Abstract
Activity rhythms in 24 h light-dark cycles, constant darkness, and constant light conditions were analyzed in four different Nasonia species for each sex separately. Besides similarities, clear differences are evident among and within Nasonia species as well as between sexes. In all species, activity in a light-dark cycle is concentrated in the photophase, typical for diurnal organisms. Contrary to most diurnal insect species so far studied, Nasonia follows Aschoff's rule by displaying long (>24 h) internal rhythms in constant darkness but short (<24 h) in constant light. In constant light, N. vitripennis males display robust circadian activity rhythms, whereas females are usually arrhythmic. In contrast to other Nasonia species, N. longicornis males display anticipatory activity, i.e. activity shortly before light-on in a light-dark cycle. As expected, N. oneida shows activity patterns similar to those of N. giraulti but with important differences in key circadian parameters. Differences in circadian activity patterns and parameters between species may reflect synchronization of specific life-history traits to environmental conditions. Scheduling mating or dispersion to a specific time of the day could be a strategy to avoid interspecific hybridization in Nasonia species that live in sympatry.
Introduction
Many traits in insects are subject to circadian oscillation. Examples are locomotor activity [1], hatching [2], eclosion [3], emergence from host puparia [4], [5], and mating [6], [7]. Many of these circadian traits are expected to have an adaptive significance [8]. Circadian and annual oscillation of many environmental parameters such as temperature, light and humidity or even predation pressure would cause synchronization of an organism's vital functions with optimal external conditions. Woelfle et al. [9] found that cyanobacteria strains with a functioning biological clock defeat strains with non-functioning clocks. Moreover, this advantage is stronger when the internal rhythm is similar to the external light-dark cycle. Considering this potential selective advantage, differences in circadian traits are also expected within species, between sexes or among populations of the same species. For instance, Drosophila species from northern and cool latitudes are preferentially active throughout the day whereas species from southern latitudes display a bimodal pattern with activities concentrated at the beginning and end of the photophase. Simunovic and Jaenike [10] found a positive correlation between the midday activity of 11 Drosophila species and the latitudinal midpoint of the species range. Furthermore, among species from the same latitudinal region, those that inhabit swampy areas and breed on skunk cabbage have significantly greater midday activity than do mycophagous species, suggesting that both latitude and breeding site influenced the evolution of their daily activity patterns. Other differences in circadian traits may be caused by selective pressure from competing organisms. In fact, exploitation of different temporal niches during the day is a way to avoid competitors. The parasitoids Eupelmus orientalis and E. vuilleti, for instance, live in sympatry and parasitize the same host. Differences observed in circadian locomotor activity between Eupelmus species are thought to reduce competition for the same resources [11].
In more closely related species, temporal isolation may also contribute to species formation. Females of Drosophila melanogaster and its sibling species D. simulans mate at different times during the day. This allochrony seems to favour reproductive isolation and may have been a cause of speciation in these Drosophila species [6]. Also Rivas et al. [12] observed small but significant differences in the phase of circadian activity patterns between two species of the sandfly Lutzomyia, the main vectors of visceral leishmaniasis in Latin America, which may contribute to their reproductive isolation.
Sex-specific variations in the temporal distribution of specific traits are also present but less studied. Since sexual activities in insects are frequently confined within a specific period of the day, synchronization between sexes is crucial to optimize reproductive success. In females of the turnip moth Agrotis segetum the circadian release of sex pheromones at the beginning of the scotophase is endogenously controlled [13] as is pheromone-mediated upwind flight in males during the same hours of the day [14]. Rivas et al. [12] found that males of the sandfly Lutzomyia initiate their activity a little earlier and have a broader activity peak than females, which tend to be more nocturnal than males. The reason for this difference is not yet known.
Species complexes displaying variations in circadian rhythms are particularly attractive for studying adaptation and the role played by clock genes herein. The parasitic wasp Nasonia vitripennis has been extensively used to study photoperiodism [15]–[17]. Recently, the genomes of four Nasonia species (N. vitripennis, N. giraulti, N. longicornis, N. oneida) were sequenced and annotated [18]. With this resource and the development of more advanced genetic tools like RNAi, Nasonia is attracting more interest for addressing evolutionary questions, like the genetic regulation of photoperiodism, which is more difficult in classic model organisms such as Drosophila melanogaster [19]. Circadian rhythms in activity and emergence but not eclosion were previously described for N. vitripennis males [5]. Here we compare circadian activity rhythms for all four Nasonia species under 24 h light-dark cycle, constant darkness and constant light conditions. We interpret the results in the context of what is known about the natural ecology of the species.
Materials and Methods
Nasonia strains
Nasonia are parasitic wasps of the family Pteromalidae (Hymenoptera) and members of the superfamily Chalcidoidea. N. vitripennis is cosmopolitan, but the other three species, N. giraulti, N. longicornis and N. oneida, are endemic to North America [20], [22]. N. longicornis occurs in northwestern North America; N. giraulti and N. oneida are sympatric and occur in eastern North America [20]. Nasonia vitripennis split first from the other species lineage between 0.2 and 1 MYA [21] and later, about 0.4–0.5 MYA, N. giraulti, N. longicornis, and N. oneida diverged from each other [20]. All species parasitize pupae of different fly species found primarily in bird nests and carcasses [22]. N. vitripennis is a more generalist and parasitizes Sarcophagidae, Muscidae and Calliphoridae species. The other Nasonia species parasitize primarily the calliphorid genus Protocalliphora (bird blowflies). The following Nasonia strains were used (when known, place and year of collection are indicated in parentheses): Nasonia giraulti RV2x(u) (Virginia, USA, 1987), NGDS (eastern North America), VA2TET (Virginia, USA, 2007, Tetracycline treated), PA233F (Pennsylvania, USA, 1989), VA1TET (Virginia, USA, 2007, Tetracycline treated); Nasonia longicornis IV7 (Utah, USA), IDB418(u) (Idaho, USA), MN8510 (Minnesota, USA), UTB316.16 (Utah, USA, 1990); N. oneida NY1136 (NY, USA, 2005); Nasonia vitripennis AsymC (Leiden, NL, 1971, cured from Wolbachia bacteria), HV3 (Hoge Veluwe, NL, 2006), Ita2 (Piedmont, Italy, 2006), LabII (Leiden, NL), Sal29 (New York, USA, 2007). Collection of Nasonia strains enjoyed permission of local authorities and did not involve endangered or protected species. Strain information comes from Darling and Werren [22], van den Assem and Jachman [23], Grillenberger et al. [24] and Raychoudhury [20]. Once in the lab, Nasonia strains are reared according to standard techniques [5].
Recording and analyses of activity rhythms
Males and females were collected at the black pupal stage and allowed to further develop in same sex groups of 10 to 15 individuals in 63×11 mm polystyrene tubes at 20°C in an 18 h∶6 h light-dark cycle. Once eclosed, virgin wasps were used immediately for recording individual activity rhythms as described in Bertossa et al. [5]. Males and females of each species were analyzed simultaneously. All animals were subjected to a protocol comprising an initial entraining phase of at least 4 full days in a 16 h∶8 h light-dark cycle (LD) followed by constant darkness (DD) for 8 days and subsequent 8 days in constant light (LL). Light intensity was approximately 30 lx. Activity data were averaged over 10 minute interval-bins and analyzed with ChronOSX v2.3.2 [25]. For average activity plots in LD, median, 25-, and 75-percentiles were calculated on the last three days of the LD entraining phase over all wasps. For the same period of time, the Centre point of Gravity (CoG, i.e. the activity peak in a fitted 24 h sine wave) was calculated in ChronOSX for each wasp.
Measuring Tau in DD and LL
The circadian period of the activity rhythm (tau, τ) in constant conditions was calculated for each wasp with ChronOSX according to the periodogram analysis by Sokolove and Bushell [26]. The first day – in DD or LL – was omitted from the calculation in order to exclude influences from the preceding light condition [5]. Double plots were first created in ChronOSX and monitored by eye to exclude dead wasps and individuals whose rhythmicity could not be clearly assessed due to e.g. presence of multiple rhythms (see figure S1). However, in order to use an unbiased method in the determination of arrhythmic animals, wasps whose ratio between the main peak value of the Qp periodogram statistics and the Qp value corresponding to the 0.01 confidence threshold (simply called “ratio-to-p”) was below 5.5 were considered arrhythmic. For examples of activity plots with increasing ratio-to-p values see figure S2.
Statistical analysis
All statistical analyses were performed in R, version 2.13.0 [27]. Differences among groups (e.g. response variables τ and CoG) were analyzed with mixed-effects models in which explanatory variables (fixed factors) were species, strains, experiments, light condition, and sex. “Strains (or species) within experiments” was fed into the model as random factor. Full models (i.e. with all factors and interactions) were simplified by removing non-significant explanatory factors. Best fit was assessed by comparing likelihoods of complex and simplified model with a Chi-squared test. Response variables were then tested for significant factors with post-hoc tests (Tukey HSD).
