Abstract
The pathophysiological mechanisms underlying the development of obesity and metabolic diseases are not well understood. To gain more insight into the genetic mediators associated with the onset and progression of diet-induced obesity and metabolic diseases, we studied the molecular changes in response to a high-fat diet (HFD) by using a mode-of-action by network identification (MNI) analysis. Oligo DNA microarray analysis was performed on visceral and subcutaneous adipose tissues and muscles of male C57BL/6N mice fed a normal diet or HFD for 2, 4, 8, and 12 weeks. Each of these data was queried against the MNI algorithm, and the lists of top 5 highly ranked genes and gene ontology (GO)-annotated pathways that were significantly overrepresented among the 100 highest ranked genes at each time point in the 3 different tissues of mice fed the HFD were considered in the present study. The 40 highest ranked genes identified by MNI analysis at each time point in the different tissues of mice with diet-induced obesity were subjected to clustering based on their temporal patterns. On the basis of the above-mentioned results, we investigated the sequential induction of distinct olfactory receptors and the stimulation of cancer-related genes during the development of obesity in both adipose tissues and muscles. The top 5 genes recognized using the MNI analysis at each time point and gene cluster identified based on their temporal patterns in the peripheral tissues of mice provided novel and often surprising insights into the potential genetic mediators for obesity progression.
Introduction
Microarray analysis has enabled the use of whole-genome expression profiling to understand the mechanisms underlying obesity and metabolic complications and to identify key genetic mediators. Statistical approaches used to analyze microarray data can be classified into 2 major categories: methods that identify differentially expressed genes [1], [2] and those that classify genes according to the functional dependency (e.g., hierarchical clustering) [3]. Although microarray analysis has yielded some promising results, it is not a very practical method considering the fact that identification of genes directly affected by a condition is difficult from the hundreds to thousands of genes that exhibit changes in expression. To overcome this problem, Berneardo et al. developed a model-based approach that accurately distinguishes a compound's targets from the indirect responders [4]. This approach, namely, the mode-of-action by network identification (MNI), involves the reverse engineering of a network model of regulatory interactions in an organism of interest by using a training dataset of whole-genome expression profiles. The MNI algorithm has been applied successfully to identify disease mediators as well as drug targets by studying gene-expression data from yeast [4], humans (A. Ergun and J.J. Collins, unpublished data), bacteria, and other organisms (X.H., unpublished data).
Differential expression can be studied from a static or temporal viewpoint. In a static experiment, the arrays are obtained irrespective of time, essentially taking a snapshot of gene expression. On the other hand, in a temporal experiment, the arrays are collected over a time course, facilitating the study of the dynamic behavior of gene expression. Most previously obtained microarray datasets were static, that is, the results obtained on the basis of the measurement of gene expression at a single time point [5]. Since the regulation of gene expression is a dynamic process, it is important to identify and characterize the changes in gene expression over time. Therefore, numerous time-series microarray experiments have been performed to study such biological processes such as abiotic stress, disease progression, and drug responses [6]–[8].
Microarray analysis for studying the mechanisms underlying obesity was first reported by Soukas et al. in 2000 [9]. They used approximately 6,500 murine genes in pairs of adipose tissues in ob/ob mice and wild-type lean mice. Subsequently, many such studies were conducted: more than 30 microarray approaches have been exploited in assessing the changes in gene expression in the adipose tissues, liver, hypothalamus, skeletal muscles, small intestines, and kidneys of lean and obese animals or human subjects. A frequent limitation of these studies is that they are not time-resolved and do not necessarily provide information of an end-point or disease stage. Considerably less is known about the key genetic mediators of HFD-induced obesity and the dynamics of changes in metabolic processes related to this condition. To gain more insight into the genetic mediators associated with the onset and progression of diet-induced obesity and metabolic diseases, we studied the molecular changes in response to the HFD by using an integrative time-resolved approach.
Materials and Methods
Ethics statement
All animal experiments were performed in accordance with the Korean Food and Drug Administration (KFDA) guidelines. Protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the Yonsei Laboratory Animal Research Center (YLARC) (Permit #: 2011-0061). All mice were maintained in the specific pathogen-free facility of the YLARC.
Animals and diets
Five-week-old male C57BL/6N mice were obtained from Orient Bio (Gyeonggi-do, South Korea). All animals were housed in specific pathogen-free conditions, with 21±2.0°C temperature, 50±5% relative humidity, and a 12 h-light/12 h-dark cycle. From a week before the diet intervention was started, all animals were fed standard chow. At the beginning of the study, mice were divided into 2 groups: (1) control group fed the normal diet (ND, n = 40) and (2) a group fed the high-fat diet (HFD, n = 40). Mice were provided food and water ad libitum. The body weight and food intake were monitored throughout the study. At 2, 4, 8, and 12 weeks after the initiation of the study, 10 animals from each group were killed. Tissues were snap-frozen immediately in liquid nitrogen and stored at −80°C until further processing.
RNA extraction for microarray analysis
Total RNA was extracted from the epididymal and subcutaneous fat tissues and gastrocnemius muscle of each mouse by using Trizol (Invitrogen, CA, USA), according to the manufacturer's recommendations. Concentrations and purity of RNA samples were determined using a Nano Drop ND-1000 spectrophotometer (Nano Drop Technologies, Inc., Wilmington, DE, USA). RNA preparations were considered suitable for array hybridization only if samples showed intact 18S and 28S rRNA bands and displayed no chromosomal peaks or RNA degradation products. The integrity of the RNA samples was determined using a Bioanalyzer 2100 System (Agilent Technologies, Palo Alto, CA, USA).
Real-time quantitative PCR
Real-time PCR amplification was performed with the SYBR Premix Ex Taq kit (Takara, Kyoto, Japan) on a Light Cycler 2 (Roche Applied Science, Indianapolis, USA). The initial denaturation step was at 95°C for 10 s, followed by 40 cycles of amplification at 95°C for 3 s and 60°C for 40 s. mRNA expression was determined using the relative standard curve method and normalized to the housekeeping gene. The primers (sense and antisense, respectively) were as follows: Gli2, 5′- GCC AAC CAG AAC AAG CAG AA-3′, 5′- CGC TTA TGA ATG GTG ATG GG -3′; Gucy2c, 5′- GTG CGG TTA CTG CTC TTC CA -3′, 5′- TTG TCC ATC ATC AGG ACG CT -3′; Olfr1181, 5′- CCT GAC AGT CAT GGC CTT TG -3′, 5′- ACC CAG GAA GCC CAG ATA AA -3′; Atp8b3, 5′- GTT TGA GCA GGA TGT GAC CG -3′, 5′- GGC TTG CAT GAA AAT GCT GT -3′; Tmem46, 5′- TTT TCC AGC AGC AGG AGC TA -3′, 5′- GCT GAG GAG AAA AGG GAT GC -3′; Pthr2, 5′- ATG CAA GGG AGA AAC CCA TC -3′, 5′- TAG ATC CTC CCA CAC AGC CA -3′; Cdh7, 5′- TGG ACT GGG CAT TTT CAA GA -3′, 5′- GGG GAT CAG CAT CTC GAT TT -3′; Mep1b, 5′- GAT GGC CAC ATA CCA TTC CA -3′, 5′- TAA GGC GAT AGC GCT CAA AA -3′; Lamc3, 5′- GAC ATG GGC TCT TGC TAC GA -3′, 5′- CGT TCT CGA ACT CAG GCA GA -3′; GAPDH, 5′- GGA GAT TGT TGC CAT CAA CG -3′, 5′- TTT GCC GTG AGT GGA GTC AT -3′.
Microarray hybridization and data analysis
Equal amounts of total RNA were pooled from 10 mice in each experimental group and subjected to microarray experiments in triplicate. For analysis, 2 μg total RNA was labeled and amplified using the Universal Linkage System antisense RNA (aRNA) labeling kit (Kreatech Diagnostics, Amsterdam, The Netherlands). The Cy5-labeled aRNAs were resuspended in 10 μL of hybridization solution (GenoCheck, Korea). The labeled aRNAs were hybridized to the NimbleGen mouse whole genome 12-plex array (Roche NimbleGen, Inc., WI, USA) that contained 60-mer probes representing 42,576 genes (average 3 probes per target). The arrays were scanned using a GenePix 4000B microarray scanner. The data were extracted from the scanned images using NimbleScan software version 2.4 (Roche NimbleGen), and the Robust Multichip Average algorithm was used to generate gene expression values. The normalized and log-transformed intensity values were then analyzed using GeneSpring GX 10 (Agilent Technologies, Santa Clara, CA, USA) and GenePlex (Istech, Inc., Seoul, South Korea). The details of labeling, hybridization, scanning, and normalization of the data are provided on the NimbleGen website (http://www.nimblegen.com). Gene expression levels between the ND and HFD samples were assessed by comparing the average expression ratios of each group. Hierarchical clustering was performed in GeneSpring GX 7.3.1 software (Agilent Technologies, Santa Clara, CA), using average gene expression values under HFD condition divided by the median of ND gene expression, per time-point.
MNI algorithm
We constructed a compendium dataset consisting of hundreds of expression profiles in the organism of interest; that the expression profiles were downloaded from the Gene Expression Omnibus, a public repository of microarray studies. The MNI algorithm was applied, using the method developed by Xing et al. [10], and was configured to output the top 200 mediators for each sample and generate the associated Z-scores for those probe sets. The Z-score for probe sets that were not within the list of the top 100 probe sets identified as mediators for a given sample were set to zero. To identify a characteristic list of genes within each group, the Z-scores across samples and probe sets for corresponding genes were averaged and ranked. The top 100 genes within that list were selected to be reported as significant genetic mediators. A higher average Z-score is an indication of higher number of occurrences of a gene on the lists generated by the MNI algorithm in each group. The 100 highest ranked genes were classified according to the biological process in which they are involved as per the criteria established by the GO.
Results
Effect of HFD feeding on visceral adiposity
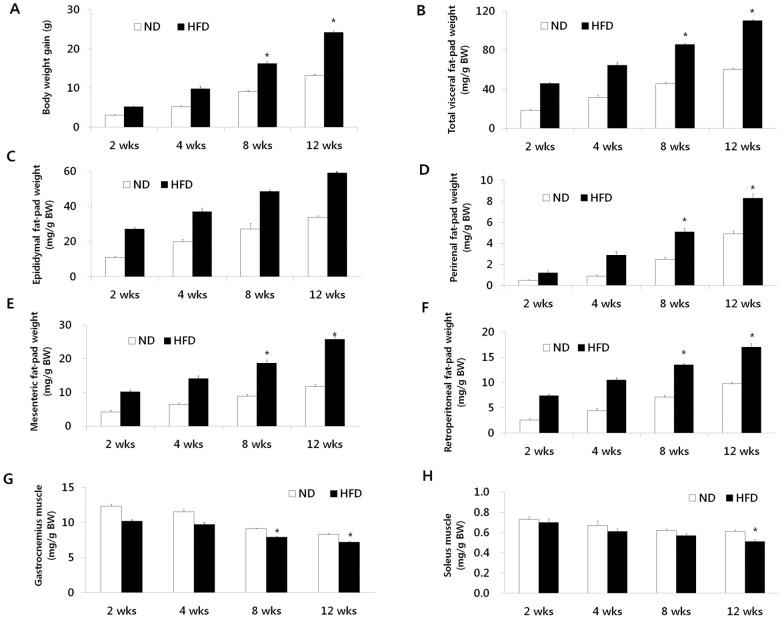
The body weight gains of mice fed the 2 diets over the 12-week period are shown in Figure 1A. The difference in body weight between the 2 groups continued to increase over the course of experimental feeding: the difference was about 45% by 12 weeks. The increase in body weight associated with the HFD was partially attributed to the expansion of visceral adipose tissues. The masses of the epididymal, perirenal, mesenteric, and retroperitoneal fat pads of the mice fed HFD for 12 weeks were 42%, 40%, 54%, and 42%, respectively; the difference in the masses was larger in the HFD-fed mice than in the ND-fed group (Figure 1B–F). Moreover, HFD-fed mice exhibited significant reductions in the wet weights of the gastrocnemius (−13%) and soleus (−16%) muscles at 12 weeks compared with those in the ND-fed mice (Figure 1G and H).
Figure 1. Changes in body weight, visceral fat-pad weights, and muscle masses over time.
(A) Body weight gain. (B) Total visceral fat-pad weight. (C) Epididymal fat-pad weight. (D) Perirenal fat-pad weight. (E) Mesenteric fat-pad weight. (F) Retroperitoneal fat-pad weight. (G) Gastrocnemius muscle mass. (H) Soleus muscle mass. Data are presented as means ± SEM. *P<0.05.
Transcription response of WAT and muscle to HFD during the 12-week time-course
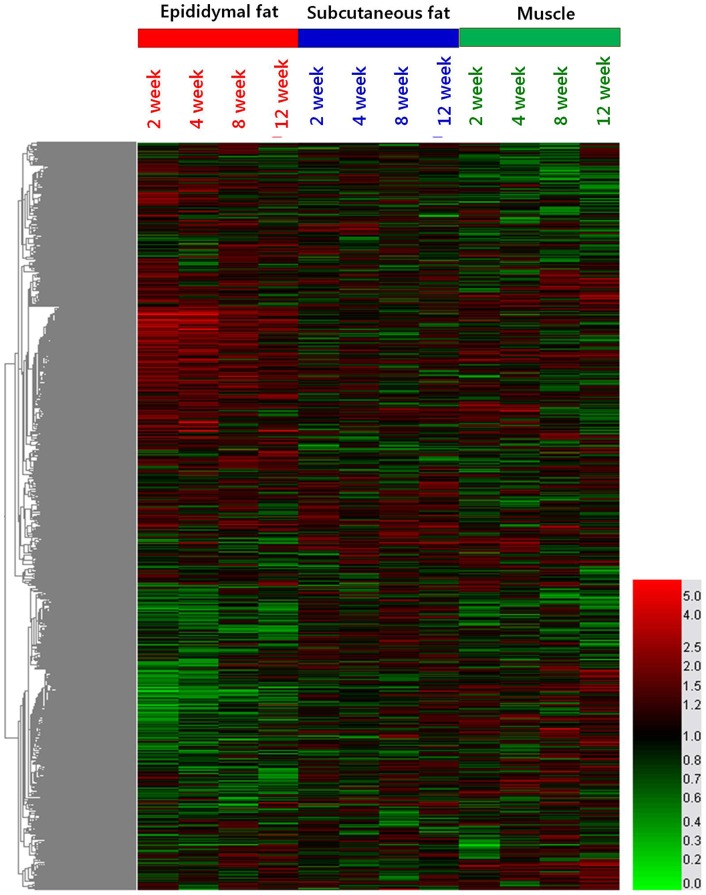
Gene expression profiling in the WAT and muscle of mice was assessed through the oligonucleotide microarray analysis. Among 25,291 genes on the NimbleGen Mouse Whole Oligo 12-plex chip used in this study, 21,890 genes (86%) were identified as known genes. After determination of the temporal effects of the HFD across 12-week time-course, we focused on dissecting the HFD specific effects on the transcriptome of epididymal and subcutaneous fats and muscle. Microarray data were analyzed by hierarchical clustering of enriched functional groups of genes (based on Gene Ontology) and the major results are graphically illustrated in a heat map (Figure 2). The HFD elicited distinct changes in gene expression in epididymal and subcutaneous fats and muscle of mice over time, and most significant changes were shown in epididymal fat tissue. Specifically, prominent expression changes were observed at the early phase (week 2 to week 4) and the enrichment of lipid metabolism and inflammatory processes were significant among the up-regulated HFD-responsive genes, whereas G-protein coupled receptor protein signaling pathway and electron transport were most significant among the down-regulated HFD-responsive genes in the epididymal fat tissue.
