Abstract
Background
Emerging evidence indicates that an endogenous autocrine/paracrine system involving γ-aminobutyric acid (GABA) is present in airways. GABAA channels, GABAB receptors and the enzyme that synthesizes GABA have been identified in airway epithelium and smooth muscle. However, the endogenous ligand itself, GABA, has not been measured in airway tissues. We sought to demonstrate that GABA is released in response to contractile agonists and tonically contributes a pro-relaxant component to contracted airway smooth muscle.
Methods
The amount and cellular localization of GABA in upper guinea pig airways under resting and contracted tone was determined by high pressure liquid chromatography and immunohistochemistry, respectively. The contribution that endogenous GABA imparts on the maintenance of airway smooth muscle acetylcholine-induced contraction was assessed in intact guinea pig airway tracheal rings using selective GABAA antagonism (gabazine) under resting or acetylcholine-contracted conditions. The ability of an allosteric agent (propofol) to relax a substance P-induced relaxation in an endogenous GABA-dependent manner was assessed.
Results
GABA levels increased and localized to airway smooth muscle following contractile stimuli in guinea pig upper airways. Acetylcholine-contracted guinea pig tracheal rings exhibited an increase in contracted force upon addition of the GABAA antagonist gabazine which was subsequently reversed by the addition of the GABAA agonist muscimol. Propofol dose-dependently relaxed a substance P contraction that was blocked by gabazine.
Conclusion
These studies demonstrate that GABA is endogenously present and increases following contractile stimuli in guinea pig upper airways and that endogenous GABA contributes a tonic pro-relaxant component in the maintenance of airway smooth muscle tone.
INTRODUCTION
Asthma is a chronic inflammatory disease of the airways that predisposes patients to episodes of severe, acute airway constriction. Despite an increasing world wide prevalence of asthma 1, new pharmacologic approaches to treat this disease are limited. Although a great deal is known regarding the mechanisms governing airway smooth muscle contraction2, relatively less is known about mechanisms of airway smooth muscle relaxation. Therefore, greater insights into the mechanisms of endogenous control of airway smooth muscle relaxation are required to identify novel therapeutic strategies.
Interestingly both volatile and intravenous anesthetics have long been recognized as potent bronchodilators 3–5, yet the exact mechanism(s) for this effect is incompletely understood and has been attributed to both neural 6,7 and direct airway smooth muscle effects 8–10. Despite the well known effect of anesthetics (e.g., propofol) as allosteric potentiators of the action of endogenous γ-aminobutyric acid (GABA) at neuronal GABAA channels 11, it has been a long standing belief that any GABAergic contribution to airway tone was largely mediated by GABAA channels in the brainstem12 or by GABAB receptors on pre-ganglionic cholinergic nerves in the lung13,14. Our recent identification of GABAA channels expressed directly on airway smooth muscle that contribute to relaxation15 raises the novel possibility that a previously unrecognized mechanism of anesthetic mediated airway smooth muscle relaxation could be anesthetics allosterically potentiating the effect of endogenous GABA at airway smooth muscle GABAA channels. In addition to GABAA channels, GABAB receptors and glutamic decarboxylase (the enzyme responsible for GABA synthesis) have also recently been identified in airway epithelial16,17 and smooth muscle cells 18 suggesting that endogenous GABA may have autocrine/paracrine functions in airways. Despite the presence of a complex GABAergic system in the airway, measurements of the endogenous ligand GABA or a tonic effect on airway tone by endogenous GABA has not yet been demonstrated.
Taken together, the expression of airway smooth muscle GABAA receptors facilitating relaxation and the clinical benefit of anesthetics on hyperreactive airway tone led us to question (1) whether endogenous GABA is present in the airway, (2) whether contractile agonists increase airway GABA release (3) whether liberated GABA tonically modulates airway smooth muscle tone, and (4) whether airway smooth muscle GABAA channels mediate a component of anesthetic (i.e., propofol)-induced airway smooth muscle relaxation. Defining and harnessing this novel relaxation pathway may identify new therapeutic options for hyperreactive airway disease.
MATERIALS AND METHODS
Reagents
Indomethacin, N-vanillyinonanamide (capsaicin analogue), pyrilamine, acetylcholine, γ–aminobutyric acid, o-phtaldialdehyde, 2-mercaptoethanol, and gabazine were obtained from Sigma (St. Louis, MO). Propofol was obtained from ICN Biomedicals and diluted in Dimethyl sulfoxide (DMSO) (Aurora, OH). Tetrodotoxin was obtained from Calbiochem (San Diego, CA).
Guinea pig tracheal rings
All animal protocols were approved by the Columbia University Animal Care and Use Committee (New York, New York). Male Hartley guinea pigs (approximately 400 g) were deeply anesthetized with intraperitoneal pentobarbital (100mg/kg). After opening the chest cavity, the entire trachea was surgically removed and promptly placed in cold (4°C) phosphate buffered saline (PBS). Each trachea was dissected under a dissecting microscope into closed rings comprised of two cartilaginous segments from which mucosa, and connective tissue were removed. Epithelium was left intact for high pressure liquid chromatography (HPLC) and immunohistochemistry studies but removed for organ bath experiments. Tissues were placed into cold Krebs-Henseleit (KH) buffer (in mM: NaCl 118, KCl 5.6, CaCl2 0.5, MgSO4 0.24, NaH2PO4 1.3, NaHCO3 25, glucose 5.6, pH 7.4) containing indomethacin 10uM (DMSO vehicle final concentration in organ baths of 0.01%) to block tone due to endogenous release of prostanoids.
