Abstract
Cancer stem cell (CSC) population in solid human breast tumor was identified by CD44+/CD24− phenotype, characterized by high tumorigenicity, invasiveness and drug resistance. In this study, we characterized drug resistant breast cancer cell line-MCF-7/Adr and a number of breast cancer cell lines using flow cytometry, immunofluorescence, mammosphere formation assay and migration assay, examining their CSC immunophenotypes, presence of CSC proteins, tumorigenicity in vitro and migratory rates, respectively. Our results show that MCF-7/Adr cells uniformly display CSC characteristics yet retain low migratory rate. They are also able to self-renew and differentiate under floating culture conditions. Furthermore, MCF-7/Adr is selectively sensitive to epigenetic drug, suberoylanilide hydroxamic acid (SAHA), losing drug resistance and changes morphology yet retaining CSC immunophenotypes. In conclusion, we show that resistant breast cancer cell line MCF-7/Adr demonstrates uniform CSC like characteristics and are sensitive to epigenetic drug treatment.
Keywords: Drug Resistance, Breast Cancer Stem Cell, Surface Markers, Epithelial Mesenchymal Transition, Epigenetic Therapy, Cancer Therapy
1. Introduction
Breast cancer is the most prevalent cancer type among women internationally. Heterogeneous by nature, breast tumor cells have a wide array of morphologies, molecular characteristics and treatment responses [1], imposing treatment difficulties. The cancer stem cell (CSC) theory states that CSCs are the only cell population in a tumor that causes tumorigenesis, cancer progression and metastasis. CSCs are able to self-renew and differentiate, possessing stem cell characteristics [2]. They are also responsible for tumor re-growth post-treatment [3]. First identified in acute myeloid leukemia, CSCs and the existence of multiple stem cell classes was evidenced by the fact that only ~1% of leukemic cells (signifying clonogenic progenitors) are able to proliferate, distinguishing from the majority of the cells [4, 5]. Soon afterward, CSCs were reported in solid tumors. Many identification systems and treatments have soon followed that focused on conquering CSCs, which are drug resistant [6–12]. However, gaps exist in the CSC theory, such as the lack of an established CSC hierarchy in a solid tumor and linking cellular lineage with their functions. Also, it is difficult to establish connections between CSCs and normal stem cells in a tissue, and trace back the cellular origin of the identified CSCs. It has been demonstrated that the selected populations possess sphere-forming ability, able to self-renew (making identical replicates), and express a high level of malignancy which allow them to resist treatment and initiate and re-initiate tumors after chemo and radiation therapy.
The most widely recognized breast cancer stem cell (BCSC) markers are CD44+/CD24−/low [13–18]. Subsequent studies have distinguished CD44−/CD24+ as luminal cells while CD44+/CD24− possess basal-like phenotype [5]. Heterogeneous (CD44+/CD24+ or CD44−/CD24−) expressions were also observed in the tumors [19]. The CD44+/CD24− sub-population of a tumor are shown to be able to initiate and propagate a tumor, and has been used to signify its ability to self-renew. The same cell population have also displayed considerable chemo-resistance [10] and distant metastasis [20] . However, other reports say that it is CD44−/CD24+ rather than CD44+/CD24− that contributes to worsened patient prognosis, Yet another study connects CD24+ with invasive breast cancer [21].
Beside CD44+/CD24−, a number of other markers have also been demonstrated to link with tumor-initiation and treatment survival, most of which have to do with cellular adhesion and mobility. Epithelial-specific antigen (ESA), CD49f and CD201 are a few that have been identified for BCSC. ESA has been shown to support migration and invasion in human metastatic cell line MDA-MB-231 [22], while CD49f is connected with cell adhesion and surface signaling. In this paper, for the first time we demonstrated the uniform and prolonged display of CD44+/CD24− phenotype along with the above-mentioned BCSC markers in a well-established multi-drug resistant breast cancer cell line MCF-7/Adr without special supplements or culture conditions, bringing new perspectives on the current knowledge of CD44+/CD24- phenotype, and that of BCSC.
Global epigenetic mutation is a landmark for cancer. Epigenetic drugs potentially can overcome and reverse cancer malignancy through differentiation therapy targeting CSCs. Epigenetic drug, suberoylanilide hydroxamic acid (SAHA), approved for cutaneous T-cell lymphoma treatment, is a strong anti-drug resistant and anti-metastatic agent [23]. Understanding the transient nature of SAHA’s therapeutic effect, we examined whether they will also exert influence on cell surface markers in the short-term, the understanding of which could profoundly affect the targeting and selecting strategies toward malignant cancer types especially utilizing epigenetic drugs as the combination therapy.
2. Materials and Methods
2.1 Materials
Human breast cancer cell lines MCF-7, MDA-MB-231, SkBr3 were obtained from ATCC (American Type Culture Collection). DCIS (ductal carcinoma in situ) was purchased from Celprogen (San Pedro, CA). MCF-7/Adr cell line was established through longterm doxorubicin incubation. Human mesenchymal stem cell (hMSC), hMSC basal medium, MSCGM bullet kit (PT-3238 & PT-4105) and hMSC trypsin/EDTA were purchased from Lonza Inc. (Walkersville, MD). APC Mouse Anti-Human CD44, PE Mouse Anti-Human CD24, FITC Mouse Anti-Human CD326 (ESA), PE Mouse Anti-Human CD201, PE-Cy™5 Mouse Anti- Human CD49f were purchased from BD Biosciences and BD Pharmingen (San Jose, CA). Human mesenchymal stromal cell marker panel (CD44, CD45, CD90 CD29 and CD105) was purchased from Abcam (Cambridge, MA). Twenty four-well ultra-low binding plates were purchased from Corning (NY, USA). Fetal bovine serum (FBS) was purchased from Gibco (Invitrogen, Milan, Italy). Crystal violet solution (HT90132-1L) and MTT reagents were purchased from Sigma-Aldrich (St. Louis, MO). B-27 Serum-Free Supplement (50X) (17504- 044) and all antibodies were obtained from Invitrogen Inc. Human recombinant fibroblast growth factor basic (GF003) was purchased from Millipore (Billerica, MA). Keratinocyte serumfree medium (K-SFM) and accompanying supplements (bovine pituitary extract, final concentration 50 µg/ml, recombinant human epidermal growth factor, final concentration 5 ng/ml) were obtained from GIBCO. Suberoylanilide hydroxamic acid (SAHA, generic name Voronistat) was purchased from Sigma-Aldrich.
2.2 Cell Culture
All cancer cell lines were cultured in DMEM supplemented with 15% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin. Cultures were incubated at 37 °C in a humidified atmosphere containing 5% CO2 using 75T cell culture flask. Cells were sub-cultured reaching 90% confluency.
hMSCs were expanded using hMSC Basal Medium containing MSCGM Bullet Kits and experiments were conducted in DMEM containing 10% FBS and 1% penicillin-streptomycin. Medium was changed every 3 days and cells were sub-cultured at 85–90% confluency. hMSCs up to passage 6 were used for experiments.
2.3 Flow Cytometric Analysis
Breast cancer cell lines were analyzed for the expression of selective BCSC markers. hMSCs were validated using hMSC surface marker panel include positive markers for CD105, CD90, and CD44, and negative markers for CD45 and CD29. In all experiments, after trypsinization, cells were washed twice with flow buffer (with 10% FBS), resuspended fluorochrome-conjugated antibody diluted in flow buffer and incubated on ice in dark for 30 min with antibody solution. Antibody concentration was consistent throughout experiments. After staining, all samples were washed thrice with PBS, re-suspended in flow buffer and tested using LSR II. Cells were shielded from light at all times. Cells from each cell line treated with the same procedure but without antibody were served as control. The samples were analyzed with FlowJo software. A minimum of 2000 events were recorded for each sample and analyzed.
