Abstract
Neuroinflammation is closely associated with the pathogenesis of Parkinson’s disease (PD) and other neurological disorders. The hallmark of neuroinflammation is microglial activation. Increasing evidence suggests that inhibition of microglia-mediated neuroinflammation might represent a promising therapeutic potential for PD and related disorders. Fluoxetine, a selective serotonin reuptake inhibitor, is commonly used for the treatment of major depression due to its tolerability and safety profiles. Recent studies have shown that fluoxetine affords robust neuroprotection in a series of neurological disease models. However, the mechanism underlying fluoxetine-mediated neuroprotection remains unclear. Here, by using rat primary midbrain neuron-glia cultures, we report that both R and S isomers of fluoxetine attenuated chronic neurodegeneration induced by a commonly used inflammogen lipopolysaccharide (LPS). Reconstituted cell culture studies further revealed that microglia were required for fluoxetine-mediated neuroprotection. Fluoxetine significantly inhibited LPS-induced activation of microglia and subsequent release of multiple pro-inflammatory and cytotoxic factors including tumor necrosis factor-α, interleukin-1β, nitric oxide, and reactive oxygen species. Furthermore, inhibition of microglial NF-κB signaling pathway participated in fluoxetine-mediated neuroprotection. Collectively, fluoxetine exerted neuroprotection against microglia-mediated neurotoxicity. Thus, fluoxetine not only can relieve depression, a common nonmotor symptom of PD, but might also hold a potential to retard inflammation-mediated chronic neurodegenerative process of this disease.
Keywords: fluoxetine, neuroinflammation, microglia, neuroprotection, anti-inflammation
1. Introduction
Extensive studies have indicated neuroinflammation participates in the pathogenesis of neurological disorders, such as stroke, depression and neurodegenerative diseases, including Parkinson’s disease (PD) [1]. The hallmark of neuroinflammation is the microglial activation [2]. Once activated, microglia secret an array of pro-inflammatory factors including chemokines, cytokines, and reactive oxygen species (ROS). The accumulation of these factors contributes to neuronal damages. Toxic soluble factors (e.g. α-synuclein) produced by the damaged neurons in turn amplify microglial activation. Accordingly, a vicious “self-propelling cycle” is created leading to prolonged neuroinflammation [2]. Therefore, inhibition of microglial activation may represent a therapeutic potential for the treatment of inflammation-related neurological disorders.
Fluoxetine [3-(p-trifluoromethylphenoxy)-N-methyl-3- phenylpropylamine], a selective serotonin reuptake inhibitor, is commonly used for the treatment of major depression [3]. Depression is the most frequent neuropsychiatric symptom in neurodegenerative diseases including PD. Additionally, several lines of evidence have shown that fluoxetine possesses potent neuroprotection against hypoxic-ischemia brain injury in rat pups [4], 3, 4-methylenedioxymethamphetamine (MDMA)-induced neurotoxicity on the serotonin transporter in rat brains [5] and kainic acid-induced neuronal death in the mouse hippocampus [6]. Moreover, fluoxetine has been found to modulate neural stem cell survival and serotoninergic differentiation through the modulation of Bcl-2 expression [7]. Recent studies indicate fluoxetine affords robust neuroprotection in the postischemic brain through its anti-inflammatory effect [8]. Further, the long-term effect of antidepressants in the adult brain has been reported to associate with increased neurogenesis, dendritic arborization and synaptogenesis [9]. However, the mechanism underlying fluoxetine-mediated neuroprotection remains unclear and warrants further study.
In the present study, primary midbrain neuron-glia cultures were used to explore neuroprotective effects of R-fluoxetine and S-fluoxetine on lipopolysaccharide (LPS)-induced neurotoxicity and to further investigate the mechanisms of fluoxetine-mediated neuroprotection.
2. Material and Methods
2.1. Animals
Timed-pregnant Fisher F344 rats were obtained from Charles River Laboratories (Raleigh, NC, USA). All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2. Reagents
LPS (Escherichia coli strain O111:B4) was purchased from Calbiochem (San Diego, CA). All other reagents were obtained from Invitrogen (Carlsbad, CA) and Sigma Chemical Co. (St. Louis, MO).