Figure 2. Heatmap of differentially expressed transcript sets.
Values used for clustering are average HFD vs. ND per time-point expression ratio. The branches of the condition tree are colored so to discriminate three subclusters with the largest distance, corresponding to three tissues of the time-course: epididymal adipose tissue (red), subcutaneous adipose tissue (blue) and gastrocnemious muscle (green). This is summarized in the color bar underneath the cluster diagram.
MNI analysis of the time course treatment with the HFD
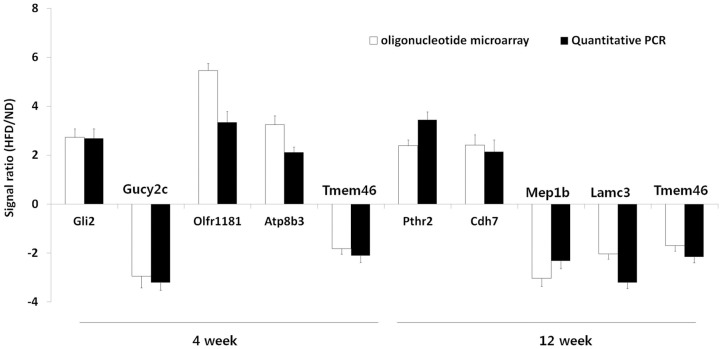
To elucidate the time course and metabolic processes underlying obesity progression induced by the HFD, we determined the gene expression profiles of the epididymal and subcutaneous fat tissues and gastrocnemius muscle of mice by using oligonucleotide microarray analysis. Each of these data was queried against the reconstructed network (MNI algorithm), and the resulting potential genetic mediators in each case were ranked according to the Z-score statistic. The lists of top 5 potential genetic mediators for obesity progression in the epididymal and subcutaneous fat tissues and gastrocnemius muscle of mice fed the HFD for 2, 4, 8, and 12 weeks are shown in Tables 1, 2, 3. The most characteristic genes across all tissues in the list were associated with cancer; the genes in this category included Nek11, Gli2, Tmem46, Mep1b, Ccdc109b, Rab23, Patz1, and Hdac9. The second representative functional theme was related to olfactory transduction, and these genes included Olfr1181, Olfr1173, Olfr855, Olfr1056, Olfr716, and Tmem16b. To validate the microarray results quantitatively, we analyzed the mRNA expression levels of top-ranked genes by real-time PCR. In all cases, a strong correspondence between the microarray data and the real-time PCR results was observed (Figure 3). We also measured the basal expression levels of selected genes including several olfactory receptors in the epididymal fat tissues of ND- or HFD-fed mice, using real-time PCR. The results indicated that the basal expression levels of highly ranked olfactory genes (Olfr1181, Olfr513, Olfr960, and Olfr1245) were comparable to those of top five genes (Gli2, Gucy2c, Atp8b3, and Tmem46) identified by MNI analysis at week 4 in the epididymal adipose tissue (Figure S1).
Table 1. List of top 5 genes identified by the MNI analysis at each time point in the epididymal fat tissue of HFD-induced obese mice.
| Rank | Gene accession No. | Gene symbol | Description | Function | Fold change | |||
| Epididymal fat tissue | ||||||||
| 2 week | 1 | NM_023173 | Dusp12 | Dual specificity phosphatase 12 | Insulin resistance | 2.52 | ||
| 2 | NM_172461 | Nek11 | NIMA (never in mitosis gene a)-related expressed kinase 11 | Cancer | 0.43 | |||
| 3 | NM_145489 | AI661453 | Expressed sequence AI661453 | Unknown | 0.23 | |||
| 4 | NM_177078 | Adrbk2 | Adrenergic receptor kinase, beta 2 | Bipolar disorder | 2.73 | |||
| 5 | NM_013679 | Svs6 | Seminal vesicle secretory protein 6 | Unknown | 0.77 | |||
| 4 week | 1 | XM_136212 | Gli2 | GLI-Kruppel family member GLI2 | Cancer | 2.73 | ||
| 2 | NM_145067 | Gucy2c | Guanylate cyclase 2c | Cancer | 0.34 | |||
| 3 | NM_001011816 | Olfr1181 | Olfactory receptor 1181 | Olfactory transduction | 5.47 | |||
| 4 | NM_026094 | Atp8b3 | ATPase, Class I, type 8B, member 3 | ATP binding | 3.25 | |||
| 5 | NM_145463 | Tmem46 | Transmembrane protein 46 | Cancer | 0.55 | |||
| 8 week | 1 | NM_199155 | Tas2r110 | Taste receptor, type 2, member 110 | Sensory perception of taste | 2.81 | ||
| 2 | AK138164 | Cntn5 | Contactin 5 | Cell adhesion | 0.33 | |||
| 3 | NM_152220 | Stx3 | Syntaxin 3 | Arachidonic acid binding | 1.81 | |||
| 4 | NM_178924 | Upk1b | Uroplakin 1B | Epithelial cell differentiation | 1.79 | |||
| 5 | NM_028622 | Lce1c | Late cornified envelope 1C | Unknown | 0.32 | |||
| 12 week | 1 | NM_139270 | Pthr2 | Parathyroid hormone receptor 2 | Parathyroid hormone receptor activity | 2.39 | ||
| 2 | NM_172853 | Cdh7 | Cadherin 7, type 2 | Calcium ion binding | 2.42 | |||
| 3 | NM_008586 | Mep1b | Meprin 1 beta | Cancer | 0.33 | |||
| 4 | NM_011836 | Lamc3 | Laminin gamma 3 | Cell adhesion | 0.49 | |||
| 5 | NM_145463 | Tmem46 | Transmembrane protein 46 | Cancer | 0.59 | |||
Table 2. List of top 5 genes identified by the MNI analysis at each time point in the subcutaneous fat tissue of HFD-induced obese mice.
| Rank | Gene accession No. | Gene symbol | Description | Function | Fold change | |||
| Subcutaneous fat tissue | ||||||||
| 2 week | 1 | NM_025779 | Ccdc109b | Coiled-coil domain containing 109B | Cancer | 0.54 | ||
| 2 | NM_001025438 | Camk2d | Calcium/calmodulin-dependent protein kinase II, delta | Calmodulin binding | 0.65 | |||
| 3 | AB211064 | L1td1 | LINE-1 type transposase domain containing 1 | Unknown | 2.61 | |||
| 4 | NM_026345 | Mansc1 | MANSC domain containing 1 | Unknown | 3.03 | |||
| 5 | NM_207566 | Olfr1173 | Olfactory receptor 1173 | Olfactory transduction | 1.97 | |||
| 4 week | 1 | NM_008529 | Ly6e | Lymphocyte antigen 6 complex, locus E | Adrenal gland development | 1.31 | ||
| 2 | NM_146524 | Olfr855 | Olfactory receptor 855 | Olfactory transduction | 1.57 | |||
| 3 | NM_018744 | Sema6a | Sema domain, transmembrane domain (TM), and cytoplasmic domain, (semaphorin) 6A | Nervous system development | 0.73 | |||
| 4 | AB211064 | L1td1 | LINE-1 type transposase domain containing 1 | Unknown | 3.13 | |||
| 5 | NM_183015 | Ccnb3 | Cyclin B3 | Cell cycle | 2.58 | |||
| 8 week | 1 | NM_153111 | Fev | FEV (ETS oncogene family) | Nervous system development | 0.39 | ||
| 2 | AB211064 | L1td1 | LINE-1 type transposase domain containing 1 | Unknown | 2.8 | |||
| 3 | NM_147018 | Olfr1056 | Olfactory receptor 1056 | Olfactory transduction | 0.73 | |||
| 4 | NM_008999 | Rab23 | RAB23, member RAS oncogene family | Cancer | 0.56 | |||
| 5 | NM_080644 | Cacng5 | Calcium channel, voltage-dependent, gamma subunit 5 | Calcium ion transport | 0.36 | |||
| 12 week | 1 | NM_001024852 | Auts2 | Autism susceptibility candidate 2 | Mental retardation | 0.53 | ||
| 2 | NM_178046 | Svil | Supervillin | Unknown | 0.57 | |||
| 3 | NM_018764 | Pcdh7 | Protocadherin 7 | Cell adhesion | 1.82 | |||
| 4 | NM_146604 | Olfr716 | Olfactory receptor 716 | Olfactory transduction | 3.08 | |||
| 5 | BC089489 | 4930474M22Rik | RIKEN cDNA 4930474M22 gene | Unknown | 1.9 | |||
Table 3. List of top 5 genes identified by the MNI analysis at each time point in the gastrocnemius muscle of HFD-induced obese mice.
| Rank | Gene accession No. | Gene symbol | Description | Function | Fold change | |
| Gastrocnemius muscle | ||||||
| 2 week | 1 | NM_019574 | Patz1 | POZ (BTB) and AT hook containing zinc finger 1 | Cancer | 1.8 |
| 2 | NM_020610 | Nrip3 | Nuclear receptor interacting protein 3 | Inflammation | 0.42 | |
| 3 | NM_024124 | Hdac9 | Histone deacetylase 9 | Cancer | 0.55 | |
| 4 | XM_975536 | Armc4 | Armadillo repeat containing 4 | Unknown | 0.54 | |
| 5 | NM_139226 | Onecut3 | One cut domain, family member 3 | DNA binding | 1.34 | |
| 4 week | 1 | NM_153589 | Tmem16b | Transmembrane protein 16B | Olfactory transduction | 0.74 |
| 2 | NM_175540 | Eda2r | Ectodysplasin A2 isoform receptor | Alopecia | 0.88 | |
| 3 | NM_008355 | Il13 | Interleukin 13 | Inflammation | 1.57 | |
| 4 | NM_010608 | Kcnk3 | Potassium channel, subfamily K, member 3 | Ion transport | 1.22 | |
| 5 | XM_129809 | Ogfrl1 | Opioid growth factor receptor-like 1 | Unknown | 0.7 | |
| 8 week | 1 | NM_024124 | Hdac9 | Histone deacetylase 9 | Cancer | 0.6 |
| 2 | NM_011990 | Slc7a11 | Solute carrier family 7 | Amino acid transport | 0.7 | |
| 3 | NM_175420 | 9330176C04Rik | RIKEN cDNA 9330176C04 gene | Unknown | 2.17 | |
| 4 | NM_016961 | Mapk9 | Mitogen activated protein kinase 9 | Insulin resistance | 0.59 | |
| 5 | NM_027462 | Wars2 | Tryptophanyl tRNA synthetase 2 (mitochondrial) | Vasculogenesis | 0.56 | |
| 12 week | 1 | NM_008114 | Gfi1 | Growth factor independent 1B | Hematopoiesis | 0.74 |
| 2 | NM_145435 | Pyy | Peptide YY | Insulin resistance | 1.49 | |
| 3 | NM_138648 | Olr1 | Oxidized low density lipoprotein (lectin-like) receptor 1 | Inflammation | 0.33 | |
| 4 | NM_177861 | Tmem67 | Transmembrane protein 67 | Mental retardation | 0.54 | |
| 5 | XM_887155 | Igsf10 | Immunoglobulin superfamily, member 10 | Unknown | 0.42 | |
Figure 3. Quantitative PCR.
Quantitative real-time PCR analysis of the mRNA expression on selected gene targets identified by MNI analysis in the epididymal fat tissues of mice. Results are presented as the average ± SEM of at least 3 separate experiments.
Functional analysis of the highly ranked genetic mediators
We next focused on the GO-annotated pathways that were significantly overrepresented among the highly ranked genetic mediators. For our analysis, we subjected the 100 highest ranked genes identified by MNI analysis in the epididymal and subcutaneous fat tissue and gastrocnemius muscle of mice with diet-induced obesity to pathway analysis based on the GO biological process annotations (Tables 4, 5, 6). We found that the olfactory transduction was highly enriched in the epididymal and subcutaneous fat tissue and gastrocnemius muscle of the HFD-fed mice compared to the ND-fed mice at all time points. Even the second representative functional theme of epididymal fat was related to cancer at all time points. The pathways thought to be associated with obesity progression in the epididymal fat as per the MNI analysis included Wnt signaling pathway, melanogenesis, chemokine signaling pathway, focal adhesion, MAPK signaling pathway, purine metabolism, regulation of actin cytoskeleton, neuroactive ligand-receptor interaction, and extracellular matrix (ECM)-receptor interaction. In the subcutaneous fat, other pathways identified by the MNI analysis for obesity progression included calcium signaling pathway, gonadotropin-releasing hormone (GnRH) signaling pathway, axon guidance, cell cycle, and tyrosine metabolism. In the gastrocnemius muscle, besides the olfactory transduction mentioned above, the over-represented groups identified according to GO biological processes for obesity progression were those involved in the various cellular processes such as neuroactive ligand-receptor interaction, cytokine-cytokine receptor interaction, pathways associated with cancer, insulin signaling pathway, pathways associated with colorectal cancer, adipocytokine signaling pathway, type II diabetes mellitus, and cell adhesion molecules.