2.4 Mammosphere culture
Cells were single-suspended at a density of 5000 cells in culture media in six-well ultra-low attachment plates (2.5 mL per plate). Two culture systems were used. One contained phenol red free serum free DMEM supplemented with 20 ng/ml human basic fibroblast growth factor, 20 ng/ml human epidermal growth factor, 1X B27, 1% penicillin-streptomycin solution. Second contained K-SFM with B Pituitary extract and 20 ng/ml epidermal growth factor, 1% penicillin-streptomycin solution. Culture media were replenished every 3 days and images were taken at day 7. After 7 days, mammospheres were collected and cells were dissociated for flow cytometry.
2.5 Migration/Invasion assay
Twenty four-well Falcon tissue culture plates with 8 micron cell culture insert were used for migration assay. Cells were first starved overnight and plated on top of the insert and allowed to migrate for 24 hours, using 5% FBS in DMEM as attractant. The assay was terminated by fixing cells with 4% paraformaldehyde on ice. Cells were stained with crystal violet and cells on top of the insert were removed with a cotton swab. Images were taken after staining. For quantification, cells migrated through the insert were lysed with RIPA buffer and lysate were quantified in 96 well plate using plate reader.
2.6 In vitro proliferation assay
MTT assays were conducted according to the manufacturer’s protocol. Cells were plated at 3000 cells/well in 96-well plates and cultured with the drugs for 5 days. After the test was completed, 26 µL of 2 mg/mL of MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; (Sigma-Aldrich) was added to each well and incubated at 37 °C for 4.5 hrs for crystallization. After crystallization, DMSO (100 µL/well) was added to each well. After crystals were dissolved completely (roughly 1 hr), 90 µL was taken out to a clean plate and the absorbance was measured using plate reading at absorbance of 570 nm (Molecular Devices, Sunnyvale, CA).
2.7 Immunofluorescence