Results
Activity rhythms were analyzed under different light settings between sexes in all four known Nasonia species. These included the standard laboratory strain of each species (Werren et al 2010) and individuals of additional strains for each species (see table 1). Standard strains were: RV2X(u) for N. giraulti, IV7 for N. longicornis, NY1136 for N. oneida, and AsymC for N. vitripennis. Wasps were initially entrained for at least four days in LD 16∶8 h. Two phases in constant conditions (8 days in DD and 8 days in LL) followed directly after the LD phase. We present the analysis for the standard strains first and comparisons within species later.
Table 1. τ and ‘ratio-to-p’ values for all Nasonia strains in constant darkness (DD).
| CONST CONSTANT DARKNESS ANT DARKNESS | |||||||||||||||
| Females | Males | ||||||||||||||
| Species | Line | Tau (τ) | SEM | ratio to p | % rhythmic | % non-rhythmic | Total wasps | Tau (τ) | SEM | ratio to p | % rhythmic | % non-rhythmic | Total wasps | ||
| N. giraulti | RV2X(u) | 23.7 | 0.11 | 14.2 | 95 | 5 | 37 | 25.0 | 0.31 | 8.1 | 68 | 32 | 31 | ||
| NGDS | 23.2 | 0.27 | 13.4 | 100 | 0 | 6 | 23.5 | 1.14 | 8.7 | 83 | 17 | 6 | |||
| VA2TET | 23.5 | 0.20 | 19.1 | 100 | 0 | 6 | 24.3 | 0.38 | 10.5 | 100 | 0 | 5 | |||
| PA233F | 23.7 | 0.24 | 16.0 | 100 | 0 | 6 | 24.4 | 0.62 | 11.0 | 100 | 0 | 6 | |||
| VA1TET | 22.5 | 0.40 | 13.6 | 100 | 0 | 5 | 23.6 | 0.65 | 6.4 | 33 | 67 | 6 | |||
| Means: | 23.5 | [23.3] | 0.10 | 14.7 | 97 | 3 | 60 | 24.6 | [24.2] | 0.25 | 8.8 | 72 | 28 | 54 | |
| N. longicornis | IV7 | 25.0 | 0.14 | 9.1 | 100 | 0 | 18 | 26.0 | 0.46 | 7.6 | 72 | 28 | 18 | ||
| IDB418(u) | 24.2 | 0.22 | 13.2 | 100 | 0 | 6 | 27.6 | 0.46 | 11.7 | 100 | 0 | 6 | |||
| MN8510 | 24.0 | 0.09 | 15.7 | 100 | 0 | 6 | 24.5 | 0.21 | 13.7 | 100 | 0 | 6 | |||
| UTB316.16 | 26.0 | 0.16 | 12.8 | 100 | 0 | 6 | 27.1 | 0.51 | 7.7 | 83 | 17 | 6 | |||
| Means: | 24.9 | [24.8] | 0.14 | 11.5 | 100 | 0 | 36 | 26.2 | [26.3] | 0.30 | 9.6 | 83 | 17 | 36 | |
| N. oneida | NY1136 | 24.6 | 0.12 | 14.6 | 100 | 0 | 19 | 26.0 | 0.20 | 12.6 | 94 | 6 | 18 | ||
| N. vitripennis | AsymC | 26.4 | 0.08 | 10.1 | 78 | 22 | 41 | 25.7 | 0.06 | 16.5 | 98 | 3 | 40 | ||
| HV3 | 24.5 | 0.26 | 11.2 | 100 | 0 | 6 | 24.4 | 0.39 | 10.5 | 100 | 0 | 6 | |||
| Ita2 | 25.4 | 0.24 | 11.8 | 83 | 17 | 6 | 25.5 | 0.16 | 12.8 | 100 | 0 | 5 | |||
| LabII | 26.2 | 0.23 | 8.9 | 80 | 20 | 5 | 26.0 | 0.11 | 18.5 | 100 | 0 | 6 | |||
| Sal29 | 25.2 | 0.29 | 9.7 | 100 | 0 | 6 | 25.8 | 0.30 | 12.6 | 80 | 20 | 5 | |||
| Means: | 25.9 | [25.5] | 0.12 | 10.3 | 83 | 17 | 64 | 25.6 | [25.5] | 0.08 | 15.5 | 97 | 3 | 62 | |
Tau (τ, in hours), ratio-to-p, standard error (SEM), and number of wasps used are indicated for each strain. Species means are indicated in bold (except for column ‘Total wasps’, which indicates the total amount of wasps per each species). Tau means are weighted and, respectively, non-weighted (in brackets). For significant differences see figures 4 and 5.
Standard Nasonia strains
Nasonia giraulti
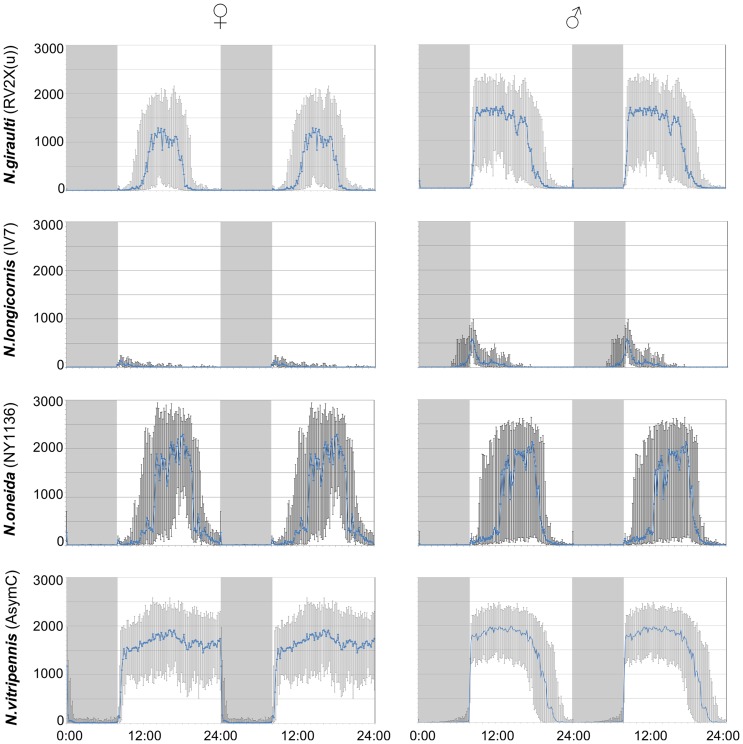
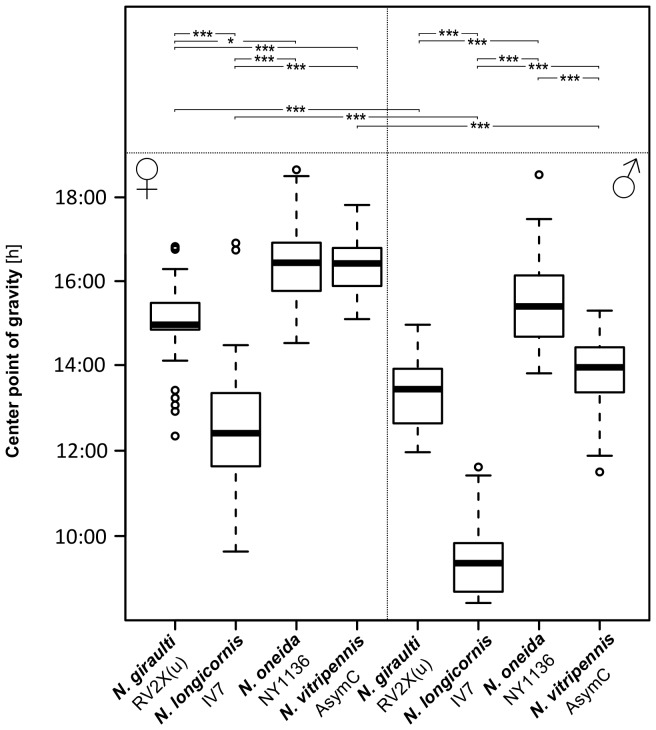
Nasonia giraulti RV2X(u) displays the greatest variation among individuals of all four species. Activity patterns can vary from short bouts of weak to sustained activity to longer bouts of intense activity (fig. 1). In males, activity begins after light-on and decreases after 9–12 h. In both constant DD and LL, on the contrary, activity is just a fraction of that of LD and irregular, with few and scattered activity bouts. An increase in activity is seen in some males at the beginning of LL however, after this, usually activity becomes again irregular as in DD. Indeed, rhythmicity is not strong in RV2X(u) males, both in DD (ratio-to-p is 8.1) and in LL (9.8). Around 67% of males are rhythmic in DD, whereas the majority (77%) is arrhythmic in LL (tables 1 and 2). Females have usually more pronounced activity patterns than males. In LD their activity is concentrated in the middle of the day (fig. 1 and 2). The peak of activity (Centre point of Gravity, CoG) can vary considerably, but this is observed in all species (fig. 3, table 3). τ of females in DD is the only one of all standard strains that is lower than 24 h (23.7 h, fig. 4). In LL, although relatively more females than males are rhythmic, the majority is non-rhythmic (57%, table 2). Their rhythmicity, however, is more evident than in males (ratio-to-p is 14.2 in DD and 10.7 in LL, tables 1 and 2).