2.3. Cell cultures
Rat primary neuron-glia, neuron-astrocyte, neuron-microglia and neuron-enriched cultures were prepared from the ventral mesencephalic tissues of embryonic day 14–15 rats as described previously [10, 11].
2.4. [3H] dopamine (DA) uptake assays
[3H] DA uptake assay was performed as described previously [10]. The specific [3H]DA uptake was calculated by subtracting the amount of radioactivity obtained in the presence of mazindol from that obtained in the absence of mazindol.
2.5. Immunocytochemical staining
Immunostaining was performed following our published protocols [10]. DA neurons were recognized with an anti-tyrosine hydroxylase (TH) antibody. Microglia were identified with an antibody specific for ionized calcium-binding adapter molecule-1 (Iba-1).
2.6. Tumour necrosis factor (TNF)-α, interleukin (IL)-1β, and nitric oxide (NO) assays
The production of NO was determined with the Griess reagent. The release of TNFα and IL-1β was measured with the immunosorbent assay kits from R&D Systems
2.7. Real-time RT-PCR and Western blot analysis
Total RNA was isolated using Trizol reagent and purified with RNeasy Kit. The primers were designed with ABI Primer Express software (Applied Biosystems, Foster City, CA). The sequences of the primers were the following: β-actin, GTATGACTCCACTCACGGCAAA (forward), GGTCTCGCTCCTGGAAGATG (reverse); iNOS, ACATCAGGTCGGCCATCACT (forward), CGTACCGGATGAGCTGTGAATT (reverse); TNFα, GACCCTCACACTCAGATCATC-TTCT (forward), CCTCCACTTGGTGGTTTGCT (reverse); IL-1 β, CTGGTGTGTGACGTTCCC-ATTA (forward), CCGACAGCACGAGGCTTT (reverse). Protein levels were quantified using BCA assay. Membranes were blocked with nonfat milk and then incubated with the primary antibodies.
2.8. Superoxide and intracellular ROS measurement
The production of extracellular superoxide was evaluated by measuring the superoxide dismutase (SOD)-inhibitable reduction of the tetrazolium salt WST-1 [12]. Intracellular ROS were determined by using the DCFH-DA assay.
2.9. Statistical analysis
Data are expressed as the mean ± S.E.M. Statistical significance was assessed by one- or two-way ANOVA with treatment or time/dose/culture type as the independent factors using GraphPad Prism software. When ANOVA showed significant differences, pairwise comparisons between means were tested by Bonferroni’s post test with correction. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Fluoxetine protected DA neurons against LPS-induced neurotoxicity
Fluoxetine is a racemic mixture (50/50) of R(−)-fluoxetine and S (+)-fluoxetine enantiomers. R-fluoxetine and S-fluoxetine are similarly effective at blocking serotonin reuptake. We found that the pretreatment of midbrain neuron-glia cultures for 30 min with R-fluoxetine and S-fluoxetine offered similar protection against LPS-induced DA neuronal damage, as shown by attenuated decreases in [3H]DA uptake and in the number of TH-positive neurons (Figure 1A, B). Morphological inspection indicated neurites of the remaining TH-positive neurons in LPS-treated cultures became fewer and shorter compared with the vehicle-treated control cultures. After R-fluoxetine and S-fluoxetine treatment, not only more DA neurons survived, but their neurites were also less damaged compared with the LPS-treated cultures (Figure 1C). Similarly, pretreatment of primary cortical neuron-glia cultures with both isomers (3 μM) significantly restored LPS-induced reduction in [3H] γ-amino butyric acid (GABA) uptake and decreases in the number of neuron-specific nuclear protein (Neu-N)-positive neurons (data not shown), which indicates the neuroprotective effect of fluoxetine is not limited to DA neurons.
Figure 1. Fluoxetine produced neuroprotection against LPS-induced neurotoxicity.