Immunofluorescence staining was performed on cell culture grown on cover slides. Cells were allowed to grow on cover-slides with or without SAHA for five days. Afterward, cells were washed with PBS and fixed with 4% paraformaldehyde (for E-cadherin and fibronectin). For vimentin staining, cells were fixed using 100% ice-cold methanol on ice for 7 min. Unspecific binding sites were blocked with 3% goat serum diluted in PBS for 15 min at room temperature. After that, the cells were stained with primary antibodies (mouse anti-human E-cadherin 1:500 v/v, mouse anti-human fibronectin 1:250 v/v, mouse anti-human vimentin 1:250 v/v) diluted in blocking buffer overnight at 4 °C, washed three times and replaced with secondary antibody (FITC conjugated goat anti-mouse IgG, IgM) for 45 min in the dark at room temperature. Secondary antibodies were diluted in 1:5000. After five washes of 10 min each, the cells were mounted with vectashield with DAPI (Vector Laboratories, Burlingame, CA) and viewed under the fluorescence microscopy.
2.8 Statistical Analysis
Statistical analysis was performed on MTT assay. Cellular density was converted to percentage of the control and represented as mean ± standard error of mean.
3. Results
3.1 Immunophenotypic characterization of breast cancer cell lines
Surface markers (CD24, CD44, CD49f, CD201, ESA) were selected according to current BCSC identification systems. We examined the presence of above markers using flow cytometry in cell lines MCF-7, MCF-7/Adr, MDA-MB-231, SkBr3, and DCIS (ductal carcinoma in situ cell line) (Fig. 1, Table 1).
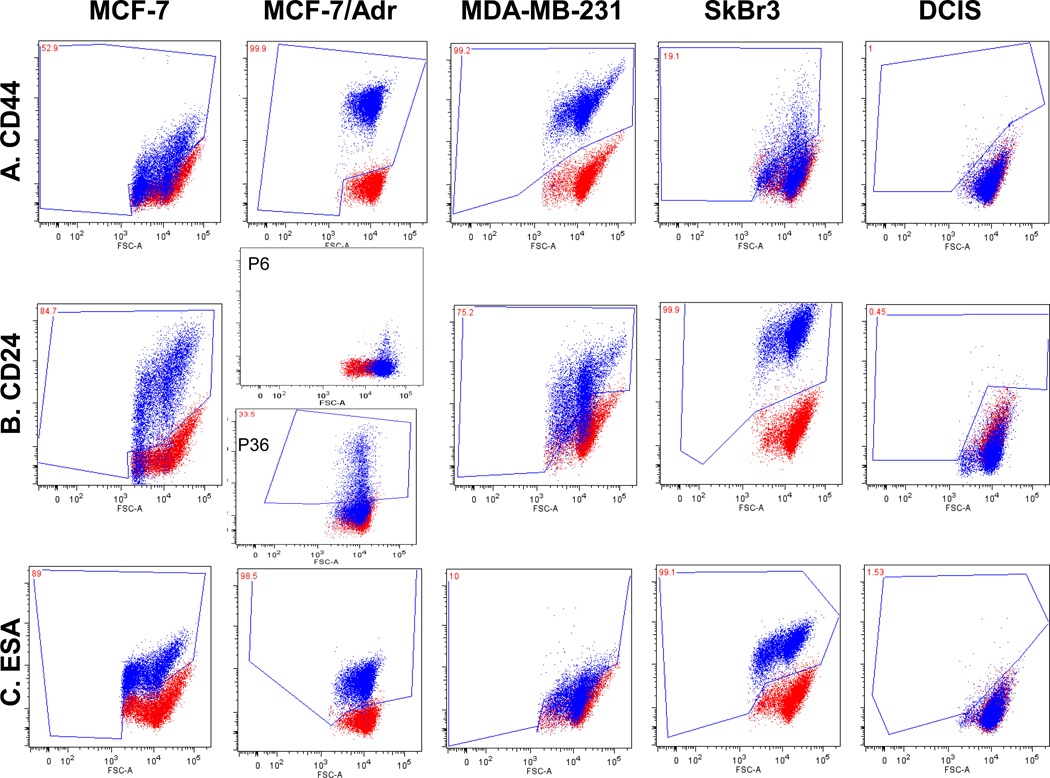
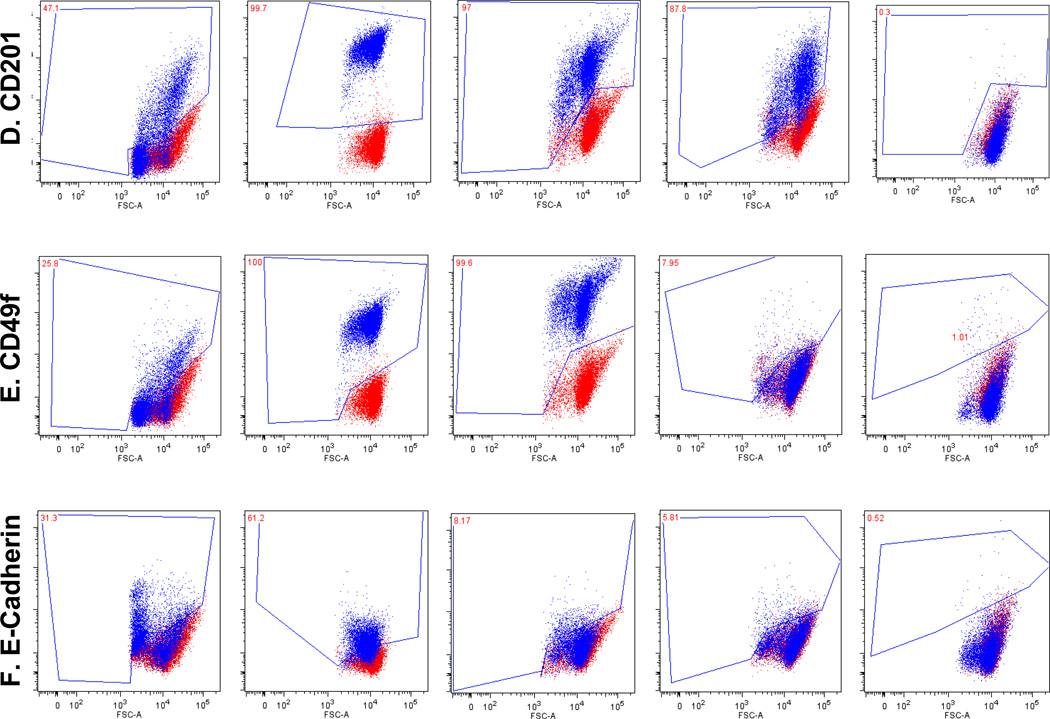
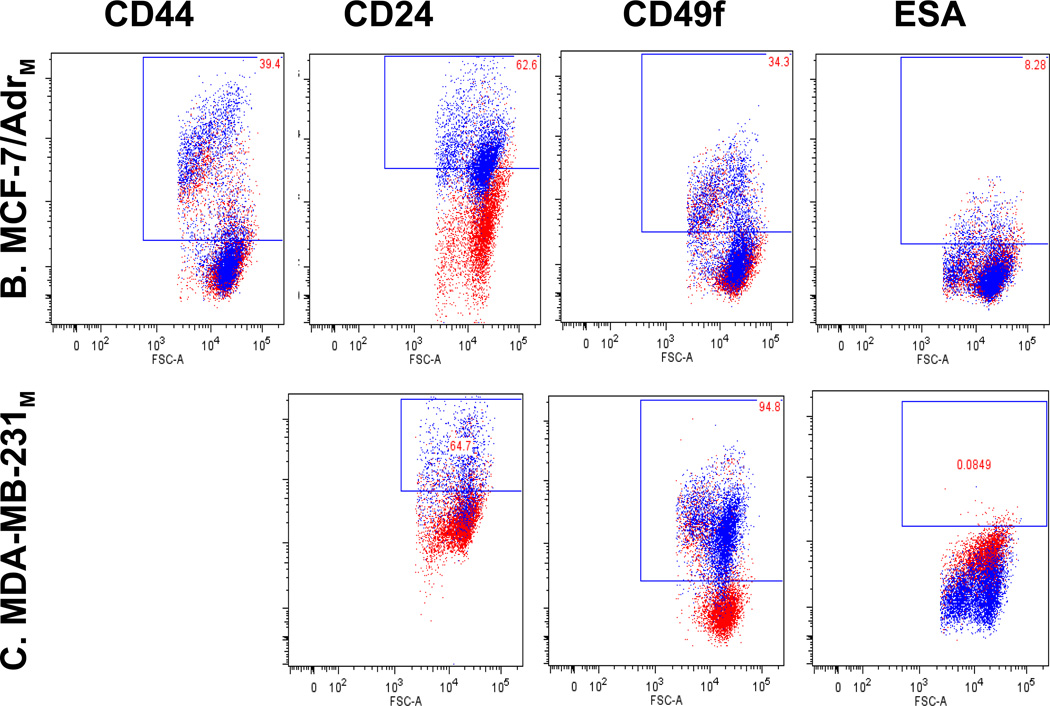
Fig. 1.
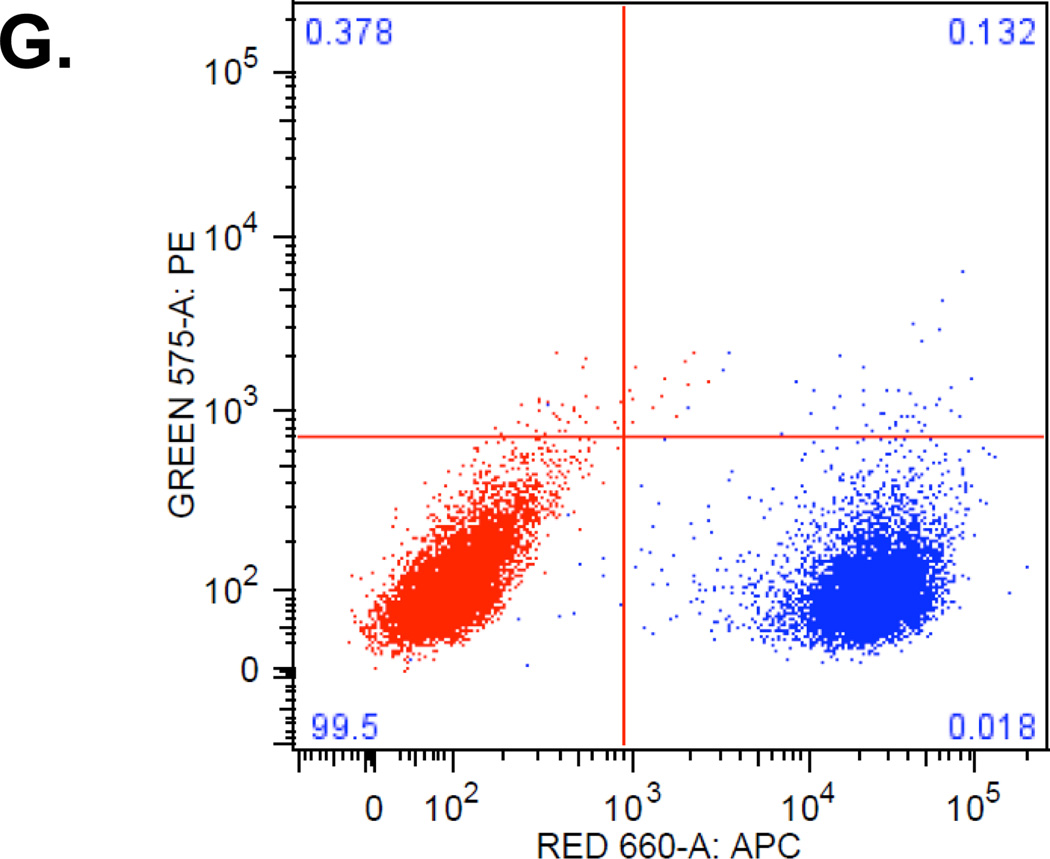
Breast cancer cell lines characterization uncovers breast cancer stem cell immunophenotypes. Red dots are unstained and blue dots are stained cells. Marker profiling of CD44 (A), CD24 (B), ESA (C), CD201 (D), CD49f (E) and E-cadherin (F) were done in cell lines MCF-7, MCF-7/Adr, MDA-MB-231, SkBr3 and DCIS by flow cytometry (see Materials and Methods). Different CD44/CD24 combinations were observed across cell lines. MCF-7/Adr showed near 100% CD44+/CD24− phenotype, commonly recognized for BCSC. MCF-7, MCF-7/Adr and MDA-MB-231 showed heterogeneity of CD24 expression, demonstrated cellular hierarchy and the existence of sub-populations in cell cultures. MCF-7/Adr’s CD24+ population varied between passages, indicative of immunophenotypic transition. (G) Double staining of CD44 (red) and CD24 (green) of MCF-7 Adr passage 6, showed 100% CD44 positive and CD24 negative.
