Abstract
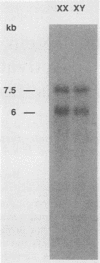
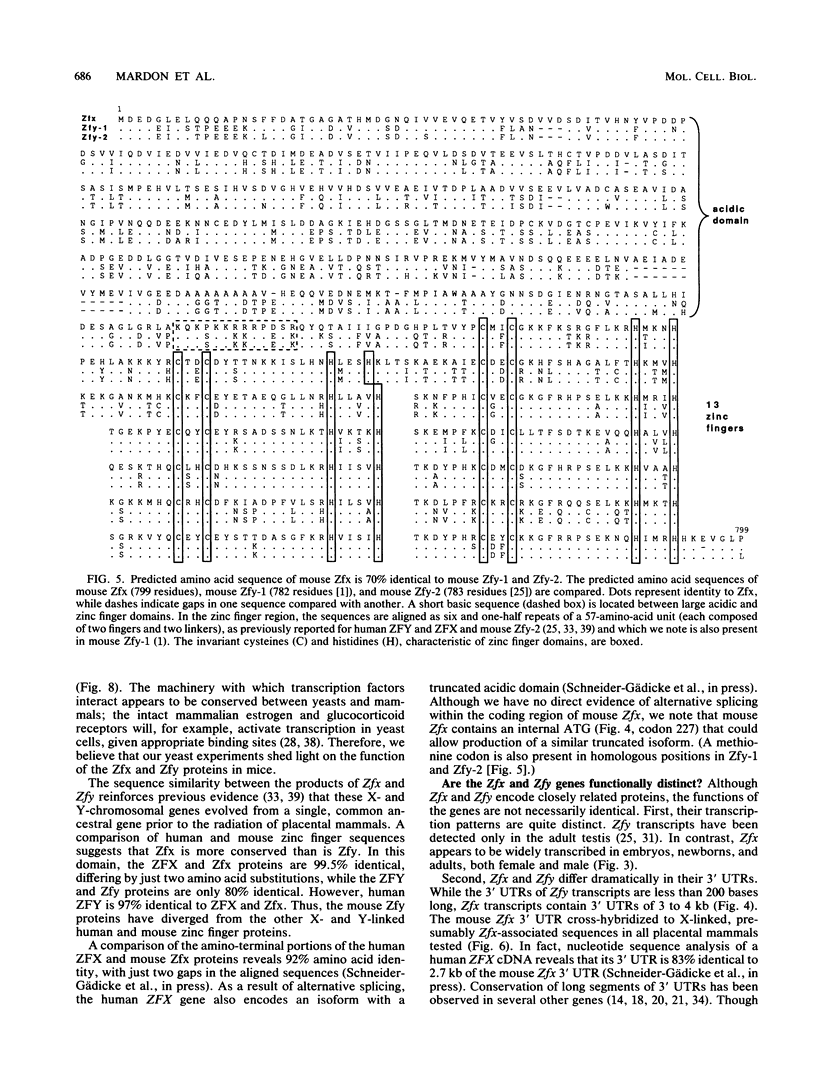
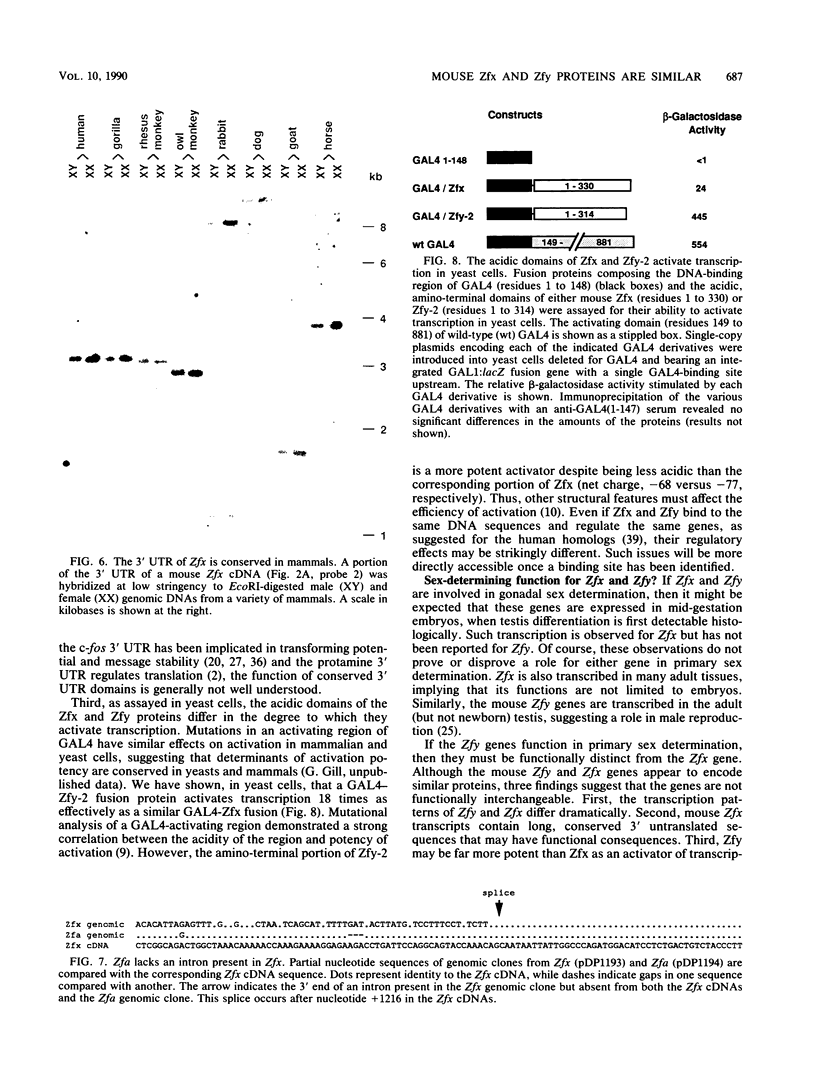
The Zfy gene is located on the Y chromosome of placental mammals and encodes a zinc finger protein which may serve as the primary sex-determining signal. A related gene, Zfx, is similarly conserved on the X chromosome. Unlike that in most mammals, the mouse genome contains four homologous zinc finger loci: Zfy-1, Zfy-2, Zfx, and Zfa (on an autosome). We report that, in contrast to the mouse Zfy genes, Zfx is widely transcribed in embryos, newborns, and adults, both male and female. Moreover, Zfx transcripts contain long 3' untranslated sequences which are phylogenetically conserved. Zfa is a processed gene derived from Zfx. An analysis of cDNA clones demonstrated that Zfx encodes a 799-amino-acid protein that is 70% identical to the mouse Zfy-1 and Zfy-2 proteins. Zfx, Zfy-1, and Zfy-2 contain highly acidic amino-terminal domains and carboxy-terminal regions containing 13 zinc fingers. When fused to the DNA-binding domain of GAL4, the acidic domains of Zfx and Zfy-2 activated transcription in yeast cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth A., Swift S., Affara N. Sequence of cDNA for murine Zfy-1, a candidate for Tdy. Nucleic Acids Res. 1989 Apr 11;17(7):2864–2864. doi: 10.1093/nar/17.7.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R. E., Peschon J. J., Behringer R. R., Brinster R. L., Palmiter R. D. Protamine 3'-untranslated sequences regulate temporal translational control and subcellular localization of growth hormone in spermatids of transgenic mice. Genes Dev. 1989 Jun;3(6):793–802. doi: 10.1101/gad.3.6.793. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. A eukaryotic transcriptional activator bearing the DNA specificity of a prokaryotic repressor. Cell. 1985 Dec;43(3 Pt 2):729–736. doi: 10.1016/0092-8674(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Brown R. S., Sander C., Argos P. The primary structure of transcription factor TFIIIA has 12 consecutive repeats. FEBS Lett. 1985 Jul 8;186(2):271–274. doi: 10.1016/0014-5793(85)80723-7. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Lee W. M. Identification of the human c-myc protein nuclear translocation signal. Mol Cell Biol. 1988 Oct;8(10):4048–4054. doi: 10.1128/mcb.8.10.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- FORD C. E., JONES K. W., POLANI P. E., DE ALMEIDA J. C., BRIGGS J. H. A sex-chromosome anomaly in a case of gonadal dysgenesis (Turner's syndrome). Lancet. 1959 Apr 4;1(7075):711–713. doi: 10.1016/s0140-6736(59)91893-8. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Gill G., Ptashne M. Mutants of GAL4 protein altered in an activation function. Cell. 1987 Oct 9;51(1):121–126. doi: 10.1016/0092-8674(87)90016-x. [DOI] [PubMed] [Google Scholar]
- Giniger E., Ptashne M. Transcription in yeast activated by a putative amphipathic alpha helix linked to a DNA binding unit. Nature. 1987 Dec 17;330(6149):670–672. doi: 10.1038/330670a0. [DOI] [PubMed] [Google Scholar]