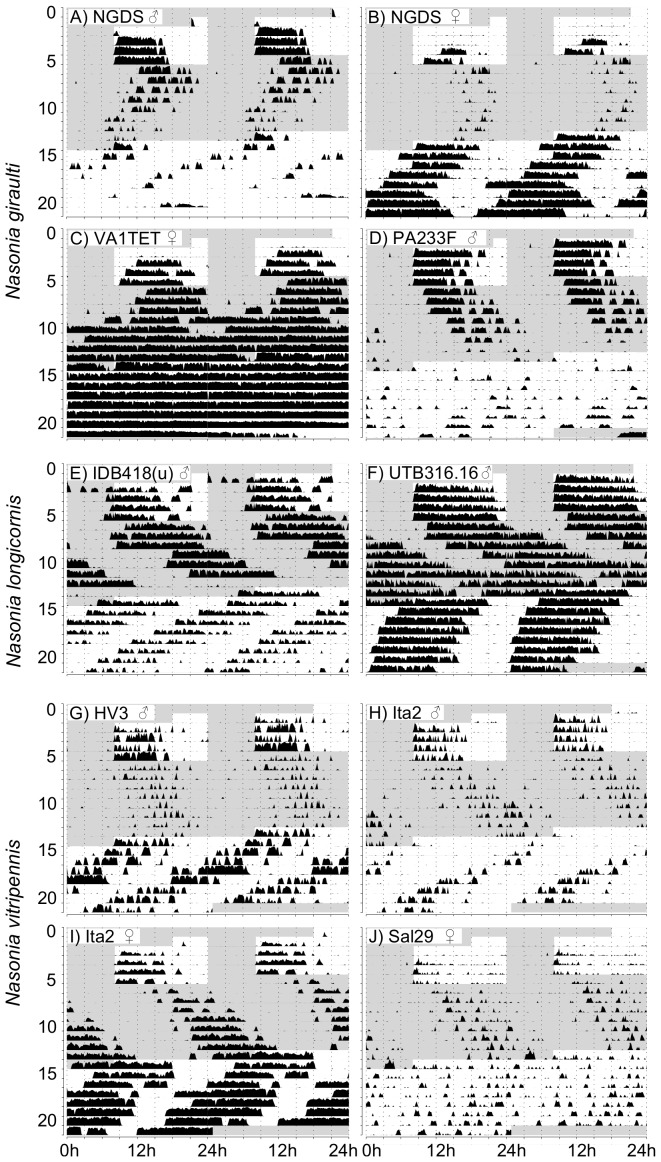
Figure 1. Typical actograms for different Nasonia species.
Representative actograms (double plotted) of male and female Nasonia are shown for standard strains in each species. Wasps were first entrained in LD 16∶8 (light phase from 8:00 to 24:00) for at least 4 days and then exposed to constant darkness (indicated with a gray background) for 8 days followed by constant light (white background). Y- and x-axes indicate time in days and hours, respectively.
Table 2. τ and ‘ratio-to-p’ values for all Nasonia strains in constant light (LL).
| CONSTANT LIGHT | |||||||||||||||
| Females | Males | ||||||||||||||
| Species | Line | Tau (τ) | SEM | ratio to p | % rhythmic | % non- rhythmic | Total wasps | Tau (τ) | SEM | ratio to p | % rhythmic | % non-rhythmic | Total wasps | ||
| N. giraulti | RV2X(u) | 21.1 | 0.20 | 10.7 | 43 | 49 | 37 | 22.0 | 0.85 | 9.8 | 33 | 67 | 15 | ||
| NGDS | 21.0 | 0.39 | 14.0 | 100 | 0 | 6 | 20.0 | 1.04 | 10.1 | 100 | 0 | 2 | |||
| VA2TET | 20.9 | 0.55 | 12.4 | 100 | 0 | 6 | 21.0 | – | 11.0 | 50 | 50 | 2 | |||
| PA233F | 21.3 | 0.51 | 16.6 | 100 | 0 | 6 | – | – | – | 0 | 100 | 3 | |||
| VA1TET | 19.4 | 0.60 | 16.2 | 80 | 20 | 5 | 21.3 | 0.54 | 8.6 | 75 | 25 | 4 | |||
| Means: | 20.9 | [20.8] | 0.18 | 13.0 | 63 | 32 | 60 | 21.4 | [21.1] | 0.47 | 9.6 | 42 | 58 | 26 | |
| N. longicornis | IV7 | 20.8 | 0.18 | 11.7 | 100 | 0 | 18 | 21.1 | 0.16 | 8.8 | 69 | 31 | 16 | ||
| IDB418(u) | 20.3 | 0.14 | 16.4 | 100 | 0 | 6 | 20.6 | 0.22 | 14.3 | 100 | 0 | 6 | |||
| MN8510 | 21.4 | 0.15 | 15.7 | 100 | 0 | 6 | 21.9 | 0.30 | 9.3 | 83 | 17 | 6 | |||
| UTB316.16 | 22.2 | 0.25 | 17.2 | 100 | 0 | 6 | 22.1 | 0.48 | 11.7 | 67 | 33 | 6 | |||
| Means: | 21.1 | [21.2] | 0.14 | 14.0 | 100 | 0 | 36 | 21.3 | [21.4] | 0.15 | 10.6 | 76 | 24 | 34 | |
| N. oneida | NY1136 | 23.5 | 0.29 | 12.6 | 87 | 13 | 15 | 23.4 | 0.48 | 8.9 | 89 | 11 | 9 | ||
| N. vitripennis | AsymC | – | – | – | 0 | 100 | 36 | 22.1 | 0.08 | 16.2 | 97 | 3 | 39 | ||
| HV3 | – | – | – | 0 | 100 | 6 | 21.1 | 0.25 | 13.2 | 100 | 0 | 5 | |||
| Ita2 | 21.9 | 0.14 | 12.1 | 60 | 40 | 5 | 21.4 | 0.59 | 11.2 | 100 | 0 | 5 | |||
| LabII | – | – | – | 0 | 100 | 3 | 22.7 | 0.31 | 16.2 | 100 | 0 | 6 | |||
| Sal29 | 23.1 | 0.46 | 6.9 | 67 | 33 | 6 | 21.8 | 0.15 | 15.2 | 100 | 0 | 5 | |||
| Means: | 22.6 | [22.5] | 0.35 | 9.1 | 13 | 88 | 56 | 22.0 | [21.9] | 0.09 | 15.4 | 98 | 2 | 60 | |
Tau (τ, in hours), ratio-to-p, standard error (SEM), and number of wasps used are indicated for each strain. Species means are indicated in bold (except for column ‘Total wasps’, which indicates the total amount of wasps per each species). Tau means are weighted and, respectively, non-weighted (in brackets). For significant differences see figures 4 and 5.
Figure 2. Average activity during LD in standard strains.
The median (with lower and upper quartiles) of the activity over the last three days in LD (double plotted) is indicated for each sex in the standard strains used for each species. Gray background indicates darkness. Y-axis: activity (pixel changes/minute); x-axis: hour of the day.