Table 4. The enriched pathways among top 100 genetic mediators identified by the MNI analysis at each time point in the epididymal fat tissue of HFD-induced obese mice.
| GO ontology | Ranked pathway genes (rank) | ||
| Epididymal fat tissue | |||
| 2 week | Olfactory transduction | Adrbk2 (4), Olfr513 (6), Olfr433 (15), Camk2g (28), Olfr1245 (29), Olfr1143 (36), Olfr996 (55), Olfr960 (57), Arrb2 (79) | |
| Wnt signaling pathway | Camk2g (28), Rhoa (31), Wnt10a (95) | ||
| Melanogenesis | Camk2g (28), Adcy5 (80), Wnt10a (95) | ||
| Chemokine signaling pathway | Rhoa (31), Arrb2 (79), Adcy5 (80) | ||
| Focal adhesion | Rhoa (31), Lamc3 (44), Bcl2 (77) | ||
| Pathways in cancer | Rhoa (31), Bcl2 (77), Wnt10a (95) | ||
| 4 week | Olfactory transduction | Camk2g (17), Olfr513 (27), Olfr960 (31), Olfr1245 (43), Arrb2 (55) | |
| Pathways in cancer | Gli2 (1), Lamc3 (9), Bcl2 (58), Fgf5 (65) | ||
| MAPK signaling pathway | Arrb2 (55), Fgf5 (65), Mapkapk5 (75) | ||
| Purine metabolism | Gucy2c (2), Nme7 (47), Cant1 (85) | ||
| 8 week | Olfactory transduction | Olfr536 (11), Olfr513 (31), Olfr654 (37), Camk2g (74), Olfr652 (99) | |
| Focal adhesion | Flnc(16), Bcl2 (64), Col6a2 (83), Rhoa (86), Mylk (96), | ||
| Regulation of actin cytoskeleton | Fgf5 (26), Rhoa (86), Mylk (96) | ||
| Pathways in cancer | Fgf5 (26), Bcl2 (64), Rhoa (86) | ||
| 12 week | Olfactory transduction | Olfr16 (6), Camk2g (10), Olfr715 (24), Olfr1245 (32), Olfr536 (36), Olfr1143 (52) | |
| Neuroactive ligand-receptor interaction | Pth2r (1), Agtrl1 (16), Vipr2 (27), P2rx6 (74) | ||
| Focal adhesion | Lamc3 (4), Bcl2 (75), Col6a2 (76), Rhoa (88) | ||
| ECM-receptor interaction | Lamc3 (4), Cd44 (59), Col6a2 (76) | ||
| Pathways in cancer | Lamc3 (4), Bcl2 (75), Rhoa (88) | ||
Table 5. The enriched pathways among top 100 genetic mediators identified by the MNI analysis at each time point in the subcutaneous fat tissue of HFD-induced obese mice.
| GO ontology | Ranked pathway genes (rank) | |
| Subcutaneous fat tissue | ||
| 2 week | Olfactory transduction | Camk2b (2), Olfr1173 (5), Olfr823 (9), Guca1a (41), Olfr411 (49), Olfr1408 (51), Olfr875 (64) |
| Calcium signaling pathway | Camk2b (2), Cacna1d (15), Htr4 (45), Ryr1 (96) | |
| GnRH signaling pathway | Camk2b (2), Cacna1d (15), Cga (48) | |
| 4 week | Olfactory transduction | Olfr855 (2), Olfr888 (13), Olfr305 (36), Olfr1173 (49), Olfr395 (52), Olfr411 (55), Olfr823 (83), Olfr1409 (89) |
| Axon guidance | Sema6a (3), Abl1 (23) | |
| Cell cycle | Ccnb3 (5), Abl1 (23) | |
| Regulation of actin cytoskeleton | Vav3 (9), Ssh2 (25) | |
| Tyrosine metabolism | Fah (26), Aoc3 (27) | |
| MAPK signaling pathway | Cacna1d (28), Hspa1a (44) | |
| 8 week | Olfactory transduction | Olfr1056 (3), Olfr960 (15), Olfr1408 (26), Olfr855 (35), Olfr609 (42), Olfr1173 (49), Olfr411 (71), Olfr205 (91), Olfr1121 (94) |
| Focal adhesion | Shc4 (63), Diap1 (77), Lamb2 (82), Vav3 (83) | |
| 12 week | Olfactory transduction | Olfr716 (4), Olfr45 (16), Olfr960 (19), Olfr1173 (32), Olfr411 (34), Olfr1408 (43), Olfr855 (45), Olfr875 (96) |
| GnRH signaling pathway | Cacna1d (29), Cga (58) | |
| Chemokine signaling pathway | Vav3 (33), Gng3 (79), Shc4 (84) | |
Table 6. The enriched pathways among top 100 genetic mediators identified by the MNI analysis at each time point in the gastrocnemius muscle of HFD-induced obese mice.
| GO ontology | Ranked pathway genes (rank) | |
| Gastrocnemius muscle | ||
| 2 week | Olfactory transduction | Clca3 (6), Olfr739 (14), Olfr488 (40), Olfr474 (55) |
| 4 week | Olfactory transduction | Olfr800 (28), Olfr978 (33), Olfr474 (49), Olfr488 (73) |
| Neuroactive ligand-receptor interaction | Oprl1 (30), Mc1r (78), Tbxa2r (89), Tspo (97) | |
| Cytokine-cytokine receptor interaction | Eda2r (2), Il13 (3), Cx3cl1 (91) | |
| Pathways in cancer | Dcc (10), Amn (71), Mlh1 (85) | |
| 8 week | Olfactory transduction | Clca3 (9), Olfr739 (48), Olfr1305 (64), Olfr1395 (80), Olfr140 (81), Olfr63 (85), Olfr689 (86) |
| Pathways in cancer | Mapk9 (4), Dcc (15), Mapk10 (78), Grb2 (87), Mitf (89) | |
| Insulin signaling pathway | Mapk9 (4), Irs3 (40), Mapk10 (78), Grb2 (87) | |
| Colorectal cancer | Mapk9 (4), Dcc (15), Mapk10 (78), Grb2 (87) | |
| Adipocytokine signaling pathway Type II diabetes mellitus | Mapk9 (4), Irs3 (40), Mapk10 (78) | |
| 12 week | Olfactory transduction | Olfr689 (22), Olfr488 (24), Olfr347 (38), Olfr1204 (69) |
| Cell adhesion molecules (CAMs) | Cd22 (23), Nlgn3 (44), Ptprm (62) | |
| Cytokine-cytokine receptor interaction | Eda2r (43), Ppbp (53), Cx3cl1 (57) | |
Representative time-course profile clusters
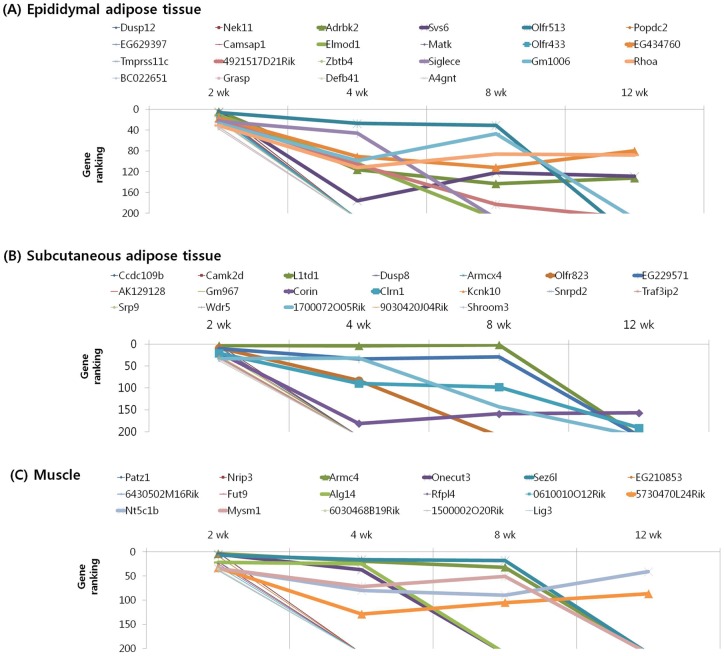
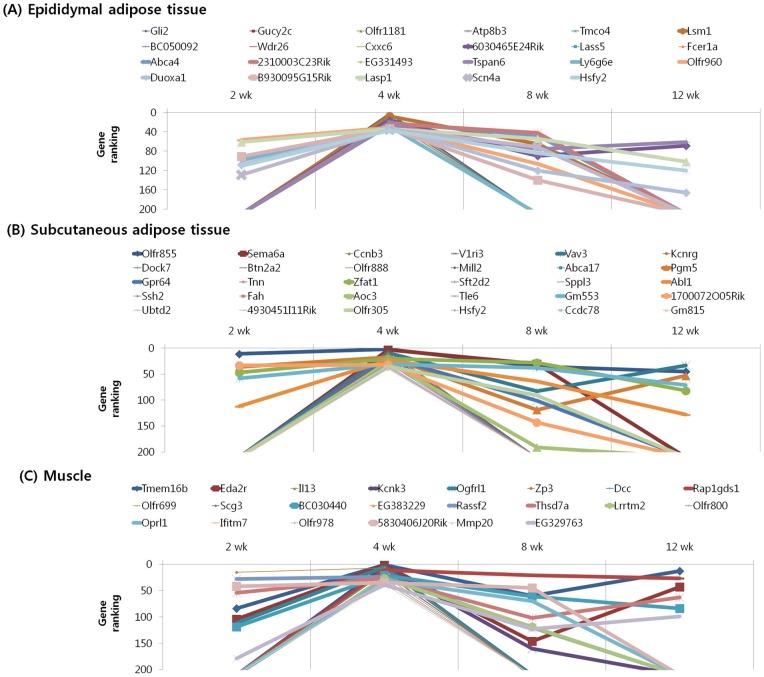
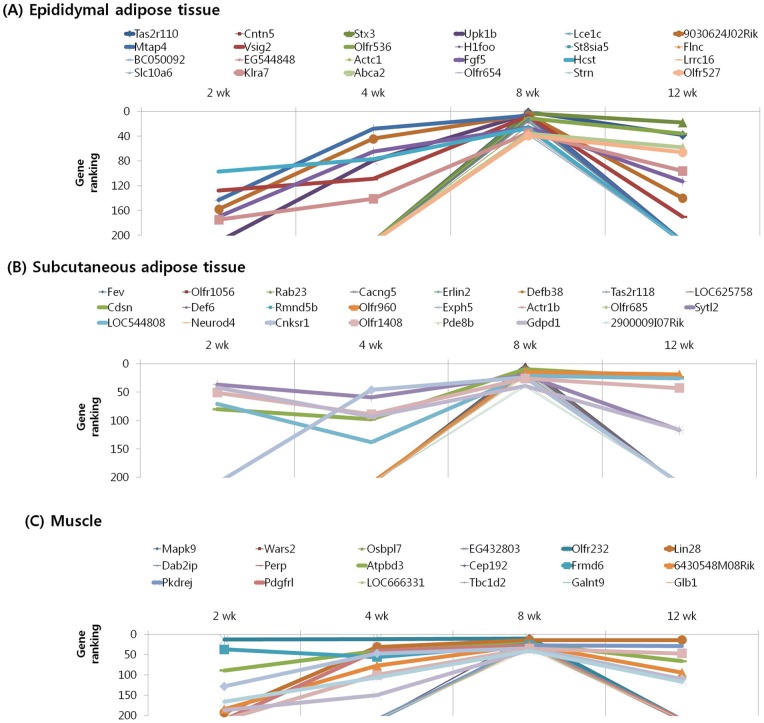
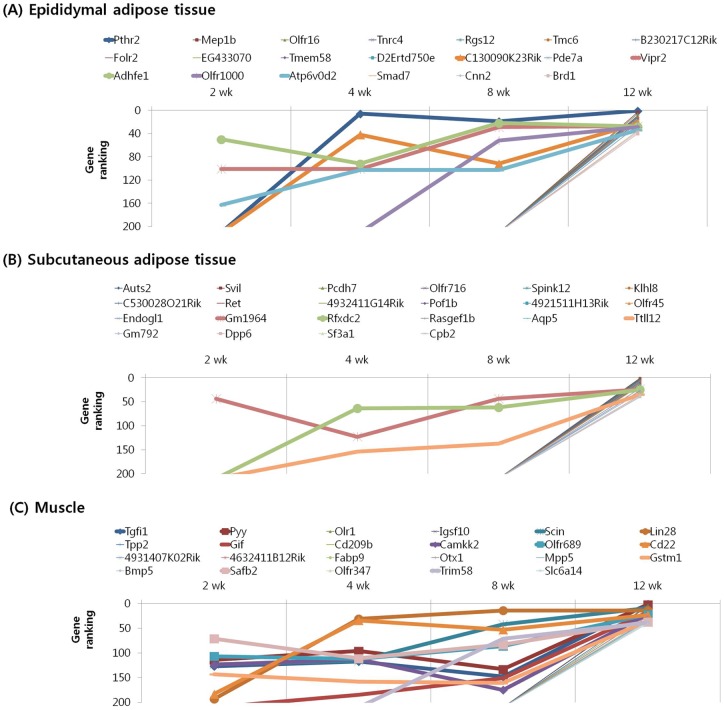
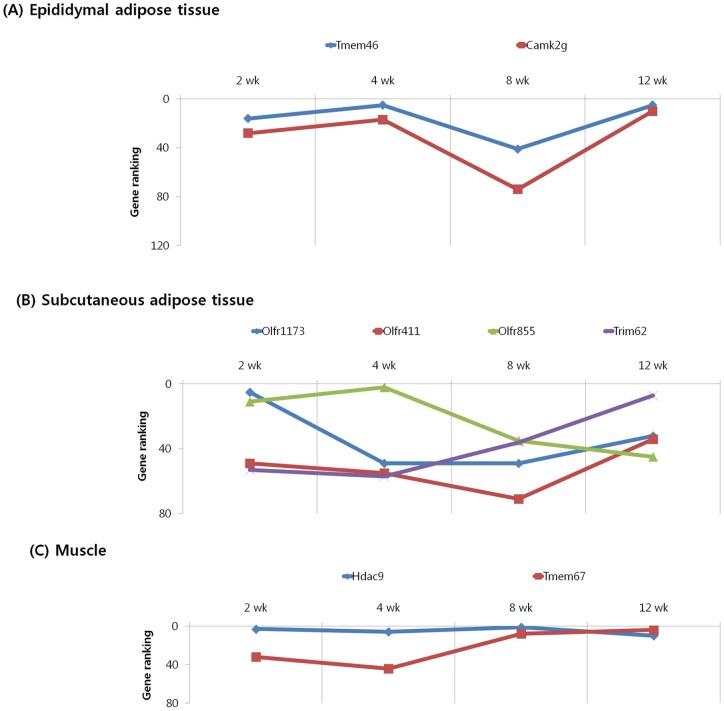
We subjected the 40 highest ranked genes identified by MNI analysis at each time point in the epididymal and subcutaneous fat tissues and gastrocnemius muscle of mice with diet-induced obesity to clustering based on their temporal pattern. Figure 4 shows genes that were observed to have decreasing ranking across time point, with a peak at 2 week. Biological processes controlled by genes in this cluster included regulation of insulin resistance (EA: Dusp12), cancer (EA: Nek11, A4gnt; SA: Srp9), inflammation (EA: Siglece; M: Nrip3, Sez6l), and olfactory transduction (EA: Olfr 513, 433; SA: Olfr 823) (Tables 7, 8, 9). The genes shown in Figures 5 and 6 exhibited the highest rank at the intermediate time points of 4 and 8 weeks, respectively. For both clusters, the majority of genes in this category were associated with cancer (EA: Gli2, Gucy2c, Lsm1, Duoxa1, Lasp1; SA: Vav3, Kcnrg, Tle6, Rab23; M: Dcc, Rassf2, Perp, Pdgfr1), inflammation (SA: Btn2a2, Def6; M: ll13, Rap1gds1), insulin resistance (SA: Neurod4; M: Mapk9), and olfactory transduction (EA: Olfr 1181, 960, 536, 654, 527; SA: Olfr 855, 888, 305, 1056, 960, 685, 1048; M: Olfr 699, 800, 978, 232, 872) (Tables 10, 11, 12, 13, 14, 15). Figure 7 shows genes that exhibited increasing ranking across all time points, with a peak at 12 week. Biological processes for genes in this cluster included regulation of adipogenesis (EA: Smad7, Adhfe1), food intake (M: Pyy), inflammation (EA: Folr2, Pde7a, Vipr2; SA: Rfxdc2, Aqp5, Cpb2), cancer (M: Lin28, Gstm1, Safb2), and olfactory transduction (EA: Olfr 16, 1000; SA: Olfr 716, 45; M: Olfr 689, 347) (Tables 16, 17, 18). The genes shown in Figure 8 exhibited a constant high MNI ranking throughout the time course. This cluster contained genes related to cancer (EA: Tmem46; SA: Trim62; M: Hdac9), insulin resistance (EA: Camk2g), hepatic fibrosis (M: Tmem67), and olfactory transduction (SA: Olfr 1173, 411, 855) (Table 19).