TABLE 1.
Human breast cancer cell lines differentially express solid breast cancer stem cell markers (% of control).
Cells have >90% of positive expression are counted are positive
| CD44 | CD24 | CD201 | CD49f | ESA | E-Cadherin | |
|---|---|---|---|---|---|---|
| MCF-7 | 53 | 85 | 47 | 26 | 89 | 31 |
| MCF-7/Adr | + | Passage 6: − Passage 33: 34% |
+ | + | + | 61 |
| MDA-MB-231 | + | 75 | + | + | 10 | − |
| SkBr3 | 19 | + | 88 | − | + | − |
| DCIS | − | − | − | − | − | − |
Cells have <10% of positive expression are counted are negative
Cell lines were found to have distinct CD44/CD24 immunophenotypes (Fig. 1a, b, Table 1). In MCF-7/Adr, differential expressions between passages were observed-passage 6 negatively expresses CD24 while passage 33 displays over 30% of CD24+ cells (Fig. 1a, b, Table 1). We suspect this relates to the gradual loss of drug resistance, thus phenotypic reversal at higher passages.
MCF-7 displayed a board range of CD24 expressions, representing the phenotypic heterogeneity within an established cell line (Fig. 1b, Table 1). However, the presence of CD49f, ESA and CD201 seem to be independent of any distinctive CD44/CD24 phenotype (Fig. 1, Table 1). Compare with its parental cell line MCF-7, MCF-7/Adr’s acquisition of drug resistance thus its malignant transformation is marked by its expression of CD44, CD49f and CD201 and Ecadherin to 100% and the complete negative expression of CD24.
The acquisition of CD24−/CD44+ phenotype, along with other BCSC markers, signifies the acquisition of stem-like properties in MCF-7/Adr cells. It has been reported that cells that undergo EMT also display a loss of E-cadherin and gaining of cellular motility. However, the contrary is observed in MCF-7/Adr when compared to its parental cell line MCF-7 (Fig. 1). Our study is the first to report the uniform and stable display of BCSC markers with an increased expression of E-cadherin in a cell line over a long period of time. The cell line is slow-dividing, highly chemo-resistant, at the same time low in migration and invasiveness. For comparison purposes DCIS cell line, which negatively expresses all the above mentioned surface markers was included in the study (Fig. 1, Table 1).
3.2 Mammosphere formation assay reveals distinct cellular properties
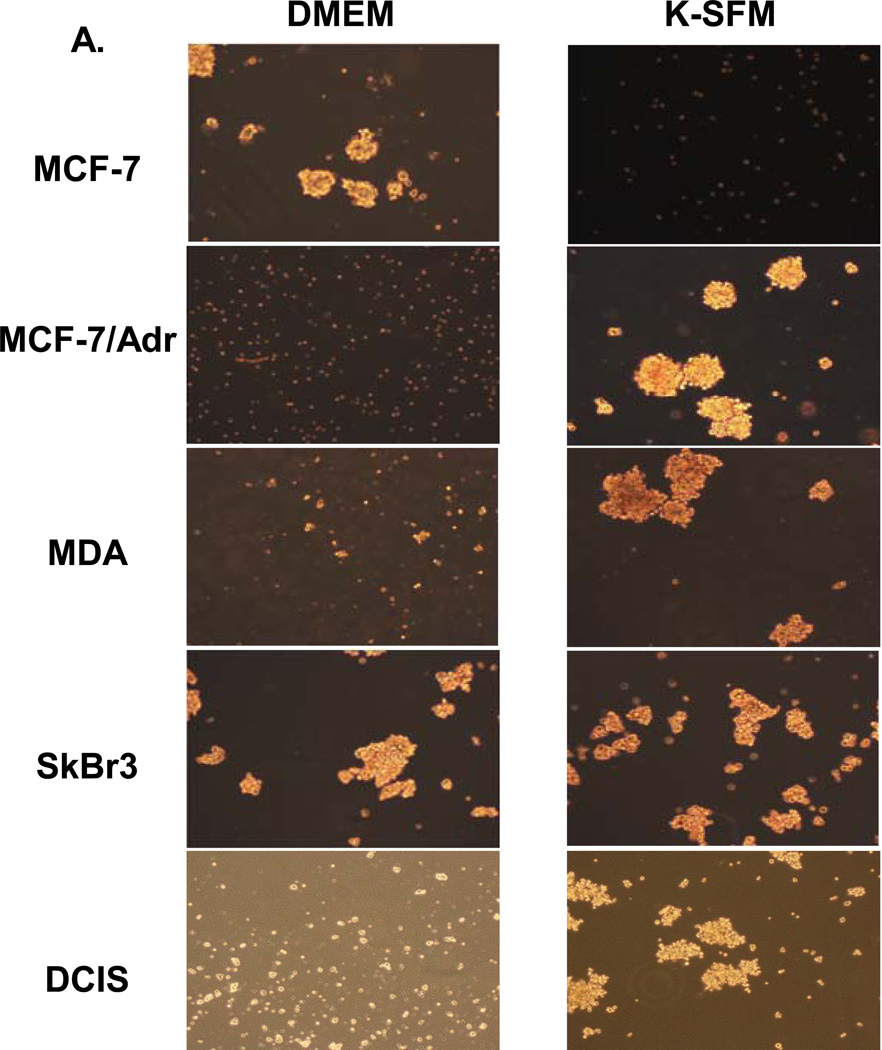
Mammosphere formation capacity to a large extent mirrors the tumor-formation capacity in vivo. We examined both cell line’s mammosphere formation ability under different culture media and the immunophenotypes of the cells dissociated from mammospheres. Mammosphere formation starts after 2–3 days and immunophenotyping was done with cells dissociated from mammospheres at day 7 (Fig. 2a, Table 3). Cell lines respond differently to different culture media. It is worth-noting the differential responses of MCF-7 and MCF-7/Adr, MCF-7/Adr, MDA and DCIS were observed to have similar responses, while MCF-7 and MCF-7/Adr responds in an opposite manner to culture conditions (Fig. 2a, Table 3). MCF-7/Adr stayed single cell suspended in DMEM which supports MCF-7 sphere formation, but grew good mammospheres in K-SFM which did not support MCF-7 mammospheres (Fig. 2a, Table 3).
Fig. 2.
Mammosphere formation assay. (A) Mammosphere formations for different cell lines require different culture conditions. MCF-7 forms mammospheres in DMEM, while MCF-7/Adr and MDA-MB-231 only form mammospheres in K-SFM, signify differences in cellular properties. Surface marker profiling of MCF-7/Adr (B) and MDA-MB-231 (C) in mammosphere culture conditions showed MCF-7/Adr’s asymmetric division. Mammosphere culture condition after 7 days showed significant reversal of CSC phenotypes in MCF-7/Adr (the positive expression of CD44, CD49f and ESA, and the negative expression of CD24). MDA-MB-231, on the contrary, did not show drastic alteration in phenotypes under mammosphere culture condition.
TABLE 3.
