Abstract
Activation of the β2 adrenergic receptor (β2AR) on immune cells has been reported to possess anti-inflammatory properties, however, the pro-inflammatory properties of β2AR activation remain unclear. In this study, using rat primary mesencephalic neuron-glia cultures, we report that salmeterol, a long-acting β2AR agonist, selectively induces dopaminergic (DA) neurotoxicity through its ability to activate microglia. Salmeterol selectively increased the production of reactive oxygen species (ROS) by NADPH oxidase (PHOX), the superoxide-producing enzyme in microglia. A key role of PHOX in mediating salmeterol-induced neurotoxicity was demonstrated by the inhibition of DA neurotoxicity in cultures pretreated with diphenylene-iodonium (DPI), an inhibitor of PHOX activity. Mechanistic studies revealed the activation of microglia by salmeterol results in the selective phosphorylation of ERK, a signaling pathway required for the translocation of the PHOX cytosolic subunit p47phox to the cell membrane. Furthermore, we found ERK inhibition, but not protein kinase A (PKA) inhibition, significantly abolished salmeterol-induced superoxide production, p47phox translocation, and its ability to mediate neurotoxicity. Together, these findings indicate that β2AR activation induces microglial PHOX activation and DA neurotoxicity through an ERK-dependent/PKA-independent pathway.
Keywords: β2AR, oxidative stress and neuroinflammation
Introduction
Catecholamines produced by the sympathetic nervous system play a significant role in the regulation of the immune responses (Felten et al. 1987). Catecholamines mediate their activities by binding to adrenergic receptors, a family of 7 transmembrane G-protein-coupled receptors (GPCR) which include two major classes, alpha (α) and beta (β) adrenergic receptors. The β2 adrenergic receptor (β2AR) is known to regulate a variety of biological functions, including the regulation of smooth muscle activity in the airway and vasculature. Recently, β2AR has also been identified to express on several different immunocompetent cell types, including macrophages, microglia, T cells, and B cells, and signaling through this receptor has been found to influence the inflammatory response of these cells (Farmer and Pugin 2000; Kin and Sanders 2006; Severn et al. 1992).
A number of studies have demonstrated that stimulation of the β2AR in macrophages has anti-inflammatory effects, which are characterized by the suppression of LPS-induced IL-1β, IL-6, and tumor necrosis factor-alpha (TNF-α) production, mainly through the inhibition of transcription factor NF-κB (Farmer and Pugin 2000; Severn et al. 1992). However, the chronic use of β2AR agonists, including salmeterol, results in increased airway reactivity, prolonged asthma exacerbations, and worsened airway inflammation (Abramson et al. 2003; McGraw et al. 2003) in asthma patients. Several studies have shown chronic stress increases susceptibility to various inflammatory conditions including peptic ulcers, ulcerative colitis, asthma, myocardial infarction and depression. In addition, recent work from our laboratory as well as others have shown that β2AR stimulation can also increase the production of pro-inflammatory mediators in both macrophages and microglial cells (Tan et al. 2007; Tomozawa et al. 1995). This pro-inflammatory effect in macrophages is mediated through an NF-κB-independent pathway which results in increased production of IL-1β and IL-6 (Tan et al. 2007).
Activation of β2AR, like most GPCRs, leads to an increase in cAMP following the activation of adenylate cyclase by Gs-proteins, and results in the stimulation of protein kinase A (PKA) (Johnson 2006). In macrophages, elevation of cAMP levels leads to the inhibition of pro-inflammatory responses, likely through inhibition of the phosphodiasterase-4 (Houslay et al. 2005). While the majority of β2AR-mediated signaling occurs through a PKA-dependent mechanism, recent evidence suggests PKA-independent pathways involved in β2AR-mediated macrophage activation, which lead to increased cytokine production in macrophages through the activation of transcription factors ATF-1 and ATF-2 (Tan et al. 2007). The relationship between the β2AR-activated PKA-dependent and independent signaling pathways, and their functional roles in regulating the inflammatory response of macrophages, are still not defined.
Microglia, the major immune cells in the central nervous system (CNS), arise from monocyte-macrophage lineage, and are known to express high levels of β2AR (Tanaka et al. 2002). Therefore, it is likely that catecholamines, which play an important role in regulating the activities of the CNS, have a major regulatory effect on microglia via β2AR activation. However, this effect on the regulation of inflammatory responses mediated by microglia in the CNS remains unclear. Increasing evidence has shown that inflammation mediated by dysregulated microglia activation can result in neurodegeneration in the CNS leading to Parkinson’s disease (PD) (Block and Hong 2005; Qian et al. 2007a; Rosi et al. 2005). The midbrain region that encompasses the substantia nigra is particularly rich in microglia (Kim et al. 2000). Recent studies have shown that activation of nigral microglia and the subsequent release of neurotoxic factors, including pro-inflammatory cytokines and reactive oxygen species (ROS), are considered key components of dopaminergic (DA) neuron degeneration in PD (Block and Hong 2005; Qian and Flood 2008). Since the activation of β2AR by its agonist has both anti- and pro-inflammatory properties on peripheral immune cells (Christensen et al. 1999; Frost et al. 2004; Mohamed-Ali et al. 2001; Rohrbach et al. 2007a; Yin et al. 2006), and β2AR agonists are also highly lipophilic and readily gain access to the brain, it is important to determine if and how β2AR activation plays a role in regulating the interplay between immune and neuronal cells during CNS inflammation.
In this study, we hypothesized that activation of β2AR on microglial cells may trigger inflammatory responses that lead to the death of DA-producing neurons, a hallmark of PD. Consequently, we tested if activation of β2AR exerts pro-inflammatory effects on microglia, and sought to elucidate the underlying cellular and molecular mechanisms. Using rodent mesencephalic neuron-glia cultures, we report that salmeterol, a widely-used β2AR agonist, produces DA neurotoxicity by activating a microglia-mediated inflammatory response through an ERK-dependent but PKA-independent signaling pathway. β2AR activation leads to enhanced NADPH oxidase (PHOX) activity and increased production of ROS from microglia. These results suggest a novel mechanism in the β2AR-mediated activation of pro-inflammatory responses in PD.
