 2012;32:1771. doi: 10.1161/ATVBAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odegaard JI, Chawla A. Science. 2013;339:172. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguzzi A, Barres BA, Bennett ML. Science. 2013;339:156. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Libby P, Ridker PM, Hansson GK. Nature. 2011;473:317. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 29.Swirski FK, Nahrendorf M. Science. 2013;339:161. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montecucco F, Mach F. Semin Immunopathol. 2009;31:127. doi: 10.1007/s00281-009-0146-7. [DOI] [PubMed] [Google Scholar]
- 31.Tabas I. Nature. 2012;487:306. doi: 10.1038/487306a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanters E, et al. J Clin Invest. 2003;112:1176. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polykratis A, van Loo G, Xanthoulea S, Hellmich M, Pasparakis M. Circulation. 2012;126:1739. doi: 10.1161/CIRCULATIONAHA.112.100339. [DOI] [PubMed] [Google Scholar]
- 34.Bornfeldt KE, Tabas I. Cell Metab. 2011;14:575. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabas I, Williams KJ, Borén J. Circulation. 2007;116:1832. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 36.Ridker PM. J Thromb Haemost. 2009;7(suppl 1):332. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 37.Ridker PM, Thuren T, Zalewski A, Libby P. Am Heart J. 2011;162:597. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Duewell P, et al. Nature. 2010;464:1357. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexander MR, et al. J Clin Invest. 2012;122:70. doi: 10.1172/JCI43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montesinos MC, et al. Arthritis Rheum. 2003;48:240. doi: 10.1002/art.10712. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, et al. Arterioscler Thromb Vasc Biol. 2009;29:1046. doi: 10.1161/ATVBAHA.109.188839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamp L, et al. Biomed Pharmacother. 2006;60:678. doi: 10.1016/j.biopha.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Montaudié H, et al. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):12. doi: 10.1111/j.1468-3083.2011.03991.x. [DOI] [PubMed] [Google Scholar]
- 44.Brito V, et al. Arterioscler Thromb Vasc Biol. 2012;32:2847. doi: 10.1161/ATVBAHA.112.300444. [DOI] [PubMed] [Google Scholar]
- 45.McPherson R, Davies RW. Can J Cardiol. 2012;28:662. doi: 10.1016/j.cjca.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Fox DA. N Engl J Med. 2012;367:565. doi: 10.1056/NEJMe1206315. [DOI] [PubMed] [Google Scholar]
- 47.Flögel U, et al. Sci Transl Med. 2012;4:146ra108. doi: 10.1126/scitranslmed.3003717. [DOI] [PubMed] [Google Scholar]
- 48.Juarez M, Filer A, Buckley CD. Swiss Med Wkly. 2012;142:w13529. doi: 10.4414/smw.2012.13529. [DOI] [PubMed] [Google Scholar]
- 49.Bennett CF, Swayze EE. Annu Rev Pharmacol Toxicol. 2010;50:259. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- 50.Lee SI, Boyle DL, Berdeja A, Firestein GS. Arthritis Res Ther. 2012;14:R38. doi: 10.1186/ar3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicodeme E, et al. Nature. 2010;468:1119. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kruidenier L, et al. Nature. 2012;488:404. doi: 10.1038/nature11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calkin AC, Tontonoz P. Arterioscler Thromb Vasc Biol. 2010;30:1513. doi: 10.1161/ATVBAHA.109.191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vucic E, et al. JACC Cardiovasc Imaging. 2012;5:819. doi: 10.1016/j.jcmg.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zelcer N, et al. Proc Natl Acad Sci USA. 2007;104:10601. doi: 10.1073/pnas.0701096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cui W, et al. Neuroscience. 2012;210:200. doi: 10.1016/j.neuroscience.2012.02.047. [DOI] [PubMed] [Google Scholar]
- 57.Spann NJ, et al. Cell. 2012;151:138. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi JH, et al. Nature. 2010;466:451. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Littman DR, Rudensky AY. Cell. 2010;140:845. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 60.Mallat Z, et al. Circ Res. 1999;85:e17. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 61.Ait-Oufella H, et al. Nat Med. 2006;12:178. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 62.Lobatto ME, Fuster V, Fayad ZA, Mulder WJ. Nat Rev Drug Discov. 2011;10:835. doi: 10.1038/nrd3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nilsson J, Hansson GK. J Intern Med. 2008;263:464. doi: 10.1111/j.1365-2796.2008.01945.x. [DOI] [PubMed] [Google Scholar]
- 64.Pan F, Fan H, Liu Z, Jiang S. Sci Signal. 2012;5:pe32. doi: 10.1126/scisignal.2003364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang L, Baban B, Johnson BA, 3rd, Mellor AL. Int Rev Immunol. 2010;29:133. doi: 10.3109/08830180903349669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morris T, et al. Proc Natl Acad Sci USA. 2010;107:8842. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clària J, Dalli J, Yacoubian S, Gao F, Serhan CN. J Immunol. 2012;189:2597. doi: 10.4049/jimmunol.1201272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lukiw WJ, et al. J Clin Invest. 2005;115:2774. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spite M, et al. Nature. 2009;461:1287. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lobatto ME, et al. Mol Pharm. 2010;7:2020. doi: 10.1021/mp100309y. [DOI] [PMC free article] [PubMed] [Google Scholar]