BCSC surface marker expression in cell lines after two weeks of SAHA treatment (% of control).
| CD44 | CD24 | CD201 | CD49f | ESA | |
|---|---|---|---|---|---|
| MCF-7 S | − | + | 34 | − | 31 |
| MCF-7/AdrS | + | 60 | 86 | + | 16 |
| MDA-MB-231 S | 70 | 34 | 48 | 59 | − |
Another interesting observation was the rapid phenotypic transition during MCF-7/Adr mammosphere culture condition, making MCF-7/Adr cells partially lose CD44, CD49f and ESA expressions, and gaining CD24+, acquiring CD44weak/CD24+/CD49weak/ESA− phenotype (Fig. 2b, Table 2). Such drastic alteration in immunophenotype was not observed in MDA-MB-231, which also formed mammospheres under K-SFM, but not DMEM culture condition (Fig. 2a, Table 3). This observation indicates that the complete loss of multiple surface markers in a short period of time in sphere culture conditions is not a common phenotype, but signifies the cell’s ability to reverse its acquired phenotype and the ability to divide differentially. The fluidity of immunophenotypic conversion observed here would open new doors to the in vitro studies of EMT and MET related processes in breast carcinoma, drawing parallels with their in vivo counterparts. The data also indicate that there is no direct correlation between a particular CD44/CD24 phenotype and a specific mammosphere culture condition (Fig. 2a, Table 3).
TABLE 2.
Alteration of BCSC markers in adherent vs. mammosphere culture conditions.
mammosphere-dissociated cells
3.3 Short-term SAHA treatment reverses drug resistance but not BCSC surface markers
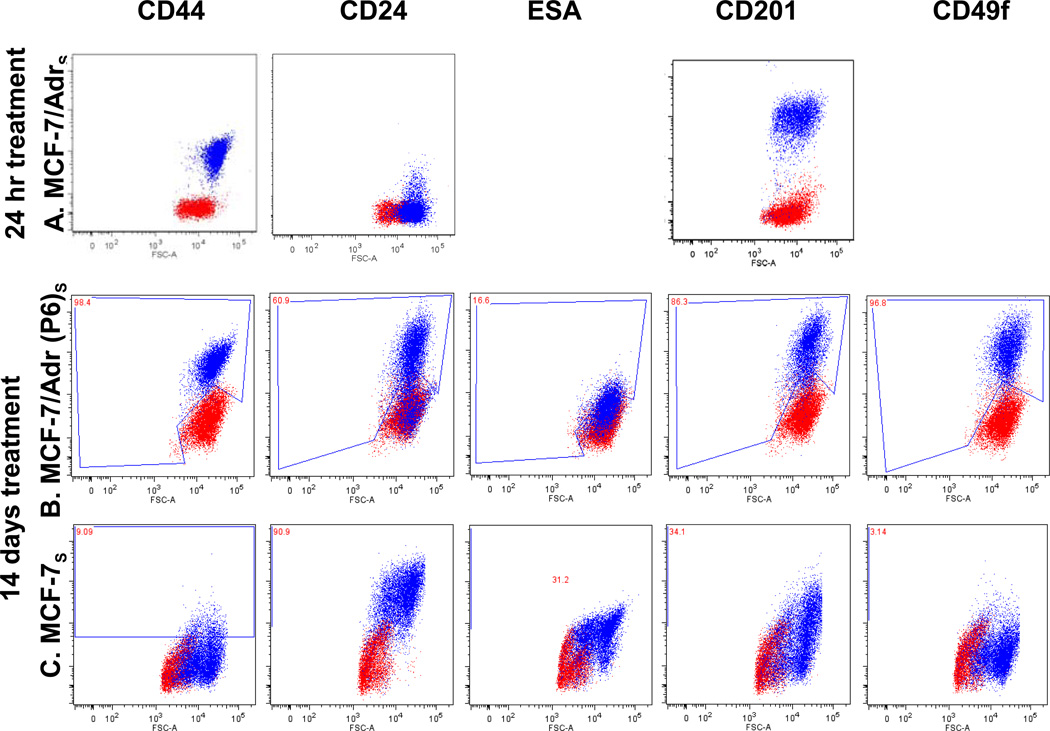
SAHA treatment was incorporated because it’s shown to counter cancer cell’s malignancy, reverse multi-drug resistance, invasiveness and metastatic potential. Cell surface marker profiling of MCF-7/Adr was done after 24 hours or 14 days of SAHA treatment, to examine if short-term drug treatment would make alterations on the surface markers. We observed no alteration in surface markers (Fig. 3a).
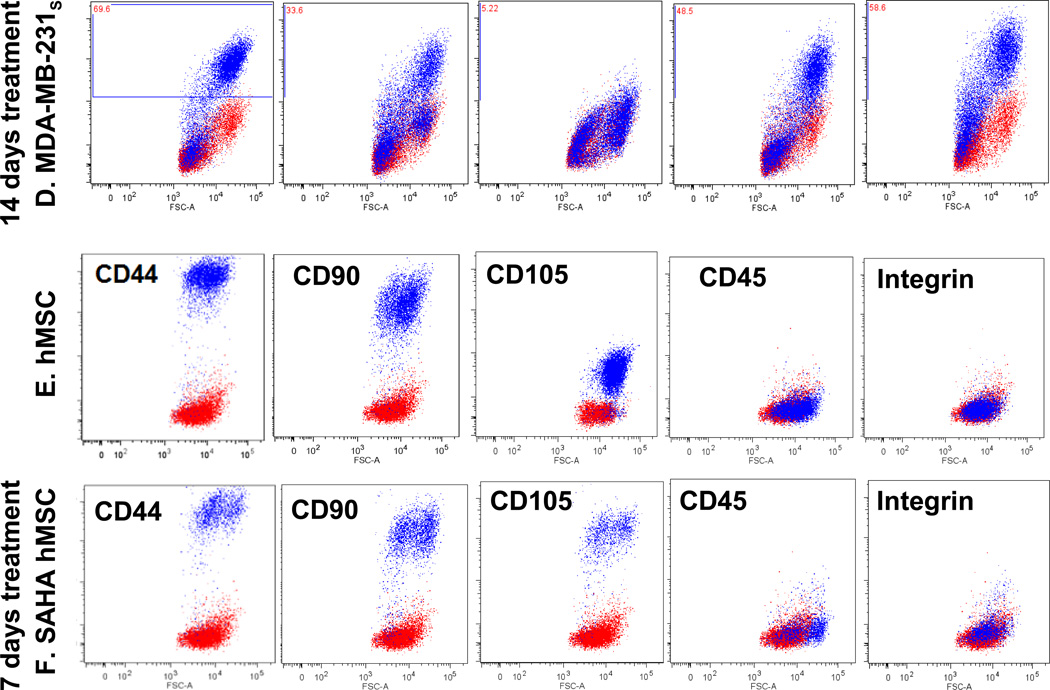
Fig. 3.
SAHA’s effect on cell surface markers. (A) 24 hours SAHA treatment, which was shown to alter cell membrane fluidity, reverse (transiently) drug resistance, and slightly increase MCF-7/Adr’s mobility, showed no effect on the expression of MCF-7/Adr surface markers. (B, C, D) 14 days of SAHA treatment on MCF-7/Adr passage 6, MCF-7 and MDA-MB-231 respectively. (E. F) human mesenchymal stem cell surface marker on hMSC before and after 7 days of SAHA treatment. SAHA treatment for 7 days (dosing every other day) had no effect on hMSC’s identification markers.
Mesenchymal stem cells were incorporated in the study to examine SAHA’s differentiation ability. The cells were treated with SAHA for 7 days (drug replenished every 2 days) and examined surface markers using MSC identification marker panel, found to retain the same set of surface markers as before treatment (Fig. 3b). However, it is possible that the drug triggers the up or down-expression of other surface markers not tested using our purchased identification kit.