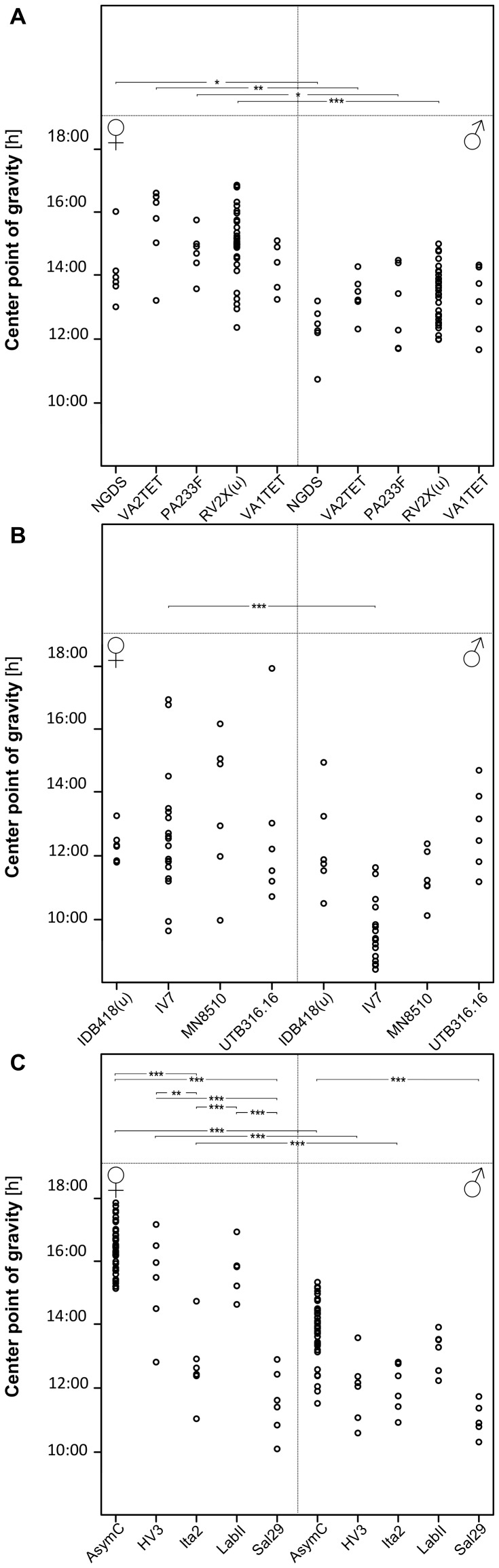
Figure 3. Centre point of gravity of main Nasonia strains.
Box-plots summarize Centre-point-of-gravity (CoG) values in LD for the standard strains used in each species. Significant differences are indicated with asterisks in the top panel: among strains (within one sex) on top, and between sexes (within each strain) at the bottom (* = p<0.05; ** = p<0.01; *** = p<0.001). Circles in box plots represent outliers. A summary of the data is given in table 3.
Table 3. CoG values for all Nasonia strains.
| Females | Males | ||||||||
| Species | Line | CoG | SEM | Total wasps | CoG | SEM | Total wasps | ||
| N. giraulti | RV2X(u) | 15:00 | 0:10 | 37 | 13:20 | 0:08 | 31 | ||
| NGDS | 14:06 | 0:24 | 6 | 12:16 | 0:20 | 6 | |||
| VA2TET | 15:34 | 0:31 | 6 | 13:22 | 0:16 | 6 | |||
| PA233F | 14:43 | 0:17 | 6 | 13:00 | 0:31 | 6 | |||
| VA1TET | 14:15 | 0:21 | 5 | 13:15 | 0:26 | 6 | |||
| Means: | 14:53 | [14:44] | 0:08 | 60 | 13:10 | [13:02] | 0:07 | 55 | |
| N. longicornis | IV7 | 12:39 | 0:27 | 18 | 9:31 | 0:13 | 18 | ||
| IDB418(u) | 12:20 | 0:13 | 6 | 12:18 | 0:38 | 6 | |||
| MN8510 | 13:30 | 0:56 | 6 | 11:19 | 0:20 | 6 | |||
| UTB316.16 | 12:46 | 1:04 | 6 | 12:52 | 0:32 | 6 | |||
| Means: | 12:45 | [12:49] | 0:19 | 36 | 10:50 | [11:30] | 0:17 | 36 | |
| N. oneida | NY1136 | 16:24 | 0:14 | 19 | 15:33 | 0:16 | 18 | ||
| N. vitripennis | AsymC | 16:24 | 0:06 | 41 | 13:58 | 0:10 | 40 | ||
| HV3 | 15:23 | 0:38 | 6 | 11:58 | 0:25 | 6 | |||
| Ita2 | 12:41 | 0:29 | 6 | 12:00 | 0:18 | 6 | |||
| LabII | 15:41 | 0:22 | 5 | 13:10 | 0:15 | 6 | |||
| Sal29 | 11:32 | 0:25 | 6 | 11:01 | 0:14 | 5 | |||
| Means: | 15:27 | [14:20] | 0:14 | 64 | 13:17 | [12:25] | 0:10 | 63 | |
CoG (hours:minutes; light phase is from 8:00 to 24:00), standard error (SEM), and number of wasps used are indicated for each strain. Species means are indicated in bold (except for columns ‘Total wasps’, which indicate the total amount of wasps per each species). CoG means are weighted and, respectively, non-weighted (in brackets). For significant differences see figures 3 and 6.
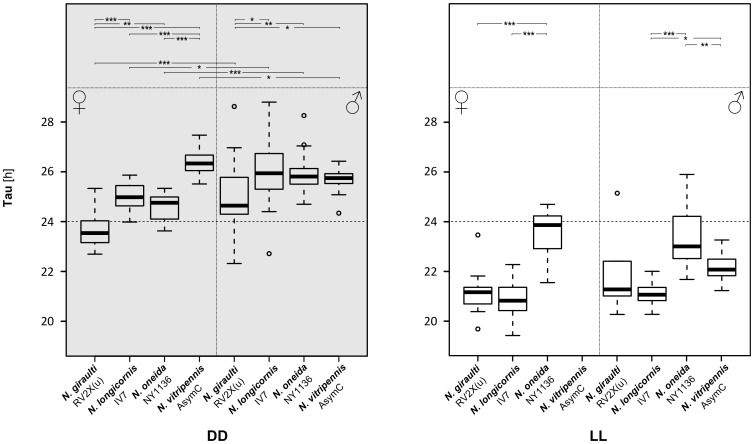
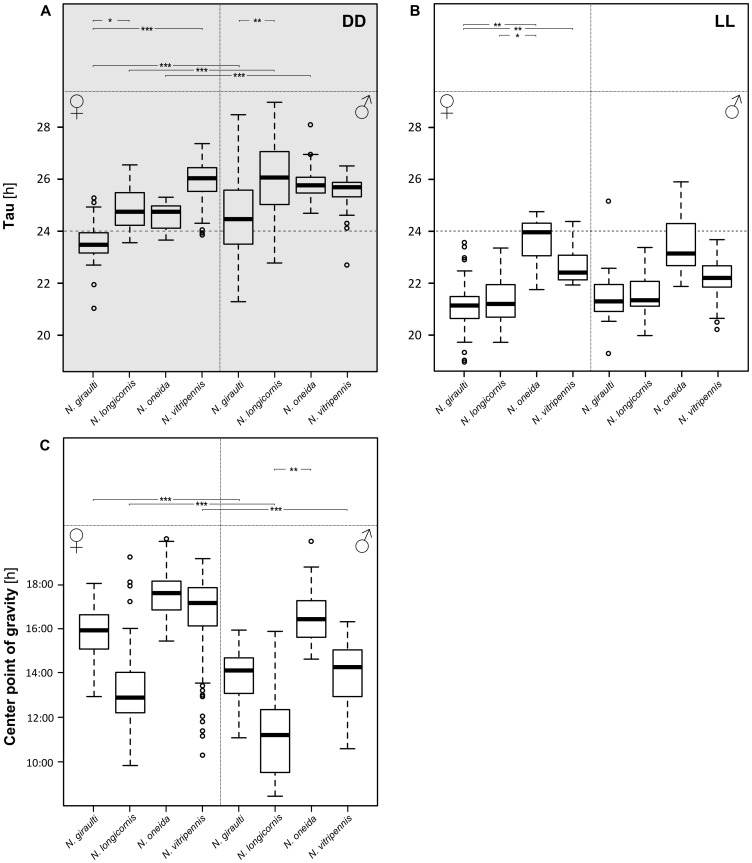
Figure 4. Τ of standard Nasonia strains.
Box-plots summarize τ values in constant darkness (DD) and constant light (LL) for the standard strain of each species. Significant differences are indicated with asterisks in the top panel: among strains (within one sex) on top, and between sexes (within each strain) at the bottom (* = p<0.05; ** = p<0.01; *** = p<0.001). Circles in box plots represent outliers. Differences between τ values in darkness and light are always significant. A summary of the data is given in tables 1 and 2.
Nasonia longicornis
N. longicornis IV7 has quite unique features compared with the other standard strains. It is the strain with the lowest levels of activity, both in males and females (fig. 2). Further, in LD both males and females have the earliest activity peak compared with other strains: CoG of males is already 1.5 hours after light-on (9∶30 h) whereas female activity peaks at 12∶40 h (fig. 3 and table 3). Notably, N. longicornis is the only species in which males show anticipatory activity before light-on in LD (fig. 1 and 2). Males and females have similar patterns of activity, concentrated in short and often scattered bouts if compared to the more prolonged bouts of N. giraulti or the even longer bouts of N. vitripennis (fig. 1). Although all females were found to be rhythmic in DD their rhythmicity is not very pronounced (ratio-to-p is 9.1). Rhythmicity of N. longicornis males is less pronounced than that of females (around 70% of males are rhythmic both in DD and LL, table 1 and 2) and is the least strong of all standard strains analyzed (ratio-to-p is 7.6 in DD and 8.8 in LL).