High Pressure Liquid Chromatography (HPLC)
To demonstrate that GABA is endogenously present in airways we utilized intact guinea pig tracheal ring segments (2 rings per sample) harvested from euthanized guinea pigs. All sample tissues were separately incubated in a 500ul volume of KH buffer (pH 7.4) on ice for 20 min followed by a 500ul KH buffer wash. Samples were separated into 3 treatment groups (no treatment, acetylcholine 10uM and β-ala Neurokinin A (NKA) fragment 4–10 (neurokinin receptor 2 agonist) 10uM) and incubated for 45min in a fresh volume of 200ul volume of KH buffer (pH 7.4) at 37°C. Following this treatment period, a 20ul aliquot was analyzed for GABA by HPLC with electrochemical detection using a method based on precolumn derivatization of the amino acid with o-phtaldialdehyde (OPA) and 2-mercaptoethanol (βME)19,20. The HPLC system used for analysis consisted of a GBC model LC1150 pump (GBC Scientific Equipment Pty Ltd, Dandenong, Australia), a Rheodyne model 9725i injector (Rheodyne, Rohnert Park, CA) and an INTRO amperometric detector (Antec, Leyeden, The Netherlands) equipped with a VT-03 (Antec) electrochemical flow cell with glassy carbon working electrode and salt bridge Ag/AgCl reference electrode. Chromatographic separation was achieved on a C18 column and a mobile phase consisting of 0.1 M Na2HPO4, 50 mg/L EDTA, and 12% methanol (pH 5.2). Separation and detection were performed at 30°C, with the cell potential set to +750 mV. Peak-heights were measured by using WinChrom chromatography software (GBC Scientific Equipment). To confirm accurate detection of a GABA peak, generation of standard curves with known concentrations of GABA preceded sample analysis (these standard curves were consistently linear over a range of 25nM to 1uM). In addition, an intra-experimental control (reanalyzing a previously processed sample spiked with a known concentration of GABA) was performed to confirm that the subsequent increase in the GABA peak is proportional to the amount of GABA added to the sample. For negative control, KH buffer only was analyzed.
Immunohistochemistry
To illustrate the localization patterns of endogenous GABA under basal (resting) and pro-contractile stimulated conditions, guinea pig tracheal rings were harvested as above and were treated with or without 10uM β-ala NKA fragment 4–10 for 15 min. Tracheal rings were immediately fixed using 4% paraformaldehyde/1% glutaraldehyde in 0.1M phosphate buffer (pH 7.4) for 4 h at 4°C for GABA immunostaining as previously described18. Briefly, tracheal rings were paraffin embedded, sectioned (5 um), dewaxed in xylene, and rehydrated in a graded alcohol series to water. Endogenous peroxidase was blocked in 0.3% hydrogen peroxide. Heat mediated antigen retrieval was performed with 10 mM sodium citrate buffer, pH 6.0 for 30 min. An avidin biotin blocking kit (Vector Laboratories, Peterborough, United Kingdom) was used (in 10% serum in PBS) to block endogenous biotin. Slides were rinsed with PBS and incubated overnight at 4°C in primary antibody against GABA (mouse, MAB316; Chemicon, Temecula, CA) at a concentration of 1:50 in 2% serum in PBS. Tracheal ring sections were also incubated with the same concentration of a mouse isotype IgG antibody (IgG1) (as a negative control) or with primary antibody directed against α–smooth muscle actin (mouse, MAB1522; Chemicon, Temecula, CA) at a concentration of 1:10,000 to identify smooth muscle (positive control). Following overnight incubation at 4°C, slides were washed with PBS, and primary antibodies were detected using biotinylated anti-mouse antibodies (Vector Laboratories) at a concentration of 1:100. The antigen antibody complex was then visualized by enzymatic reduction of 3,3-diaminobenzidine tetrahydrochloride. Sections were counterstained with hematoxylin and dried, and cover slides were mounted using Poly-mount (Polysciences, Warrington, PA).
Organ baths
Closed guinea pig tracheal rings were suspended in organ baths as previously described21. Briefly, tissues were attached with silk thread inferiorly to a fixed tissue hook in a water-jacketed (37oC) 2-ml organ bath (Radnoti Glass Technology, Inc., Monrovia, CA) and superiorly to a Grass FT03 force transducer (Grass Telefactor, West Warwick, RI) coupled to a computer via BioPac hardware and Acqknowledge 7.3.3 software (Biopac Systems, Inc., Goleta, CA) for continuous digital recording of muscle force. Tissues were secured such that muscle contraction would align with the vertical plane between the anchoring hook below and transducer above. KH buffer was continuously bubbled with 95% oxygen and 5% carbon dioxide and tissues were allowed to equilibrate at 1g isotonic force for 1 hour with fresh KH buffer changes every 15 min.
Preliminary contractile challenges
Following equilibration, the capsaicin analog N-vanillylnonanamide (10 μM final) was added to the organ baths containing guinea pig tracheal rings to first activate and then deplete nonadrenergic, noncholinergic nerves. After N-vanillylnonanamide induced force had returned to baseline (~50 min), the KH buffer in the organ baths were changed 6 times to wash out added or liberated mediators. Tracheal rings were then subjected to two cycles of increasing cumulative concentrations of acetylcholine (0.1 μM to 1 mM) with 6 buffer changes and resetting of the resting tension between cycles. The resulting concentration response curves were then used to determine the EC50 concentrations of acetylcholine required for each individual ring. Individual tissues have variable sensitivity to contractile agonists (EC50) and variation in the magnitude of contraction (Emax). To avoid bias between treatment groups, tissues were contracted to individually calculated EC50‘s for acetylcholine and tissues with similar Emax values were randomly assigned to treatments within individual experiments. Following preliminary contractile challenges, tissues were subjected to extensive KH buffer changes (8–9 times) and allowed to stabilize at their respective isotonic resting tensions (1.0 g). To remove confounding effects of other pro-contractile pathways each airway ring received a complement of antagonists 20 minutes prior to subsequent contractile challenge. The antagonists included pyrilamine (10 μM; H1 histamine receptor antagonist), and tetrodotoxin (1μM; antagonist of endogenous neuronal-mediated cholinergic or C-fiber effects).