Materials and methods
Animals
Timed-pregnant Fisher F344 rats were obtained from Charles River Laboratories (Raleigh, NC). Housing of the animals were performed in strict accordance with the National Institutes of Health guidelines.
Reagents and cell line
Salmeterol (>99% pure) and ICI118,551 were obtained from Tocris Cookson, Ballwin, MO. U0126 and Rp-cAMPS were purchased from Biomol (Plymouth Meeting, PA). LPS (E.coli strain O111:B4) was purchased from Calbiochem (San Diego, CA). [3H]-DA was obtained from Perkin-Elmer Life Sciences (Boston, MA). The polyclonal anti-tyrosine hydroxylase antibody was a generous gift from Dr. John Reinhard (GlaxoSmithKline, Research Triangle Park, NC). The Vectastain ABC kit and biotinylated secondary antibodies were purchased from Vector Laboratories (Burlingame, CA). The fluorescence probe Dichlorodihydro- fluorescein Diacetate (DCFH-DA) was obtained from Calbiochem (La Jolla, CA). Rabbit anti-p47phox was purchased from Upstate (Lake Placid, NY). Rabbit anti-GAPDH was obtained from Abcam (Cambridge, MA). Mouse anti-gp91phox was purchased from BD Transduction Laboratories (San Jose, CA). Anti-phospho-ERK1/2 Ab, anti-ERK1/2 Ab, anti-phospho-p38 Ab, anti-p38 Ab, anti-phospho-JNK Ab or anti-JNK Ab were purchased from Cell Signaling Technology (Danvers, MA). The rat microglia HAPI cell line was a generous gift from Dr. James R. Connor (Cheepsunthorn et al. 2001).
Primary mesencephalic neuron-glia cultures
Neuron-glia cultures were prepared from the ventral mesencephalic tissues of embryonic day 14–15 rats, as described previously (Liu et al. 2000a). At the time of treatment, immunocytochemical analysis indicated that the rat neuron-glia cultures were made up of 11% microglia, 48% astrocytes, 41% neurons, and 1% tyrosine hydroxylase immunoreacitve (TH-IR) neurons.
Primary mesencephalic neuron-enriched cultures
Midbrain neuron-enriched cultures were established as described previously (Qian et al. 2006). Routinely, the 7-day-old neuron-enriched cultures normally contain less than 0.1% microglia, and less than 3–5% astrocytes, were used for treatment.
Primary microglia-enriched cultures
Rat microglia-enriched cultures with a purity of >98%, were prepared from whole brains of 1-day-old Fischer 344 rat pups as described previously (Liu et al. 2000b).
Primary midbrain neuron-astroglia cocultures
Rat primary neuron-astroglia cocultures were obtained by suppressing microglial proliferation with 1.5 mM L-leucine methyl ester 24 h after seeding the cells, as described previously (Qian et al. 2006). The cultures stained with F4/80 antibody showed less than 0.1% microglia.
[3H]-DA Uptake assays
DA Uptake assays were performed as described previously (Qian et al. 2007b) and the specific [3H]-DA uptake was calculated by subtracting the mazindole counts from the wells without the uptake inhibitors.
Immunocytochemistry
Immunostaining was performed as previously described (Gao et al. 2002). For visual counting of TH-IR neurons, nine representative areas per well of the 24-well plate were counted under the microscope at 100 × magnification by three individuals. The average of these scores was reported.
Superoxide assay
The production of superoxide was determined by measuring the superoxide dismutase (SOD)-inhibitable reduction of the tetrazolium salt WST-1 (Peskin and Winterbourn 2000; Tan and Berridge 2000). Microglia-enriched cultures were incubated at 37°C for 30 min with vehicle control or salmeterol. Then, 50 μl of HBSS with and without SOD (50 U/ml) was added to each well along with 50 μl of WST-1 (1 mM) in HBSS, and 50 μl of vehicle or salmeterol. Fifteen minutes later, absorbance at 450 nm was read with a SpectraMax Plus microplate spectrophotometer (Molecular Devices Corp, Sunnyvale, CA). The difference in absorbance observed in the presence and absence of SOD was considered to be the amount of superoxide produced, and results were expressed as the percentage of vehicle-treated control cultures.
Intracellular ROS assay
Intracellular ROS were determined by using a 2′,7′-dichlorodi-hydrofluorescein (DCFH-DA) assay as described previously with minor modifications (Liu et al. 2001). The cells were exposed to DCFH-DA for 1 h and then treated with HBSS containing the corresponding concentrations of LPS for 2 h. The fluorescence was read immediately at wavelengths of 485 nm for excitation and 530 nm for emission using a SpectraMax Gemini XS fluorescence microplate reader(Molecular Devices). The experimental value minus the value of the control group was interpreted as the increase in intracellular ROS.
Cytosol and membrane fractionation and western blot analysis
Cytosol and membrane fractionation was performed as described (Qian et al. 2008). Membranes were blocked and incubated with rabbit anti-p47phox antibody or mouse anti-gp91phox for overnight at 4°C. Horse radish peroxidase-linked anti-rabbit or mouse IgG for 1 h at 25°C, ECL+Plus reagents (Amersham Biosciences Inc., Piscataway, NJ) were used as a detection system. For detection the phosphorylation of mitogen activated protein kinase (MAPK), membranes were blocked and incubated with either antibodies against phosphorylated MAPK or total MAPK overnight at 4°C. Anti-rabbit horse radish peroxidase-linked secondary antibody was incubated for 1h at 25°C. The same detection system as above was used.
