Abstract
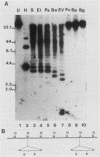
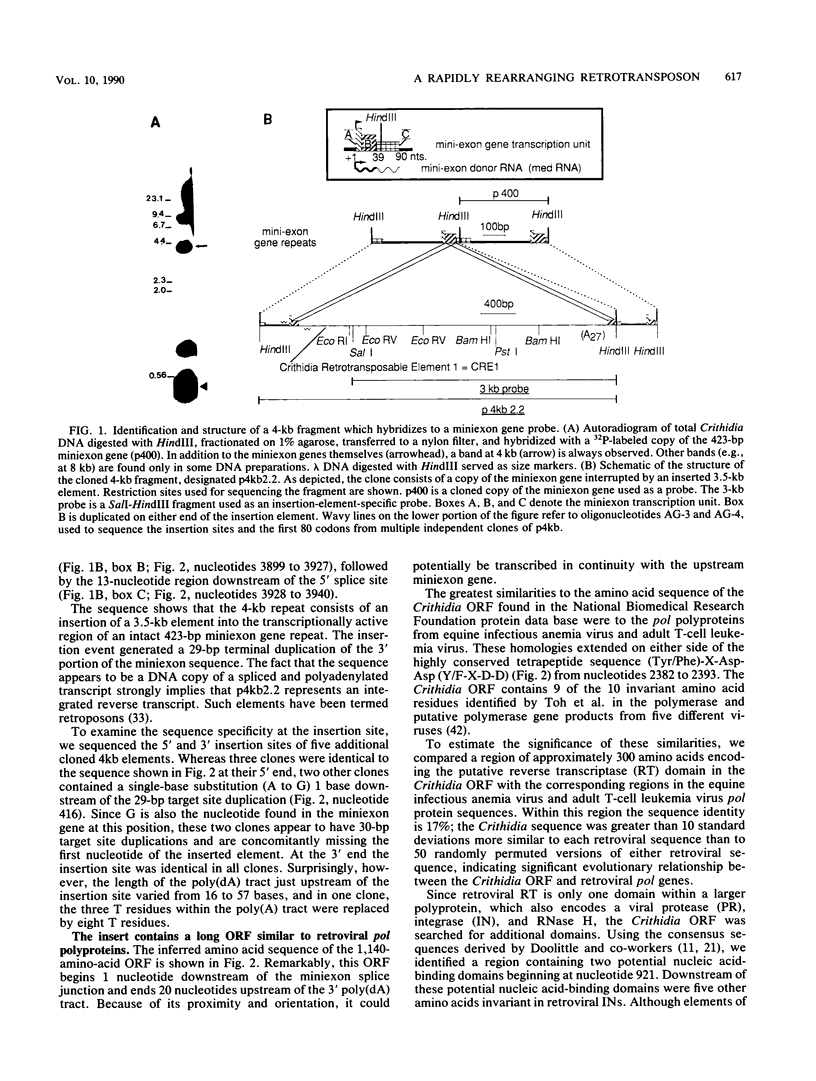
The tandemly arrayed miniexon genes of the trypanosomatid Crithidia fasciculata are interrupted at specific sites by multiple copies of an inserted element. The element, termed Crithidia retrotransposable element 1 (CRE1), is flanked by 29-base-pair target site duplications and contains a long 3'-terminal poly(dA) stretch. A single 1,140-codon reading frame is similar in sequence to the integrase and reverse transcriptase regions of retroviral pol polyproteins. Cloned lines derived from a stock of C. fasciculata have unique arrangements of CRE1s. In different cloned lines, CRE1s, in association with miniexon genes, are located on multiple chromosomes. By examining the arrangement of CRE1s in subclones, we estimate that the element rearranges at a rate of ca. 1% per generation. These results indicate that the C. fasciculata miniexon locus is the target for a novel retrotransposon.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksoy S., Lalor T. M., Martin J., Van der Ploeg L. H., Richards F. F. Multiple copies of a retroposon interrupt spliced leader RNA genes in the African trypanosome, Trypanosoma gambiense. EMBO J. 1987 Dec 1;6(12):3819–3826. doi: 10.1002/j.1460-2075.1987.tb02718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellofatto V., Cooper R., Cross G. A. Discontinuous transcription in Leptomonas seymouri: presence of intact and interrupted mini-exon gene families. Nucleic Acids Res. 1988 Aug 11;16(15):7437–7456. doi: 10.1093/nar/16.15.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Corces V. G. Transcription and reverse transcription of retrotransposons. Annu Rev Microbiol. 1989;43:403–434. doi: 10.1146/annurev.mi.43.100189.002155. [DOI] [PubMed] [Google Scholar]
- Burke W. D., Calalang C. C., Eickbush T. H. The site-specific ribosomal insertion element type II of Bombyx mori (R2Bm) contains the coding sequence for a reverse transcriptase-like enzyme. Mol Cell Biol. 1987 Jun;7(6):2221–2230. doi: 10.1128/mcb.7.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. A., Thornton D. A., Boothroyd J. C. Apparent discontinuous transcription of Trypanosoma brucei variant surface antigen genes. 1984 Sep 27-Oct 3Nature. 311(5984):350–355. doi: 10.1038/311350a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington M., Roditi I., Williams R. O. The structure and transcription of an element interspersed between tandem arrays of mini-exon donor RNA genes in Trypanosoma brucei. Nucleic Acids Res. 1987 Dec 23;15(24):10179–10198. doi: 10.1093/nar/15.24.10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs G., Maxson R., Cohn R. H., Kedes L. Orphons: dispersed genetic elements derived from tandem repetitive genes of eucaryotes. Cell. 1981 Mar;23(3):651–663. doi: 10.1016/0092-8674(81)90428-1. [DOI] [PubMed] [Google Scholar]
- Cornelissen A. W., Verspieren M. P., Toulmé J. J., Swinkels B. W., Borst P. The common 5' terminal sequence on trypanosome mRNAs: a target for anti-messenger oligodeoxynucleotides. Nucleic Acids Res. 1986 Jul 25;14(14):5605–5614. doi: 10.1093/nar/14.14.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S., McClure M. A. Origins and evolutionary relationships of retroviruses. Q Rev Biol. 1989 Mar;64(1):1–30. doi: 10.1086/416128. [DOI] [PubMed] [Google Scholar]
- Englund P. T. The replication of kinetoplast DNA networks in Crithidia fasciculata. Cell. 1978 May;14(1):157–168. doi: 10.1016/0092-8674(78)90310-0. [DOI] [PubMed] [Google Scholar]