Nasonia oneida
Only one strain of N. oneida was analyzed (NY1136). Not surprisingly it has many features in common with N. giraulti, from which it is assumed to have recently split [20]. For instance, activity patterns – with variably sized bouts of intense activity – resemble those of N. giraulti (fig. 1) and females have activity concentrated in the middle of the light phase in LD (fig. 1 and 2). However, contrary to giraulti, also oneida males have activity concentrated in the middle of the day (fig. 1 and 2) and, in LD, activities peak also at different times than observed in giraulti (fig. 3 and table 3). Under constant conditions, most male and female N. oneida are rhythmic in both DD and LL and, in LL, have the largest τ of all standard strains (23.5 h for females and 23.4 h for males, fig. 4 and table 2). Rhythms are usually readily apparent in both sexes and the three light conditions.
Nasonia vitripennis
In N. vitripennis wasps of strain AsymC both females and males display intense and sustained activity in the light phase of LD and, typically, also in constant conditions (fig. 1 and 2). In LD, males begin activity immediately after light-on and cease activity after about 10–13 h (fig. 1 and 2), although some individuals may continue for the whole 16 h of light (not shown). After light-on in LD, females do not usually start activity as fast as males but are active throughout the light phase (fig. 1 and 2). Furthermore, females appear the only ones to consistently display a residual activity during the dark phase in LD (fig. 1 and 2). In LD, activity peaks at 16∶24 h in females and 13∶58 h in males (fig. 3 and table 3). In constant conditions, activity bouts are frequently very sharp, with low to absent background activity. In DD, τ is 25.7 h in males and 26.4 h in females – the largest of all female groups in DD (fig. 4; table 1) and more males (98%) than females (78%) are rhythmic. In LL, males maintain rhythmicity albeit with a τ of 22.15 h while females are arrhythmic (table 2).
Additional strains
Nasonia giraulti
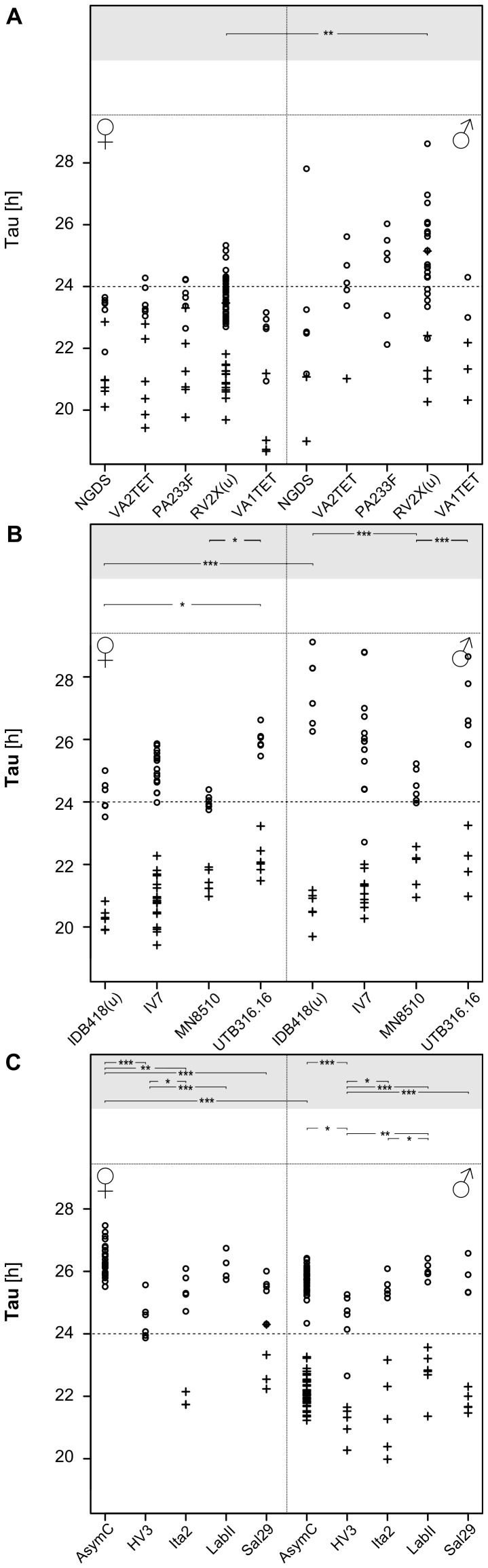
Four additional N. giraulti strains were analysed. No significant differences among strains were found in τ (fig. 5A), CoG (fig. 6A), or activity patterns in LD (fig. S3). However, sexes (within the same strain) differ in their LD activity peaks in four out of five strains (fig. 6A). In contrast, for τ in constant DD no differences between sexes were observed except in the standard strain RV2X(u) (fig. 5A). Note however the large variation observed within certain strains. For instance, for the standard strain, τ of male wasps in DD varied between 22.3 and 28.6 h. Females, instead, show less variation of τ in DD, are mostly rhythmic and their rhythms are quite pronounced, with ratio-to-p ranging from 13.4 to 19.1 (table 1). Also in LL, females of most N. giraulti strains are rhythmic with well supported rhythms while fewer males are rhythmic in LL (although a high mortality among males reduced available samples, table 2). CoG of N. giraulti females is at around 14∶50 h; that of males at 13∶10 h. Representative double-plots of N. giraulti strains are presented in figure 7. In general, activity patterns resemble those of main strain RV2X(u).
Figure 5. Τ in all tested strains for each Nasonia species.
τ values are indicated for all strains used in N. giraulti (A), N. longicornis (B), and N. vitripennis (C). Circles (o) and crosses (+) are used to distinguish τ values in DD an LL, respectively. Significant differences (among strains within each sex, and between sexes within each strain) are indicated in the top panels with asterisks (* = p<0.05; ** = p<0.01; *** = p<0.001). Background is gray for DD and white for LL, respectively. Differences in τ between constant darkness and constant light are always significant. A summary of the data is given in tables 1 and 2.
Figure 6. CoG for all tested strains of four Nasonia species.
CoG values are indicated for all strains used in N. giraulti (A), N. longicornis (B), and N. vitripennis (C). Significant differences are indicated in the top panel with asterisks: among strains (within each sex) on top, and between sexes (within each strain) at the bottom (* = p<0.05; ** = p<0.01; *** = p<0.001).A summary of the data is given in table 3.
Figure 7. Actograms of additional Nasonia strains.
N. giraulti: NGDS males can show strong activity at the beginning (A); conspicuous LL activity is seen in some NGDS females (B); an example of VA1TET female with strong activity pattern (C); a male PA233F starting activity at light-on in LD (D). N. longicornis: IDB418(u) males can have robust and sustained activity similar to that observed in AsymC males (E, anticipation in LD is evident); an example of a UTB316.16 male showing strong activity but no anticipation in LD (F). N. vitripennis: in some vitripennis strains males can be less active in DD than are AsymC males (G and H) and females of some vitripennis strains can be rhythmic in LL (I and J).
Nasonia longicornis
Although within single N. longicornis strains τ varies less than within N. giraulti strains, greater differences are observed among longicornis strains used here and some are significant (fig. 5B). In these strains τ in DD is generally greater than in giraulti strains, both in males (26.2 h) and in females (24.9 h). In LL however, τ are similar to those observed in giraulti strains (table 2 and fig. 5). Similar to N. giraulti, N. longicornis strains show usually more evident rhythms in females than in males, in both light conditions (tables 1 and 2). For instance, the average ratio-to-p in DD is above 11 for females while that of males is below 10 (table 1). This difference is even more accentuated in LL (table 2). Female LD activity peaks roughly 2 h earlier than in N. giraulti (12∶49 h) while that of males is maximal at around 11∶30 h, with exception of strain IV7 which peaks earlier (9∶31 h, table 3 and fig. 6B). Activity patterns of the additional N. longicornis strains are quite similar to those of the standard IV7 strain. However, some important differences can be noted. For instance, males in some strains can have robust and sustained activity similar to that observed in AsymC (e.g. fig. 7E and F, and S3) and have frequently also a pronounced rhythmicity (tables 1 and 2). Even if lower than compared to other species, LD activity in females of N. longicornis strains is not as low as observed for IV7 females (compare fig. 2 with S3). Anticipation is seen in most males of N. longicornis strains (fig. 7 and S3). Other strains have an activity pattern similar to that of IV7 (not shown).
Nasonia vitripennis
Most significant differences are observed among N. vitripennis strains (fig. 5C, 6C and S3). However, differences between sexes are significant only for CoG but not τ (except for AsymC). In general, in DD females display a τ greater than observed in other species (table 1). Furthermore, in most strains females are clearly non-rhythmic in LL with the exception of Ita2 and Sal29 (table 2). Males are usually highly rhythmic in both DD and LL. Activity patterns of HV3 are similar to those of AsymC. Despite having a strong supported rhythmicity (table 1), HV3 and Ita2 males are less active in DD than AsymC males (fig. 7, G and H). Ita2 females can be rhythmic in LL (fig. 7I). Sal29 females show rhythmicity in LL but this is not strongly supported (fig. 7J). AsymC and LabII activity patterns are very similar. Continuous activity throughout the light phase does not seem to be the rule in N. vitripennis females (fig. S3). Additionally, night activity is only present in laboratory lines (AsymC and LabII).