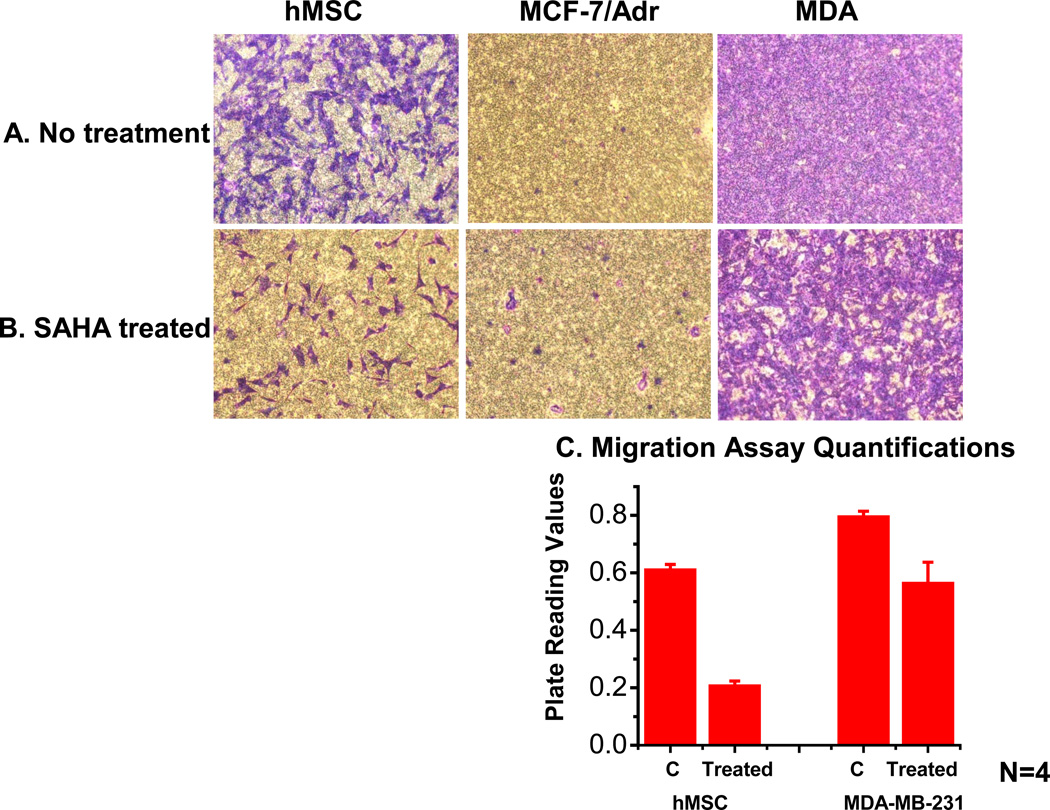
3.4 SAHA displays differential drug effect on cellular migratory ability
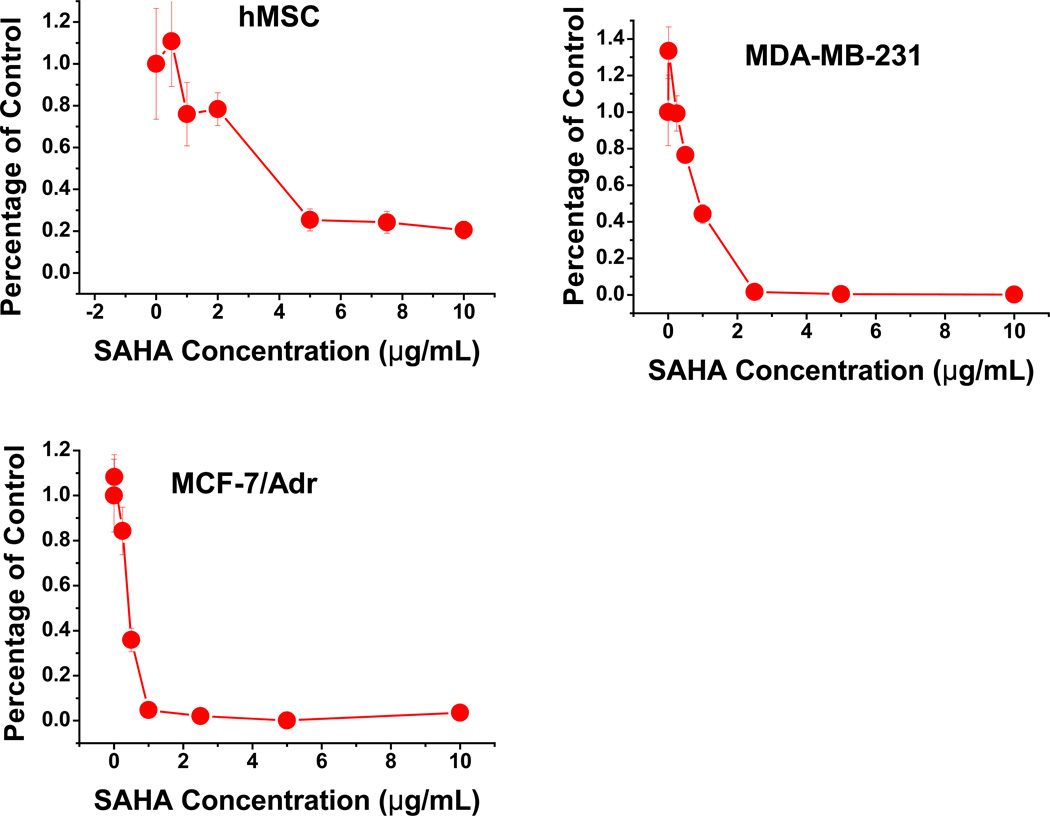
Migration assay was performed on the highly migratory cell lines hMSCs and MDA-MB-231, and highly adherent MCF-7/Adr with or without SAHA treatment. For both migratory cell lines, SAHA was found to significantly lower migratory ability (Fig. 4). On the other hand, MCF- 7/Adr remains non-migratory, uninfluenced by SAHA treatment (Fig. 4). This observation was possibly due to drug effect on MCF-7/Adr’s membrane properties, increasing membrane fluidity and decreasing cell-cell contact and adhesion ability, thus making them more mobile. MTT assay showed MCF-7/Adr to be much more vulnerable to SAHA than both MDA-MB-231 and hMSC (Fig. 5).
Fig. 4.
Migration Assay assesses SAHA’s migratory potential in 24 hours. 24 hours SAHA treatment during migration assay dramatically reduced cellular motility for highly migratory cell lines hMSC and MDA-MB-231. MCF-7/Adr, a strongly adherent and non-migratory cell line, did not demonstrate significant change due to SAHA treatment. (C) Migratory rate quantification showed significant change in migration rate in both hMSC and MDA-MB-231.
Fig. 5.
SAHA resistance in different cell lines assessed by MTT assay. hMSC has the greatest resistance to SAHA, while MCF-7/Adr, which shows multi-drug resistant phenotype, has the least SAHA resistance.
