Abstract
The DCCT/EDIC (Diabetes Control and Complications Trial/ Epidemiology of Diabetes Interventions and Complications) provides a comprehensive characterization of the natural history of diabetic neuropathy in patients with type 1 diabetes and provides insight into the impact of intensive insulin therapy in disease progression. The lessons learned about the natural history of distal symmetrical polyneuropathy and cardiovascular autonomic neuropathy and the impact of glycemic control on neuropathy are discussed in this review.
Keywords: Distal symmetrical polyneuropathy, Cardiovascular autonomic neuropathy, Nerve conduction studies, Heart rate variability studies, Glycemic control
Introduction
The DCCT (Diabetes Control and Complications Trial) began recruitment in 1983 and enrolled 1,441 patients with type 1 diabetes by 1989. Patients were randomly assigned to intensive or conventional insulin therapy. In 1993, after an average of 6.5 years of follow-up, the investigators reported that intensive therapy significantly reduced the incidence of diabetic retinopathy, nephropathy, and neuropathy [1-3]. This finding triggered fundamental changes in the standard of care for patients with diabetes. The contribution of the DCCT cohort, however, did not end in 1993. At DCCT closeout, all subjects were encouraged to adopt intensive treatment and most agreed to participate in the observational EDIC (Epidemiology of Diabetes Interventions and Complications) study [4]. Subsequent EDIC evaluations demonstrated long-term benefits of prior intensive glycemic control on microvascular complications [5-7] and cardiovascular disease [8]. This beneficial effect of prior intensive glucose control is termed “metabolic memory” [7].
Neuropathy Evaluations During DCCT and EDIC
The methods used during the DCCT to assess distal symmetrical polyneuropathy and autonomic neuropathy have been reported elsewhere and will be described only briefly [1, 2].
Neuropathy Assessments During DCCT
Distal Symmetrical Polyneuropathy
A diagnosis of clinical neuropathy required at least two positive responses among symptoms, sensory signs, or ankle reflexes (diminished or absent) consistent with a distal symmetrical polyneuropathy and without causal explanation aside from diabetes, as assessed clinically by a neurologist who was masked to the treatment group assignment. Nerve conduction studies (NCSs) were performed on the dominant side and included evaluation of median motor and sensory, peroneal motor, and sural sensory nerves. Electrophysiologic evidence of neuropathy required an absolute abnormality of amplitude, conduction velocity, or F-wave latency in at least two anatomically distinct nerves. The primary outcome, confirmed clinical neuropathy, was defined as the combination of clinical neuropathy and NCS abnormalities involving ≥ two nerves among the median, peroneal, and sural nerves [1, 2]. The individual components of the clinical and nerve conduction examinations were secondary outcome measures.
Cardiovascular Autonomic Neuropathy
Measures of cardiovascular autonomic neuropathy (CAN) included R-R response to paced breathing (R-R variation), R-R response to Valsalva maneuver (Valsalva ratio), and postural changes in blood pressure [3]. CAN was defined as an R-R variation less than 15 or an R-R variation between 15 to 19.9 in combination with a Valsalva ratio ≤ 1.5 or a decrease of more than 10 mm Hg in diastolic blood pressure [3]. Secondary outcomes included changes in the continuous measures of R-R variation and Valsalva ratio during EDIC between the former intensive and conventional therapy cohort.
Neuropathy Assessments During EDIC
Although retinopathy and nephropathy evaluations in EDIC were identical to those performed during the DCCT, only screening neuropathy evaluations consisting of the Michigan Neuropathy Screening Instrument (MNSI) were performed during the first 12 years of EDIC [9]. Although MNSI results suggested that the metabolic memory phenomenon applied to new-onset (incident) neuropathy, this instrument has neither the sensitivity nor specificity of the more comprehensive neurologic and electrodiagnostic evaluations used to evaluate neuropathy in the original DCCT cohort. No measures of CAN were assessed during the first 12 years of EDIC, so the incidence and prevalence of neither distal symmetrical polyneuropathy nor autonomic neuropathy had not been evaluated in this cohort since the conclusion of the DCCT.