Figure 4. Results of MNI analysis at week 2.
Genes that exhibited decreasing ranking across time points as revealed by the MNI analysis, with a peak at 2 week in the peripheral tissues of mice. (A) Epididymal adipose tissue. (B) Subcutaneous adipose tissue. (C) Muscle.
Table 7. List of genes that exhibited decreasing ranking across time point as revealed by the MNI analysis, with a peak at 2 week in the epididymal adipose tissue of mice.
| Gene accession No. | Gene symbol | Description | Fold change (HFD/ND) | |||
| 2wk | 4wk | 8wk | 12wk | |||
| Epididymal adipose tissue | ||||||
| Insulin resistance | ||||||
| NM_023173 | Dusp12 | Dual specificity phosphatase 12 | 2.5 | 1.8 | 2.1 | 1.7 |
| Inflammation | ||||||
| NM_031181 | Siglece | Sialic acid binding Ig-like lectin E | 2.1 | 1.9 | 1.7 | 1.7 |
| Cancer | ||||||
| NM_172461 | Nek11 | NIMA (never in mitosis gene a)-related expressed kinase 11 | 0.4 | 0.5 | 0.6 | 0.6 |
| XM_286168 | A4gnt | Alpha-1,4-N-acetylglucosaminyltransferase | 2.0 | 1.7 | 1.6 | 1.5 |
| Olfactory transduction | ||||||
| NM_146723 | Olfr513 | Olfactory receptor 513 | 0.3 | 0.3 | 0.3 | 0.4 |
| NM_146717 | Olfr433 | Olfactory receptor 433 | 2.1 | 2.0 | 1.9 | 1.5 |
| Others | ||||||
| NM_022318 | Popdc2 | Popeye domain containing 2 | 2.6 | 2.0 | 1.8 | 1.9 |
| XM_901428 | EG629397 | Predicted gene, EG629397 | 1.8 | 1.7 | 1.5 | 1.4 |
| XM_899101 | Camsap1 | Calmodulin regulated spectrin-associated protein 1-like 1 | 3.4 | 2.2 | 2.3 | 2.6 |
| NM_177769 | Elmod1 | ELMO domain containing 1 | 1.7 | 1.6 | 1.5 | 1.4 |
| NM_010768 | Matk | Megakaryocyte-associated tyrosine kinase | 2.3 | 1.8 | 1.7 | 1.8 |
| NM_177078 | Adrbk2 | Adrenergic receptor kinase, beta 2 | 2.7 | 2.1 | 2.2 | 2.1 |
| NM_013679 | Svs6 | Seminal vesicle secretory protein 6 | 0.8 | 0.7 | 0.7 | 0.7 |
| XM_486653 | EG434760 | Predicted gene, EG434760 | 0.4 | 0.5 | 0.4 | 0.4 |
| NM_001030297 | Tmprss11c | Transmembrane protease, serine 11c | 1.9 | 1.5 | 2.0 | 1.7 |
| NM_026338 | 4921517D21Rik | RIKEN cDNA 4921517D21 gene | 2.9 | 2.5 | 2.8 | 2.0 |
| NM_133879 | Zbtb4 | Zinc finger and BTB domain containing 48 | 0.4 | 0.2 | 0.3 | 0.5 |
| NM_001034875 | Gm1006 | Gene model 1006, (NCBI) | 2.1 | 2.1 | 1.7 | 2.0 |
| NM_016802 | Rhoa | Ras homolog gene family, member A | 2.1 | 2.1 | 1.7 | 2.0 |
| NM_177887 | BC022651 | cDNA sequence BC022651 | 1.8 | 1.4 | 1.6 | 1.5 |
| NM_207670 | Grasp | GRIP1 associated protein | 1.4 | 1.2 | 1.3 | 1.3 |
| NM_183124 | Defb41 | Defensin beta 41 | 0.7 | 0.8 | 0.8 | 0.7 |
Table 8. List of genes that exhibited decreasing ranking across time point as revealed by the MNI analysis, with a peak at 2 week in the subcutaneous adipose tissue of mice.
| Gene accession No. | Gene symbol | Description | Fold change (HFD/ND) | |||
| 2wk | 4wk | 8wk | 12wk | |||
| Subcutaneous adipose tissue | ||||||
| Cancer | ||||||
| NM_012058 | Srp9 | Signal recognition particle 9 | 0.5 | 0.6 | 0.7 | 0.5 |
| Olfactory transduction | ||||||
| NM_146673 | Olfr823 | Olfactory receptor 823 | 0.6 | 0.6 | 0.7 | 0.7 |
| Others | ||||||
| NM_025779 | Ccdc109b | Coiled-coil domain containing 109B | 0.5 | 0.7 | 0.7 | 0.6 |
| NM_001025438 | Camk2d | Calcium/calmodulin-dependent protein kinase II, delta | 0.8 | 0.8 | 0.8 | 0.8 |
| AB211064 | L1td1 | LINE-1 type transposase domain containing 1 | 3.1 | 2.8 | 1.9 | 1.9 |
| NM_008748 | Dusp8 | Dual specificity phosphatase 8 | 0.6 | 0.6 | 0.8 | 0.8 |
| XM_905633 | Armcx4 | Armadillo repeat containing, X-linked 4 | 1.9 | 2.0 | 2.4 | 2.4 |
| NM_001034860 | EG229571 | Predicted gene, EG229571 | 1.6 | 1.5 | 1.4 | 1.4 |
| XM_898433 | AK129128 | cDNA sequence AK129128 | 2.3 | 1.8 | 1.9 | 1.4 |
| XM_355152 | Gm967 | Gene model 967, (NCBI) | 0.5 | 0.6 | 0.6 | 0.7 |
| NM_016869 | Corin | Corin | 0.7 | 0.7 | 0.8 | 0.8 |
| NM_153386 | Clrn1 | Clarin 1 | 1.3 | 1.2 | 1.3 | 1.3 |
| NM_029911 | Kcnk10 | Potassium channel, subfamily K, member 10 | 0.7 | 0.7 | 0.8 | 0.8 |
| NM_026943 | Snrpd2 | Small nuclear ribonucleoprotein D2 | 0.6 | 0.8 | 0.7 | 0.7 |
| NM_134000 | Traf3ip2 | Traf3 interacting protein 2 | 1.5 | 1.3 | 1.2 | 1.2 |
| NM_023790 | Wdr5 | WD repeat domain 54 | 1.8 | 1.3 | 1.3 | 0.9 |
| XM_987216 | 1700072O05Rik | RIKEN cDNA 1700072O05 gene | 0.6 | 0.6 | 0.6 | 0.7 |
| XM_146632 | 9030420J04Rik | RIKEN cDNA 9030420J04 gene | 2.5 | 2.1 | 2.0 | 1.5 |
| NM_015756 | Shroom3 | Shroom family member 3 | 1.9 | 1.4 | 1.3 | 1.5 |
Table 9. List of genes that exhibited decreasing ranking across time point as revealed by the MNI analysis, with a peak at 2 week in the muscle of mice.
| Gene accession No. | Gene symbol | Description | Fold change (HFD/ND) | |||
| 2wk | 4wk | 8wk | 12wk | |||
| Muscle | ||||||
| Inflammation | ||||||
| NM_020610 | Nrip3 | Nuclear receptor interacting protein 3 | 0.4 | 0.5 | 0.7 | 0.5 |
| BC065117 | Sez6l | Seizure related 6 homolog like | 1.7 | 1.7 | 1.7 | 1.3 |
| Others | ||||||
| NM_019574 | Patz1 | POZ (BTB) and AT hook containing zinc finger 1 | 1.8 | 1.4 | 1.3 | 1.5 |
| XM_975536 | Armc4 | Armadillo repeat containing 4 | 0.5 | 0.4 | 0.5 | 0.6 |
| NM_139226 | Onecut3 | One cut domain, family member 3 | 1.3 | 1.4 | 1.3 | 1.2 |
| NM_177596 | EG210853 | Predicted gene, EG210853 | 0.4 | 0.6 | 0.7 | 0.7 |
| NM_175455 | 6430502M16RIk | RIKEN cDNA 6430502M16 gene | 2.3 | 1.6 | 1.7 | 1.7 |
| NM_010243 | Fut9 | Fucosyltransferase 9 | 0.8 | 0.9 | 0.9 | 0.9 |
| NM_024178 | Alg14 | Asparagine-linked glycosylation 14 homolog (yeast) | 0.4 | 0.5 | 0.6 | 0.6 |
| NM_138954 | Rfpl4 | Ret finger protein-like 4 | 0.6 | 0.8 | 0.7 | 0.7 |
| XM_900215 | 0610010O12Rik | RIKEN cDNA 0610010O12 gene | 1.6 | 1.3 | 1.4 | 1.3 |
| NM_025679 | 5730470L24Rik | RIKEN cDNA 5730470L24 gene | 0.8 | 0.8 | 0.8 | 0.8 |
| NM_027588 | Nt5c1b | 5′-nucleotidase, cytosolic IB | 0.7 | 0.7 | 0.7 | 0.6 |
| NM_177239 | Mysm1 | Myb-like, SWIRM and MPN domains 1 | 0.5 | 0.6 | 0.5 | 0.7 |
| XM_126537 | 6030468B19Rik | RIKEN cDNA 6030468B19 gene | 1.6 | 1.4 | 1.3 | 1.2 |
| NM_028047 | 1500002O20Rik | RIKEN cDNA 1500002O20 gene | 1.3 | 1.2 | 1.3 | 1.2 |
| U66058 | Lig3 | Ligase III, DNA, ATP-dependent | 0.8 | 0.8 | 0.8 | 0.8 |
Figure 5. Results of MNI analysis at week 4.
Genes that exhibited the highest rank at the intermediate time point of 4 week as revealed by the MNI analysis in the peripheral tissues of mice. (A) Epididymal adipose tissue. (B) Subcutaneous adipose tissue. (C) Muscle.
Figure 6. Results of MNI analysis at week 8.
Genes that exhibited the highest rank at the intermediate time point of 8 week as revealed by the MNI analysis in the peripheral tissues of mice. (A) Epididymal adipose tissue. (B) Subcutaneous adipose tissue. (C) Muscle.