- Feagin J. E., Abraham J. M., Stuart K. Extensive editing of the cytochrome c oxidase III transcript in Trypanosoma brucei. Cell. 1988 May 6;53(3):413–422. doi: 10.1016/0092-8674(88)90161-4. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gabriel A., Sisodia S. S., Cleveland D. W. Evidence of discontinuous transcription in the trypanosomatid Crithidia fasciculata. J Biol Chem. 1987 Nov 25;262(33):16192–16199. [PubMed] [Google Scholar]
- Harbers K., Soriano P., Müller U., Jaenisch R. High frequency of unequal recombination in pseudoautosomal region shown by proviral insertion in transgenic mouse. Nature. 1986 Dec 18;324(6098):682–685. doi: 10.1038/324682a0. [DOI] [PubMed] [Google Scholar]
- Hasan G., Turner M. J., Cordingley J. S. Complete nucleotide sequence of an unusual mobile element from trypanosoma brucei. Cell. 1984 May;37(1):333–341. doi: 10.1016/0092-8674(84)90329-5. [DOI] [PubMed] [Google Scholar]
- Hasan G., Turner M. J., Cordingley J. S. Ribosomal RNA genes of Trypanosoma brucei. Cloning of a rRNA gene containing a mobile element. Nucleic Acids Res. 1982 Nov 11;10(21):6747–6761. doi: 10.1093/nar/10.21.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Royle N. J., Wilson V., Wong Z. Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature. 1988 Mar 17;332(6161):278–281. doi: 10.1038/332278a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Individual-specific 'fingerprints' of human DNA. Nature. 1985 Jul 4;316(6023):76–79. doi: 10.1038/316076a0. [DOI] [PubMed] [Google Scholar]
- Johnson M. S., McClure M. A., Feng D. F., Gray J., Doolittle R. F. Computer analysis of retroviral pol genes: assignment of enzymatic functions to specific sequences and homologies with nonviral enzymes. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7648–7652. doi: 10.1073/pnas.83.20.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel B. E., ole-MoiYoi O. K., Young J. R. Ingi, a 5.2-kb dispersed sequence element from Trypanosoma brucei that carries half of a smaller mobile element at either end and has homology with mammalian LINEs. Mol Cell Biol. 1987 Apr;7(4):1465–1475. doi: 10.1128/mcb.7.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambowitz A. M. Infectious introns. Cell. 1989 Feb 10;56(3):323–326. doi: 10.1016/0092-8674(89)90232-8. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Marini J. C., Levene S. D., Crothers D. M., Englund P. T. Bent helical structure in kinetoplast DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7664–7668. doi: 10.1073/pnas.79.24.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhich M. L., Hughes D. E., Simpson A. M., Simpson L. The monogenetic kinetoplastid protozoan, Crithidia fasciculata, contains a transcriptionally active, multicopy mini-exon sequence. Nucleic Acids Res. 1987 Apr 10;15(7):3141–3153. doi: 10.1093/nar/15.7.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy N. B., Pays A., Tebabi P., Coquelet H., Guyaux M., Steinert M., Pays E. Trypanosoma brucei repeated element with unusual structural and transcriptional properties. J Mol Biol. 1987 Jun 20;195(4):855–871. doi: 10.1016/0022-2836(87)90490-6. [DOI] [PubMed] [Google Scholar]
- Murphy W. J., Watkins K. P., Agabian N. Identification of a novel Y branch structure as an intermediate in trypanosome mRNA processing: evidence for trans splicing. Cell. 1986 Nov 21;47(4):517–525. doi: 10.1016/0092-8674(86)90616-1. [DOI] [PubMed] [Google Scholar]