Important questions about the impact of former intensive therapy on diabetic distal symmetrical polyneuropathy and autonomic neuropathy among the EDIC cohort remained unanswered. For this reason, additional evaluations of peripheral and autonomic neuropathy (collectively termed NeuroEDIC) were performed during the 13th and 14th years of EDIC. In NeuroEDIC, as in DCCT, the assessment of distal symmetrical polyneuropathy and CAN included the same comprehensive measures performed during DCCT and described above. The outcome measures were also consistent with those used in the DCCT.
Of the 1,274 subjects from the original DCCT cohort who were active in EDIC at the time NeuroEDIC was initiated, 1186 agreed to participate in the distal symmetrical polyneuropathy evaluations and 1226 agreed to participate in the CAN evaluations. Figure 1 shows a summary of subject participation.
Fig. 1.
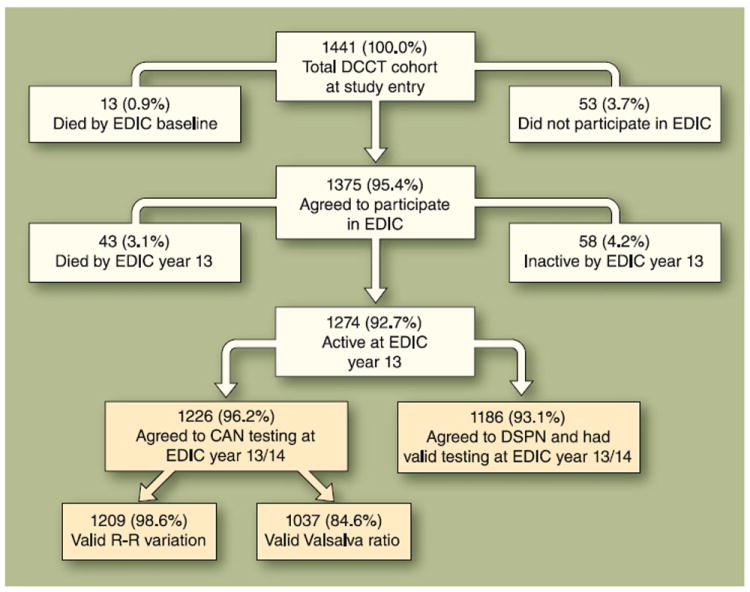
Flow diagram of NeuroEDIC (neuropathy assessments during EDIC) participation. The four gold-colored boxes illustrate the subjects who had evaluations in EDIC year 13/14. CAN—cardiovascular autonomic neuropathy; DCCT—Diabetes Control and Complications Trial; DSPN—distal symmetrical polyneuropathy; EDIC—Epidemiology of Diabetes Interventions and Complications
Natural History of Distal Symmetrical Polyneuropathy and CAN
The DCCT included a primary prevention cohort and a secondary intervention cohort. The primary prevention cohort had diabetes for 1–5 years (mean, 2.6 years) and no retinopathy at baseline. The secondary intervention cohort had diabetes for 1–15 years (mean, 8.7 years) and mild to moderate retinopathy at baseline [1]. At DCCT baseline, there were only minor differences in the clinical and electrodiagnostic measures of neuropathy between the two cohorts. The secondary intervention cohort had a higher prevalence of confirmed clinical neuropathy and of abnormal R-R variation at DCCT baseline than the primary prevention cohort (9.4% vs 3.5% and 6.2% vs 1.6 %, respectively) [2, 3]. In the combined DCCT cohort, there were no significant between-treatment group differences at baseline for any of the neuropathy measures. At DCCT closeout, hemoglobin A1c (HbA1c) was 7.4% in the intensive therapy group and 9.1% in the conventional therapy group (P<0.001). The differences in glycemic control between the treatment groups during the DCCT reflected the protocol-directed interventions; during the DCCT, 97% of DCCT study time was spent on assigned therapy.
At DCCT closeout (median, 5 years of treatment), the treatment groups differed in most measures of neuropathy, with a 64% reduction in the incidence of confirmed clinical neuropathy in the intensive compared to conventional therapy group [1, 2]. Numerous significant differences in NCS results were also observed between groups, all favoring better function (faster sensory and motor conduction velocities and shorter F-wave latencies) in the intensive treatment group. The 64% reduction in the incidence of neuropathy attributed to intensive therapy during DCCT reflected mainly measures of distal symmetrical polyneuropathy. However, the incidence of CAN also was reduced by 45%, albeit involving a small percentage of the cohort (4% intensive vs 9% conventional) [1-3]. Abnormalities in both measures of distal symmetrical polyneuropathy and CAN were more prevalent in subjects from the secondary intervention cohort and among patients followed for longer periods [2, 3].
