Abstract
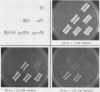
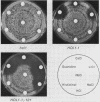
Saccharomyces cerevisiae histidine auxotrophs are unable to use L-histidinol as a source of histidine even when they have a functional histidinol dehydrogenase. Mutations in the hol1 gene permit growth of His- cells on histidinol by enhancing the ability of cells to take up histidinol from the medium. Second-site mutations linked to HOL1-1 further increase histidinol uptake. HOL1 double mutants and, to a lesser extent, HOL1-1 single mutants show hypersensitivity to specific cations added to the growth medium, including Na+, Li+, Cs+, Be2+, guanidinium ion, and histidinol, but not K+, Rb+, Ca2+, or Mg2+. The Na(+)-hypersensitive phenotype is correlated with increased uptake and accumulation of this ion. The HOL1-1-101 gene was cloned and used to generate a viable haploid strain containing a hol1 deletion mutation (hol1 delta). The uptake of cations, the dominance of the mutant alleles, and the relative inability of hol1 delta cells to take up histidinol or Na+ suggest that hol1 encodes an ion transporter. The novel pattern of ion transport conferred by HOL1-1 and HOL1-1-101 mutants may be explained by reduced selectivity for the permeant ions.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartley W., Broomhead V. Properties of yeast grown anaerobically in media limiting in potassium. Biochem J. 1971 Feb;121(3):461–467. doi: 10.1042/bj1210461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Borst-Pauwels G. W. Ion transport in yeast. Biochim Biophys Acta. 1981 Dec;650(2-3):88–127. doi: 10.1016/0304-4157(81)90002-2. [DOI] [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Crabeel M., Grenson M. Regulation of histidine uptake by specific feedback inhibition of two histidine permeases in Saccharomyces cerevisiae. Eur J Biochem. 1970 May 1;14(1):197–204. doi: 10.1111/j.1432-1033.1970.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Donahue T. F., Farabaugh P. J., Fink G. R. The nucleotide sequence of the HIS4 region of yeast. Gene. 1982 Apr;18(1):47–59. doi: 10.1016/0378-1119(82)90055-5. [DOI] [PubMed] [Google Scholar]
- Gaber R. F., Styles C. A., Fink G. R. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Jul;8(7):2848–2859. doi: 10.1128/mcb.8.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Hou C., Crabeel M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. IV. Evidence for a general amino acid permease. J Bacteriol. 1970 Sep;103(3):770–777. doi: 10.1128/jb.103.3.770-777.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Schild D. Genetic map of Saccharomyces cerevisiae, edition 9. Microbiol Rev. 1985 Sep;49(3):181–213. doi: 10.1128/mr.49.3.181-213.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D. D. Biochemical Mutants in the Smut Fungus Ustilago Maydis. Genetics. 1949 Sep;34(5):607–626. doi: 10.1093/genetics/34.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Navarro A., Ramos J. Dual system for potassium transport in Saccharomyces cerevisiae. J Bacteriol. 1984 Sep;159(3):940–945. doi: 10.1128/jb.159.3.940-945.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Rothstein A. Relationship of cation influxes and effluxes in yeast. J Gen Physiol. 1974 Nov;64(5):608–621. doi: 10.1085/jgp.64.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M. B., Roth J. R. Genetic methods for analysis and manipulation of inversion mutations in bacteria. Genetics. 1983 Nov;105(3):517–537. doi: 10.1093/genetics/105.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M. B., Roth J. R. Selection and endpoint distribution of bacterial inversion mutations. Genetics. 1983 Nov;105(3):539–557. doi: 10.1093/genetics/105.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J., Fink G. R. The histidine permease gene (HIP1) of Saccharomyces cerevisiae. Gene. 1985;38(1-3):205–214. doi: 10.1016/0378-1119(85)90219-7. [DOI] [PubMed] [Google Scholar]
- Wang A., Roth J. R. Activation of silent genes by transposons Tn5 and Tn10. Genetics. 1988 Dec;120(4):875–885. doi: 10.1093/genetics/120.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F., Chumley F., Fink G. R. Eviction and transplacement of mutant genes in yeast. Methods Enzymol. 1983;101:211–228. doi: 10.1016/0076-6879(83)01016-2. [DOI] [PubMed] [Google Scholar]