Statistical Analysis
Data is presented as the means ± SE. For multiple comparisons of groups, two-way ANOVA was used. Statistical significance between groups was assessed by paired Student’s t test, followed by Bonferroni correction using the JMP program (SAS Institute, Cary, NC, USA). A value of P < 0.05 was considered statistically significant.
Results
β2AR agonist salmeterol induces selective toxicity to DA neurons through β2AR
To discern the effect of the β2AR agonist salmeterol on DA neurotoxicity, mesencephalic neuron-glia cultures were exposed to the drug (0.1–10 μM), and the function and viability of DA neurons were measured. Salmeterol treatment reduced the capacity of the cultures to take up DA in a dose-dependent fashion as shown by a [3H]-DA uptake assay (Fig. 1A). The viability of DA neurons determined by numeration of the TH-IR neurons show similar results; i.e. cell counts of TH-IR neurons show significant loss of DA neurons (Fig. 1B). However, salmeterol-mediated toxicity appear to be specific to DA neurons, as direct cell counting of total neurons after Neu-N staining reveal no significant difference between cells treated with or without salmeterol at 0.1 to 3 μM (Fig. 1C). Increasing salmeterol concentration to 10 μM resulted in nonspecific toxicity as evidenced by the loss of both TH-IR and Neu-N neurons. The chemical specifications for the preparation of salmeterol used showed that this preparation was highly purified (>99% pure), suggesting that the effects reported in this study are due to exposure to salmeterol alone and no other activating stimuli were provided. Taking together, these data demonstrate that salmeterol at 1μM is selectively toxic to DA neurons. Morphological inspection confirmed that salmeterol treatment not only decreased the number of TH-IR neurons, but also resulted in a loss of neuronal process. However, treatment did not affect the number or morphology of Neu-N at concentrations less than 3 μM (Fig. 1D). It is worth noting that TH-IR neurons only represent 1% of total neurons in these neuron-glia cultures, and consequently a significant loss of TH-IR neurons does not translate into a significant over-all loss of total neurons as measured by Neu-N staining.
FIGURE 1.
Salmeterol produces a dose-dependent and selective toxicity on DA-producing neurons. Rat primary mesencephalic neuron-glia cultures were treated with indicated concentrations of salmeterol. After seven days, the salmeterol-induced DA neurotoxicity was quantified by the [3H]-DA uptake assay (A) and TH neuron count (B). The effect of salmeterol on overall neuron number was determined at 7 days post-treatment with immunocytochemical staining using Neu-N antibody (C). Representative light micrographs of the immunostained TH neurons or total neurons were shown in (D); Results in (A–C) were expressed as a percentage of the vehicle-treated control cultures and were the mean ± SE. from three independent experiments in triplicate. **P<0.01 when compared with the vehicle-treated cultures. Bar = 50 μm.
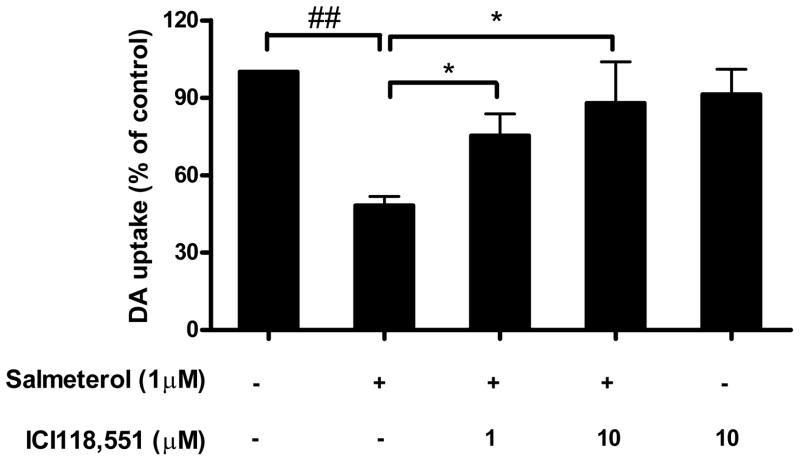
In order to determine if salmeterol-induced neurotoxicity was mediated through the β2AR. Neuron-glia cultures were pretreated with ICI118,551, a specific β2AR antagonist, for 30 min prior to adding salmeterol. Neurotoxicity was determined by the DA uptake assay. Our data show that ICI118,551 significantly inhibited the neurotoxicity of salmeterol at both 1 and 10 μM, while had no effect when added alone (Fig. 2). These results indicate that salmeterol-mediated DA neurotoxicity is β2AR dependent.
FIGURE 2.
Salmeterol-induced neurotoxicity is through β2AR. ICI118,551was added to rat mesencephalic midbrain neuron-glia cultures for 30 min prior to the addition of salmeterol. DA neurotoxicity was quantified on day 7 using the [3H]-DA uptake assay. Data are percentage of control cultures, and are presented as the mean ± SE of 3 separate experiments. *P<0.05 compared with the salmeterol-alone treated cultures without ICI118,551, ##P<0.01 when compared with the vechicle-treated cultures.
Microglia mediate salmeterol-induced neurotoxicity
To further investigate the cell type that mediates the neurotoxic effect of salmeterol, we treated cultures of rat midbrain neuron-glia cell (NG), cultures lacking all glial cells (N, neuron enriched cultures) or cultures containing neurons and astrocytes but not microglia (NA, neuron-astrocyte enriched cultures) and measured DA uptake 7 days after treatment. Fig. 3 shows that while salmeterol has no significant DA neurotoxic effect on NA and N cultures, significant toxicity is seen in NG cultures which contain normal numbers of microglia cells. These data indicate that microglia are indispensable in β2AR agonist-induced DA neurotoxicity.
FIGURE 3.