At the end of the DCCT, the care of all subjects was returned to their own physicians and intensive therapy was recommended for all. This resulted in more intensified treatment in the former conventional group that was equivalent to the treatment approach for the former intensive group during the EDIC follow-up. As a consequence, at the first EDIC study examination, HbA1c separation between former DCCT intensive and conventional groups narrowed substantially to 7.9% versus 8.3% at EDIC year 1 and was no longer statistically significant by EDIC year 5 [10••]. The distribution of HbA1c values over time in the DCCT/EDIC conventional and intensive treatment groups reflects the different study goals during the DCCT and subsequently the universal recommendation for intensive therapy during the EDIC follow-up.
During the 13 years to 14 years of EDIC follow-up, the prevalence of confirmed clinical neuropathy in the combined cohorts increased substantially [11••], whereas the difference between the former treatment groups remained significant (P<0.001) (Table 1). A persistent treatment group effect was also observed for most NCS measures including all peroneal motor and sural sensory measures. Compared to DCCT closeout, the treatment group differences and level of significance were smaller at EDIC year 13 years to 14 (Table 1) [11••]. Additionally, the primary prevention cohort showed a significant treatment group difference only in the prevalence of abnormal NCS results, whereas the secondary intervention cohort showed significant treatment group differences in the prevalence of all measures of neuropathy except abnormal reflexes.
Table 1.
Prevalence of distal symmetrical neuropathy and CAN outcomes at DCCT baseline, DCCT closeout, and EDIC year 13 to 14
| Test | Group | DCCT baseline | DCCT closeout | EDIC year 13/14 |
|---|---|---|---|---|
| Confirmed clinical neuropathy, No. (%) | INT | 39 (7) | 52 (9)a | 152 (25)a |
| CONV | 31 (5) | 97 (17) | 204 (35) | |
| Abnormal NCS, No. (%) | INT | 185 (31) | 164 (28)a | 326 (54)a |
| CONV | 196 (34) | 288 (50) | 401 (69) | |
| Clinical neuropathy, No. (%) | INT | 57 (10) | 88 (15)a | 204 (34)a |
| CONV | 48 (8) | 128 (22) | 240 (41) | |
| CAN composite definition, No. (%)b | INT | 24 (4) | 43 (7) | 179 (29)c |
| CONV | 31 (5) | 57 (10) | 208 (35) | |
| R-R variation <15, No. (%) | INT | 20 (3) | 39 (7) | 147 (24)c |
| CONV | 25 (4) | 53 (10) | 178 (30) | |
| Adjusted R-R variation, mean ± SDd | INT | 49±21 | 42±19c | 30±17e |
| CONV | 47±21 | 39±19 | 26±17 |
CAN cardiac autonomic neuropathy, CONV conventional, DCCT Diabetes Control and Complications Trial, EDIC Epidemiology of Diabetes Interventions and Complications, INT intensive, NCS nerve conduction study
P<0.001 for treatment group differences by the Wilcoxon rank-sum test or chi-square test comparing INT and CONV treatment groups
CAN prevalence is defined as any one of the following conditions: R-R variation < 15; R-R variation < 20 in combination with Valsalva ratio ≤ 1.5, or postural hypotension
P<0.05 for treatment group differences by the Wilcoxon rank-sum test or chi-square test comparing INT and CONV treatment groups
Means adjusted for DCCT baseline age, sex, cohort assignment, and duration in the DCCT study
P<0.01 for treatment group differences by the Wilcoxon rank-sum test or chi-square test comparing INT and CONV treatment groups
With respect to CAN, the prevalence of CAN and of abnormal R-R variation (R-R < 15) at EDIC year 13 to 14 was significantly lower in the former intensive group compared to former conventional group in pooled data from the primary prevention and secondary intervention cohorts (28.9% vs 35.2%; P=0.018 and 30.2% vs 23.8%, P =0.012, respectively). The analysis of the changes in the continuous measures of CAN also showed that the adjusted R-R variation was significantly higher in the former intensive group compared to the conventional group (P< 0.001) (Table 1) [10••]. Similarly with distal symmetrical neuropathy, the categorical and continuous measures of CAN showed poorer performance in the secondary intervention cohort compared to the primary prevention cohort at EDIC year 13 to 14.