- Parsons M., Nelson R. G., Stuart K., Agabian N. Variant antigen genes of Trypanosoma brucei: genomic alteration of a spliced leader orphon and retention of expression-linked copies during differentiation. Proc Natl Acad Sci U S A. 1984 Feb;81(3):684–688. doi: 10.1073/pnas.81.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons M., Nelson R. G., Watkins K. P., Agabian N. Trypanosome mRNAs share a common 5' spliced leader sequence. Cell. 1984 Aug;38(1):309–316. doi: 10.1016/0092-8674(84)90552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard M. A., Dura J. M., Pélisson A., Bucheton A., Finnegan D. J. A cloned I-factor is fully functional in Drosophila melanogaster. Mol Gen Genet. 1988 Nov;214(3):533–540. doi: 10.1007/BF00330491. [DOI] [PubMed] [Google Scholar]
- Rogers J. Retroposons defined. Nature. 1983 Feb 10;301(5900):460–460. doi: 10.1038/301460e0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Saffran W., Welsh J., Haas R., Goldenberg M., Cantor C. R. New techniques for purifying large DNAs and studying their properties and packaging. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):189–195. doi: 10.1101/sqb.1983.047.01.024. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. M., Feagin J. E., Stuart K., Simpson L. Editing of kinetoplastid mitochondrial mRNAs by uridine addition and deletion generates conserved amino acid sequences and AUG initiation codons. Cell. 1988 May 6;53(3):401–411. doi: 10.1016/0092-8674(88)90160-2. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Sutton R. E., Boothroyd J. C. Evidence for trans splicing in trypanosomes. Cell. 1986 Nov 21;47(4):527–535. doi: 10.1016/0092-8674(86)90617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Wu R. Unequal crossing over in the ribosomal DNA of Saccharomyces cerevisiae. Nature. 1980 Apr 3;284(5755):426–430. doi: 10.1038/284426a0. [DOI] [PubMed] [Google Scholar]
- Toh H., Hayashida H., Miyata T. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. 1983 Oct 27-Nov 2Nature. 305(5937):827–829. doi: 10.1038/305827a0. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Schwartz D. C., Cantor C. R., Borst P. Antigenic variation in Trypanosoma brucei analyzed by electrophoretic separation of chromosome-sized DNA molecules. Cell. 1984 May;37(1):77–84. doi: 10.1016/0092-8674(84)90302-7. [DOI] [PubMed] [Google Scholar]
- Varmus H. Retroviruses. Science. 1988 Jun 10;240(4858):1427–1435. doi: 10.1126/science.3287617. [DOI] [PubMed] [Google Scholar]
- Walder J. A., Eder P. S., Engman D. M., Brentano S. T., Walder R. Y., Knutzon D. S., Dorfman D. M., Donelson J. E. The 35-nucleotide spliced leader sequence is common to all trypanosome messenger RNA's. Science. 1986 Aug 1;233(4763):569–571. doi: 10.1126/science.3523758. [DOI] [PubMed] [Google Scholar]
- Xiong Y. E., Eickbush T. H. Functional expression of a sequence-specific endonuclease encoded by the retrotransposon R2Bm. Cell. 1988 Oct 21;55(2):235–246. doi: 10.1016/0092-8674(88)90046-3. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Eickbush T. H. Similarity of reverse transcriptase-like sequences of viruses, transposable elements, and mitochondrial introns. Mol Biol Evol. 1988 Nov;5(6):675–690. doi: 10.1093/oxfordjournals.molbev.a040521. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Eickbush T. H. The site-specific ribosomal DNA insertion element R1Bm belongs to a class of non-long-terminal-repeat retrotransposons. Mol Cell Biol. 1988 Jan;8(1):114–123. doi: 10.1128/mcb.8.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]