Microglia mediate salmeterol-induced DA neurotoxicity. Mesencephalic midbrain neuron-glia cultures (open bars, NG), neuron-astrocyte (solid bars, NA) and neuron-enriched cultures (cross-hatched bars, N) were treated with either vehicle or 1 μM salmeterol. DA neurotoxicity was measured at 7 days post-treatment using the [3H]-DA uptake assay. Data are percentage of control cultures, presented as the mean ± SE of 3 separate experiments. *P<0.05 when compared with the salmeterol-treated NG cultures, ##P<0.01 when compared with the vechicle-treated cultures.
β2AR activation by salmeterol increased the production of ROS from microglia
In order to determine whether ROS from microglia participate in salmeterol-induced neurotoxicity, extracellular superoxide and intracellular ROS were measured in primary microglia-enriched cultures. As shown in Fig. 4A and 4B, salmeterol treatment increased both extracellular superoxide and intracellular ROS production from microglia in a dose-dependent fashion. Conversely, no measureable production of IL-1β, TNFα, and nitric oxide (NO) was seen from these cultures after salmeterol alone treatment (data not shown). These results demonstrate that the β2AR agonist salmeterol induces microglia to generate ROS, which are among the major factors leading to DA neurotoxicity.
FIGURE 4.
Salmeterol increases production of ROS from purified microglia. Microglia-enriched cultures were treated with either vehicle or indicated concentrations of salmeterol for 30 min. Production of superoxide was measured by the superoxide dismutase (SOD) inhibitable reduction of WST-1 (A). Production of intracellular ROS was measured 1 h post-stimulation using DCFDA (B). The results are the mean ± SE and the average of 3 separate experiments. *P<0.05 compared with the vehicle alone-treated cultures.
PHOX plays an important role in β2AR agonist-mediated DA neurotoxicity
To determine whether PHOX plays an important role in β2AR activation-induced neurotoxicity, DPI was used to pharmacologically inhibit this enzyme in primary rat mesencephalic neuron-glia cultures. Cultures were pretreated with or without DPI (0.01 μM) for 30 min prior to the addition of salmeterol (1 μM), our data show that DPI pretreatment significantly attenuated salmeterol-induced neurotoxicity (Fig. 5).
FIGURE 5.
NADPH oxidase plays an important role in salmeterol-induced DA neurotoxicity. DA neurotoxicity was determined by the [3H]-DA uptake assay in neuron-glia cultures 7 days after pretreatment with DPI (0.01 μM) for 30 min and treatment with salmeterol (1 μM). The results are the mean ± SE, and values are the average of 3 separate experiments. *P<0.05 salmeterol + DPI group compared with salmeterol alone-treated cultures, ##P<0.01 salmeterol only group compared with the vechicle alone-treated cultures.
β2AR-mediated DA neurotoxicity is PKA-independent but ERK-dependent
β2AR activation normally leads to activation of the catalytic subunit of PKA, which in turn phosphorylates cAMP-responsive element binding protein (CREB), allowing it to bind to the CRE sites (Rockman et al. 2002). Recent evidence from our laboratory has shown that a second, PKA-independent MAPK-dependent pathway of β2AR activation can lead to a pro-inflammatory response in macrophages (Tan et al. 2007). Therefore, we sought to determine whether β2AR activation induced by salmeterol operates through a PKA-dependent or independent pathway. Surprisingly, neuron-glia cells pretreated with the PKA inhibitor Rp-cAMPS, showed no protective effects against salmeterol-induced DA neurotoxicity (Fig. 6A). We further detect whether this inhibitor can affect salmeterol-induced superoxide production. Consistently, pretreatment with Rp-cAMPS did not show any effects on salmeterol-induced superoxide either (Fig. 6B). These findings indicate that the activation of PHOX and the subsequent neurotoxicity induced by salmeterol is not through the conventional PKA pathway.
FIGURE 6.
Salmeterol-induced neurotoxicity is PKA independent. Mesencephalic midbrain neuron-glia cultures were pretreated with Rp-cAMPS (25 and 50 μM) for 30 min prior to the addition of salmeterol (1 μM). Neurotoxicity was assessed by DA uptake 7 days after salmeterol addition (A). Superoxide production was measured 30 min after salmeterol addition in purified microglia cultures (B). The results are the mean ± SE, and values are the average of 3 separate experiments. #P<0.05, ##P<0.01 compared with the vehicle alone-treated cultures.
Next, we determined whether microglia activation by salmeterol can lead to the activation of the MAPK signaling pathways. Our results show that salmeterol significantly enhanced the phosphorylation of ERK, but not p38 and JNK in enriched microglia (Fig. 7A, quantified in Fig. 7B). Meanwhile, we tested how several other β2AR agonists, including bambuterol, formoterol, and clenbuterol, affect the phosphorylation levels of ERK, it is clear that all these three agonists significantly induced the phosphorylation of ERK in primary microglia (Fig. 7C, quantified in Fig. 7D). To further verify the importance of ERK in the β2AR activation and subsequent neurotoxicity, we used the specific ERK inhibitor U0126. We found that U0126 significantly reduced salmeterol-elicited DA neurotoxicity and superoxide production in a dose-dependent fashion (Fig. 7C and 7D, respectively). These results suggest that salmeterol-induced increases in superoxide production and subsequent DA neurotoxicity are ERK dependent.
FIGURE 7.
ERK activation is required for salmeterol-mediated neurotoxicity and superoxide production. Purified microglia were incubated with 1 μM salmeterol for 15 min. The cells lysates were subjected to western blot. The levels of phosphorylated MAPK relative to total MAPK were determined by western blot using specific antibodies against phosphorylated or total ERK, p38 or JNK respectively. Representative western blots are shown from 3 independent experiments (A). ImageJ software was used to quantitate the intensity of the phosphorylated MAPK and total MAPK bands in western blot, respectively, normalized to the vehicle-treated control (B). Effects of additional three long-acting β2AR agonists, including bambuterol, formoterol, and clenbuterol, on the phosphorylation levels of ERK were studied. The representative pictures and quantitative data are shown (C and D). Neuron-glia cultures were pretreated with U0126 for 30 min prior to the addition of salmeterol. Neurotoxicity was assessed by DA uptake 7 days after salmeterol addition (E). Superoxide production was measured 30 min after salmeterol stimulation in purified microglia cultures (F). Results in B, C and D are the means ± SE, and are the average of 3 separate experiments. ##P<0.01, #P<0.05 compared with the vehicle alone-treated cultures, *P<0.05, **P<0.01, compared with the salmeterol alone-treated cultures.