In evaluating the natural history of diabetic complications, exposure to chronic hyperglycemia must be balanced against other risk covariates that could influence various microvascular complication-prone organs. Orchard et al. [12] argued that use of a cumulative glycemic exposure variable that combines both the degree and duration of hyperglycemia did not enhance prediction of complications any better than does use of the individual components. Dyck et al. [13] demonstrated that a combination of HbA1c, duration of diabetes, and age at onset of diabetes correlates significantly with occurrence of complications and predicts late complications better than the single components. Therefore, the information on the natural history of diabetic neuropathy among the DCCT/EDIC cohort provided an ideal opportunity to evaluate the contribution of glycemic control on neuropathy as measured by the HbA1c throughout the DCCT/EDIC. We modeled the incidence and prevalence of distal symmetrical polyneuropathy and CAN as a function of the mean HbA1c level during DCCT and during EDIC, independent of prior treatment group assignment. The odds ratio of developing or having the particular measure of neuropathy was determined for a one percentage point increase in the HbA1c, with all other variables held constant.
The mean HbA1c level during the DCCT and during EDIC was associated with the incidence of confirmed clinical neuropathy and with the prevalence of all measures of neuropathy at EDIC year 13 to 14 [11••]. The odds of having confirmed clinical neuropathy at EDIC year 13 to 14 (prevalence) increased significantly per one percentage point increase in mean HbA1c during DCCT (OR, 1.35; 95% CI, 1.35–1.50) and during EDIC (OR, 1.80; 95% CI, 1.56–2.07) [11••]. The incidence of CAN also was associated with higher mean HbA1c levels during the DCCT and during EDIC. After adjusting for both the mean HbA1c levels during DCCT and during EDIC, treatment group differences were no longer significant [10••]. The proportion of the DCCT treatment group effect explained by the group differences in HbA1c in DCCT and EDIC was 77.9% [10••]. Therefore, the incidence and prevalence of both distal symmetrical neuropathy and CAN reflect differences in HbA1c during the DCCT and the subsequent convergence of HbA1c after DCCT closeout.
To place our data into perspective, the EURODIAB (European Diabetes Prospective Complications Study), an observational study that included 1,172 subjects with type 1 diabetes from 31 centers across Europe, evaluated the epidemiology of complications in type 1 diabetes [14]. Although the assessment of distal symmetrical polyneuropathy was less comprehensive than the one performed in NeuroEDIC, EURODIAB found that after only 7.3 years of follow-up, neuropathy developed in 24% of subjects who had no evidence of neuropathy at baseline. Although the differences in study design do not allow for precise comparisons between the true incidence of neuropathy in the DDCT/EDIC and the EURODIAB cohorts, a 29% incidence of clinical neuropathy found in the former DCCT intensive treatment cohort after an average follow-up of 13 years would argue for a better outcome in this group. EURODIAB also reported that the cumulative incidence of neuropathy was related to the HbA1c value over the follow-up. In addition to glycemic control and duration of diabetes, EURODIAB also found that hypertension, smoking, obesity, and triglycerides were independent risk factors for neuropathy.
Metabolic Memory
The persistent long-term benefits demonstrated during EDIC of the prior intensive glycemic control during DCCT on retinopathy and nephropathy complications were termed “metabolic memory” [7]. We assessed whether prior intensive glycemic control influenced the incidence of confirmed clinical neuropathy, abnormal NCS results, CAN function, and abnormal R-R variation at EDIC year 13 to 14 among participants with no evidence of these abnormalities at DCCT closeout.
Unadjusted analysis demonstrated a 30% reduction in the risk of confirmed clinical neuropathy (OR, 0.70; 95% CI, 0.52–0.93; P=0.0125) with intensive versus conventional therapy among those subjects without confirmed clinical neuropathy at DCCT closeout (Table 2). In a separate analysis, analytic models of incident neuropathy that adjusted for subclinical differences in NCS measures at DCCT closeout showed that the differences between the intensive and conventional therapy groups were no longer statistically significant at EDIC year 13 to 14 (Table 2) [11••]. These analyses suggest that among those without clinically evident neuropathy, the between group differences in incident neuropathy were explained in part by differences between the groups at DCCT closeout [11••]. Additional analysis of the NCS results that adjusted for the DCCT closeout NCS results showed nominally better performance for three of the ten NCS measures, all in the lower extremity and among those most likely to be abnormal in a mild diabetic neuropathy.