- Godowski P. J., Picard D., Yamamoto K. R. Signal transduction and transcriptional regulation by glucocorticoid receptor-LexA fusion proteins. Science. 1988 Aug 12;241(4867):812–816. doi: 10.1126/science.3043662. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Evans R. M. Multiple and cooperative trans-activation domains of the human glucocorticoid receptor. Cell. 1988 Dec 2;55(5):899–906. doi: 10.1016/0092-8674(88)90145-6. [DOI] [PubMed] [Google Scholar]
- Hope I. A., Struhl K. Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell. 1986 Sep 12;46(6):885–894. doi: 10.1016/0092-8674(86)90070-x. [DOI] [PubMed] [Google Scholar]
- Hsu C. Y., Frankel F. R. Conserved and unique sequences in the 3'-untranslated region of rat smooth-muscle alpha-actin mRNA. Gene. 1988 Sep 30;69(2):345–348. doi: 10.1016/0378-1119(88)90445-3. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS P. A., STRONG J. A. A case of human intersexuality having a possible XXY sex-determining mechanism. Nature. 1959 Jan 31;183(4657):302–303. doi: 10.1038/183302a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krawetz S. A., Connor W., Dixon G. H. Cloning of bovine P1 protamine cDNA and the evolution of vertebrate P1 protamines. DNA. 1987 Feb;6(1):47–57. doi: 10.1089/dna.1987.6.47. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., White R. G., Dunham R. G., Kanda P. Effect of basic and nonbasic amino acid substitutions on transport induced by simian virus 40 T-antigen synthetic peptide nuclear transport signals. Mol Cell Biol. 1988 Jul;8(7):2722–2729. doi: 10.1128/mcb.8.7.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. M., Lin C., Curran T. Activation of the transforming potential of the human fos proto-oncogene requires message stabilization and results in increased amounts of partially modified fos protein. Mol Cell Biol. 1988 Dec;8(12):5521–5527. doi: 10.1128/mcb.8.12.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire C., Heilig R., Mandel J. L. The chicken dystrophin cDNA: striking conservation of the C-terminal coding and 3' untranslated regions between man and chicken. EMBO J. 1988 Dec 20;7(13):4157–4162. doi: 10.1002/j.1460-2075.1988.tb03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewinger L., McKeon F. Mutations in the nuclear lamin proteins resulting in their aberrant assembly in the cytoplasm. EMBO J. 1988 Aug;7(8):2301–2309. doi: 10.1002/j.1460-2075.1988.tb03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Ptashne M. Deletion analysis of GAL4 defines two transcriptional activating segments. Cell. 1987 Mar 13;48(5):847–853. doi: 10.1016/0092-8674(87)90081-x. [DOI] [PubMed] [Google Scholar]
- Mardon G., Mosher R., Disteche C. M., Nishioka Y., McLaren A., Page D. C. Duplication, deletion, and polymorphism in the sex-determining region of the mouse Y chromosome. Science. 1989 Jan 6;243(4887):78–80. doi: 10.1126/science.2563173. [DOI] [PubMed] [Google Scholar]