In vitro assessment of the functional contribution endogenous GABA imparts on resting and pre-contracted (acetylcholine EC50) airway smooth muscle tone
Following equilibration, tracheal rings were randomly assigned to one of four groups: +/− gabazine with rings at resting tension (1g) or contracted with an EC50 concentration of acetylcholine which were allowed to achieve a steady-state plateau of increased force (typically 15 min). To determine the functional effect of endogenous GABA on airway smooth muscle GABAA channels, a single dose of the selective GABAA channel antagonist gabazine (100uM) was added to the resting or acetylcholine-precontracted tracheal rings. Changes in muscle force were recorded over 15 minutes. For all groups, changes in muscle force were analyzed as the percent of change in muscle force from an initial baseline or acetylcholine-induced muscle force measured 15 min after treatments. In a separate set of experiments, to confirm the effect of gabazine was not attributable to non-specific actions (a non-GABAA channel effect), a single dose of the selective GABAA channel antagonist gabazine (100uM or 200uM) was added to acetylcholine-precontracted tracheal rings. After 15 minutes treatment groups then received muscimol (200uM) to reverse the effect of gabazine-induced airway smooth muscle GABAA channel blockade.
In vitro determination of dose responses for airway smooth muscle GABAA channel antagonism and dose response for reversal of this GABAA channel antagonism by muscimol
To illustrate that functional antagonism of endogenous GABA at the airway smooth muscle GABAA channel occurs in a dose dependent fashion we performed a dose response study utilizing cumulative gabazine concentrations (0–800uM) administered following an EC50 acetylcholine contraction. Changes in muscle force were analyzed as the percent of change in muscle force from an initial acetylcholine EC50 contraction measured at 15 min intervals following each treatment. Furthermore to compare relative potency between gabazine and muscimol, we also performed a dose response of muscimol (0–800uM) following a fixed concentration of gabazine (200uM) that was given after an acetylcholine EC50 contraction. For these experiments, changes in muscle force mediated by muscimol were analyzed as the percent of change in muscle force from the preceding gabazine (200uM) mediated increase in contractile force.
In vitro assessment of an allosteric GABAA channel agent (i.e. propofol) effect on airway smooth muscle relaxation following contraction with substance P
To illustrate the importance of endogenous GABA acting at GABAA channels to mediate relaxation of airway smooth muscle, we assessed the ability of an allosteric activator of GABA (propofol) to relax airway smooth muscle following a 1uM substance P contraction. Using the paradigm outlined above, guinea pig rings underwent preliminary contractile challenges with the capsaicin analog and acetylcholine. Following extensive washing and a resetting of baseline resting tone (1.0g), tissues were randomly assigned to one of three groups: time control (no treatment), vehicle treatment only (DMSO), and propofol treatment. Guinea pig tracheal rings were then treated with substance P (1uM), allowed to reach a plateau in muscle force, and then were treated with cumulatively increasing concentrations (0.5, 5, 10, 20, 50 and 100uM) of either propofol or vehicle (DMSO) equivalents in 6 minute increments. In separate experiments substance P-induced contracted guinea pig tracheal rings were pretreated with or without 5uM gabazine 15 min before substance P and with or without cumulatively increasing concentrations (5, 10, 50, 100uM) of propofol at 6 minute increments. For all groups, changes in relaxation were analyzed as a percentage of retained muscle force from the initial substance P contraction and were compared to controls.
Statistical Analysis
Each experimental permutation included intra-experimental controls. Dose response curves were constructed using a sigmoidal dose response analysis function in Prism 4.0 software (GraphPad, San Diego CA) which employs a four-parameter logistic equation according to the Hill model: Y = minimum + (Emax − minimum)/(1 + 10(dose-logEC50)), where the minimum represents the initial resting muscle tension. Data was analyzed by one way ANOVA with Bonferroni posttest comparisons between appropriate groups. Data is presented as mean ± SEM; p < 0.05 is considered significant.