Table 2.
Incidence of distal symmetrical neuropathy and CAN outcomes at EDIC year 13 to 14 assessing treatment group differences among subjects with intact function at DCCT closeout
| EDIC year 13–14 | ||
|---|---|---|
| DSPN measures | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI)a |
| Clinical neuropathy | 0.77 (0.59–1.01) | 0.99 (0.73–1.34) |
| Age and height,b | ||
| average rank of 3 leg NCS measures † | ||
| Abnormal NCS | 0.76 (0.57–1.03) | 1.01 (0.72–1.41) |
| Weightc | ||
| average rank of all 10 NCS measures ‡ | ||
| Confirmed clinical neuropathy | 0.70 (0.52–0.93) | 1.17 (0.84–1.63) |
| Age and heightc | ||
| average rank of all 10 NCS measures ‡ | ||
| CAN Measures | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI)d |
| R-R variation < 15e | 0.76 (0.57–1.02) | 0.70 (0.51–0.96) |
| Valsalva ratio ≤ 1.5 | 0.92 (0.69–1.23) | 0.85 (0.62–1.16) |
| Abnormal CAN functionf | 0.76 (0.59–0.995) | 0.69 (0.51–0.93) |
CAN cardiac autonomic neuropathy, DCCT Diabetes Control and Complications Trial, DSPN distal symmetrical polyneuropathy, EDIC Epidemiology of Diabetes Interventions and Complications, HbA1c hemoglobin A1c, NCS nerve conduction study
Covariates that entered into the models using a stepwise selection are indicated in italics with the OR (95% CI) for each model
Adjusted for the average rank over three leg NCS measures at DCCT closeout: peroneal (amplitude and conduction velocity), sural (amplitude)
Adjusted for the average rank over all ten NCS measures at DCCT closeout: median motor (amplitude, conduction velocity, and F-wave latency), median sensory (amplitude and conduction velocity), peroneal (amplitude, conduction velocity, and F-wave latency), and sural (amplitude and conduction velocity)
Logistic regression models were adjusted for DCCT baseline age, sex, cohort assignment, and duration in the DCCT study
Models for R-R variation < 15 were also adjusted for R-R variation at DCCT closeout, models for Valsalva ratio ≤ 1.5 adjusted for Valsalva ratio at DCCT closeout, and models for abnormal CAN function adjusted for both quantitative measures. HbA1c models include both the mean HbA1c level during DCCT and during EDIC
Abnormal CAN function was defined as any one of the following conditions: R-R variation < 15; R-R variation < 20 in combination with Valsalva ratio ≤ 1.5, or postural hypotension
Intensive insulin therapy during DCCT reduced the risk of incident CAN by 31% (OR, 0.69; 95% CI, 0.51–0.93) and of abnormal R-R variation by 30% (OR, 0.70; 95% CI, 0.51–0.96) after adjusting for DCCT baseline age, sex, cohort assignment, duration in the DCCT study, and the level of R-R variation at DCCT closeout (Table 2) [10••]. There were persistent beneficial effects of intensive versus conventional therapy on CAN after 13 years to 14 years of follow-up in EDIC (Tables 1 and 2). Analytic models that adjusted for mean HbA1c levels during DCCT and EDIC showed that treatment group differences were no longer significant. Thus, the differences in the DCCT and EDIC mean HbA1c levels between groups explained virtually all of the difference between treatment groups in the incidence of CAN [10••].
These results support the impact of “metabolic memory” on CAN as previously observed for retinopathy and nephropathy. However, for distal symmetrical polyneuropathy, the metabolic memory effect is less evident and may be mediated by subclinical treatment group differences in NCS results at closeout.
Nevertheless, significant differences in measures of distal symmetrical polyneuropathy and CAN continued to favor the former intensive treatment group over the former conventional treatment group throughout EDIC follow-up. At EDIC year 13 to 14, 25% and 35% of the former intensive and conventional treatment groups had confirmed clinical neuropathy. During EDIC, CAN also progressed substantially in both treatment groups. However, in contrast with confirmed clinical neuropathy, there was a persistent benefit of the former intensive therapy resulting in a 31% and 30% reduction in the risk of developing CAN and abnormal R-R variation, respectively, during EDIC after adjusting for the level of autonomic measurements or presence of CAN at the end of the DCCT. These differences in the incidence of CAN and confirmed clinical neuropathy may suggest possible differences in the susceptibility of small and large nerve fibers to the metabolic memory effects of hyperglycemia.