Strains pooling and comparison among species
Data of all strains within a species were pooled in order to assess differences among species. (fig. 8). Difference of τ between DD and LL is significant for all species (mixed-effects model, effect of light: χ 2 = 726.15, p<2.2e-16). Furthermore, in DD but not in LL, females have a significantly lower τ compared to males (except N. vitripennis). In females τ differs significantly also between N. giraulti and two other species: N. longicornis and N. vitripennis in DD, and N. oneida and N. vitripennis in LL. As for CoG, differences between sexes are in most species significant (except N. oneida).
Figure 8. τ and CoG differences among Nasonia species.
Data from distinct strains within one species were pooled in order to find differences among Nasonia species for τ and CoG. Significant differences are indicated with asterisks in the top panel: among strains (within one sex) on top, and between sexes (within each strain) at the bottom (* = p<0.05; ** = p<0.01; *** = p<0.001).Circles in box plots represent outliers. Differences are based on a model that accounts for different amount of data per strain. Despite pooling, significant differences are found among species and between sexes. Differences between τ values in darkness and light are always significant.
Circadian activity rhythms in Nasonia species display some characteristic hallmarks and can be formally classified by general features as well as elements typical of LD and constant light conditions. General features are: type of activity pattern (i.e. length and intensity of activity bouts), activity patterns at phase changes, and regular pattern vs. presence of abrupt changes (over days or conditions). Elements specific for LD are: Centre point of Gravity, activity distribution over the light phase, onset of activity (i.e. at light-on or delayed), presence of background activity in the dark phase, and anticipation. Finally, activity in constant conditions can be rhythmic (i.e. meaningful τ) or not. With these elements in mind, activity patterns of Nasonia species have been classified in table S1. Representative double plots for Nasonia species are shown in Figure S4.
Discussion
Nasonia species offer a unique opportunity for studying species formation because, under special circumstances, they can be readily crossed [18]. Furthermore, species exhibit consistent differences in important life history traits, such as host preference [28], learning and memory [29], mating or aggressive behaviour [20], [30]–[32]. Circadian rhythms are a class of traits which also show species-specific differences in insects and have been clearly associated with species formation [33]. Variations in endogenous clock genetics and connected phenotypic outputs – such as circadian rhythms – deserve therefore particular attention for understanding how populations differentiate and form new species. We have previously shown that Nasonia vitripennis wasps display robust circadian rhythms [5]. Here we have reported on activity rhythms of both sexes of several strains of Nasonia wasps. The protocol used comprised an entraining phase of at least four days in LD 16:8 h followed by eight days in DD and completed by eight days in LL. A longer protocol was not possible due to the restricted lifespan of the wasps. We chose not to provide additional food as it would disturb their circadian rhythms and the automatic registration. A different protocol could be recording activity in DD and LL separately, both after an entraining period in LD, as done by Stelzer et al. in a study on bumblebees [34]. Another important restriction in our experiments was the use of virgin wasps in order to standardize settings. Circadian rhythms in insects may be also influenced by mating status [35], as well as several other factors, like temperature and social environment [36].
Nasonia wasps obey Aschoff's rule
An initial observation is that in all Nasonia species τ in DD is always significantly greater than in LL. Furthermore, with the exception of N. giraulti females, τ in DD is larger than 24 h, while in LL it is always smaller than 24 h (fig. 4, 5 and 8). According to Aschoff's rule, this situation is characteristic for diurnal animals [37] and, besides the bumblebee, which has a τ around 24 h in DD and shorter than 24 h in LL [34], Nasonia is the only diurnal insect so far which strictly follows Aschoff's rule [38]. τ in the blow fly Calliphora vicina, for instance, lengthens upon transfer from DD to LL when light intensity is below 2 lx while more and more flies become arrhythmic above 2 lx [39]. The same is true even in the honey bee A. mellifera which is, as Nasonia, a hymenopteran [40]. Although several strains of Nasonia are rhythmic under approximately 30 lx, a shift in τ under different light intensities cannot be ruled out as the same light intensity was used throughout all experiments.
Similarities and differences among Nasonia species and sexes
A τ smaller than 24 h in DD could suggest nocturnal-type behaviour in N. giraulti females as does a τ near 24 h in LL in N. oneida females. However, double-plots of both N. giraulti and N. oneida in LD do not indicate nocturnal activity in females of these species. The fact that N. giraulti wasps mate within the host puparium [30], [31], before emergence, a place somewhat protected against direct external light, may make them less affected by light fluctuations. Bertossa et al. [5] found that, in LD 16∶8, emergence of N. vitripennis AsymC virgin males from their host puparium is rhythmic and happens around light-on while eclosion is arrhythmic. Assuming that the latter extends to all Nasonia species, one could speculate that mating in N. giraulti, if correlated with an arrhythmic eclosion, would be arrhythmic too, i.e. not influenced by the LD cycle, despite adult activity being rhythmic. Conversely, mating in N. vitripennis, which occurs after emergence from the host puparium [30], [31], is expected to follow a circadian rhythm. Another interpretation could be derived from Pittendrigh's hypothesis: a circadian clock tracking dawn would have a τ larger than 24 h in DD, while it would be shorter than 24 h if it would track dusk [41]. If this could underlie the differences in τ between N. vitripennis and N. giraulti females in DD (fig. 4 and 8) which traits would be affected? More specific experiments, aimed at testing associations between activity rhythms and important life-history traits, are needed to answer this question.
Clear differences in male anticipation in LD are apparent among Nasonia species (females show virtually no anticipation). N. longicornis displays the most pronounced anticipation (and correlated early CoG, table 3), followed by N. vitripennis, whereas N. giraulti and N. oneida have virtually no anticipation (fig. 2 and S3). Anticipation in N. longicornis may be a strategy to compete against N. vitripennis males with which they live in sympatry. However, N. longicornis males do not differ much from N. vitripennis males in terms of within-host-mating, dispersion and aggression in contrast to N. giraulti males which do disperse [31]. So, whether male anticipation has a particular adaptive significance remains to be determined. In that respect, the difference observed between a strong anticipation in male emergence [5] and a negligible one in activity (fig. 2) shows that circadian rhythms can appear quite different depending on the conditions in which an animal's activity is recorded and the type of behaviour measured: in this case whether the circadian activity is of emerging males vs. simple locomotion (without e.g. presence of hosts). Males inside the host may be influenced by other factors, like presence of other males, particular odours, light coming through the puparium wall, or age.
A clear difference between males and females is the onset of activity at light-on in N. giraulti. This happens abruptly in males, whereas a delay is observed in females (fig. 2 and S3). This difference could be correlated either with male dispersion and/or within-host-mating, both present in N. giraulti but not in other species [31]. Dispersal may be safer in the middle part of the light phase, while searching for and parasitizing hosts at the place of emergence has to be maximized and can hence be done as soon as and as long as there is light. In that respect it would be highly informative to know whether there are differences in dispersal at different moments in an LD light protocol. Even if in N. giraulti mating occurs within the host, a premature onset of activity in females and possible emergence before mating may be prevented by their delayed activity onset. The fact that virtually all AsymC females are arrhythmic in LL and show residual nocturnal activity may have to do with their prolonged exposure to laboratory conditions. In fact, this is not observed in more recent strains obtained from the wild, such as Ita2 and Sal29 (fig. 5 and S3).