Rat primary mesencephalic neuron-glia cultures were pretreated with fluoxetine for 30 min before the addition of LPS. Seven days later, the LPS-induced dopaminergic neurotoxicity was determined by [3H]DA uptake assay (A) and the quantification of cell number (B) and morphological assessment of TH-immunoreactive DA neurons (C). Scale bar, 200 μm. Results were the mean ± S.E.M. from three independent experiments performed in triplicate. #p < 0.05 compared with control cultures; *p < 0.05 compared with LPS-treated cultures.
3.2. Fluoxetine attenuated LPS-induced activation of microglia and production of pro- inflammatory factors
We next elucidated the cellular and molecular mechanisms underlying the neuroprotection produced by fluoxetine. In four types of reconstituted culture systems, MPP+ (the active metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine [MPTP] that causes Parkinsonism in humans) reduced DA uptake capacity by approximately 40% compared with the corresponding control cultures. However, the neurotoxicity was differentially attenuated by fluoxetine. In neuron-glia cultures that contain neurons, astrocytes and microglia and neuron-microglia cultures, but not in either neuron-enriched or neuron-astrocyte cultures, fluoxetine-mediated neuroprotection was detected (Figure 2A). These results demonstrated microglia, but not astroglia, were required for fluoxetine-mediated neuroprotection.
Figure 2. Fluoxetine attenuated LPS-induced microglial activation.
Reconstituted cell cultures were pretreated with fluoxetine followed by MPP+ treatment. The [3H]DA uptake analysis was performed 7 days later (A). Mesencephalic neuron-glia cultures were pretreated with fluoxetine for 30 min before stimulated with LPS (10 ng/ml). At 1, 3, 5 and 7 days after LPS treatment, the protein level of Iba-1 was determined by Western blotting (B). Results were the mean ± S.E.M. from three independent experiments performed in triplicate. #p < 0.05 compared with control cultures; *p < 0.05 compared with LPS-treated or MPP+-treated cultures. Seven days after LPS treatment, the cultures were immunostained with anti-Iba-1 antibody (C). Scale bar, 200 μm.
The elevated expression of Iba-1, a microglial marker, started to manifest from day 1 after LPS stimulation. At 5-day after LPS treatment, up to 3-fold increase of Iba-1 protein expression was observed. The inhibitory effects of fluoxetine on LPS-induced upregulation of Iba-1 were discerned from day 5 after LPS application (Figure 2B). As seen in Figure 2C, activated microglia revealed larger cell body, thicker processes and irregular shapes; these morphological changes were significantly attenuated by the pretreatment with fluoxetine.
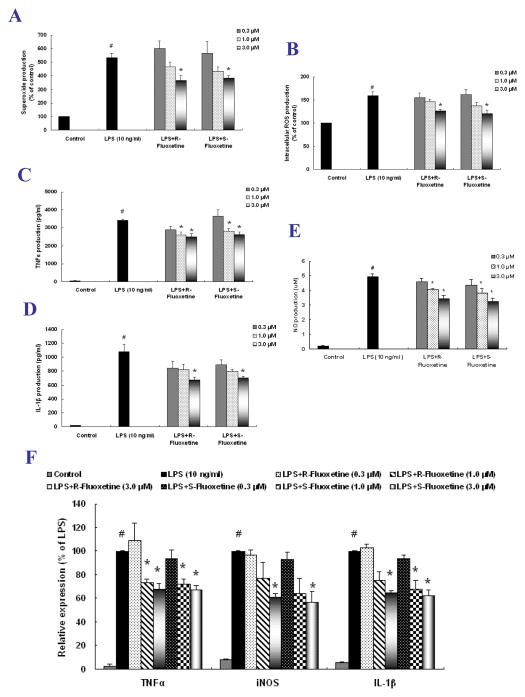
We then found that fluoxetine suppressed the production of multiple pro-inflammatory and cytotoxic factors in microglia-enriched and neuron-glia cultures. Both R-fluoxetine and S-fluoxetine attenuated LPS-induced extracellular superoxide release and intracellular ROS production (Figure 3A and B). Treatment with both isomers resulted in a significant reduction in LPS-induced mRNA expression and extracellular release of TNFα, NO and IL-1β (Figure 3C–F).
Figure 3. Fluoxetine reduced pro-inflammatory factor production.