The NeuroEDIC findings should be interpreted in light of substantial changes in glycemic control after DCCT closeout and the lack of glycemic separation between the former treatment groups for nearly a decade during EDIC. The longitudinal analyses of overall glycemic control demonstrated a significant association between mean HbA1c and measures of incident and prevalent confirmed clinical neuropathy. In addition, in DCCT and EDIC, physiologic measures such as NCSs to assess subclinical changes in peripheral nerve were not available for the retina or kidney. Therefore, it remains unclear whether the metabolic memory effects on these outcomes could also be a reflection of early subclinical changes. An additional consideration is that the opportunity to study distal symmetrical polyneuropathy and CAN at earlier times after DCCT closeout was missed until EDIC year 13 to 14. Recent data show that the magnitude of the effect of prior intensive treatment on diabetic retinopathy has diminished over time [15••]. It is therefore possible that evaluation of distal symmetrical polyneuropathy and CAN at earlier time points after DCCT closeout may have demonstrated a more robust effect of metabolic memory on neuropathy.
Conclusions
The DCCT/EDIC studies provide a comprehensive characterization of the natural history of neuropathy in patients with type 1 diabetes. A reproducible neurologic testing protocol repeated over time, robust definitions to define neuropathy, and the large sample size enhance the validity of the results. The DCCT demonstrated that intensive control designed to achieve near-normal glycemia was essential to preventing or delaying progression of distal symmetric polyneuropathy and CAN. The strong relationship between hyperglycemia and development of diabetic neuropathy was sustained after 13 years to 14 years of EDIC. Our optimism about the DCCT results was tempered by the subsequent high frequency of both emergent and prevalent confirmed clinical neuropathy and CAN 13 years to 14 years after DCCT closeout. Although the incidence of both confirmed clinical neuropathy and CAN during DCCT was low, both distal symmetrical polyneuropathy and CAN emerged during EDIC, suggesting the attainable intensive glycemic control is necessary but perhaps insufficient to prevent adverse nervous system effects.
The EDIC study also confirmed that the long-term maintenance of near-normal glycemic control is an elusive goal for many individuals with type 1 diabetes, even after the significant improvement in knowledge and technologies for insulin delivery.
The concept of metabolic memory appears to apply to CAN measures in our model, but the data are less conclusive for distal symmetrical polyneuropathy, suggesting possible differences in the susceptibility to the metabolic memory effects of glucose control between the small and the large nerve fibers. Most EDIC findings involving neuropathy are explained by group differences in NCS results existing at DCCT closeout, suggesting that the metabolic memory effect may be explained in part by a continuum of prior neurotoxicity that emerges at a later time in association with age- and diabetes-related neuronal attrition, as opposed to a programmed group of events that express their effect at a later time. Nevertheless, it is also possible that we missed the time when metabolic memory might have been observed because of the long interval between the DCCT closeout and NeuroEDIC assessments
The EDIC study continues to provide unique observational data for a large cohort of patients with type 1 diabetes mellitus, with 92% of the baseline cohort (96% of the surviving cohort) followed for a mean of 19 years (range, 16–22 years), use of uniform, standardized methods for collecting data across all 28 centers in Canada and the United States, and objective measures of outcomes. Although it remains unproven whether good glycemic control can reverse pre-existing peripheral and autonomic nervous system damage caused by type 1 diabetes, the earlier we implement intensive therapy, the more effectively we prevent later complications, including neuropathy. These findings complement the reported data of other microvascular and macrovascular complications of type 1 diabetes, expanding the depth of our understanding of the natural history of type 1 diabetes.
Acknowledgments
Participating neurologists and electromyographers are listed in the Disclosure section. A complete list of participants in the DCCT/EDIC research group can be found in Archives of Ophthalmology 2008, 126:1713.