- Mardon G., Page D. C. The sex-determining region of the mouse Y chromosome encodes a protein with a highly acidic domain and 13 zinc fingers. Cell. 1989 Mar 10;56(5):765–770. doi: 10.1016/0092-8674(89)90680-6. [DOI] [PubMed] [Google Scholar]
- McCarrey J. R., Thomas K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature. 1987 Apr 2;326(6112):501–505. doi: 10.1038/326501a0. [DOI] [PubMed] [Google Scholar]
- Meijlink F., Curran T., Miller A. D., Verma I. M. Removal of a 67-base-pair sequence in the noncoding region of protooncogene fos converts it to a transforming gene. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4987–4991. doi: 10.1073/pnas.82.15.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger D., White J. H., Chambon P. The human oestrogen receptor functions in yeast. Nature. 1988 Jul 7;334(6177):31–36. doi: 10.1038/334031a0. [DOI] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M., Simon D., Affara N., Ferguson-Smith M., Avner P., Bishop C. Localization of murine X and autosomal sequences homologous to the human Y located testis-determining region. Genetics. 1989 Apr;121(4):803–809. doi: 10.1093/genetics/121.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine C. M., Chan K. M., Kozak C. A., Lau Y. F. Chromosome mapping and expression of a putative testis-determining gene in mouse. Science. 1989 Jan 6;243(4887):80–83. doi: 10.1126/science.2563174. [DOI] [PubMed] [Google Scholar]
- Page D. C. Is ZFY the sex-determining gene on the human Y chromosome? Philos Trans R Soc Lond B Biol Sci. 1988 Dec 1;322(1208):155–157. doi: 10.1098/rstb.1988.0123. [DOI] [PubMed] [Google Scholar]
- Page D. C., Mosher R., Simpson E. M., Fisher E. M., Mardon G., Pollack J., McGillivray B., de la Chapelle A., Brown L. G. The sex-determining region of the human Y chromosome encodes a finger protein. Cell. 1987 Dec 24;51(6):1091–1104. doi: 10.1016/0092-8674(87)90595-2. [DOI] [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Schönthal A., Angel P., Litfin M., Rüther U., Herrlich P. Posttranscriptional regulation of c-fos mRNA expression. Nucleic Acids Res. 1987 Feb 25;15(4):1643–1659. doi: 10.1093/nar/15.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schena M., Yamamoto K. R. Mammalian glucocorticoid receptor derivatives enhance transcription in yeast. Science. 1988 Aug 19;241(4868):965–967. doi: 10.1126/science.3043665. [DOI] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Beer-Romero P., Brown L. G., Nussbaum R., Page D. C. ZFX has a gene structure similar to ZFY, the putative human sex determinant, and escapes X inactivation. Cell. 1989 Jun 30;57(7):1247–1258. doi: 10.1016/0092-8674(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Welshons W. J., Russell L. B. THE Y-CHROMOSOME AS THE BEARER OF MALE DETERMINING FACTORS IN THE MOUSE. Proc Natl Acad Sci U S A. 1959 Apr;45(4):560–566. doi: 10.1073/pnas.45.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]