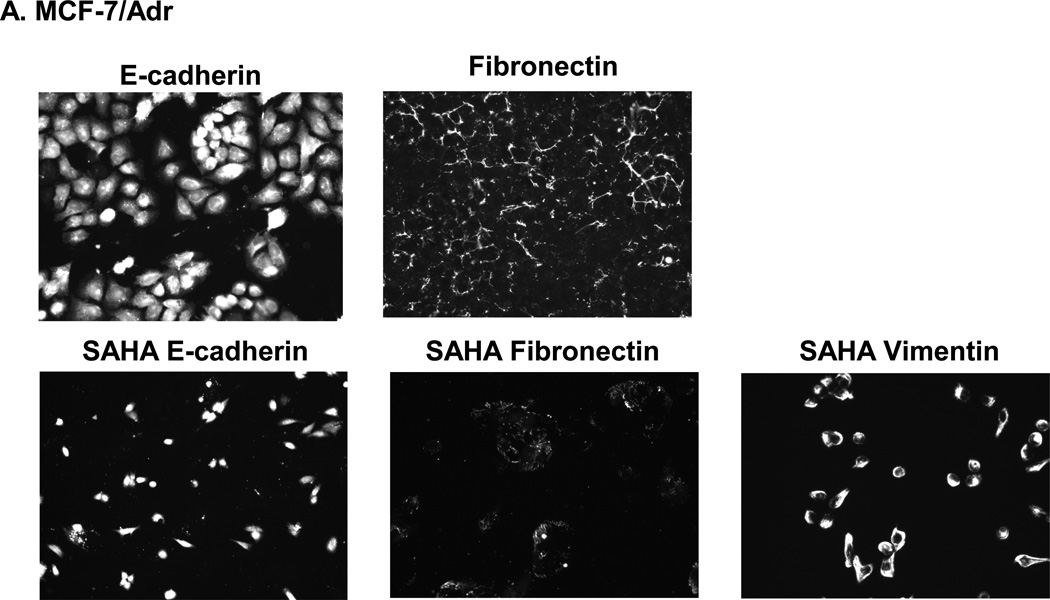
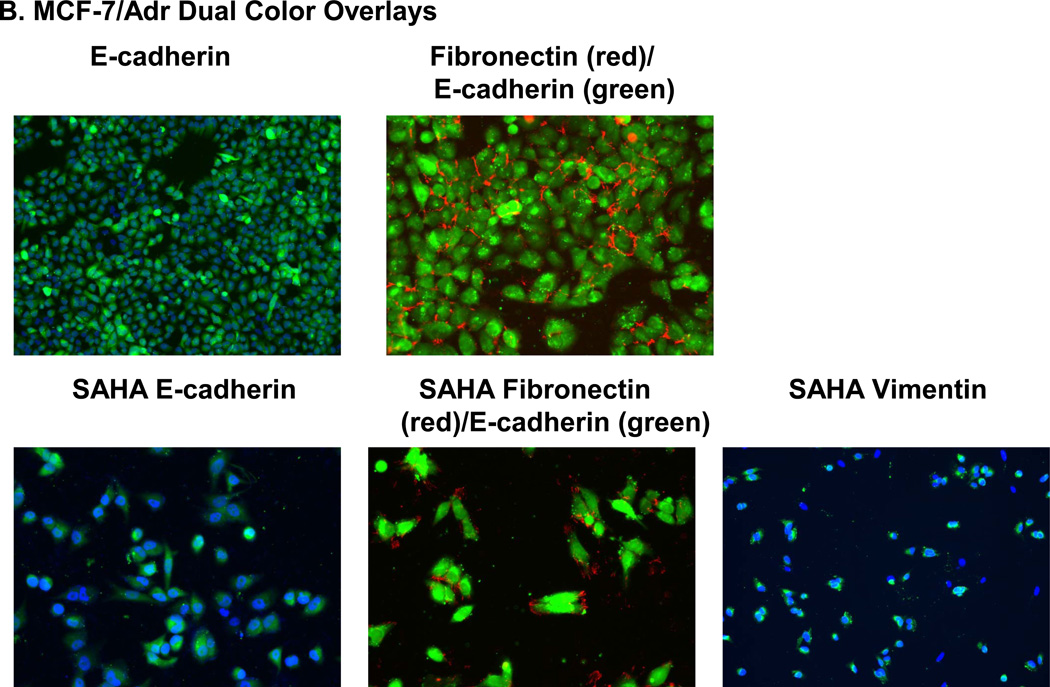
3.5 Expression of E-cadherin, fibronectin and vimentin in MCF-7/Adr cells
All three proteins, E-cadherin, fibronectin and vimentin, were positively expressed by MCF-7/Adr both before and after 5 days of SAHA treatment (Fig. 6).
Fig. 6.
(A) SAHA effect on E-cadherin, fibronectin and vimentin in MCF-7/Adr. Immunofluorescence staining examines the presence of E-cadherin, fibronectin and vimentin with or without 7 days of SAHA treatment in MCF-7/Adr. There is evident expression of Ecadherin, fibronectin and vimentin both with and without SAHA treatment. (B) Dual color imaging of both untreated and SAHA treated MCF-7/Adr. Blue: nucleus, green: E-cadherin or vimentin, red: fibronectin.
4. Discussion
4.1 CD44+/CD24− phenotype
It has been shown that cancer stem cells should be able to self-renew and differentiate, being drug/radiation-resistant and highly in metastatic/invasive [2]. CD44/CD24 combinations categorize breast cancer cells into luminal, basal A and basal B subtypes [24]. High CD44+/CD24− expression represents basal/mesenchymal phenotype, while CD44−/CD24+ signatures luminal/epithelial phenotype [24]. We observed all four combinations in our cell lines, having metastatic MDA-MB-231 cell line positively expresses CD44 and have 70% CD24+ expression. On the other hand, ductal carcinoma have uniform CD44−/CD24− phenotype (Fig. 1), whose tumorigenic and metastatic properties we know very little about. For the first time, a cell line with uniform CD44+/CD24− phenotype was reported, which possesses high drug resistance but poor migratory and proliferation potential. A number of other BCSC surface markers, include CD201, CD49f, and ESA, were also positively identified in MCF-7/Adr (Fig. 1).
4.2 Malignant Transformation
The malignant transformation the parental cell line MCF-7 experiences to acquire its drug resistance is likely through epithelial-mesenchymal transition (EMT). EMT genes in MCF- 7/Adr were analyzed in detail by Ozlem et al, and were confirmed to be up-regulated comparing to its parental cell line [25]. Similar observations were made by Arumugam et al. have reported that cells acquire drug resistance through EMT in pancreatic cancer [26]. However, differ from traditional views on EMT, which has been closely related to tumour progression, increase in cell motility and invasion [27], MCF-7/Adr demonstrated decreased motility and a stronger cellular adhesion compare to its parental cell line MCF-7 (Fig. 4). Also, it showed no E-cadherin repression (Table 1) and no increase in tumorigenicity.
Previous studies have identified CD44+/CD24− sub-population in a number of cell lines include MDA-MB-231, MDA-MB-436, and SUM204, and was sought to have higher invasiveness and metastatic potential than the rest of cell population [27]. We have also observed heterogeneity in our MDA-MB-231-luc cell line, containing roughly 25% of CD24− phenotype (Fig. 1). The entire MDA-MB-231-luc cell line positively expresses CD201, CD49f, but negatively expresses ESA and E-cadherin, which relates to cell’s migratory ability. Since many therapies are formulated based on cancer cell’s molecular profiles [28], it is important to precisely correlate immunophenotypes with cellular properties. Fillmore et al. also showed that CD44+/CD24− phenotype in the culture has high metastatic ability and invasiveness [28]. Since CD44+/CD24− phenotype is also evident in our MCF-7/Adr cell line, which possesses no metastatic or invasive properties, we suspect either the CD44+/CD24− phenotype represent different properties which are cell-line specific, or there are other markers which are greater indicators of metastatic potentials than CD44/CD24. Furthermore, no study has yet shown an entire cell-line possessing CD44+/CD24−/ESA+ phenotype able to be maintained stably for a long period of time without reversing back to its parental phenotype.