RESULTS
Activation of the neurokinin receptor 2 enhances GABA immunostaining over airway smooth muscle
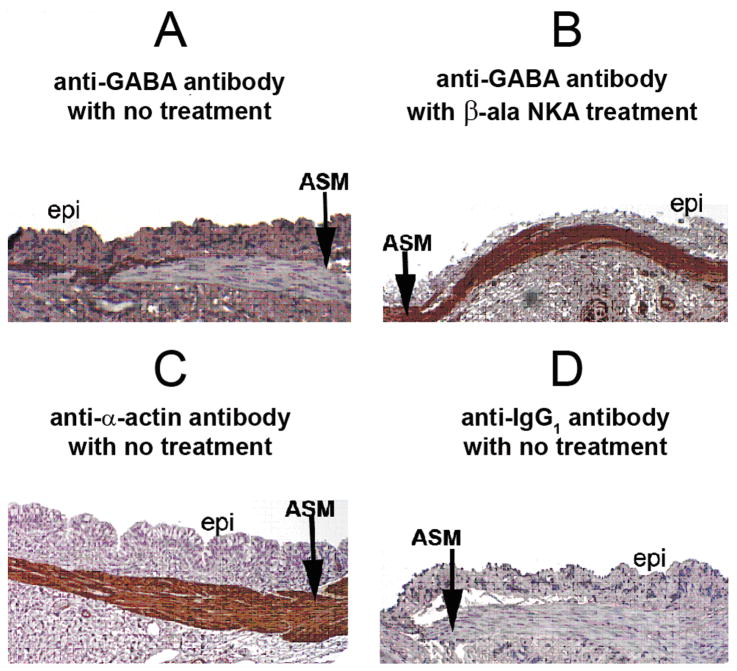
Under unstimulated conditions, endogenous GABA was immunohistochemically detectable at the interface between airway smooth muscle and the adjacent epithelium in guinea pig tracheal rings (fig. 1A). Limited staining in small punctate areas within the airway smooth muscle itself was discernable at high magnification (data not shown). In contrast, 15 minute exposure to the selective neurokinin receptor 2 agonist, β-ala NKA fragment 4–10 (10uM), enhanced GABA staining throughout the airway smooth muscle layer (fig. 1B), suggesting that either GABA re-localizes or demonstrates enhanced release within the airway smooth muscle layer itself. The identity of the airway smooth muscle layer was confirmed using an anti-α-actin antibody (fig. 1C) and a control for primary/secondary antibody non-specific staining was performed using mouse-specific anti-IgG1 antibody (fig. 1D).
Figure 1. Immunohistochemical staining for γ-aminobutyric acid (GABA) in guinea pig tracheal rings.
Representative immunohistochemical GABA staining in guinea pig tracheal rings. (A) Untreated tracheal segment illustrates limited staining for GABA (brown) in airway smooth muscle (ASM), with the majority of staining localized to the interface between the ASM and adjacent epithelium (epi). (B) Following 15 min treatment with a neurokinin 2 agonist (10uM β-ala neurokinin A fragment 4–10) a dramatic increase in GABA staining occurs over airway smooth muscle. (C) Immunohistochemical staining for α–actin confirms the identity of the airway smooth muscle layer. (D) Antibody negative control: Isotype specific (IgG1) negative control antibody for the mouse anti-GABA antibody used in panels A and B reveals no staining in airway smooth muscle.
Endogenous GABA eluted from guinea pig tracheal rings is detectable by HPLC and increased GABA levels are detected following stimulation with β-ala NKA fragment 4–10 or acetylcholine
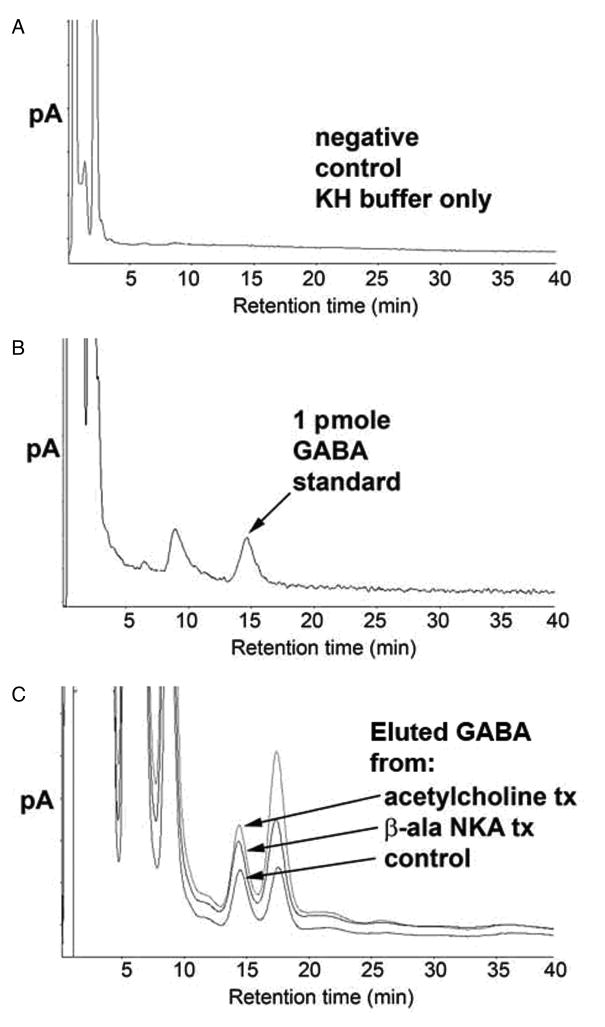
To correlate our immunohistochemical findings, we assessed eluted airway GABA levels using HPLC. GABA has not been previously demonstrated in isolated airway segments. Therefore, representative HPLC controls are first demonstrated in figure 2. A representative chromatogram of KH buffer alone is illustrated in figure 2A. Figure 2B illustrates a representative chromatogram of a 1 pmole standard of GABA in KH buffer. The GABA peak had a retention time of 14.7 min and showed a linear increase in magnitude over a range of GABA standard concentrations (0.25–10 pmole)(data not shown). GABA eluted from unstimulated or stimulated guinea pig rings in 200ul KH buffer at 37°C over a 45 min period is illustrated in figure 2C. Untreated tracheal rings in KH buffer for 45 min eluted measurable amounts of GABA but in tracheal rings treated for 45 min with 10uM β-ala NKA fragment 4–10 or acetylcholine, the eluted GABA increased. Figure 2D demonstrates the quantitative differences in GABA eluted during 45 min at 37°C in 200ul KH buffer. The pro-contractile stimuli acetylcholine and β-ala NKA fragment 4–10 significantly increased eluted GABA (n=6) (p < 0.01 compared to control).