Similarities and differences among strains
Circadian rhythms dynamics are readily affected by adaptation to local climatic conditions, such as latitude, altitude or humidity [10], [42], [43] and may explain differences in τ and CoG observed among Nasonia species, as discussed above, but also among strains. A trait related to circadian rhythms in Nasonia is photoperiodism, apparent in the form of diapause induction in short days [15], [44], [45]. Whether the circadian system in insects, apparent in overt circadian rhythms, and photoperiodism rely on a unique genetic system comprising canonical clock genes or whether these affect both phenotypes through different pathways is still unclear [19], [46], [47]. While it is undisputed that both systems share components (i.e. clock genes), the magnitude of communality also depends on the insect system being considered, indicating that the genetic architecture(s) underlying circadian and seasonal systems is evolutionarily plastic [48], [49]. So far, evidence in Nasonia indicates that photoperiod changes are measured by a circadian timing system comprising a double oscillator [16]. Since in Nasonia also temperature influences diapause [50], differences in propensity to enter diapause may correlate with shifts in τ according to e.g. latitude. Differences in τ among N. giraulti strains tested in this study are virtually non-existent and may be explained by the fact that N. giraulti apparently contains very low genetic variation [20]. Although more variation is present among N. longicornis and N. vitripennis strains, no correlation is found between τ and latitude. The only correlation is seen between CoG in N. vitripennis females: strains from lower latitudes (Ita2 and Sal29, 45.7°N and 40.7°N, respectively) have a peak in activity earlier in the day than strains from higher latitudes (AsymC and HV3, 52.2°N). Despite finding consistent variation of circadian parameters (Tau, CoG) in populations of the cricket D. fascipes collected from 8°S to 43°N, Shimizu and Masaki [51] did not find any correlation with latitude. On the other hand, Joshi [43] found a correlation between circadian parameters in strains of Drosophila ananassae and the latitude at which these were collected, ranging from 6° to 34°N. Therefore, although our study did not aim directly at testing latitudinal clines in circadian parameters, results may depend on several factors like the species and the particular latitudinal range considered. However, since also induction of photoperiodism in N. vitripennis is known to correlate with latitude [45], more work is needed to understand whether also other circadian parameters follow a latitudinal gradient.
Other explanations for differences among strains are also plausible. Founder effects and rearing in the laboratory may influence circadian rhythms as discussed by others [52]. Additionally, since genes influencing circadian rhythms influence also other traits (e.g. developmental time, courtship behaviour) and rearing in the laboratory may affect these traits, a correlated effect of these causes on circadian rhythms is also expected.
Conclusions
Our results confirm and extend previous findings to all four known Nasonia species. Remarkable differences are found in activity rhythms both among and within species and between sexes. Some differences, especially those among strains, may be caused by differences in geographical origin (i.e. different climatic conditions) or prolonged laboratory breeding. Differences among species that live in sympatry may contribute to temporal shifts in important life-history traits, such as mating or dispersal, and reduce the risk of interspecific hybridizations. Differences between sexes suggest that some sex-specific traits (e.g. emergence, mating) may be differently synchronized with circadian and annual rhythms. In conclusion, our results support the existence of chronotypic life-history differences among Nasonia species, strains and sexes but the functional significance of these differences requires further study.
Supporting Information
Examples of activity plots with rhythm splits. These double-plots are examples in which activities have more than one internal period or display splits in the activity period. If the main period is sufficiently supported (see materials and methods) it is included in the analysis. N. giraulti RV2x(u) female with a main internal period (21.8 h) and a secondary period at roughly 25 h in LL (A). Two periods (23 and 26 h) are apparent in a N. vitripennis Sal29 female in LL (B). N. longicornis IV7R2 male with an unclear rhythm split in LL (C). Some N. giraulti VA1TET males show a 24 h-like rhythm in the first days in DD but then split. In this example, however, a main 22.6 h period can be seen from day 6 to 13 (D). ‘Wandering’ rhythms in a N. vitripennis HV3 male in DD: 22.5 h from day 6 to 10; 26 h from day 10 to 13 (E). Red dashed lines highlight multiple rhythms.
(TIF)
Examples of activity plots with increasing ratio-to-p values. In order to use a quantitative estimate to distinguish rhythmic vs. non-rhythmic activity plots, the ‘ratio-to-p’ value – the main peak value of the Qp statistics for rhythmicity (over the τ period tested) divided by the Qp value corresponding to the 0.01 probability threshold – was used as cut-off value. Activity plots with a ratio-to-p equal or lower than 5.5 were considered non-rhythmic. Here, examples of activity plots with increasing ratio-to-p values are shown. The values in the inset correspond to τ (in hours) and the ratio-to-p value, respectively, for the LL phase.
(TIF)
Average activity in LD for additional Nasonia strains. The median (with lower and upper quartiles) of the activity over the last three days in LD (double plotted) is indicated for each sex in additional Nasonia strains used for each species. Gray background indicates darkness. Y-axis: activity (pixel changes/minute); x-axis: hour of the day.
(TIF)
Representative activity rhythms for Nasonia species. Representative actograms to distinguish among Nasonia species and sexes are shown. For a description of activity patterns see Table S1. Additional actograms can be found in figures 1 and 7.
(TIF)
Classification of Nasonia species according to activity patterns and parameters in different light conditions.
(TIF)
Acknowledgments
We thank M. Giesbers, S. Paolucci, and T. Schwander for useful suggestions on statistical analyses and an anonymous reviewer for helpful comments on a previous version of the manuscript.
Funding Statement
RCB was supported by a grant (ALW 817.02.020) from the Netherlands Organization for Scientific Research (NWO) URL: http://www.nwo.nl/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Helfrich-Förster C (2001) The locomotor activity rhythm of Drosophila melanogaster is controlled by a dual oscillator system. J Insect Physiol 47: 877–887. [Google Scholar]
- 2. Itoh MT, Sumi Y (2000) Circadian clock controlling egg hatching in the cricket (Gryllus bimaculatus). J Biol Rhythms 15: 241–245. [DOI] [PubMed] [Google Scholar]
- 3. Pittendrigh CS (1954) On temperature independence in the clock system controlling emergence time in Drosophila . Proc Natl Acad Sci U S A 40: 1018–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reznik SY, Voinovich ND, Vaghina NP, Karpova SG (2008) Arrhythmic adult ecdysis but rhythmic emergence from the host chorion in Trichogramma embryophagum (hymenoptera: Trichogrammatidae). Eur J Entomol 105: 81–85. [Google Scholar]
- 5. Bertossa RC, van Dijk J, Beersma DG, Beukeboom LW (2010) Circadian rhythms of adult emergence and activity but not eclosion in males of the parasitic wasp Nasonia vitripennis . J Insect Physiol 56: 805–812. [DOI] [PubMed] [Google Scholar]
- 6. Sakai T, Ishida N (2001) Circadian rhythms of female mating activity governed by clock genes in Drosophila . Proc Natl Acad Sci U S A 98: 9221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rymer J, Bauernfeind AL, Brown S, Page TL (2007) Circadian rhythms in the mating behavior of the cockroach, Leucophaea maderae . J Biol Rhythms 22: 43–57. [DOI] [PubMed] [Google Scholar]
- 8. Sharma VK (2003) Adaptive significance of circadian clocks. Chronobiol Int 20: 901–919. [DOI] [PubMed] [Google Scholar]
- 9. Woelfle MA, Ouyang Y, Phanvijhitsiri K, Johnson CH (2004) The adaptive value of circadian clocks: An experimental assessment in cyanobacteria. Curr Biol 14: 1481–1486. [DOI] [PubMed] [Google Scholar]
- 10. Simunovic A, Jaenike J (2006) Adaptive variation among Drosophila species in their circadian rhythms. Evol Ecol Res 8: 803–811. [Google Scholar]
- 11. Ndoutoume-Ndong A, Rojas-Rousse D, Allemand R (2006) Locomotor activity rhythms in two sympatric parasitoid insects: Eupelmus orientalis and Eupelmus vuilleti (hymenoptera, eupelmidae). C R Biol 329: 476–482. [DOI] [PubMed] [Google Scholar]
- 12. Rivas GBS, Souza NA, Peixoto AA (2008) Analysis of the activity patterns of two sympatric sandfly siblings of the Lutzomyia longipalpis species complex from Brazil. Med Vet Entomol 22: 288–290. [DOI] [PubMed] [Google Scholar]
- 13. Rosen WQ (2002) Endogenous control of circadian rhythms of pheromone production in the turnip moth, Agrotis segetum . Arch Insect Biochem 50: 21–30. [DOI] [PubMed] [Google Scholar]
- 14. Rosen WQ, Han GB, Lofstedt C (2003) The circadian rhythm of the sex-pheromone-mediated behavioral response in the turnip moth, Agrotis segetum, is not controlled at the peripheral level. J Biol Rhythms 18: 402–408. [DOI] [PubMed] [Google Scholar]
- 15. Saunders DS (1968) Photoperiodism and time measurement in the parasitic wasp, Nasonia vitripennis . J Insect Physiol 14: 433–450. [Google Scholar]
- 16. Saunders DS (1974) Evidence for 'dawn' and 'dusk' oscillators in the Nasonia photoperiodic clock. J Insect Physiol 20: 77–88. [Google Scholar]
- 17. Saunders DS (2012) Insect photoperiodism: Seeing the light. Physiol Entomol 37: 207–218. [Google Scholar]
- 18. Werren JH, Richards S, Desjardins CA, Niehuis O, Gadau J, et al. (2010) Functional and evolutionary insights from the genomes of three parasitoid Nasonia Species. Science 327: 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saunders DS, Bertossa RC (2011) Deciphering time measurement: The role of circadian ‘Clock’ genes and formal experimentation in insect photoperiodism. J Insect Physiol 57: 557–566. [DOI] [PubMed] [Google Scholar]
- 20. Raychoudhury R, Desjardins CA, Buellesbach J, Loehlin DW, Grillenberger BK, et al. (2010) Behavioral and genetic characteristics of a new species of Nasonia . Heredity 104: 278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Campbell BC, Steffen-Campbell JD, Werren JH (1993) Phylogeny of the Nasonia species complex (hymenoptera: Pteromalidae) inferred from an internal transcribed spacer (ITS2) and 28 S rDNA sequences. Insect Mol Biol 2: 225–237. [DOI] [PubMed] [Google Scholar]
- 22. Darling D, Werren J (1990) Biosystematics of Nasonia (hymenoptera, pteromalidae) - 2 new species reared from birds nests in north-America. Ann Entomol Soc Am 83: 352–370. [Google Scholar]
- 23. van den Assem J, Jachmann F (1999) Changes in male perseverance in courtship and female readiness to mate in a strain of the parasitic wasp Nasonia vitripennis over a period of 20 years. Neth J Zool 49: 125–137. [Google Scholar]
- 24. Grillenberger BK, Koevoets T, Burton-Chellew MN, Sykes EM, Shuker DM, et al. (2008) Genetic structure of natural Nasonia vitripennis populations: Validating assumptions of sex-ratio theory. Mol Ecol 17: 2854–2864. [DOI] [PubMed] [Google Scholar]
- 25. Roenneberg T, Taylor W (2000) Automated recordings of bioluminescence with special reference to the analysis of circadian rhythms. Method Enzymol 305: 104–19. [DOI] [PubMed] [Google Scholar]
- 26. Sokolove PG, Bushell WN (1978) Chi square periodogram - its utility for analysis of circadian-rhythms. J Theor Biol 72: 131–160. [DOI] [PubMed] [Google Scholar]
- 27.R Core Team. (2012) A language and environment for statistical computing. Available: http://www.R-project.org via the Internet.