Activation of ERK but not PKA is required for β2AR activation-induced p47phox translocation
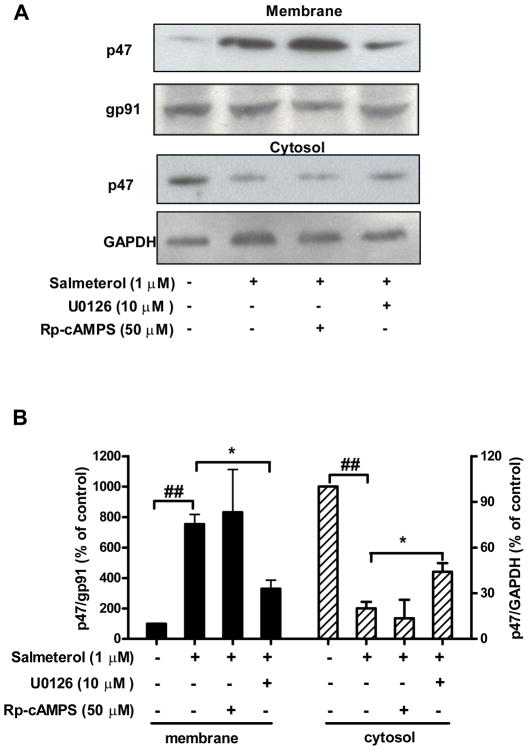
Phosphorylation and translocation of the cytosolic component p47phox to the plasma membrane is required for the activation of PHOX (Groemping and Rittinger 2005). Above studies (Fig. 6B and 7D) demonstrate that ERK, but not PKA play a central role in salmeterol-mediated superoxide production. We further tested the role of ERK and PKA on salmeterol-induced p47phox translocation by using ERK inhibitor U0126 and PKA inhibitor Rp-cAMPS. HAPI microglia cells were stimulated with salmeterol, then isolated their membrane and cytosolic fractions for western blot analysis. While the majority of p47phox protein is found in the cytosol of inactivated HAPI cells, most of the p47phox protein was located in the cellular membrane fraction following salmeterol stimulation. Pretreatment with U0126, but not Rp-cAMPS prevented salmeterol-induced p47phox translocation to the cellular membrane (Fig. 8). In contrast, the level of gp91 found in cellular membrane fraction was unaltered by U0126 treatment, as were the cytosolic levels of GAPDH. These results suggest that ERK phosphorylation is required for β2AR activation-induced p47phox translocation to the cellular membrane, and ultimately for cellular ROS production, resulting in significant toxicity on DA-producing neurons.
FIGURE 8.
Salmeterol-induced cytosolic p47phox protein translocation is ERK dependent, but PKA independent. HAPI cells were pretreated with vehicle, U0126 (10 μM) or Rp-cAMPS (50 μM) for 30 min prior to the addition of salmeterol (1 μM). Thirty minutes after salmeterol addition, subcellular fractions were isolated for western blot analysis. Representative western blots are shown from 3 independent experiments (A). ImageJ software was used to quantitate the intensity of the bands in western blots, results given in B represents the percentage difference of the ratio of membrane p47phox protein compared with membrane gp91phox protein (left panel, solid bars), cytosolic p47phox protein compared with cytosolic GAPDH (right panel, cross-hatched bars), respectively, normalized to the vehicle-treated control (B).
Discussion
The main purpose of this study was to elucidate the cellular and molecular mechanisms underlying β2AR-mediated pro-inflammatory and neurotoxic effects in a well-established model of PD. Our results show that salmeterol, a long-actingβ2AR agonist, selectively induces microglia-mediated DA neurotoxicity through a β2AR-dependent, but cAMP/PKA independent pathway. Mechanistic studies demonstrate that enhanced oxidative neuronal damage is due to the increase in the production of superoxide through the activation of PHOX. Further studies reveal that salmeterol activates PHOX by inducing the translocation of its cytosolic subunit p47phox to the cellular membrane through an ERK-dependent pathway.
Salmeterol is a long-acting β2AR agonist used primarily to treat asthma and pulmonary disorders (Oppenheimer and Nelson 2008). Numerous studies with salmeterol and other β2AR agonists have shown potent anti-inflammatory effects at doses between 1–100 nM (Farmer and Pugin 2000; Hung et al. 2008; Severn et al. 1992; van der Poll et al. 1994). Conversely, data from our laboratory and others have demonstrated that β2AR agonists such as salmeterol can cause pro-inflammatory reactions at doses of 1–10 μM in several peripheral cells (Christensen et al. 1999; Frost et al. 2004; Mohamed-Ali et al. 2001; Rohrbach et al. 2007b; Tan et al. 2007; Yin et al. 2006). Given the standard clinical dose of the drug commonly used (50–100 μg), initial concentrations of salmeterol in the fluid lining the airways after inhalation of a 50 μg puff would range from 1.2 to 12 μM (Ottonello et al. 1996). The pro-inflammatory effects of salmeterol can not only affect airway inflammation, but may also enter the bloodstream and contribute to other inflammation-related disorders. We showed salmeterol alone produced ROS by activating microglia and produced subsequent inflammation and caused neurotoxicity. Our findings further support the important role of ROS and other pro-inflammatory factors in the airway inflammation after long-term use of salmeterol, and suggest that chronic use of long-acting β2-adrenergic therapy to inhibit inflammation may actually lead to increased inflammatory responses from microglia and ultimately to the progression of inflammatory CNS conditions such as PD.