Figure 2. High pressure liquid chromatography (HPLC) detection of γ-aminobutyric acid (GABA) in buffer eluates from guinea pig tracheal rings.
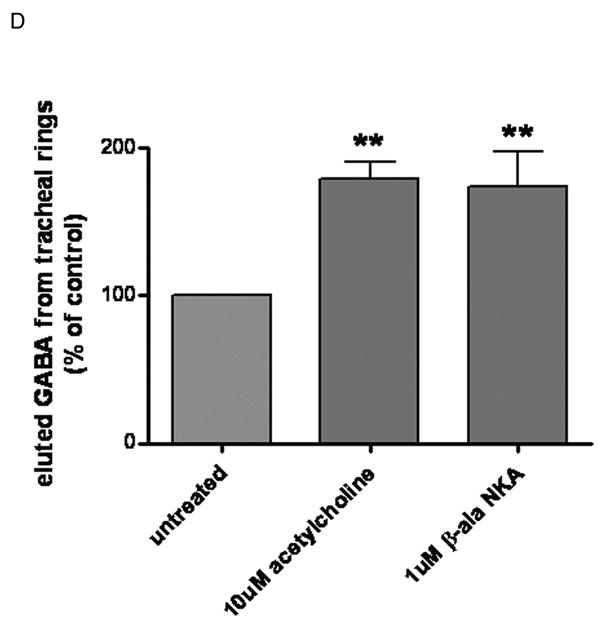
Panel A: Representative chromatograms of GABA eluted from guinea pig tracheal rings. Negative control (buffer only); Panel B: Positive control (buffer spiked with 1pmol GABA): indicated peak at 15 min retention time linearly increased with standard GABA concentrations of 0.5–10 pmoles; Panel C: Superimposed chromatograms of GABA eluted from untreated guinea pig tracheal ring, guinea pig tracheal ring treated with β-ala neurokinin A (10uM), and guinea pig tracheal ring treated with acetylcholine (10uM). Eluted airway GABA levels are detectable by HPLC under baseline (unstimulated) conditions, and increase following treatment (tx) with pro-contractile stimuli. Panel D: Endogenous GABA levels normalized to tissue weight and expressed as percentage of the control group (no treatment). Eluted GABA levels demonstrate a significant increase above baseline levels following treatment with 10uM acetylcholine or 10uM β-ala neurokinin A fragment 4–10 (n=6). ** p<0.01
Blockade of endogenous GABAA channels increases guinea pig airway smooth muscle force which is greater following an acetylcholine EC50 contraction compared to baseline resting tension
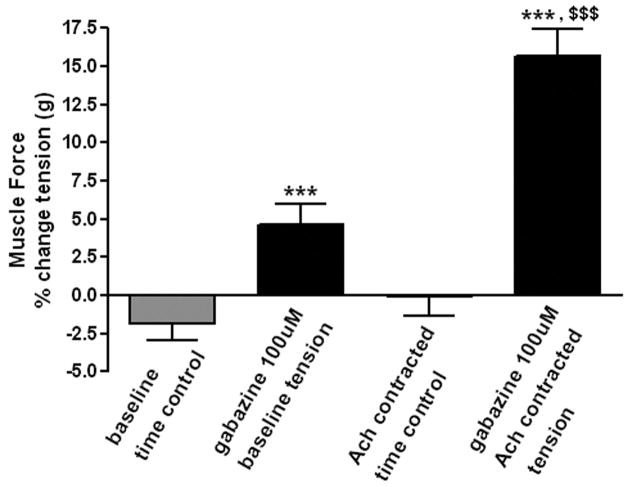
We next investigated whether antagonism of GABAA channels in intact guinea pig tracheal rings modulated airway smooth muscle force. Guinea pig tracheal rings suspended in organ baths under optimal resting tension (1.0g) were treated with 100 uM gabazine which resulted in a small increase in muscle force when compared to untreated controls in parallel organ baths (gabazine: 4.6 ± 1.35 % of baseline tone (n=8), versus control: −1.91 ± 1.02 % of baseline tone (n=7), respectively, p<0.01). In contrast, when 100uM gabazine was added to the organ baths during a sustained contraction with acetylcholine (EC50 concentration), the augmentation of airway smooth muscle force was significantly greater (gabazine: 15.67 ± 1.79 % increase above acetylcholine EC50 plateau force (n=15) versus control: 4.61 ± 1.35 % increase above acetylcholine EC50 plateau force (n=8), p<0.001, fig. 3).
Figure 3. The effect of selective γ-aminobutyric acid channel subtype A (GABAA) antagonism on airway smooth muscle force under baseline or contracted (EC50 acetylcholine) conditions.
100uM gabazine effect on airway smooth muscle force under baseline or acetylcholine (EC50)-contracted conditions. Blockade of endogenous GABAA channels results in a functional increase in muscle force compared to time controls. (n=7) *** p < 0.001 compared to time controls; $$$ p < 0.001 compared to baseline tension.