Disclosure The DCCT/EDIC project is supported by contracts with the Division of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases, National Eye Institute, National Institute of Neurological Disorders and Stroke, the General Clinical Research Centers Program and the Clinical and Translational Science Awards Program, National Center for Research Resources, and by Genentech through a Cooperative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases.
Contributors of free or discounted supplies and/or equipment include the following: Lifescan, Roche, Aventis, Eli Lilly, OmniPod, Can-Am, Beckton-Dickinson, Animas, Medtronic, Medtronic Minimed, Bayer (donation one time in 2008), and Omron.
Dr. Rodica Pop-Busui is also supported by the American Diabetes Association Grant 1-08-CR-48, the Juvenile Diabetes Research Foundation Grant 1-2008-1025, and the Juvenile Diabetes Research Foundation for the Study of Complications of Diabetes Grant 4-200-421.
Footnotes
No other potential conflicts of interest relevant to this article were reported.
Contributor Information
Rodica Pop-Busui, Email: rpbusui@umich.edu, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI, USA.
William H. Herman, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI, USA
Eva L. Feldman, Department of Neurology, University of Michigan Medical School, Ann Arbor, MI, USA
Phillip A. Low, Department of Neurology, Mayo Clinic, Rochester, MN, USA
Catherine L. Martin, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI, USA
Patricia A. Cleary, The DCCT/EDIC Research Group, Box DCCT/EDIC, The Biostatistics Center, George Washington University, Rockville, MD, USA
Barbara H. Waberski, The DCCT/EDIC Research Group, Box DCCT/EDIC, The Biostatistics Center, George Washington University, Rockville, MD, USA
John M. Lachin, The DCCT/EDIC Research Group, Box DCCT/EDIC, The Biostatistics Center, George Washington University, Rockville, MD, USA
James W. Albers, Department of Neurology, University of Michigan Medical School, Ann Arbor, MI, USA
References
Papers of particular interest, published recently, have been highlighted as:
-
••
Of major importance
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. no authors listed. [DOI] [PubMed] [Google Scholar]
- 2.Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol. 1995;38:869–880. doi: 10.1002/ana.410380607. no authors listed. [DOI] [PubMed] [Google Scholar]
- 3.The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT) Diabetologia. 1998;41:416–423. doi: 10.1007/s001250050924. no authors listed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. no authors listed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. no authors listed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–2569. doi: 10.1001/jama.287.19.2563. no authors listed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group: Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159–2167. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin CL, Albers J, Herman WH, et al. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care. 2006;29:340–344. doi: 10.2337/diacare.29.02.06.dc05-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Pop-Busui R, Low PA, Waberski BH, et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC) Circulation. 2009;119:2886–2893. doi: 10.1161/CIRCULATIONAHA.108.837369. This paper evaluated the effects of prior intensive insulin therapy on the prevalence and incidence of cardiac autonomic neuropathy in former DCCT intensive and conventional therapy subjects 13 to 14 years after DCCT closeout. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Albers JW, Herman WH, Pop-Busui R, et al. Effect of prior intensive insulin treatment during the Diabetes Control And Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions, and Complications (EDIC) Study. Diabetes Care. 2010 Feb 11; doi: 10.2337/dc09-1941. [Epub ahead of print]. This paper evaluated the impact of former intensive versus conventional insulin treatment on distal symmetrical neuropathy in DCCT intensive and conventional treatment subjects with type 1 diabetes 13 to 14 years after DCCT closeout. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orchard TJ, Forrest KY, Ellis D, et al. Cumulative glycemic exposure and microvascular complications in insulin-dependent diabetes mellitus. The glycemic threshold revisited. Arch Intern Med. 1997;157:1851–1856. [PubMed] [Google Scholar]
- 13.Dyck PJ, Davies JL, Clark VM, et al. Modeling chronic glycemia exposure variables as correlates and predictors of microvascular complications of diabetes. Diabetes Care. 2006;29:2282–2288. doi: 10.2337/dc06-0525. [DOI] [PubMed] [Google Scholar]
- 14.Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352:341–350. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 15••.White NH, Sun W, Cleary PA, et al. Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the Diabetes Control and Complications Trial. Arch Ophthalmol. 2008;126:1707–1715. doi: 10.1001/archopht.126.12.1707. This paper examined differences in the persistence of the benefits of intensive therapy on retinopathy 10 years after completion of the DCCT. [DOI] [PMC free article] [PubMed] [Google Scholar]