Extensive studies from our laboratory and others convincingly point out a critical role of microglia-based, inflammation-related neurodegeneration. The release of a series of pro-inflammatory factors such as pro-inflammatory cytokines, free radicals and prostaglandins are among the major players in mediating inflammation-elicited neuronal damage. It is interesting to note that one of the major inflammatory factors released from salmeterol-activated microglia is superoxide and its related ROS (Fig. 4). In contrast, levels of TNFα, IL-1β and nitric oxide are too low to be measured. Previous reports from our laboratory and others indicate that the production of superoxide and related ROS by cells of the substantia nigra is mainly accomplished by microglia (Qin et al. 2004). The higher sensitivity of DA neurons than other type of neurons in the substantia nigra to oxidative damage has been mainly attributed to their reduced antioxidant capacity, increased accumulation of iron, and the high concentration of dopamine neurotransmitter that is prone to oxidative modification (Greenamyre et al. 1999; Jenner 1998). To determine type of neuronal death, neuron-glia cultures were treated with salmeterol (1 μM) for 7 days. Immunohistochemical analysis showed very few annexin V-positive staining cells were detected during the entire treatment periods. In addition, we pretreated neuron-glia culture with a pan caspase inhibitor, Z-VAD-FMK, prior to the addition of salmeterol, and the degeneration of DA neurons was then determined by the numeration of the TH-IR neurons. Our data showed that Z-VAD-FMK failed to protect DA neurotoxicity induced by salmeterol (data not shown). This observation is consistent with our previous reports indicating that inflammation-related neuronal death, which is mediated through microglia activation, is not through the apoptotic pathway. It is likely that DA neuronal death induced by salmeterol mainly from necrosis, which also provides the source of toxic factors, and causes the continued activation of reactive microgliosis we see in the culture system.
While the activation of the β2AR normally results in the production of cAMP and the activation of the PKA signaling pathway, it is clear that the salmeterol-induced DA neurotoxicity we observed is PKA independent (Fig. 6). For this reason, we looked for an alternative pathway mediating salmeterol-related toxic effects.β2AR activity is known to activate MAPKs via both Gs-dependent and Gs-independent mechanisms, and we have previously found that a Gs-independent increase in phosphorylation of ERK occurred following salmeterol treatment in RAW264 macrophage cells (Tan et al. 2007). Consistent with this finding, data from this study showed salmeterol specifically enhanced the phophoryaltion of ERK, but not p38 and JNK (Fig. 7A). In addition, salmeterol had no effect on the activation of NF-κB or the production of inflammatory mediators normally under NF-κB regulation, such as TNFα and NO. Since our previous results have shown that the activation of PHOX in microglia requires the phosphorylation of p47phox by ERK (Qian et al. 2008), we proposed that β2AR activation by salmeterol enhances the activity of ERK, which then increases the phosphorylation of p47phox to initiate the translocation of the cytosolic subunits to the cell membrane and trigger the activation of PHOX, followed by the production of superoxide. Studies using a specific ERK inhibitor, U0126, show that inhibition of ERK activity strongly reduced salmeterol-induced neurodegeneration, superoxide production and p47phox translocation. These findings suggest that this neurotoxicity may be mediated by ERK-dependent, PKA and NF-κB-independent pathway in microglia.
Activation of ERK by β2AR has been shown to occur through both a PKA-dependent and PKA-independent pathway (Daaka et al. 1997; Friedman et al. 2002; Shenoy et al. 2006). PKA-dependent ERK phorphorylation has been shown to occur rapidly after activation, peaking within 2–5 min. A second PKA-independent signal leading to ERK phosphorylation is slower in onset and lasts more than 30 min (Shenoy et al. 2006). Previous studies with salmeterol have shown that this drug leads to a PKA-dependent anti-inflammatory response in macrophages involving inhibition of NF-κB activity, likely through the rapidly activated ERK-dependent pathway. In the present study, ERK activation is independent of PKA, suggesting the slower, more sustained signal induced by salmeterol results in superoxide production. We have previously found this pathway to be active in β2AR-mediated increase in cytokine production by salmeterol in macrophages (Tan et al. 2007). The scaffolding protein B-raf is believed to be the main regulator of ERK activity in PKA-independent β2AR-mediated signaling (Houslay and Kolch 2000). It is possible that the long-acting nature of salmeterol, coupled with the culture conditions we used to activate microglia, leads to a switch from the rapid to the slower activation pathway, one involving the PKA-independent EPAC-Rap1-mediated activation of B-raf and the downstream activation of ERK (Tan et al. 2007). However, when we used the EPAC agonist, 8CPT-2′-O-Me-cAMP (CPTOMe; 1~10μM) to stimulate primary microglia, we failed to observe the activation of ERK, which suggests that this β2AR activation-induced ERK phosphorylation is not through PKA or EPAC pathway (data not shown). Therefore, it appears as though the activation ERK, leading to a neurotoxic inflammatory response, in primary microglia occurs via a PKA and EPAC-independent signaling pathway. Experiments to test the role of the EPAC-independent Rap1/B-raf signaling pathway in the activation of pro-inflammatory responses in microglia are currently underway.
Our studies provide a novel pathway which is different from the conventional PKA/NF-κB-dependent pathway mediated by β2AR activation. These findings also introduce potential opportunities for novel therapeutic approaches in inflammation-mediated CNS disorders such as PD. It is important to recognize that, long-term stimulation of β2AR may actually exacerbate chronic inflammatory conditions (Abramson et al. 2003; McGraw et al. 2003). In PD, for example, long-term stimulation of β2AR may actually exacerbate the inflammatory response of microglial cells leading to increased DA neurotoxicity. Our studies also propose that different intracellular signaling pathways are responsible for the β2AR-mediated bronchodilatory and pro-inflammatory effects. While bronchodilation occurs via the classical β2AR-mediated PKA/CREB pathway (Hussein et al. 2005), the pro-inflammatory effects of β2AR agonists are likely mediated by a novel PKA/CREB and EPAC-independent/ERK-dependent signaling cascade. These results suggest that β2AR target therapy can be improved by selectively blocking the pro-inflammatory side effects of β2AR activation. Consequently, further definition of the dual effects of β2AR activation on the inflammatory response of macrophages and microglia cells will likely lead to more effective therapies in the treatment of chronic inflammatory conditions.