Gabazine mediated antagonism of airway smooth muscle GABAA channels is reversed by the selective GABAA channel agonist muscimol
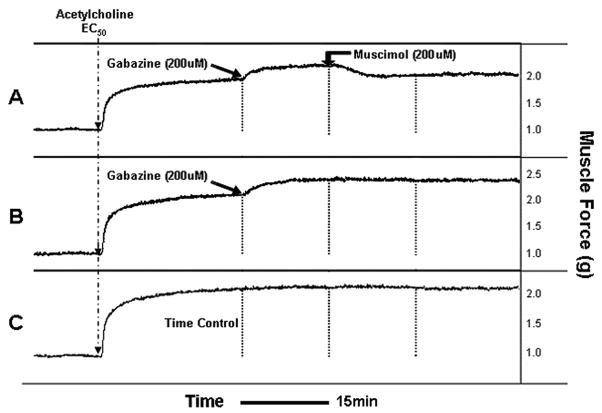
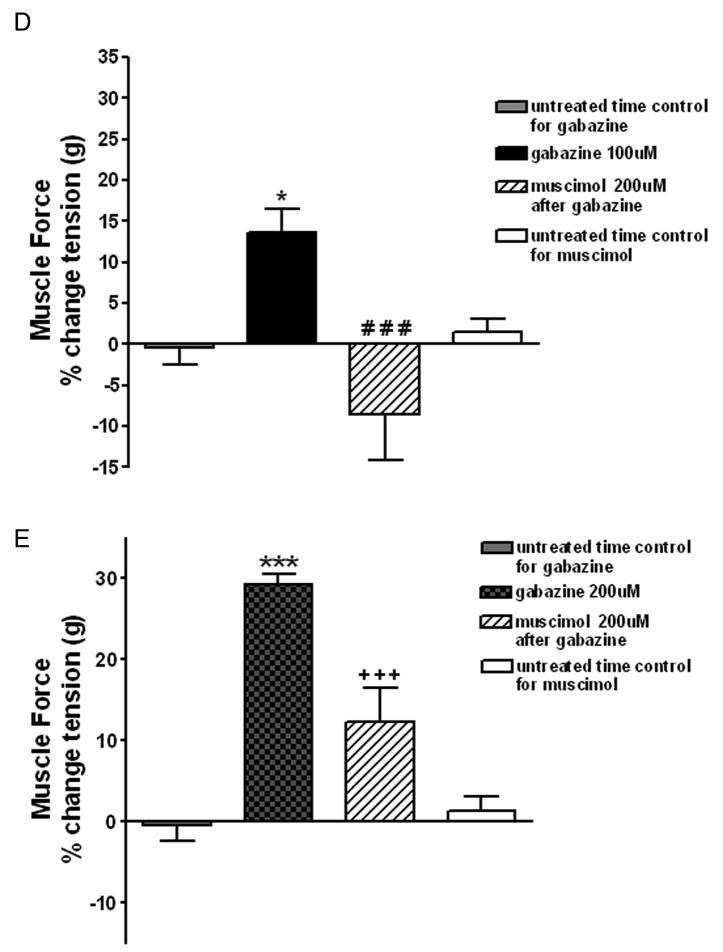
A separate set of experiments were performed to confirm that gabazine’s effect occurs selectively at endogenous GABAA channels. Representative force tracings are shown in figure 4A–C for 200uM gabazine treatment of a sustained acetylcholine contraction (EC50) and reversal by 200uM muscimol. We used 100uM or 200uM gabazine during a sustained acetylcholine (EC50) contraction and then attempted to reverse both concentrations of gabazine with 200uM of muscimol (a specific GABAA agoinst). As before, 100uM gabazine during a sustained acetylcholine (EC50)-induced contraction resulted in a significant increase in airway smooth muscle force compared to untreated acetylcholine-contracted control tracheal rings in parallel organ baths (gabazine: 13.56± 3.03 % increase above acetylcholine EC50 plateau force (n=8) versus control: −0.44 ± 1.99% increase above acetylcholine EC50 plateau force (n=8, p<0.05). This 100uM gabazine-induced increase in muscle force was completely reversed by 200uM muscimol (−8.54 ± 5.58 % increase above acetylcholine EC50 plateau force (n=8), p<0.001 compared to 100uM gabazine alone (fig. 4D). 200uM gabazine during the sustained phase of an EC50 acetylcholine contraction resulted in an even greater increase in airway smooth muscle tension compared to untreated acetylcholine-contracted controls in parallel organ baths or compared to the effect of 100uM gabazine (200uM gabazine: 29.33 ± 1.30 % increase above acetylcholine EC50 plateau force, n=7, p<0.001; fig. 4E), and this increase was significantly attenuated by subsequent treatment with 200uM muscimol (12.35 ± 4.23 % increase above acetylcholine EC50 plateau force (n=7), (p<0.001 compared to 200uM gabazine alone).
Figure 4. Following an EC50 acetylcholine contraction, selective γ-aminobutyric acid subtype A channel (GABAA) antagonism increases airway smooth muscle force that is reversed by the GABAA channel agonist muscimol.
Representative tracings in force/time of guinea pig tracheal rings contracted with an EC50 concentration of acetylcholine and treated with; Panel A: 200uM gabazine followed 15 min later by 200uM muscimol; Panel B: 200uM gabazine; or Panel C: nothing. Blockade of GABAA channels with gabazine results in a sustained increase in muscle force which is reversed by the selective GABAA agonist muscimol. Panel D: Muscle force in guinea pig tracheal rings contracted to an EC50 with acetylcholine and then subjected to nothing, 100uM gabazine or 200uM gabazine followed by 200uM muscimol. 100uM gabazine significantly increases muscle force compared to time control (n=8) (* p<0.05 compared to untreated time control), and this effect is reversed by 200uM muscimol (n=8) (### p<0.001 compared to 100uM gabazine). Panel E: 200uM gabazine significantly increases muscle force compared to time control (n=8) (*** p<0.001 compare to untreated time control) and this effect is significantly attenuated by 200uM muscimol (n=7) (+++ p<0.001 compared to 200uM gabazine).