Table 10. List of genes that exhibited the highest ranking at the intermediate time point of 4 week as revealed by the MNI analysis in the epididymal adipose tissue of mice.
| Gene accession No. | Gene symbol | Description | Fold change (HFD/ND) | |||
| 2wk | 4wk | 8wk | 12wk | |||
| Epididymal adipose tissue | ||||||
| cancer | ||||||
| XM_136212 | Gli2 | GLI-Kruppel family member GLI2 | 2.2 | 2.7 | 2.4 | 1.8 |
| NM_145067 | Gucy2c | Guanylate cyclase 2c | 0.4 | 0.3 | 0.5 | 0.5 |
| NM_138721 | Lsm1 | U7 snRNP-specific Sm-like protein LSM10 | 0.3 | 0.9 | 0.8 | 0.6 |
| NM_145395 | Duoxa1 | Dual oxidase maturation factor 1 | 1.7 | 1.7 | 1.7 | 1.5 |
| NM_010688 | Lasp1 | LIM and SH3 protein 1 | 2.1 | 2.5 | 2.8 | 2.3 |
| olfacroty transduction | ||||||
| NM_001011816 | Olfr1181 | Olfactory receptor 1181 | 3.1 | 5.5 | 2.9 | 3.9 |
| NM_146279 | Olfr960 | Olfactory receptor 960 | 2.4 | 2.3 | 2.1 | 1.8 |
| Others | ||||||
| NM_026094 | Atp8b3 | ATPase, Class I, type 8B, member 3 | 2.5 | 3.3 | 2.5 | 2.1 |
| NM_029857 | Tmco4 | Transmembrane and coiled-coil domains 4 | 0.5 | 0.3 | 0.6 | 0.4 |
| NM_181419 | BC050092 | cDNA sequence BC050092 | 2.2 | 3.4 | 2.8 | 2.6 |
| NM_145514 | Wdr26 | WD repeat domain 26 | 0.4 | 0.3 | 0.4 | 0.4 |
| XM_125673 | Cxxc6 | CXXC finger 6 | 2.4 | 2.9 | 2.2 | 1.8 |
| BC019404 | 6030465E24Rik | RIKEN cDNA 6030465E24 gene | 1.4 | 1.7 | 1.6 | 1.6 |
| NM_028015 | Lass5 | Longevity assurance homolog 5 (S. cerevisiae) | 2.4 | 3.5 | 2.4 | 2.3 |
| NM_010184 | Fcer1a | Fc receptor, IgE, high affinity I, alpha polypeptide | 0.6 | 0.5 | 0.7 | 0.6 |
| NM_007378 | Abca4 | ATP-binding cassette, sub-family A (ABC1), member 4 | 1.7 | 1.9 | 1.8 | 1.5 |
| NM_029607 | 2310003C23Rik | RIKEN cDNA 2310003C23 gene | 0.4 | 0.3 | 0.4 | 0.5 |
| NM_001033541 | EG331493 | Predicted gene, EG331493 | 1.3 | 1.5 | 1.4 | 1.4 |
| NM_019656 | Tspan6 | Tetraspanin 6 | 2.0 | 2.8 | 2.4 | 2.2 |
| NM_027366 | Ly6g6e | Lymphocyte antigen 6 complex, locus G6E | 1.7 | 1.5 | 1.5 | 1.4 |
| BC096543 | B930095G15Rik | RIKEN cDNA B930095G15 gene | 3.6 | 3.8 | 2.7 | 2.7 |
| NM_133199 | Scn4a | Sodium channel, voltage-gated, type IV, alpha | 0.4 | 0.4 | 0.5 | 0.5 |
| NM_027661 | Hsfy2 | Heat shock transcription factor, Y linked 2 | 1.4 | 1.4 | 1.5 | 1.3 |
Table 11. List of genes that exhibited the highest ranking at the intermediate time point of 4 week as revealed by the MNI analysis in the subcutaneous adipose tissue of mice.
| Gene accession No. | Gene symbol | Description | Fold change (HFD/ND) | |||
| 2wk | 4wk | 8wk | 12wk | |||
| Subcutaneous adipose tissue | ||||||
| Inflammation | ||||||
| NM_175938 | Btn2a2 | Butyrophilin, subfamily 2, member A2 | 1.6 | 2.4 | 1.8 | 1.5 |
| Cancer | ||||||
| NM_020505 | Vav3 | Vav 3 oncogene | 1.3 | 1.6 | 1.4 | 1.5 |
| NM_206974 | Kcnrg | Potassium channel regulator | 1.3 | 1.7 | 1.5 | 1.3 |
| NM_053254 | Tle6 | Transducin-like enhancer of split 6, homolog of drosophila E(spl) | 0.7 | 0.6 | 0.8 | 0.7 |
| olfactory transduction | ||||||
| NM_146524 | Olfr855 | Olfactory receptor 855 | 1.7 | 1.6 | 2.0 | 1.6 |
| NM_146424 | Olfr888 | Olfactory receptor 888 | 0.6 | 0.5 | 0.6 | 0.7 |
| NM_146616 | Olfr305 | Olfactory receptor 305 | 0.7 | 0.6 | 0.6 | 0.7 |
| Others | ||||||
| NM_018744 | Sema6a | Sema domain, transmembrane domain (TM), and cytoplasmic domain, (semaphorin) 6A | 0.8 | 0.7 | 0.7 | 0.8 |
| NM_183015 | Ccnb3 | Cyclin B3 | 1.8 | 2.6 | 1.5 | 1.8 |
| NM_134220 | V1ri3 | Vomeronasal 1 receptor, I3 | 1.6 | 2.3 | 1.6 | 1.7 |
| NM_026082 | Dock7 | Dedicator of cytokinesis 7 | 1.6 | 2.2 | 1.5 | 1.4 |
| NM_153761 | Mill2 | MHC I like leukocyte 2 | 0.7 | 0.6 | 0.7 | 0.8 |
| NM_001031621 | Abca17 | ATP-binding cassette, sub-family A (ABC1), member 17 | 1.3 | 1.7 | 1.4 | 1.4 |
| NM_175013 | Pgm5 | Phosphoglucomutase 5 | 1.5 | 1.5 | 1.5 | 1.3 |
| NM_178712 | Gpr64 | G protein-coupled receptor 64 | 1.3 | 1.6 | 1.6 | 1.4 |
| NM_177839 | Tnn | Tenascin N | 0.8 | 0.7 | 0.8 | 0.9 |
| NM_198644 | Zfat1 | ZFAT zinc finger 1 | 2.1 | 2.7 | 2.1 | 2.0 |
| NM_145512 | Sft2d2 | SFT2 domain containing 2 | 1.2 | 1.5 | 1.4 | 1.3 |
| NM_029012 | Sppl3 | Signal peptide peptidase 3 | 0.7 | 0.5 | 0.7 | 0.7 |
| NM_009594 | Abl1 | v-abl Abelson murine leukemia oncogene 1 | 0.7 | 0.7 | 0.7 | 0.8 |
| NM_177710 | Ssh2 | Slingshot homolog 2 (Drosophila) | 0.7 | 0.6 | 0.8 | 0.7 |
| NM_010176 | Fah | Fumarylacetoacetate hydrolase | 0.7 | 0.7 | 0.8 | 0.8 |
| NM_009675 | Aoc3 | Amine oxidase, copper containing 3 | 1.2 | 1.4 | 1.3 | 1.3 |
| XM_149023 | Gm553 | Gene model 553, (NCBI) | 2.0 | 1.9 | 1.8 | 1.6 |
| XM_987216 | 1700072O05Rik | RIKEN cDNA 1700072O05 gene | 0.6 | 0.6 | 0.6 | 0.7 |
| NM_173784 | Ubtd2 | Ubiquitin domain containing 2 | 1.6 | 2.0 | 1.6 | 1.4 |
| NM_183131 | 4930451I11Rik | RIKEN cDNA 4930451I11 gene | 1.8 | 2.2 | 1.8 | 1.4 |
| NM_027661 | Hsfy2 | Heat shock transcription factor, Y linked 2 | 1.9 | 3.0 | 0.5 | 0.9 |
| XM_354998 | Ccdc78 | Coiled-coil domain containing 78 | 1.2 | 1.5 | 1.3 | 1.2 |
| NM_001033407 | Gm815 | Gene model 815, (NCBI) | 0.7 | 0.5 | 0.7 | 0.7 |
Table 12. List of genes that exhibited the highest ranking at the intermediate time point of 4 week as revealed by the MNI analysis in the muscle of mice.
| Gene accession No. | Gene symbol | Description | Fold change (HFD/ND) | |||
| 2wk | 4wk | 8wk | 12wk | |||
| Muscle | ||||||
| Inflammation | ||||||
| NM_008355 | Il13 | Interleukin 13 | 1.3 | 1.6 | 1.3 | 1.3 |
| NM_145544 | Rap1gds1 | RAP1, GTP-GDP dissociation stimulator 1 | 1.3 | 1.5 | 1.5 | 1.4 |
| Cancer | ||||||
| NM_007831 | Dcc | Deleted in colorectal carcinoma | 0.8 | 0.6 | 0.6 | 0.7 |
| NM_175445 | Rassf2 | Ras association (RalGDS/AF-6) domain family 2 | 0.6 | 0.5 | 0.7 | 0.7 |
| olfactory transduction | ||||||
| NM_153589 | Tmem16b | Transmembrane protein 16B | 0.7 | 0.7 | 0.7 | 0.7 |
| NM_001011862 | Olfr699 | Olfactory receptor 699 | 0.5 | 0.4 | 0.6 | 0.7 |
| NM_146548 | Olfr800 | Olfactory receptor 800 | 0.7 | 0.5 | 0.6 | 0.6 |
| NM_147105 | Olfr978 | Olfactory receptor 978 | 0.7 | 0.5 | 0.5 | 0.7 |
| Others | ||||||
| NM_175540 | Eda2r | Ectodysplasin A2 isoform receptor | 0.8 | 0.9 | 0.8 | 0.9 |
| NM_010608 | Kcnk3 | Potassium channel, subfamily K, member 3 | 1.1 | 1.2 | 1.2 | 1.2 |
| XM_129809 | Ogfrl1 | Opioid growth factor receptor-like 1 | 0.8 | 0.7 | 0.8 | 0.8 |
| NM_011776 | Zp3 | Zona pellucida glycoprotein 3 | 1.8 | 2.0 | 1.5 | 1.4 |
| NM_009130 | Scg3 | Secretogranin III | 0.6 | 0.5 | 0.7 | 0.8 |
| NM_173732 | BC030440 | cDNA sequence BC030440 | 1.3 | 1.2 | 1.2 | 1.2 |
| XM_356935 | EG383229 | Predicted gene, EG383229 | 1.3 | 1.5 | 1.2 | 1.3 |
| XM_992003 | Thsd7a | Thrombospondin, type I, domain containing 7A | 1.5 | 1.3 | 1.4 | 1.4 |
| NM_178005 | Lrrtm2 | Leucine rich repeat transmembrane neuronal 2 | 0.8 | 0.7 | 0.7 | 0.8 |
| NM_011012 | Oprl1 | Opioid receptor-like 1 | 1.3 | 1.6 | 1.5 | 1.4 |
| NM_028968 | Ifitm7 | Interferon induced transmembrane protein 7 | 0.6 | 0.4 | 0.6 | 0.6 |
| NM_175204 | 5830406J20Rik | RIKEN cDNA 5830406J20 gene | 1.6 | 1.9 | 1.7 | 1.5 |
| NM_013903 | Mmp20 | Matrix metallopeptidase 20 (enamelysin) | 0.7 | 0.7 | 0.8 | 0.8 |
| NM_177860 | EG329763 | Predicted gene, EG329763 | 0.8 | 0.7 | 0.7 | 0.7 |
Table 13. List of genes that exhibited the highest ranking at the intermediate time point of 8 week as revealed by the MNI analysis in the epididymal adipose tissue of mice.
| Gene accession No. | Gene symbol | Description | Fold change (HFD/ND) | |||
| 2wk | 4wk | 8wk | 12wk | |||
| Epididymal adipose tissue | ||||||
| Olfactory transduction | ||||||
| NM_146520 | Olfr536 | Olfactory receptor 536 | 0.6 | 0.6 | 0.5 | 0.5 |
| NM_146379 | Olfr654 | Olfactory receptor 654 | 1.9 | 2.4 | 2.8 | 1.9 |
| NM_001011776 | Olfr527 | Olfactory receptor 527 | 1.7 | 1.5 | 1.8 | 1.8 |
| Others | ||||||
| NM_199155 | Tas2r110 | Taste receptor, type 2, member 110 | 1.9 | 2.5 | 2.8 | 2.8 |
| AK138164 | Cntn5 | Contactin 5 | 0.4 | 0.4 | 0.3 | 0.5 |
| NM_152220 | Stx3 | Syntaxin 3 | 1.8 | 1.4 | 1.8 | 1.8 |
| NM_178924 | Upk1b | Uroplakin 1B | 1.4 | 1.8 | 1.8 | 1.7 |
| NM_028622 | Lce1c | Late cornified envelope 1C | 0.5 | 0.4 | 0.3 | 0.5 |
| NM_027815 | 9030624J02Rik | RIKEN cDNA 9030624J02 gene | 0.7 | 0.7 | 0.8 | 0.7 |
| NM_008633 | Mtap4 | Microtubule-associated protein 4 | 1.4 | 1.4 | 1.4 | 1.2 |
| NM_020518 | Vsig2 | V-set and immunoglobulin domain containing 2 | 2.0 | 1.8 | 2.1 | 1.8 |
| NM_138311 | H1foo | H1 histone family, member O, oocyte-specific | 0.6 | 0.6 | 0.5 | 0.5 |
| NM_153124 | St8sia5 | ST8 alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 5 | 0.8 | 0.7 | 0.7 | 0.7 |
| XM_898823 | Flnc | Filamin C, gamma (actin binding protein 280) | 0.5 | 0.5 | 0.4 | 0.4 |
| NM_181419 | BC050092 | cDNA sequence BC050092 | 2.2 | 3.4 | 2.8 | 2.6 |
| XM_001004783 | EG544848 | Predicted gene, EG544848 | 0.5 | 0.6 | 0.5 | 0.5 |
| NM_009608 | Actc1 | Actin, alpha, cardiac | 1.8 | 2.3 | 2.7 | 2.3 |
| NM_010203 | Fgf5 | Fibroblast growth factor 5 | 0.5 | 0.5 | 0.5 | 0.5 |
| NM_011827 | Hcst | Hematopoietic cell signal transducer | 2.0 | 2.2 | 2.0 | 1.7 |
| NM_026825 | Lrrc16 | Leucine rich repeat containing 16 | 1.4 | 1.5 | 1.7 | 1.5 |
| NM_029415 | Slc10a6 | Solute carrier family 10 (sodium/bile acid cotransporter family), member 6 | 0.5 | 0.4 | 0.3 | 0.5 |
| U10093 | Klra7 | Killer cell lectin-like receptor, subfamily A, member 7 | 1.2 | 1.2 | 1.2 | 1.2 |
| NM_007379 | Abca2 | ATP-binding cassette, sub-family A (ABC1), member 2 | 0.5 | 0.6 | 0.5 | 0.4 |
| NM_001039878 | Strn | Striatin, calmodulin binding protein 4 | 0.6 | 0.6 | 0.5 | 0.6 |
Table 14. List of genes that exhibited the highest ranking at the intermediate time point of 8 week as revealed by the MNI analysis in the subcutaneous adipose tissue of mice.