Primary microglia-enriched cultures were pretreated with fluoxetine for 30 min before LPS treatment. Extracellular superoxide release was detected by SOD-inhibitable reduction of WST-1 (A) and levels of intracellular ROS were measured with DCFH-DA (B). Primary neuron-glia cultures were pretreated with fluoxetine for 30 min and then treated with LPS. The extracellular release and the mRNA expression of pro-inflammatory factors were measured by Griess reagent and ELISA (C–E) and real-time RT-PCR (F). Results were the mean ± S.E.M. from three independent experiments performed in triplicate. #p < 0.05 compared with control cultures; *p < 0.05 compared with LPS-treated cultures.
3.3. Fluoxetine suppressed LPS-induced activation of NF-κB signaling pathway in microglia
As shown in Figure 4, R-fluoxetine and S-fluoxetine significantly attenuated LPS-induced phosphorylation of p65 and IKKβ in the primary microglia-enriched cultures. Moreover, LPS induced a rapid phosphorylation of cytosolic IκBα followed by the degradation of IκBα; these changes were ameliorated by the pretreatment with fluoxetine.
Figure 4. Fluoxetine suppressed LPS-induced activation of NF-κB signaling pathway in microglia.
Primary microglia-enriched cultures were pretreated with fluoxetine for 30 min and then incubated with LPS for 15 min. The whole cell lysates were analyzed by western blotting. Results were the mean ± S.E.M. from three independent experiments. #p < 0.05 compared with control cultures; *p < 0.05 compared with LPS-treated cultures.
4. Discussion
This study demonstrated that both R-fluoxetine and S-fluoxetine produced microglia-dependent neuroprotection against LPS- and MPP+-induced neuronal damage. Fluoxetine significantly inhibited LPS-induced activation of microglia and the subsequent release of pro-inflammatory factors such as TNFα, IL-1β, NO and ROS. Furthermore, inhibition of microglial NF-κB signaling pathway was involved in fluoxetine-mediated neuroprotection.
We previously reported that LPS directly induced microglial activation and the removal of microglia prevented LPS-induced neurotoxicity; MPP+, although incapable of directly activating microglia, caused reactive microgliosis (a secondary response to neuronal damages) after targeted DA neurons [13]. The present study indicated that the presence of microglia is essential for fluoxetine to attenuate chronic neurotoxicity induced by both LPS and MPP+ (Figure 1 and 2).
A wealth of evidence supports that excessive amount of pro-inflammatory factors released by activated microglia become deleterious to neurons [2]. Postmorten brain analysis reveals microglial activation and elevated production of pro-inflammatory mediators in patients with neurodegenerative diseases [14]. Reduction in these inflammatory factors by pharmacological inhibition or genetic deletion provides neuroprotective effects in various experimental settings. In this study, we observed that fluoxetine exhibited potent inhibitory effects on LPS-induced production of various pro-inflammatory factors such as TNFα, IL-1β, NO and ROS. These results were consistent with the previous study that fluoxetine suppressed the inflammatory process in cultured microglia [Liu, 2011] and the postischemic brain [8]. Taken together, fluoxetine-mediated neuroprotection was attributed to the inhibition of microglial activation and the reduction of various pro-inflammatory factors.
NF-κB is the most important transcription factor in inflammatory responses and regulates the production of various pro-inflammatory factors [15]. NF-κB activation was observed in MPTP-induced PD animal model and in the PD brains of patients [16]. Of great interest is discerned colocalization of p65 with CD11b-positive activated microglia in the substantial nigra of postmortem PD brains [17]. In addition, selective inhibition of NF-κB activation suppressed microglial activation and prevented DA neuronal loss against MPTP-injured PD mouse model [17]. Here, we found fluoxetine significantly inhibited LPS-induced NF-κB activation, which might be responsible for the decreased production of pro-inflammatory factors and consequent neuroprotection. However, whether fluoxetine at concentrations used in this study (0.3–3.0 μM) also targets other inflammation-associated signaling pathways warrants further investigation.