Gabazine mediated GABAA channel antagonism following an EC50 acetylcholine contraction is dose dependent
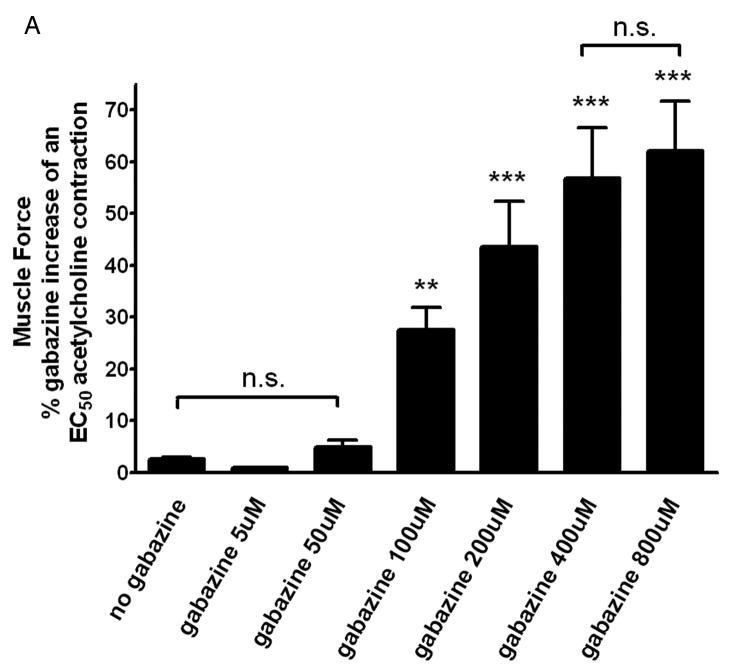
Additional organ bath experiments were performed to demonstrate cumulatively increasing concentrations of gabazine (from 0 to 800uM) administered following an EC50 acetylcholine contraction results in dose dependent increase in muscle force, and exhibits an IC50 of 163uM (fig. 5A).
Figure 5. Gabazine-induced increase in muscle force following an EC50 acetylcholine contraction and antagonism of gabazine’s effect by theγ-aminobutyric acid channel subtype A channel (GABAA) agonist muscimol are dose dependent.
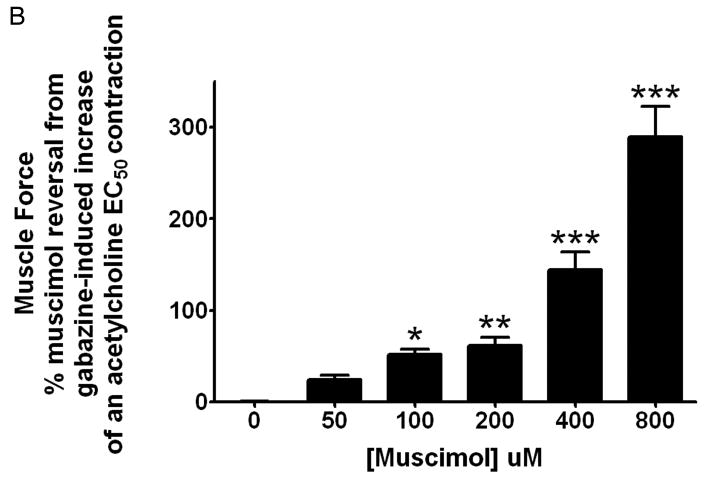
Panel A: Cumulatively increasing concentrations of gabazine (from 0 to 800uM) result in a significant increase in muscle force above a sustained EC50 acetylcholine contraction with an IC50 of 163uM. (n = 12) (** p<0.01 compared to time controls (no gabazine treatment); and *** p<0.001 compared to time controls). Panel B: Cumulatively increasing concentrations of the GABAA channel agonist muscimol (0–800uM) following a fixed dose of gabazine (200uM) demonstrates significant reversal of the achieved GABAA channel antagonism in a dose dependent fashion. (n = 4–8) (* p<0.05, ** p<0.01, and *** p<0.001 compared to no treatment (0uM Muscimol)).
Reversal of gabazine mediated GABAA channel antagonism following an EC50 acetylcholine contraction also displays dose dependency
Cumulatively increasing concentrations of the GABAA channel agonist muscimol (0–800uM) following a fixed dose of gabazine (200uM) demonstrates significant reversal of the achieved GABAA channel antagonism in a dose dependent fashion, beginning at a concentration of 100uM (n = 4) p<0.05 compared to no treatment (0uM Muscimol, n = 8). Full reversal of the increase in muscle force of 200uM required a concentration of muscimol >200uM demonstrating that gabazine and muscimol are not equally potent under these conditions (fig. 5B).
An allosteric agent at GABAA channels enhances endogenous GABA effect of relaxing substance P-induced contraction in guinea pig tracheal rings
The significant increase in muscle force induced by gabazine during the sustained phase of an acetylcholine contraction suggests that endogenous GABA is present and counter-balances induced contractile muscle force. To illustrate the endogenous presence and contribution of GABA to decreasing muscle force, substance P induced contractions were treated with an allosteric agent at GABAA channels in the absence and presence of a GABAA antagonist. Guinea pig tracheal rings were contracted with 1uM substance P and then subjected to cumulatively increasing concentrations of propofol (5, 10, 20, 50, or 100 uM), or the appropriate concentrations of the vehicle for propofol (DMSO) in the absence or presence of the selective GABAA antagonist gabazine (5uM). Propofol (20–100uM) had a significant effect on relaxation compared to the spontaneous decay of an untreated time control contraction, with no significant effect of the vehicle (n=10 in each group) (p<0.05 for 20uM propofol compared to time control; p < 0.001 for 50 or 100uM propofol compared to time control) (fig. 6A). To demonstrate that a component of the propofol-induced relaxation was due to propofol allosterically enhancing endogenous GABA effects at GABAA channels, substance P-induced contractions were relaxed with propofol (5, 10, 50, 100uM) in the absence or presence of 5uM gabazine. Gabazine partially but significantly reversed propofol-induced relaxation with gabazine alone having no significant effect on the substance P-induced contraction (n=8 in each group) (p<0.01 for propofol+gabazine compared to propofol alone; fig. 6B).