| Gene accession No. | Gene symbol | Description | Fold change (HFD/ND) | |||
| 2wk | 4wk | 8wk | 12wk | |||
| Subcutaneous adipose tissue | ||||||
| Insulin resistance | ||||||
| NM_007501 | Neurod4 | Neurogenic differentiation 4 | 0.8 | 0.8 | 0.6 | 0.7 |
| Inflammation | ||||||
| NM_027185 | Def6 | Differentially expressed in FDCP 6 | 1.9 | 2.0 | 3.0 | 1.7 |
| Cancer | ||||||
| NM_008999 | Rab23 | RAB23, member RAS oncogene family | 0.8 | 0.8 | 0.6 | 0.7 |
| olfactory transduction | ||||||
| NM_147018 | Olfr1056 | Olfactory receptor 1056 | 0.8 | 0.9 | 0.7 | 0.8 |
| NM_146279 | Olfr960 | Olfactory receptor 960 | 1.4 | 1.6 | 1.7 | 1.9 |
| NM_001011857 | Olfr685 | Olfactory receptor 685 | 0.8 | 0.8 | 0.7 | 0.9 |
| NM_146764 | Olfr1408 | Olfactory receptor 1408 | 0.6 | 0.6 | 0.7 | 0.7 |
| Others | ||||||
| NM_153111 | Fev | FEV (ETS oncogene family) | 0.7 | 0.5 | 0.4 | 0.6 |
| NM_080644 | Cacng5 | Calcium channel, voltage-dependent, gamma subunit 5 | 0.6 | 0.6 | 0.4 | 0.5 |
| NM_153592 | Erlin2 | ER lipid raft associated 2 | 1.3 | 1.3 | 1.6 | 1.3 |
| NM_183036 | Defb38 | Defensin beta 38 | 1.6 | 2.1 | 2.8 | 2.0 |
| NM_207022 | Tas2r118 | Taste receptor, type 2, member 118 | 2.0 | 1.6 | 2.9 | 2.1 |
| XM_132900 | LOC625758 | Hypothetical LOC625758 | 2.0 | 2.4 | 2.6 | 1.5 |
| NM_001008424 | Cdsn | Corneodesmosin | 0.8 | 0.7 | 0.6 | 0.7 |
| NM_025346 | Rmnd5b | Required for meiotic nuclear division 5 homolog B (S. cerevisiae) | 1.6 | 1.9 | 2.8 | 2.0 |
| NM_176846 | Exph5 | Exophilin 5 | 0.5 | 0.7 | 0.5 | 0.5 |
| NM_146107 | Actr1b | ARP1 actin-related protein 1 homolog B (yeast) | 0.5 | 0.6 | 0.3 | 0.6 |
| XM_986681 | Sytl2 | Synaptotagmin-like 2 | 1.3 | 1.5 | 1.3 | 1.4 |
| XM_618920 | LOC544808 | Hypothetical LOC544808 | 1.7 | 1.6 | 1.7 | 1.9 |
| XM_110525 | Cnksr1 | Connector enhancer of kinase suppressor of Ras 1 | 1.3 | 1.5 | 1.4 | 1.3 |
| NM_172263 | Pde8b | Phosphodiesterase 8B | 0.8 | 0.7 | 0.6 | 0.8 |
| NM_025638 | Gdpd1 | Glycerophosphodiester phosphodiesterase domain containing 1 | 0.3 | 0.4 | 0.5 | 0.4 |
| NM_026520 | 2900009I07Rik | RIKEN cDNA 2900009I07 gene | 0.8 | 0.7 | 0.5 | 0.7 |
Table 15. List of genes that exhibited the highest ranking at the intermediate time point of 8 week as revealed by the MNI analysis in the muscle of mice.
| Gene accession No. | Gene symbol | Description | Fold change (HFD/ND) | |||
| 2wk | 4wk | 8wk | 12wk | |||
| Muscle | ||||||
| Insulin resistance | ||||||
| NM_016961 | Mapk9 | Mitogen activated protein kinase 9 | 0.8 | 0.7 | 0.6 | 0.8 |
| Cancer | ||||||
| NM_022032 | Perp | PERP, TP53 apoptosis effector | 0.6 | 0.7 | 0.5 | 0.7 |
| NM_026840 | Pdgfrl | Platelet-derived growth factor receptor-like | 0.8 | 0.7 | 0.7 | 0.8 |
| Olfactory transduction | ||||||
| NM_146686 | Olfr232 | Olfactory receptor 232 | 0.6 | 0.6 | 0.6 | 0.7 |
| NM_146560 | Olfr872 | Olfactory receptor 872 | 1.3 | 1.3 | 1.3 | 1.2 |
| Others | ||||||
| NM_027462 | Wars2 | Tryptophanyl tRNA synthetase 2 (mitochondrial) | 0.7 | 0.8 | 0.6 | 0.7 |
| XM_903020 | Osbpl7 | Oxysterol binding protein-like 7 | 1.2 | 1.4 | 1.6 | 1.4 |
| XM_484317 | EG432803 | Predicted gene, EG432803 | 0.7 | 0.8 | 0.6 | 0.8 |
| NM_001031772 | Lin28 | Lin-28 homolog B (C. elegans) | 0.7 | 0.7 | 0.7 | 0.7 |
| NM_001001602 | Dab2ip | Disabled homolog 2 (Drosophila) interacting protein | 1.5 | 1.9 | 2.2 | 1.5 |
| NM_145582 | Atpbd3 | ATP binding domain 3 | 0.7 | 0.6 | 0.6 | 0.6 |
| AK014527 | Cep192 | Centrosomal protein 192 | 1.3 | 1.4 | 1.6 | 1.3 |
| NM_028127 | Frmd6 | FERM domain containing 6 | 0.6 | 0.7 | 0.6 | 0.8 |
| NM_172286 | 6430548M08Rik | RIKEN cDNA 6430548M08 gene | 1.2 | 1.2 | 1.2 | 1.1 |
| NM_011105 | Pkdrej | Polycystic kidney disease (polycystin) and REJ (sperm receptor for egg jelly, sea urchin homolog)-like | 1.5 | 1.9 | 2.0 | 2.2 |
| XM_983096 | LOC666331 | Hypothetical protein LOC666331 | 1.2 | 1.3 | 1.6 | 1.4 |
| NM_024196 | Tbc1d2 | TBC1 domain family, member 20 | 0.8 | 0.8 | 0.7 | 0.8 |
| NM_198306 | Galnt9 | UDP-N-acetyl-alpha-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase 9 | 0.8 | 0.6 | 1.0 | 0.7 |
| AK014852 | Glb1 | Galactosidase, beta 1 like 3 | 1.4 | 1.4 | 1.4 | 1.5 |
| NM_001004182 | EG434008 | Predicted gene, EG434008 | 0.8 | 0.7 | 0.7 | 0.7 |
| NM_011284 | Rpa2 | Replication protein A2 | 1.6 | 1.5 | 1.9 | 1.4 |
| NM_025381 | Atp6v1f | ATPase, H+ transporting, lysosomal V1 subunit F | 1.4 | 1.3 | 1.3 | 1.4 |
| NM_010571 | Irs3 | Insulin receptor substrate 3 | 0.6 | 0.7 | 0.6 | 0.8 |
Figure 7. Results of MNI analysis at week 12.
Genes that exhibited increasing ranking across time points as revealed by the MNI analysis, with a peak at 12 week in the peripheral tissues of mice. (A) Epididymal adipose tissue. (B) Subcutaneous adipose tissue. (C) Muscle.
Table 16. List of genes that exhibited increasing ranking across time points as revealed by the MNI analysis, with a peak at 12 week in the epididymal adipose tissue of mice.
| Gene accession No. | Gene symbol | Description | Fold change (HFD/ND) | |||
| 2wk | 4wk | 8wk | 12wk | |||
| Epididymal adipose tissue | ||||||
| NM_175236 | Adhfe1 | Alcohol dehydrogenase, iron containing, 1 | 0.6 | 0.6 | 0.7 | 0.6 |
| AF015260 | Smad7 | MAD homolog 7 (Drosophila) | 0.7 | 0.6 | 0.7 | 0.5 |
| Inflammation | ||||||
| NM_008035 | Folr2 | Folate receptor 2 (fetal) | 0.7 | 0.7 | 0.7 | 0.6 |
| NM_008802 | Pde7a | Phosphodiesterase 7A | 2.1 | 1.9 | 2.2 | 2.7 |
| NM_009511 | Vipr2 | Vasoactive intestinal peptide receptor 2 | 2.2 | 2.0 | 2.0 | 1.8 |
| Cancer | ||||||
| NM_008586 | Mep1b | Meprin 1 beta | 0.5 | 0.5 | 0.5 | 0.3 |
| NM_181321 | Tmc6 | Transmembrane channel-like gene family 6 | 1.8 | 1.8 | 1.7 | 2.3 |
| Olfactory transduction | ||||||
| NM_008763 | Olfr16 | Olfactory receptor 16 | 1.4 | 2.5 | 1.2 | 2.3 |
| NM_001011695 | Olfr1000 | Olfactory receptor 1000 | 2.2 | 2.2 | 2.5 | 3.0 |
| Others | ||||||
| NM_139270 | Pthr2 | Parathyroid hormone receptor 2 | 2.2 | 3.1 | 2.8 | 2.4 |
| NM_172434 | Tnrc4 | Trinucleotide repeat containing 4 | 1.3 | 1.5 | 1.4 | 1.5 |
| NM_173402 | Rgs12 | Regulator of G-protein signaling 12 | 0.7 | 0.7 | 0.7 | 0.5 |
| XM_977897 | B230217C12Rik | RIKEN cDNA B230217C12 gene | 1.8 | 1.4 | 1.8 | 1.8 |
| EG433070 | EG433070 | Predicted gene, EG433070 | 1.7 | 1.8 | 2.0 | 2.5 |
| NM_175259 | Tmem58 | Transmembrane protein 58 | 1.5 | 1.3 | 1.5 | 1.6 |
| NM_026412 | D2Ertd750e | DNA segment, Chr 2, ERATO Doi 750, expressed | 0.7 | 0.6 | 0.7 | 0.6 |
| NM_181323 | C130090K23Rik | RIKEN cDNA C130090K23 gene | 1.5 | 1.4 | 1.8 | 1.5 |
| NM_175406 | Atp6v0d2 | ATPase, H+ transporting, lysosomal V0 subunit D2 | 1.4 | 1.4 | 1.5 | 1.3 |
| NM_007725 | Cnn2 | Calponin 2 | 0.6 | 0.6 | 0.6 | 0.5 |
| NM_001033274 | Brd1 | Bromodomain containing 1 | 1.7 | 2.2 | 2.2 | 2.3 |
Table 17. List of genes that exhibited increasing ranking across time points as revealed by the MNI analysis, with a peak at 12 week in the subcutaneous adipose tissue of mice.
| Gene accession No. | Gene symbol | Description | Fold change (HFD/ND) | |||
| 2wk | 4wk | 8wk | 12wk | |||
| Subcutaneous adipose tissue | ||||||
| Inflammation | ||||||
| NM_001024852 | Auts2 | Autism susceptibility candidate 2 | 0.8 | 0.9 | 0.8 | 0.5 |
| NM_001033536 | Rfxdc2 | Regulatory factor X domain containing 2 homolog (human) | 1.2 | 1.4 | 1.3 | 1.4 |
| NM_009701 | Aqp5 | Aquaporin 5 | 0.7 | 0.7 | 0.8 | 0.6 |
| XM_285901 | Gm792 | Gene model 792, (NCBI) | 0.7 | 0.6 | 0.5 | 0.4 |
| NM_019775 | Cpb2 | Carboxypeptidase B2 (plasma) | 1.7 | 1.6 | 1.5 | 2.5 |
| Cancer | ||||||
| NM_009050 | Ret | Ret proto-oncogene | 1.2 | 1.3 | 1.2 | 1.5 |
| Olfactory transduction | ||||||
| NM_146604 | Olfr716 | Olfactory receptor 716 | 1.9 | 1.7 | 2.0 | 3.1 |
| NM_146963 | Olfr45 | Olfactory receptor 45 | 1.6 | 1.5 | 1.9 | 2.2 |
| Others | ||||||
| NM_178046 | Svil | Supervillin | 0.7 | 0.8 | 0.7 | 0.6 |
| NM_018764 | Pcdh7 | Protocadherin 7 | 1.5 | 1.4 | 1.3 | 1.8 |
| NM_030061 | Spink12 | Serine peptidase inhibitor, Kazal type 12 | 1.4 | 1.4 | 1.3 | 1.7 |
| NM_178741 | Klhl8 | Kelch-like 8 (Drosophila) | 1.3 | 1.3 | 1.3 | 1.6 |
| NM_175696 | C530028O21Rik | RIKEN cDNA C530028O21 gene | 1.3 | 1.3 | 1.2 | 1.5 |
| NM_177711 | 4932411G14Rik | RIKEN cDNA 4932411G14 gene | 0.7 | 0.6 | 0.7 | 0.5 |
| NM_181579 | Pof1b | Premature ovarian failure 1B | 1.7 | 1.8 | 1.6 | 2.2 |
| XM_489612 | 4921511H13Rik | RIKEN cDNA 4921511H13 gene | 1.3 | 1.4 | 1.2 | 1.6 |
| NM_172456 | Endogl1 | Endonuclease G-like 1 | 1.6 | 1.9 | 1.4 | 2.2 |
| NM_001033488 | Gm1964 | Gene model 1964, (NCBI) | 1.5 | 1.4 | 1.5 | 1.5 |
| NM_181318 | Rasgef1b | RasGEF domain family, member 1B | 0.7 | 0.8 | 0.8 | 0.6 |
| NM_183017 | Ttll12 | Tubulin tyrosine ligase-like family, member 12 | 0.8 | 0.6 | 0.7 | 0.7 |
| NM_010075 | Dpp6 | Dipeptidylpeptidase 6 | 1.1 | 1.2 | 1.2 | 1.3 |
| NM_026175 | Sf3a1 | Splicing factor 3a, subunit 1 | 1.5 | 1.4 | 1.7 | 1.9 |
Table 18. List of genes that exhibited increasing ranking across time points as revealed by the MNI analysis, with a peak at 12 week in the muscle of mice.