At present, the main treatment for PD – dopamine replacement – can only temporarily alleviate motor symptoms but fails to retard PD progression or to relieve concomitant nonmotor symptoms (e.g. depression, anxiety and dementia). The anti-inflammation and neuroprotective effects of fluoxetine observed in this study may contribute to its approved anti-depression effect and potential retardation of chronic neurodegeneration in PD. Given its tolerability and safety profiles, fluoxetine may become a promising therapeutics for the treatment of both motor and nonmotor dysfunction in PD.
In conclusion, fluoxetine protected neurons against microglia-mediated neurotoxicity. This neuroprotection was at least partially mediated by the inhibition of microglial NF-κB activation and the consequent decrease in the production of pro-inflammatory factors. Thus, fluoxetine might possess a potential benefit for the treatment of PD and other inflammation-related neurological disorders.
Acknowledgments
This work was supported by the Intramural Research Program of the NIH/NIEHS and the Science and Technology Foundation of Guizhou Province of China (Grant No. 20107030 and 20112316).
References
- 1.Kim YJ, Park HJ, Lee G, Bang OY, Ahn YH, Joe E, et al. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009;57:13–23. doi: 10.1002/glia.20731. [DOI] [PubMed] [Google Scholar]
- 2.Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:57–65. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac) Nat Rev Drug Discov. 2005;4:764–74. doi: 10.1038/nrd1821. [DOI] [PubMed] [Google Scholar]
- 4.Chang YC, Tzeng SF, Yu L, Huang AM, Lee HT, Huang CC, et al. Early-life fluoxetine exposure reduced functional deficits after hypoxic-ischemia brain injury in rat pups. Neurobiol Dis. 2006;24:101–13. doi: 10.1016/j.nbd.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Li IH, Huang WS, Shiue CY, Huang YY, Liu RS, Chyueh SC, et al. Study on the neuroprotective effect of fluoxetine against MDMA-induced neurotoxicity on the serotonin transporter in rat brain using micro-PET. Neuroimage. 2010;49:1259–70. doi: 10.1016/j.neuroimage.2009.07.072. [DOI] [PubMed] [Google Scholar]
- 6.Jin Y, Lim CM, Kim SW, Park JY, Seo JS, Han PL, et al. Fluoxetine attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. Brain Res. 2009;1281:108–16. doi: 10.1016/j.brainres.2009.04.053. [DOI] [PubMed] [Google Scholar]
- 7.Chen SJ, Kao CL, Chang YL, Yen CJ, Shui JW, Chien CS, et al. Antidepressant administration modulates neural stem cell survival and serotoninergic differentiation through bcl-2. Curr Neurovasc Res. 2007;4:19–29. doi: 10.2174/156720207779940707. [DOI] [PubMed] [Google Scholar]
- 8.Lim CM, Kim SW, Park JY, Kim C, Yoon SH, Lee JK. Fluoxetine affords robust neuroprotection in the postischemic brain via its anti-inflammatory effect. J Neurosci Res. 2009;87:1037–45. doi: 10.1002/jnr.21899. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, et al. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002;22:3673–82. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu B, Du L, Hong JS. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther. 2000;293:607–17. [PubMed] [Google Scholar]
- 11.Zhang W, Shin EJ, Wang T, Lee PH, Pang H, Wie MB, et al. 3- Hydroxymorphinan, a metabolite of dextromethorphan, protects nigrostriatal pathway against MPTP-elicited damage both in vivo and in vitro. Faseb J. 2006;20:2496–511. doi: 10.1096/fj.06-6006com. [DOI] [PubMed] [Google Scholar]
- 12.Tan AS, Berridge MV. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immuno methods. 2000;238:59–68. doi: 10.1016/s0022-1759(00)00156-3. [DOI] [PubMed] [Google Scholar]
- 13.Gao HM, Liu B, Zhang W, Hong JS. Novel anti-inflammatory therapy for Parkinson’s disease. Trends Pharmacol Sci. 2003;24:395–401. doi: 10.1016/S0165-6147(03)00176-7. [DOI] [PubMed] [Google Scholar]
- 14.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–91. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 15.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 16.Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, et al. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc Natl Acad Sci U S A. 1997;94:7531–36. doi: 10.1073/pnas.94.14.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, et al. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2007b;104:18754–9. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]