- 28. Desjardins CA, Perfectti F, Bartos JD, Enders LS, Werren JH (2010) The genetic basis of interspecies host preference differences in the model parasitoid Nasonia . Heredity 104: 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoedjes KM, Steidle JL, Werren JH, Vet LE, Smid HM (2012) High-throughput olfactory conditioning and memory retention test reveal variation in Nasonia parasitic wasps. Genes Brain Behav 11: 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drapeau MD, Werren JH (1999) Differences in mating behaviour and sex ratio between three sibling species of Nasonia . Evol Ecol Res 1: 223–234. [Google Scholar]
- 31. Leonard JE, Boake CRB (2006) Site-dependent aggression and mating behaviour in three species of Nasonia (hymenoptera: Pteromalidae). Anim Behav 71: 641–647. [Google Scholar]
- 32. van den Assem J, Werren JH (1994) A comparison of the courtship and mating-behavior of three species of Nasonia (hymenoptera, pteromalidae). J Insect Behav 7: 53–66. [Google Scholar]
- 33. Tauber E, Roe H, Costa R, Hennessy JM, Kyriacou CP (2003) Temporal mating isolation driven by a behavioral gene in Drosophila . Curr Biol 13: 140–5. [DOI] [PubMed] [Google Scholar]
- 34. Jurgen Stelzer R, Stanewsky R, Chittka L (2010) Circadian foraging rhythms of bumblebees monitored by radio-frequency identification. J Biol Rhythms 25: 257–267. [DOI] [PubMed] [Google Scholar]
- 35. Johnson JN, Hardgrave E, Gill C, Moore D (2010) Absence of consistent diel rhythmicity in mated honey bee queen behavior. J Insect Physiol 56: 761–773. [DOI] [PubMed] [Google Scholar]
- 36. Levine JD, Funes P, Dowse HB, Hall JC (2002) Resetting the circadian clock by social experience in Drosophila melanogaster . Science 298: 2010–2. [DOI] [PubMed] [Google Scholar]
- 37. Aschoff J (1960) Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Sym 25: 11–28. [DOI] [PubMed] [Google Scholar]
- 38.Saunders DS. (2002) Insect clocks. Amsterdam: Elsevier Science. 560 p.
- 39. Saunders DS, Cymborowski B (2008) Light-induced behavioural effects on the locomotor activity rhythm of the blow fly, Calliphora vicina (diptera: Calliphoridae). Eur J Entomol 105: 585–590. [Google Scholar]
- 40.Bloch G. (2009) Plasticity in the circadian clock and the temporal organi zation of insect societies. In: Gadau J, Fewell J, editors. Organization of Insect Societies. Harvard: Harvard University Press. pp. 402-431. [Google Scholar]
- 41. Pittendrigh CS (1993) Temporal organization: Reflections of a darwinian clock-watcher. Annu Rev Physiol 55: 16–54. [DOI] [PubMed] [Google Scholar]
- 42. Vanlalhriatpuia K, Chhakchhuak V, Moses SK, Iyyer SB, Kasture MS, et al. (2007) Effects of altitude on circadian rhythm of adult locomotor activity in himalayan strains of Drosophila helvetica . J Circadian Rhythms 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Joshi D (1999) Latitudinal variation in locomotor activity rhythm in adult Drosophila ananassae . Can J Zoolog 77: 865–870. [Google Scholar]
- 44. Saunders DS (1970) Circadian clock in insect photoperiodism. Science 168: 601–603. [DOI] [PubMed] [Google Scholar]
- 45.Paolucci S, van de Zande L, Beukeboom LW. (2013) Adaptive latitudinal cline of photoperiodic diapause induction in the parasitoid Nasonia vitripennis in Eur J Evol Biol: in press. [DOI] [PubMed] [Google Scholar]
- 46. O'Brien C, Bradshaw WE, Holzapfel CM (2011) Testing for causality in covarying traits: Genes and latitude in a molecular world. Mol Ecol 20: 2471–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bradshaw WE, Emerson KJ, Holzapfel CM (2012) Genetic correlations and the evolution of photoperiodic time measurement within a local population of the pitcher-plant mosquito, Wyeomyia smithii . Heredity 108: 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kostal V (2011) Insect photoperiodic calendar and circadian clock: Independence, cooperation, or unity? J Insect Physiol 57: 538–556. [DOI] [PubMed] [Google Scholar]
- 49. Goto SG (2013) Roles of circadian clock genes in insect photoperiodism. Entomol Sci 16: 1–16. [Google Scholar]
- 50. Saunders DS (1973) Thermoperiodic control of diapause in an insect: Theory of internal coincidence. Science 181: 358–360. [DOI] [PubMed] [Google Scholar]
- 51. Shimizu T, Masaki S (1997) Geographical and species variation in circadian rhythm parameters in nemobiine crickets. Physiol Entomol 22: 83–93. [Google Scholar]
- 52. Harano T, Miyatake T (2010) Genetic basis of incidence and period length of circadian rhythm for locomotor activity in populations of a seed beetle. Heredity 105: 268–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Examples of activity plots with rhythm splits. These double-plots are examples in which activities have more than one internal period or display splits in the activity period. If the main period is sufficiently supported (see materials and methods) it is included in the analysis. N. giraulti RV2x(u) female with a main internal period (21.8 h) and a secondary period at roughly 25 h in LL (A). Two periods (23 and 26 h) are apparent in a N. vitripennis Sal29 female in LL (B). N. longicornis IV7R2 male with an unclear rhythm split in LL (C). Some N. giraulti VA1TET males show a 24 h-like rhythm in the first days in DD but then split. In this example, however, a main 22.6 h period can be seen from day 6 to 13 (D). ‘Wandering’ rhythms in a N. vitripennis HV3 male in DD: 22.5 h from day 6 to 10; 26 h from day 10 to 13 (E). Red dashed lines highlight multiple rhythms.
(TIF)
Examples of activity plots with increasing ratio-to-p values. In order to use a quantitative estimate to distinguish rhythmic vs. non-rhythmic activity plots, the ‘ratio-to-p’ value – the main peak value of the Qp statistics for rhythmicity (over the τ period tested) divided by the Qp value corresponding to the 0.01 probability threshold – was used as cut-off value. Activity plots with a ratio-to-p equal or lower than 5.5 were considered non-rhythmic. Here, examples of activity plots with increasing ratio-to-p values are shown. The values in the inset correspond to τ (in hours) and the ratio-to-p value, respectively, for the LL phase.
(TIF)
Average activity in LD for additional Nasonia strains. The median (with lower and upper quartiles) of the activity over the last three days in LD (double plotted) is indicated for each sex in additional Nasonia strains used for each species. Gray background indicates darkness. Y-axis: activity (pixel changes/minute); x-axis: hour of the day.
(TIF)
Representative activity rhythms for Nasonia species. Representative actograms to distinguish among Nasonia species and sexes are shown. For a description of activity patterns see Table S1. Additional actograms can be found in figures 1 and 7.
(TIF)
Classification of Nasonia species according to activity patterns and parameters in different light conditions.
(TIF)