| Gene accession No. | Gene symbol | Description | Fold change (HFD/ND) | |||
| 2wk | 4wk | 8wk | 12wk | |||
| Muscle | ||||||
| Insulin resistance | ||||||
| NM_145435 | Pyy | Peptide YY | 1.4 | 1.4 | 1.3 | 1.5 |
| Inflammation | ||||||
| NM_138648 | Olr1 | Oxidized low density lipoprotein (lectin-like) receptor 1 | 0.5 | 0.6 | 0.5 | 0.3 |
| NM_009132 | Scin | Scinderin | 0.6 | 0.6 | 0.7 | 0.7 |
| Cancer | ||||||
| NM_145833 | Lin28 | Lin-28 homolog (C. elegans) | 0.7 | 0.7 | 0.7 | 0.7 |
| NM_010358 | Gstm1 | Glutathione S-transferase, mu 1 | 1.3 | 1.4 | 1.4 | 1.3 |
| NM_001029979 | Safb2 | Scaffold attachment factor B2 | 0.7 | 0.7 | 0.7 | 0.6 |
| Olfactory transduction | ||||||
| NM_146750 | Olfr689 | Olfactory receptor 689 | 0.6 | 0.7 | 0.7 | 0.5 |
| NM_146943 | Olfr347 | Olfactory receptor 347 | 1.2 | 1.3 | 1.2 | 1.5 |
| Others | ||||||
| NM_009372 | Tgif1 | TG interacting factor 1 | 1.2 | 1.1 | 0.8 | 0.6 |
| XM_887155 | Igsf10 | Immunoglobulin superfamily, member 10 | 0.7 | 0.5 | 0.6 | 0.4 |
| NM_009418 | Tpp2 | Tripeptidyl peptidase II | 1.2 | 1.4 | 1.2 | 1.5 |
| NM_008118 | Gif | Gastric intrinsic factor | 0.8 | 0.7 | 0.7 | 0.8 |
| NM_026972 | Cd209b | CD209b antigen | 0.9 | 0.8 | 0.6 | 0.7 |
| NM_145358 | Camkk2 | Calcium/calmodulin-dependent protein kinase kinase 2, beta | 1.2 | 1.2 | 1.3 | 1.2 |
| BC051526 | Cd22 | CD226 antigen | 0.8 | 0.9 | 0.9 | 0.7 |
| NM_029946 | 4931407K02Rik | RIKEN cDNA 4931407K02 gene | 1.3 | 1.1 | 1.2 | 1.3 |
| NM_172652 | 4632411B12Rik | RIKEN cDNA 4632411B12 gene | 1.7 | 1.4 | 1.4 | 1.9 |
| NM_011598 | Fabp9 | Fatty acid binding protein 9, testis | 0.8 | 0.8 | 0.9 | 0.7 |
| NM_011023 | Otx1 | Orthodenticle homolog 1 (Drosophila) | 0.7 | 0.7 | 0.7 | 0.5 |
| NM_019579 | Mpp5 | Membrane protein, palmitoylated 5 | 0.5 | 0.5 | 0.6 | 0.3 |
| NM_007555 | Bmp5 | Bone morphogenetic protein 5 | 0.9 | 0.9 | 0.8 | 0.8 |
| NM_001039047 | Trim58 | Tripartite motif-containing 58 | 1.3 | 1.2 | 1.5 | 1.5 |
| NM_020049 | Slc6a14 | Solute carrier family 6 (neurotransmitter transporter), member 14 | 0.8 | 0.6 | 0.7 | 0.6 |
Figure 8. Results of MNI analysis throughout the time course.
Genes that exhibited a constant high MNI ranking throughout the time course as revealed by the MNI analysis in the peripheral tissues of mice. (A) Epididymal adipose tissue. (B) Subcutaneous adipose tissue. (C) Muscle.
Table 19. List of genes that exhibited a constant high MNI ranking throughout the time course as revealed by the MNI analysis in the peripheral tissues of mice.
| Gene accession No. | Gene symbol | Description | Fold change (HFD/ND) | |||
| 2wk | 4wk | 8wk | 12wk | |||
| Epididymal adipose tissue | ||||||
| Insulin resistance | ||||||
| NM_001039138 | Camk2g | Calcium/calmodulin-dependent protein kinase II gamma | 0.6 | 0.5 | 0.6 | 0.5 |
| Others | ||||||
| NM_145463 | Tmem46 | Transmembrane protein 46 | 0.6 | 0.6 | 0.7 | 0.6 |
| Subcutaneous adipose tissue | ||||||
| olfactory transduction | ||||||
| NM_207566 | Olfr1173 | Olfactory receptor 1173 | 2.0 | 2.3 | 1.9 | 2.7 |
| NM_146709 | Olfr411 | Olfactory receptor 411 | 1.5 | 1.4 | 1.4 | 1.6 |
| NM_146524 | Olfr855 | Olfactory receptor 855 | 1.7 | 1.6 | 2.0 | 1.6 |
| Others | ||||||
| NM_178110 | Trim62 | Tripartite motif-containing 62 | 1.6 | 1.8 | 1.6 | 1.8 |
| Muscle | ||||||
| Cancer | ||||||
| NM_024124 | Hdac9 | Histone deacetylase 9 | 0.6 | 0.5 | 0.6 | 0.6 |
| Others | ||||||
| NM_177861 | Tmem67 | Transmembrane protein 67 | 0.7 | 0.6 | 0.7 | 0.5 |
Discussion
Animals use their olfactory system to monitor the chemical environment for molecules that reveal food sources or toxic substances and signal the presence of predators [11]. There are numerous olfactory receptors of different types, with as many as 1,000 in the mammalian genome that represent approximately 3% of the human genomeentire genetic information [12]. The information related to odor gathered by these olfactory receptors is funneled through a common signaling pathway. When an olfactory receptor binds to its odorant, it activates a single species of G protein, the olfactory trimeric G protein (Golf), which in turn activates the olfactory isoform of adenylate cyclase (AC3) [12]. Converging evidence has demonstrated that the olfactory system is a target for hormones related to metabolism and food-intake regulation; it adapts its function to nutritional needs by promoting or inhibiting food foraging [13]. Recent studies have found that obese patients display decreased olfactory acuity [14] and are significantly more likely to have absolute olfactory dysfunction or anosmia [15]. Furthermore, Simchen et al. showed that the abilities to detect and identify odors have been found to decrease as body mass index (BMI) increases in subjects less than 65 years old, independent of any linkage to food odor or gender [16]. Recently, the elements of olfactory-like chemosensory signaling have been found to also present in nonolfactory tissues such as testis [17], brain [18], and heart [19]. To our knowledge, this is the first study that shows a differential mRNA expression and high MNI ranking of olfactory receptors in the epididymal and subcutaneous fat tissues and muscles between ND and HFD-fed mice (Tables 1, 2, 3). These results imply that the olfactory receptors and the molecules involved in olfactory transduction might be the mediators of HFD-induced obesity progression in the peripheral tissues. This hypothesis is supported by the fact that the increased cAMP production by AC3 activates cAMP responsive element binding (CREB) protein, leading to increased adipogenesis in an obese mouse model. Furthermore, mice lacking AC3, which is a downstream regulator of olfactory receptors, exhibit obesity that is apparently caused by low locomotor activity, hyperphagia, and leptin insensitivity [20]. In future studies, it will be intriguing to further investigate the role of individual olfactory receptors in peripheral tissues, such as the pancreas, liver, muscle, and fat, to better understand the activation process of these signaling pathways and their physiological roles.
Cancer-related genes such as Nek11, A4gnt, Srp9, Gli2, Gucy2c, Lsm1, Duoxa1, Lasp1, Ret, Bex2, Vav3, Kcnrg, Tle6, Rab23, Dcc, Rassf2, Perp, Pdgfr1, Lin28, Gstm1, Safb2, Tmem46, and Hdac9 were remarkably overrepresented in time-course clusters identified by the MNI analysis in the epididymal and subcutaneous fat tissues and gastrocnemius muscle of mice with diet-induced obesity (Figs. 4, 5, 6, 7, 8). Of these 23 genes, 6 are known breast cancer-related genes (Lsm1, Duoxa1, Ret, Bex2, Rassf2, and Safb2). Lsm1 is a transforming oncogene that is amplified and overexpressed in breast cancer [21] and might affect either cell cycle progression or apoptosis [22]. Duoxa1, which was originally identified as a numb-interacting protein, was recently shown to function as a maturation factor in breast cancer [23]. Ret exhibits both estrogen- and retinoic acid-dependent transcriptional modulation in breast cancer [24]. Bex2 has a significant role in promoting cell survival and growth in breast cancer cells [25], [26], and Rassf2 might function as a tumor suppressor gene in in vitro cell migration and cell cycle progression [27]. The expression of Safb2 protein, which functions as estrogen receptor co-repressor and growth inhibitor, was lost in approximately 20% of breast cancers [28]. Many studies have attempted to determine the relationship between diet and breast cancer. Dietary fat is a source of endogenous estrogen and has been suggested as a possible risk factor for breast cancer [29]. To our knowledge, this is the first study showing an association between these 6 genes involved in breast cancer development and HFD-induced obesity in a rodent model.
Colon cancer-related genes such as Nek11, Gucy2c, Srp9, Tle6, and Pdgfrl were also overrepresented in the time-course clusters identified by the MNI analysis in the epididymal and subcutaneous fat tissues and gastrocnemius muscle of mice with diet-induced obesity (Figs. 4, 5, 6, 7, 8). Nek11, a member of the NIMA-related kinase family, phosphorylates Cdc25a and controls its degradation; Cdc25a phosphorylation is required for cell cycle progression in colorectal cancer cells [30]. Gucy2c and Srp9 have been shown to be overexpressed in colorectal cancer cells and were recently shown to function as a candidate biomarker for colon cancer [31], [32]. Tle6 is recurrently overexpressed in human colon cancer and enhances cell proliferation, colony formation, migration, and xenograft tumorigenicity [33]. Pdgfrl acts as a tumor suppressor and inhibits the growth of colorectal cancer cells [34]. Epidemiological studies indicate that both high body weight and high body mass index (BMI) were significantly associated with an increased colon cancer risk. Intra-abdominal visceral obesity, high plasma glucose levels, HbA1C, and C-peptide were also found to be associated with increased risk of colorectal cancer [35]–[38]. The current study showed that the above-mentioned genes that are involved in the regulation of colon cancer might play a genetic role in the development of obesity. No mechanistic insights have been reported to explain the relationship between the regulation of cancer-related genes in the adipose tissue or muscle and cancer susceptibility. It could be probable that the changes in the expression of cancer-related genes in the adipose tissue may accompany the regulation of same genes in epithelial tissues such as breast or colon.
Genes that were found to have the highest rank at the early phase and return to baseline after several weeks might be considered genetic mediators of acute-phase response in metabolic processes related to HFD-induced obesity. Dusp12 was one of the 58 genes that were observed to have decreasing ranking during the development of obesity, with a peak at 2 week. Previous studies identified several single nucleotide polymorphisms in this gene associated with type 2 diabetes in different populations, including Caucasians and Chinese [39]. Dusp12 is a glucokinase-associated protein that participates in glycolysis in the liver and dephosphorylation of cytoplasmic glucokinase in the pancreatic beta cells [40]. Therefore, Dusp12 might play a role in the regulation of glycolysis during the early stages of obesity. When glycolysis was decreased, whole-body glucose disposal was also reduced, indicating a decrease in glucose utilization in the peripheral tissues in response to the HFD. The latter likely results from an impaired glucose transport that precedes impaired insulin signaling.
Def6 and Mapk9 were one of the 145 genes that were found to have the highest rank at the intermediate time points of 4 or 8 weeks during the development of obesity. Def6, a novel type of activator for Rho GTPase, is expressed in myeloid cells, and disruption of Def6 expression leads to defects in toll-like receptor 4 (TLR4) signaling and innate immune responses [41]. Rho GTPases have been shown to be recruited to the cytosolic domain of TLR and the closely related interleukin 1 receptor (IL-1R) and to regulate the production of proinflammatory cytokines [42], [43]. In the present study, the high MNI ranking of Def6 in the subcutaneous adipose tissue of HFD-fed mice suggested that it might participate in the regulation of obesity-induced inflammation through TLR4 signaling. Mapk9, which is ubiquitously expressed, can invoke transcription factors such as c-Jun and many other apoptosis-related proteins [44]. Interestingly, recent studies have shown that the knockdown of Mapk9 leads to reduced serum levels of glucose, insulin, and homeostatic model assessment and therefore reverses insulin resistance in HFD-fed mice [45]. These findings provide supporting evidences to the high MNI ranking of Mapk9 associated with HFD-induced obesity observed in the present study. However, further studies are required to elucidate the precise function of Mapk9 in the development of HFD-induced type 2 diabetes.
Smad7, Adhfe1, and Pyy are one of the 65 genes that showed increasing ranking during the development of obesity, with a peak at 12 weeks. Smad7 was initially characterized as a factor induced by shear stress in vascular endothelial cells [46]. Only recently, new functions of Smad7 were elucidated: it inhibits transforming growth factor-β (TGF-β)-activated responses [46]. TGF-β is known to inhibit adipose differentiation of preadipocyte cell lines and primary cultures [47] and to block adipogenesis in vivo [48]. This suggests that Smad7 enhances adipogenesis through the inhibition of TGF-β signaling. Adhfe1 was characterized as a hydroxyacid-oxoacid transhydrogenase that catalyzes the conversion of γ-hydroxybutyrate to succinic semialdehyde [49]. Recently, Adhfe1 was suggested to play a role in adipocyte differentiation. The expression of Adhfe1 transcript is tightly linked to the phenotype of mature adipocytes both in vivo and in vitro, although the mechanisms underlying Adhfe1-mediated regulation of adipogenesis remain poorly understood [50]. Pyy, which is expressed and secreted in endocrine intestinal cells, plays a role in reducing appetite and caloric intake [51]. Recently, plasma Pyy concentrations were found to be decreased in both obese humans [52] and diet-induced obese mice [53]. These studies might suggest that Smad7 and Adhfe1 play a role in obesity by amplifying the aggressive effect of adipogenesis.
Camk2g and Tmem67 are one of the 8 genes that exhibited a constant high MNI ranking from 2 to 12 weeks. The increase of cytosolic Ca2+ in the beta cells is central to the initiation of insulin secretion under physiological conditions [54]. Recent findings suggest that Camk2g involved in the regulation of calcium in the islet beta cells is a candidate gene for type 2 diabetes [55]. The Tmem67 gene mediates a fundamental developmental stage of ciliary formation and epithelial morphogenesis [56]. In addition, defects in the Tmem67 gene resulted in Meckel syndrome type 3, Joubert syndrome type 6, and nephronophthisis 11, which show many clinical phenotypic similarities, including hepatic fibrosis [56], [57]. Consumption of fat-rich diets seems to play an important role in the pathogenesis of hepatic steatosis and its progression to fibrosis [58]. The constantly high MNI ranking of Tmem67 from 2 to 12 weeks associated with a HFD suggests that Tmem67 might participate in the development of hepatic fibrosis.
In summary, this study is the most comprehensive investigation of the gene expression patterns conducted using a time-resolved approach to gain insight into the development of HFD-induced obesity in a mouse model. A reverse-engineered gene network was used for the first time for the identification of key genetic mediators and pathways that have been implicated in the initiation and advancement of obesity. We highlighted the sequential induction of distinct olfactory receptors and stimulation of cancer-related genes during the development of obesity. To our knowledge, the proposed changes in the olfactory transduction machinery as per the MNI ranking have not been previously reported. These putative mechanisms clearly need further investigation. The top 5 genes recognized through the MNI analysis at each time points (2, 4, 8, and 12 weeks) and gene clusters identified based on their temporal patterns in the 3 different tissues (visceral and subcutaneous adipose tissues and muscle) of mice need special attention as potential genetic mediators for obesity progression.