Fibronectin and vimentin are both EMT proteins. Vimentin signifies basal phenotype, while E-cadherin represents luminal phenotype [24]. Our study shows that MCF-7/Adr expresses a high level of E. Cadherin, at the same time positively expressing vimentin and fibronectin (Fig. 6). This observation is uniquely important in revolutionizing the established perceptions on BCSCs and EMT. It was reported that a number of genes induced in iPS transformation were well-established oncogenes (c-myc and SOX2, for example) [29–31]. It is therefore reasonable to make the connection between the acquisition of drug resistance (the up-regulation of oncogenes) and the transition into BCSC-like cells.
4.3 On mammosphere differentiation
Mammosphere culture mirrors in vitro tumorigenic capacity. It has been shown that MCF-7 undergoes EMT through mammosphere culture condition, generating sub-population with CD44+/CD24− mesenchymal phenotype, E-cadherin down-regulation and more tumorigenic cells formation [32]. We report the reverse of EMT in MCF-7/Adr through mammosphere culture conditions, which loses its acquired BCSC markers in 7 days (Fig. 2b, Table 2). MDA-MB-231 mammospheres were also characterized and examined for comparison purposes, and no drastic phenotypic alterations were observed. It is possible that at in vivo implantation, similar transition takes place resulting in a heterogeneous tumor with partial chemo-resistance.
4.4 Implications for Breast Cancer and in vitro CSC studies
The difficulties of sorting CSC subpopulation from tumors give rise to the need of a reliable system to study CSCs in vitro. Inconsistency across reported studies, especially in the characterizations of CSCs in primary tumors is not uncommon. Also, it is yet unknown whether the gaining of CD44+/CD24− phenotype is an exclusive indication of EMT. Nevertheless, this study provided a new perspective on breast CSC phenotypes through the display of uniform CD44+/CD24− phenotype in a cell line, which has great implications on the in vitro studies of transitions between epithelial and mesenchymal phenotypes.
5. Conclusions
This study for the first time identified a cell line with uniform BCSC immunophenotype, possessing drug resistant characteristics, can be maintained stably overtime and undergoes environment-driven MET within 7 days. Phenotypically, it is high in drug resistance and low in metastasis. It positively expresses E-cadherin, vimentin and fibronectin. Its drug resistance can be reversed by short-term SAHA treatment yet retaining its BCSC immunophenotype. The unique characteristics presented by this cell line opens new doors for the in vitro characterization of BCSC-like phenotypes, and for drawing parallels with similar in vivo observations.
Acknowledgements
This study was funded by grant R01 CA149359-01 (to VL) from the National Cancer Institute of the National Institutes of Health.
List of Abbreviations
- BCSC
breast cancer stem cell
- SAHA
suberoylanilide hydroxamic acid
- hMSC
human mesenchymal stem cell
- ESA
epithelial-specific antigen
References
- 1.Dick JE. Breast cancer stem cells revealed. Proc Natl Acad Sci U S A. 2003;100(7):3547–3549. doi: 10.1073/pnas.0830967100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dick JE. Stem cells: Self-renewal writ in blood. Nature. 2003;423(6937):231–233. doi: 10.1038/423231a. [DOI] [PubMed] [Google Scholar]
- 3.Li W, Liu F, Lei T, Xu X, Liu B, Cui L, et al. The clinicopathological significance of CD44+/CD24−/low and CD24+ tumor cells in invasive micropapillary carcinoma of the breast. Pathol Res Pract. 2010;206(12):828–834. doi: 10.1016/j.prp.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Griffin JD, Lowenberg B. Clonogenic cells in acute myeloblastic leukemia. Blood. 1986;68(6):1185–1195. [PubMed] [Google Scholar]
- 5.McCulloch EA. Stem cells in normal and leukemic hemopoiesis (Henry Stratton Lecture, 1982) Blood. 1983;62(1):1–13. [PubMed] [Google Scholar]
- 6.Charafe-Jauffret E, Ginestier C, Birnbaum D. Breast cancer stem cells: tools and models to rely on. BMC Cancer. 2009;9:202. doi: 10.1186/1471-2407-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuthapisith S, Eremin J, El-Sheemey M, Eremin O. Breast cancer chemoresistance: emerging importance of cancer stem cells. Surg Oncol. 2010;19(1):27–32. doi: 10.1016/j.suronc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Dontu G. Breast cancer stem cell markers - the rocky road to clinical applications. Breast Cancer Res. 2008;10(5):110. doi: 10.1186/bcr2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26(17):2813–2820. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 11.Morrison BJ, Schmidt CW, Lakhani SR, Reynolds BA, Lopez JA. Breast cancer stem cells: implications for therapy of breast cancer. Breast Cancer Res. 2008;10(4):210. doi: 10.1186/bcr2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98(24):1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 13.Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11(3):1154–1159. [PubMed] [Google Scholar]
- 14.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauerschmitz GJ, Ranki T, Kangasniemi L, Ribacka C, Eriksson M, Porten M, et al. Tissue-specific promoters active in CD44+CD24−/low breast cancer cells. Cancer Res. 2008;68(14):5533–5539. doi: 10.1158/0008-5472.CAN-07-5288. [DOI] [PubMed] [Google Scholar]
- 16.Hill RP, Perris R. "Destemming" cancer stem cells. J Natl Cancer Inst. 2007;99(19):1435–1440. doi: 10.1093/jnci/djm136. [DOI] [PubMed] [Google Scholar]
- 17.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317(5836):337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 18.Snyder EL, Bailey D, Shipitsin M, Polyak K, Loda M. Identification of CD44v6(+)/CD24− breast carcinoma cells in primary human tumors by quantum dot-conjugated antibodies. Lab Invest. 2009;89(8):857–866. doi: 10.1038/labinvest.2009.54. [DOI] [PubMed] [Google Scholar]
- 19.Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, et al. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10(3):R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mylona E, Giannopoulou I, Fasomytakis E, Nomikos A, Magkou C, Bakarakos P, et al. The clinicopathologic and prognostic significance of CD44+/CD24(−/low) and CD44−/CD24+ tumor cells in invasive breast carcinomas. Hum Pathol. 2008;39(7):1096–1102. doi: 10.1016/j.humpath.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Baumann P, Cremers N, Kroese F, Orend G, Chiquet-Ehrismann R, Uede T, et al. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res. 2005;65(23):10783–10793. doi: 10.1158/0008-5472.CAN-05-0619. [DOI] [PubMed] [Google Scholar]
- 22.Osta WA, Chen Y, Mikhitarian K, Mitas M, Salem M, Hannun YA, et al. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004;64(16):5818–5824. doi: 10.1158/0008-5472.CAN-04-0754. [DOI] [PubMed] [Google Scholar]
- 23.An Z, Gluck CB, Choy ML, Kaufman LJ. Suberoylanilide hydroxamic acid limits migration and invasion of glioma cells in two and three dimensional culture. Cancer Lett. 2010;292(2):215–227. doi: 10.1016/j.canlet.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME, Neve RM, et al. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25(6):629–642. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- 25.Iseri OD, Kars MD, Arpaci F, Atalay C, Pak I, Gunduz U. Drug resistant MCF-7 cells exhibit epithelial-mesenchymal transition gene expression pattern. Biomed Pharmacother. 2011;65(1):40–45. doi: 10.1016/j.biopha.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69(14):5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, et al. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8(5):R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10(2):R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41(11):1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 32.Guttilla IK, Phoenix KN, Hong X, Tirnauer JS, Claffey KP, White BA. Prolonged mammosphere culture of MCF-7 cells induces an EMT and repression of the estrogen receptor by microRNAs. Breast Cancer Res Treat. 2012;132(1):75–85. doi: 10.1007/s10549-011-1534-y. [DOI] [PubMed] [Google Scholar]