Acknowledgments
We thank Drs. Thaddeus Schug at NIEHS, Lynda Peterson and Tyra Thomas at UNC for their helpful suggestions in editing this paper.
This work was supported by NIH grant DE-13079 from the National Institute for Dental and Craniofacial Research, and was also supported in part by the Intramural Research Program of the NIH/NIEHS.
Abbreviations used in this paper
- DA
dopaminergic
- PD
Parkinson’s disease
- ROS
reactive oxygen species
- iNOS
inducible nitric oxide synthase
- TH-IR
tyrosine hydroxylase-immunoreactive
- NO
nitritc oxide
- PHOX
NADPH oxidase
- DCFH-DA
dichlorodihydrofluorescein Diacetate
- SOD
superoxide dismutase
- TNF-α
tumornecrosis factor-alpha
- MAPK
mitogen activated protein kinase
- iROS
intracellular ROS
- CREB
cAMP-responsive element binding protein
- GPCR
G-protein-coupled receptors
- DPI
diphenylene-iodonium
- β2AR
β2 adrenergic receptor
- CNS
central nervous system
- PKA
Protein Kinase A
References
- Abramson MJ, Walters J, Walters EH. Adverse effects of beta-agonists: are they clinically relevant? Am J Respir Med. 2003;2(4):287–97. doi: 10.1007/BF03256657. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog Neurobiol. 2005;76(2):77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Cheepsunthorn P, Radov L, Menzies S, Reid J, Connor JR. Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia. 2001;35(1):53–62. doi: 10.1002/glia.1070. [DOI] [PubMed] [Google Scholar]
- Christensen JD, Hansen EW, Frederiksen C, Molris M, Moesby L. Adrenaline influences the release of interleukin-6 from murine pituicytes: role of beta2-adrenoceptors. Eur J Pharmacol. 1999;378(1):143–8. doi: 10.1016/s0014-2999(99)00448-3. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390(6655):88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- Farmer P, Pugin J. beta-adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IkappaB/NF-kappaB pathway. Am J Physiol Lung Cell Mol Physiol. 2000;279(4):L675–82. doi: 10.1152/ajplung.2000.279.4.L675. [DOI] [PubMed] [Google Scholar]
- Felten DL, Felten SY, Bellinger DL, Carlson SL, Ackerman KD, Madden KS, Olschowki JA, Livnat S. Noradrenergic sympathetic neural interactions with the immune system: structure and function. Immunol Rev. 1987;100:225–60. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- Friedman J, Babu B, Clark RB. Beta(2)-adrenergic receptor lacking the cyclic AMP-dependent protein kinase consensus sites fully activates extracellular signal-regulated kinase 1/2 in human embryonic kidney 293 cells: lack of evidence for G(s)/G(i) switching. Mol Pharmacol. 2002;62(5):1094–102. doi: 10.1124/mol.62.5.1094. [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Lang CH. Epinephrine stimulates IL-6 expression in skeletal muscle and C2C12 myoblasts: role of c-Jun NH2-terminal kinase and histone deacetylase activity. Am J Physiol Endocrinol Metab. 2004;286(5):E809–17. doi: 10.1152/ajpendo.00560.2003. [DOI] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem. 2002;81(6):1285–97. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- Greenamyre JT, MacKenzie G, Peng TI, Stephans SE. Mitochondrial dysfunction in Parkinson’s disease. Biochem Soc Symp. 1999;66:85–97. doi: 10.1042/bss0660085. [DOI] [PubMed] [Google Scholar]
- Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386(Pt 3):401–16. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD, Kolch W. Cell-type specific integration of cross-talk between extracellular signal-regulated kinase and cAMP signaling. Mol Pharmacol. 2000;58(4):659–68. [PubMed] [Google Scholar]
- Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10(22):1503–19. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- Hung CH, Chu YT, Hua YM, Hsu SH, Lin CS, Chang HC, Lee MS, Jong YJ. Effects of formoterol and salmeterol on the production of Th1- and Th2-related chemokines by monocytes and bronchial epithelial cells. Eur Respir J. 2008;31(6):1313–21. doi: 10.1183/09031936.00121406. [DOI] [PubMed] [Google Scholar]
- Hussein A, Al-Wadei N, Takahashi T, Schuller HM. Theophylline stimulates cAMP-mediated signaling associated with growth regulation in human cells from pulmonary adenocarcinoma and small airway epithelia. Int J Oncol. 2005;27(1):155–60. [PubMed] [Google Scholar]
- Jenner P. Oxidative mechanisms in nigral cell death in Parkinson’s disease. Mov Disord. 1998;13(Suppl 1):24–34. [PubMed] [Google Scholar]
- Johnson M. Molecular mechanisms of beta(2)-adrenergic receptor function, response, and regulation. J Allergy Clin Immunol. 2006;117(1):18–24. doi: 10.1016/j.jaci.2005.11.012. quiz 25. [DOI] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci. 2000;20(16):6309–16. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. J Leukoc Biol. 2006;79(6):1093–104. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- Liu B, Du L, Hong JS. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther. 2000a;293(2):607–17. [PubMed] [Google Scholar]
- Liu B, Du L, Kong LY, Hudson PM, Wilson BC, Chang RC, Abel HH, Hong JS. Reduction by naloxone of lipopolysaccharide-induced neurotoxicity in mouse cortical neuron-glia co-cultures. Neuroscience. 2000b;97(4):749–56. doi: 10.1016/s0306-4522(00)00057-9. [DOI] [PubMed] [Google Scholar]