Supporting Information
The basal expression levels of some target genes identified by MNI analysis. Quantitative real-time PCR analysis of the basal expression on highly ranked olfactory genes and top 5 genes at week 4 in the epididymal adipose tissues of (A) ND- or (B) HFD-fed mice. Results are presented as the average ± SEM of at least 3 separate experiments.
(TIF)
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. 2012-0000643). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jain N, Thatte J, Braciale T, Ley K, O'Connell M, et al. (2003) Local-pooled-error test for identifying differentially expressed genes with a small number of replicated microarrays. Bioinformatics 19: 1945–1951. [DOI] [PubMed] [Google Scholar]
- 2. Storey JD, Tibshirani R (2003) Statistical methods for identifying differentially expressed genes in DNA microarrays. Methods in molecular biology 224: 149–157. [DOI] [PubMed] [Google Scholar]
- 3. Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proceedings of the National Academy of Sciences of the United States of America 95: 14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. di Bernardo D, Thompson MJ, Gardner TS, Chobot SE, Eastwood EL, et al. (2005) Chemogenomic profiling on a genome-wide scale using reverse-engineered gene networks. Nature biotechnology 23: 377–383. [DOI] [PubMed] [Google Scholar]
- 5. Kerr MK, Martin M, Churchill GA (2000) Analysis of variance for gene expression microarray data. Journal of computational biology: a journal of computational molecular cell biology 7: 819–837. [DOI] [PubMed] [Google Scholar]
- 6. Cobb JP, Mindrinos MN, Miller-Graziano C, Calvano SE, Baker HV, et al. (2005) Application of genome-wide expression analysis to human health and disease. Proceedings of the National Academy of Sciences of the United States of America 102: 4801–4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsu KL, Pilobello KT, Mahal LK (2006) Analyzing the dynamic bacterial glycome with a lectin microarray approach. Nature chemical biology 2: 153–157. [DOI] [PubMed] [Google Scholar]
- 8. McAdams HH, Shapiro L (2003) A bacterial cell-cycle regulatory network operating in time and space. Science 301: 1874–1877. [DOI] [PubMed] [Google Scholar]
- 9. Soukas A, Cohen P, Socci ND, Friedman JM (2000) Leptin-specific patterns of gene expression in white adipose tissue. Genes & development 14: 963–980. [PMC free article] [PubMed] [Google Scholar]
- 10. Xing H, Gardner TS (2006) The mode-of-action by network identification (MNI) algorithm: a network biology approach for molecular target identification. Nature protocols 1: 2551–2554. [DOI] [PubMed] [Google Scholar]
- 11. Zhang X, Rogers M, Tian H, Zou DJ, Liu J, et al. (2004) High-throughput microarray detection of olfactory receptor gene expression in the mouse. Proceedings of the National Academy of Sciences of the United States of America 101: 14168–14173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gilad Y, Lancet D (2003) Population differences in the human functional olfactory repertoire. Molecular biology and evolution 20: 307–314. [DOI] [PubMed] [Google Scholar]
- 13.Palouzier-Paulignan B, Lacroix MC, Aime P, Baly C, Caillol M, et al.. (2012) Olfaction Under Metabolic Influences. Chem Senses. [DOI] [PMC free article] [PubMed]
- 14. Richardson BE, Vander Woude EA, Sudan R, Thompson JS, Leopold DA (2004) Altered olfactory acuity in the morbidly obese. Obes Surg 14: 967–969. [DOI] [PubMed] [Google Scholar]
- 15. Richardson BE, Vanderwoude EA, Sudan R, Leopold DA, Thompson JS (2012) Gastric bypass does not influence olfactory function in obese patients. Obes Surg 22: 283–286. [DOI] [PubMed] [Google Scholar]
- 16. Simchen U, Koebnick C, Hoyer S, Issanchou S, Zunft HJ (2006) Odour and taste sensitivity is associated with body weight and extent of misreporting of body weight. Eur J Clin Nutr 60: 698–705. [DOI] [PubMed] [Google Scholar]
- 17. Parmentier M, Libert F, Schurmans S, Schiffmann S, Lefort A, et al. (1992) Expression of members of the putative olfactory receptor gene family in mammalian germ cells. Nature 355: 453–455. [DOI] [PubMed] [Google Scholar]
- 18. Mombaerts P (1999) Molecular biology of odorant receptors in vertebrates. Annual review of neuroscience 22: 487–509. [DOI] [PubMed] [Google Scholar]
- 19. Young JM, Trask BJ (2002) The sense of smell: genomics of vertebrate odorant receptors. Human molecular genetics 11: 1153–1160. [DOI] [PubMed] [Google Scholar]
- 20. Wang Z, Li V, Chan GC, Phan T, Nudelman AS, et al. (2009) Adult type 3 adenylyl cyclase-deficient mice are obese. PloS one 4: e6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelley JR, Fraser MM, Hubbard JM, Watson DK, Cole DJ (2003) CaSm antisense gene therapy: a novel approach for the treatment of pancreatic cancer. Anticancer research 23: 2007–2013. [PubMed] [Google Scholar]
- 22. Fraser MM, Watson PM, Fraig MM, Kelley JR, Nelson PS, et al. (2005) CaSm-mediated cellular transformation is associated with altered gene expression and messenger RNA stability. Cancer research 65: 6228–6236. [DOI] [PubMed] [Google Scholar]
- 23. Ostrakhovitch EA, Li SS (2010) NIP1/DUOXA1 expression in epithelial breast cancer cells: regulation of cell adhesion and actin dynamics. Breast cancer research and treatment 119: 773–786. [DOI] [PubMed] [Google Scholar]
- 24. Ahmad N, Kumar R (2011) Steroid hormone receptors in cancer development: a target for cancer therapeutics. Cancer letters 300: 1–9. [DOI] [PubMed] [Google Scholar]
- 25. Naderi A, Teschendorff AE, Beigel J, Cariati M, Ellis IO, et al. (2007) BEX2 is overexpressed in a subset of primary breast cancers and mediates nerve growth factor/nuclear factor-kappaB inhibition of apoptosis in breast cancer cell lines. Cancer research 67: 6725–6736. [DOI] [PubMed] [Google Scholar]
- 26. Naderi A, Liu J, Bennett IC (2010) BEX2 regulates mitochondrial apoptosis and G1 cell cycle in breast cancer. International journal of cancer Journal international du cancer 126: 1596–1610. [DOI] [PubMed] [Google Scholar]
- 27. Vos MD, Ellis CA, Elam C, Ulku AS, Taylor BJ, et al. (2003) RASSF2 is a novel K-Ras-specific effector and potential tumor suppressor. The Journal of biological chemistry 278: 28045–28051. [DOI] [PubMed] [Google Scholar]
- 28. Oesterreich S (2003) Scaffold attachment factors SAFB1 and SAFB2: Innocent bystanders or critical players in breast tumorigenesis? Journal of cellular biochemistry 90: 653–661. [DOI] [PubMed] [Google Scholar]
- 29. Ruggeri BA, Klurfeld DM, Kritchevsky D, Furlanetto RW (1989) Caloric restriction and 7,12-dimethylbenz(a) anthracene-induced mammary tumor growth in rats: alterations in circulating insulin, insulin-like growth factors I and II, and epidermal growth factor. Cancer research 49: 4130–4134. [PubMed] [Google Scholar]
- 30. Sorensen CS, Melixetian M, Klein DK, Helin K (2010) NEK11: linking CHK1 and CDC25A in DNA damage checkpoint signaling. Cell cycle 9: 450–455. [DOI] [PubMed] [Google Scholar]
- 31. Mejia A, Schulz S, Hyslop T, Weinberg DS, Waldman SA (2009) GUCY2C reverse transcriptase PCR to stage pN0 colorectal cancer patients. Expert review of molecular diagnostics 9: 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rho JH, Qin S, Wang JY, Roehrl MH (2008) Proteomic expression analysis of surgical human colorectal cancer tissues: up-regulation of PSB7, PRDX1, and SRP9 and hypoxic adaptation in cancer. Journal of proteome research 7: 2959–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen PC, Kuraguchi M, Velasquez J, Wang Y, Yang K, et al. (2008) Novel roles for MLH3 deficiency and TLE6-like amplification in DNA mismatch repair-deficient gastrointestinal tumorigenesis and progression. PLoS genetics 4: e1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo FJ, Zhang WJ, Li YL, Liu Y, Li YH, et al. (2010) Expression and functional characterization of platelet-derived growth factor receptor-like gene. World journal of gastroenterology: WJG 16: 1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahmed RL, Schmitz KH, Anderson KE, Rosamond WD, Folsom AR (2006) The metabolic syndrome and risk of incident colorectal cancer. Cancer 107: 28–36. [DOI] [PubMed] [Google Scholar]
- 36. Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, et al. (2006) Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). Journal of the National Cancer Institute 98: 920–931. [DOI] [PubMed] [Google Scholar]
- 37. Saydah SH, Platz EA, Rifai N, Pollak MN, Brancati FL, et al. (2003) Association of markers of insulin and glucose control with subsequent colorectal cancer risk. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 12: 412–418. [PubMed] [Google Scholar]
- 38. Ma J, Giovannucci E, Pollak M, Leavitt A, Tao Y, et al. (2004) A prospective study of plasma C-peptide and colorectal cancer risk in men. Journal of the National Cancer Institute 96: 546–553. [DOI] [PubMed] [Google Scholar]
- 39. Hu C, Zhang R, Wang C, Ma X, Wang J, et al. (2011) Lack of association between genetic polymorphisms within DUSP12 – ATF6 locus and glucose metabolism related traits in a Chinese population. BMC medical genetics 12: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Munoz-Alonso MJ, Guillemain G, Kassis N, Girard J, Burnol AF, et al. (2000) A novel cytosolic dual specificity phosphatase, interacting with glucokinase, increases glucose phosphorylation rate. The Journal of biological chemistry 275: 32406–32412. [DOI] [PubMed] [Google Scholar]
- 41. Chen Q, Gupta S, Pernis AB (2009) Regulation of TLR4-mediated signaling by IBP/Def6, a novel activator of Rho GTPases. Journal of leukocyte biology 85: 539–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arbibe L, Mira JP, Teusch N, Kline L, Guha M, et al. (2000) Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nature immunology 1: 533–540. [DOI] [PubMed] [Google Scholar]
- 43. Chen LY, Zuraw BL, Liu FT, Huang S, Pan ZK (2002) IL-1 receptor-associated kinase and low molecular weight GTPase RhoA signal molecules are required for bacterial lipopolysaccharide-induced cytokine gene transcription. Journal of immunology 169: 3934–3939. [DOI] [PubMed] [Google Scholar]
- 44. Jaeschke A, Rincon M, Doran B, Reilly J, Neuberg D, et al. (2005) Disruption of the Jnk2 (Mapk9) gene reduces destructive insulitis and diabetes in a mouse model of type I diabetes. Proceedings of the National Academy of Sciences of the United States of America 102: 6931–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, et al. (2009) Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology 49: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Topper JN, Cai J, Qiu Y, Anderson KR, Xu YY, et al. (1997) Vascular MADs: two novel MAD-related genes selectively inducible by flow in human vascular endothelium. Proceedings of the National Academy of Sciences of the United States of America 94: 9314–9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ignotz RA, Massague J (1985) Type beta transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proceedings of the National Academy of Sciences of the United States of America 82: 8530–8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Clouthier DE, Comerford SA, Hammer RE (1997) Hepatic fibrosis, glomerulosclerosis, and a lipodystrophy-like syndrome in PEPCK-TGF-beta1 transgenic mice. The Journal of clinical investigation 100: 2697–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lyon RC, Johnston SM, Panopoulos A, Alzeer S, McGarvie G, et al. (2009) Enzymes involved in the metabolism of gamma-hydroxybutyrate in SH-SY5Y cells: identification of an iron-dependent alcohol dehydrogenase ADHFe1. Chemico-biological interactions 178: 283–287. [DOI] [PubMed] [Google Scholar]
- 50. Kim JY, Tillison KS, Zhou S, Lee JH, Smas CM (2007) Differentiation-dependent expression of Adhfe1 in adipogenesis. Archives of biochemistry and biophysics 464: 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xu J, McNearney TA, Chen JD (2011) Impaired postprandial releases/syntheses of ghrelin and PYY (3–36) and blunted responses to exogenous ghrelin and PYY (3–36) in a rodent model of diet-induced obesity. Journal of gastroenterology and hepatology 26: 700–705. [DOI] [PubMed] [Google Scholar]
- 52. Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, et al. (2003) Inhibition of food intake in obese subjects by peptide YY3-36. The New England journal of medicine 349: 941–948. [DOI] [PubMed] [Google Scholar]
- 53. le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, et al. (2006) Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 147: 3–8. [DOI] [PubMed] [Google Scholar]
- 54. Gloyn AL, Desai M, Clark A, Levy JC, Holman RR, et al. (2002) Human calcium/calmodulin-dependent protein kinase II gamma gene (CAMK2G): cloning, genomic structure and detection of variants in subjects with type II diabetes. Diabetologia 45: 580–583. [DOI] [PubMed] [Google Scholar]
- 55. Easom RA (1999) CaM kinase II: a protein kinase with extraordinary talents germane to insulin exocytosis. Diabetes 48: 675–684. [DOI] [PubMed] [Google Scholar]
- 56. Dawe HR, Smith UM, Cullinane AR, Gerrelli D, Cox P, et al. (2007) The Meckel-Gruber Syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Human molecular genetics 16: 173–186. [DOI] [PubMed] [Google Scholar]
- 57. Seeman T, Seemanova E, Nuernberg G, Nuernberg P, Janssen S, et al. (2010) Polycystic kidney and hepatic disease with mental retardation is nephronophthisis 11 caused by MKS3/TMEM67 mutations. Pediatric nephrology 25: 2375–2376. [DOI] [PubMed] [Google Scholar]
- 58. Barbuio R, Milanski M, Bertolo MB, Saad MJ, Velloso LA (2007) Infliximab reverses steatosis and improves insulin signal transduction in liver of rats fed a high-fat diet. The Journal of endocrinology 194: 539–550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The basal expression levels of some target genes identified by MNI analysis. Quantitative real-time PCR analysis of the basal expression on highly ranked olfactory genes and top 5 genes at week 4 in the epididymal adipose tissues of (A) ND- or (B) HFD-fed mice. Results are presented as the average ± SEM of at least 3 separate experiments.
(TIF)