- Liu J, Shen HM, Ong CN. Role of intracellular thiol depletion, mitochondrial dysfunction and reactive oxygen species in Salvia miltiorrhiza-induced apoptosis in human hepatoma HepG2 cells. Life Sci. 2001;69(16):1833–50. doi: 10.1016/s0024-3205(01)01267-x. [DOI] [PubMed] [Google Scholar]
- McGraw DW, Almoosa KF, Paul RJ, Kobilka BK, Liggett SB. Antithetic regulation by beta-adrenergic receptors of Gq receptor signaling via phospholipase C underlies the airway beta-agonist paradox. J Clin Invest. 2003;112(4):619–26. doi: 10.1172/JCI18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed-Ali V, Flower L, Sethi J, Hotamisligil G, Gray R, Humphries SE, York DA, Pinkney J. beta-Adrenergic regulation of IL-6 release from adipose tissue: in vivo and in vitro studies. J Clin Endocrinol Metab. 2001;86(12):5864–9. doi: 10.1210/jcem.86.12.8104. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J, Nelson HS. Safety of long-acting beta-agonists in asthma: a review. Curr Opin Pulm Med. 2008;14(1):64–9. doi: 10.1097/MCP.0b013e3282f1980b. [DOI] [PubMed] [Google Scholar]
- Ottonello L, Morone P, Dapino P, Dallegri F. Inhibitory effect of salmeterol on the respiratory burst of adherent human neutrophils. Clin Exp Immunol. 1996;106(1):97–102. doi: 10.1046/j.1365-2249.1996.d01-804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskin AV, Winterbourn CC. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1) Clin Chim Acta. 2000;293(1–2):157–66. doi: 10.1016/s0009-8981(99)00246-6. [DOI] [PubMed] [Google Scholar]
- Qian L, Block ML, Wei SJ, Lin CF, Reece J, Pang H, Wilson B, Hong JS, Flood PM. Interleukin-10 protects lipopolysaccharide-induced neurotoxicity in primary midbrain cultures by inhibiting the function of NADPH oxidase. J Pharmacol Exp Ther. 2006;319(1):44–52. doi: 10.1124/jpet.106.106351. [DOI] [PubMed] [Google Scholar]
- Qian L, Flood PM. Microglial cells and Parkinson’s disease. Immunol Res. 2008 doi: 10.1007/s12026-008-8018-0. [DOI] [PubMed] [Google Scholar]
- Qian L, Tan KS, Wei SJ, Wu HM, Xu Z, Wilson B, Lu RB, Hong JS, Flood PM. Microglia-mediated neurotoxicity is inhibited by morphine through an opioid receptor-independent reduction of NADPH oxidase activity. J Immunol. 2007a;179(2):1198–209. doi: 10.4049/jimmunol.179.2.1198. [DOI] [PubMed] [Google Scholar]
- Qian L, Wei SJ, Zhang D, Hu X, Xu Z, Wilson B, El-Benna J, Hong JS, Flood PM. Potent Anti-Inflammatory and Neuroprotective Effects of TGF-{beta}1 Are Mediated through the Inhibition of ERK and p47phox-Ser345 Phosphorylation and Translocation in Microglia. J Immunol. 2008;181(1):660–8. doi: 10.4049/jimmunol.181.1.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Xu Z, Zhang W, Wilson B, Hong JS, Flood PM. Sinomenine, a natural dextrorotatory morphinan analog, is anti-inflammatory and neuroprotective through inhibition of microglial NADPH oxidase. J Neuroinflammation. 2007b;4:23. doi: 10.1186/1742-2094-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279(2):1415–21. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- Rohrbach S, Engelhardt S, Lohse MJ, Werdan K, Holtz J, Muller-Werdan U. Activation of AP-1 contributes to the beta-adrenoceptor-mediated myocardial induction of interleukin-6. Mol Med. 2007a doi: 10.2119/2007-00071.Rohrbach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach S, Engelhardt S, Lohse MJ, Werdan K, Holtz J, Muller-Werdan U. Activation of AP-1 contributes to the beta-adrenoceptor-mediated myocardial induction of interleukin-6. Mol Med. 2007b;13(11–12):605–14. doi: 10.2119/2007-00071.Rohrbach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J Neurosci. 2005;25(3):723–31. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severn A, Rapson NT, Hunter CA, Liew FY. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J Immunol. 1992;148(11):3441–5. [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281(2):1261–73. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- Tan AS, Berridge MV. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods. 2000;238(1–2):59–68. doi: 10.1016/s0022-1759(00)00156-3. [DOI] [PubMed] [Google Scholar]
- Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal. 2007;19(2):251–60. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Tanaka KF, Kashima H, Suzuki H, Ono K, Sawada M. Existence of functional beta1- and beta2-adrenergic receptors on microglia. J Neurosci Res. 2002;70(2):232–7. doi: 10.1002/jnr.10399. [DOI] [PubMed] [Google Scholar]
- Tomozawa Y, Yabuuchi K, Inoue T, Satoh M. Participation of cAMP and cAMP-dependent protein kinase in beta-adrenoceptor-mediated interleukin-1 beta mRNA induction in cultured microglia. Neurosci Res. 1995;22(4):399–409. doi: 10.1016/0168-0102(95)00922-g. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Jansen J, Endert E, Sauerwein HP, van Deventer SJ. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect Immun. 1994;62(5):2046–50. doi: 10.1128/iai.62.5.2046-2050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Wang YY, Du JH, Li C, Lu ZZ, Han C, Zhang YY. Noncanonical cAMP pathway and p38 MAPK mediate beta2-adrenergic receptor-induced IL-6 production in neonatal mouse cardiac fibroblasts. J Mol Cell Cardiol. 2006;40(3):384–93. doi: 10.1016/j.yjmcc.2005.12.005. [DOI] [PubMed] [Google Scholar]