Figure 6. Relaxation of a substance P-induced contraction is enhanced by the selective positive allosteric effect of propofol on the γ-aminobutyric acid subtype A channel (GABAA).
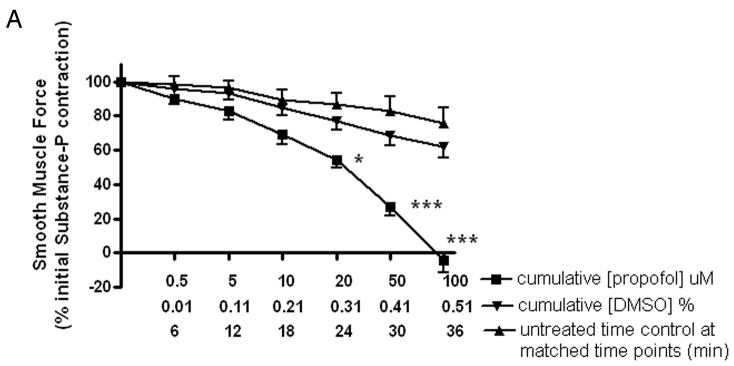
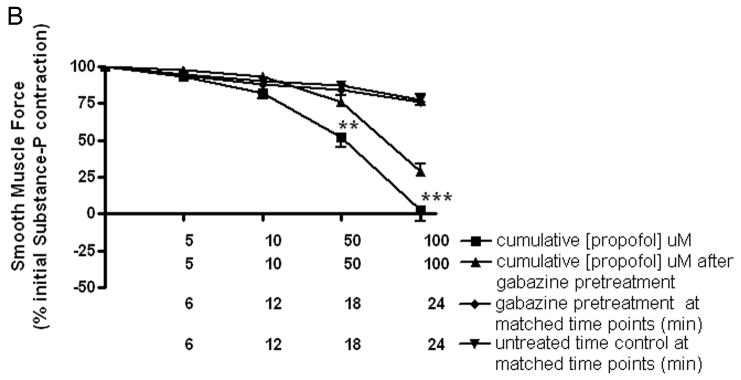
Panel A: Cumulatively increasing concentration response curves expressed as a percent of initial force following a substance P contraction for propofol (■), Dimethyl sulfoxide (DMSO) vehicle (▼;) or time control (▲). Relaxation of guinea pig tracheal rings contracted with 1uM substance P was significantly enhanced by propofol (≥ 20uM). * p < 0.05 compared to time control; *** p < 0.001 compared to time control. Panel B: Cumulatively increasing concentration response curves expressed as a percent of initial force following a substance P contraction for propofol (■) or with 5uM gabazine pretreatment (▲) before propofol. Partial but significant reversal of propofol-induced relaxation occurred with low dose gabazine (5uM) pretreatment (at 50uM and 100uM propofol ** p < 0.01 and *** p < 0.001 respectively).. Gabazine alone (◆) was not different from untreated time controls (▼;).
Discussion
The major finding of this study is that endogenous GABA exists in the guinea pig airway and plays an important role in modulating airway smooth muscle tone by facilitating smooth muscle relaxation via activation of airway smooth muscle GABAA channels. To establish the contribution of endogenous GABA in regulating airway tone, we used both descriptive (HPLC, immunohistochemistry) as well as functional studies (in vitro organ baths), and demonstrate the importance of GABA by testing its pro-relaxant effect against two different contractile stimuli (muscarinic and neurokinin receptor 2 agonists). In addition, to illustrate that the GABA effects were specific to GABAA channels we performed studies demonstrating a pro-contractile effect by GABAA antagonism (gabazine) which was reversed by GABAA agonism (muscimol). This is the first demonstration that measurable amounts of endogenous GABA are present in the airway, that GABA release and cellular localization changes in response to pro-contractile stimuli and that following smooth muscle contraction GABA promotes airway smooth muscle relaxation.
Many anesthetic agents that facilitate bronchodilation are also positive allosteric agents at GABAA channels, which function to augment the effects of endogenous GABA. Despite these properties, the exact mechanism(s) responsible for anesthetics’ bronchodilatory effect are incompletely understood. It has been a long standing belief that any potential GABAergic contribution to airway tone was largely mediated by GABAA channels in the brainstem12 or by GABAB channels on preganglionic cholinergic nerves in the lung13,14. However, there is emerging evidence that a far more complex airway GABAergic system exists, one which involves the presence of GABAA channels not only on airway nerves, but also on airway epithelium and airway smooth muscle where they facilitate relaxation16–18. In addition, since the enzyme responsible for GABA synthesis (glutamic acid decarboxylase) is also present in airway epithelium16,17 and airway smooth muscle17, it is possible that the bronchodilatory effect of anesthetic agents (which require GABA present to exert their effect) is also attributable to an allosteric effect at airway smooth muscle GABAA channels.
The expression of GABAB receptors on postganglionic parasympathetic nerves terminating in the airways has been known for years,14 and we recently described the expression of GABAB receptors on airway smooth muscle18 and airway epithelial cells17 coupled to Gi signaling pathways. Therefore, in addition to its target on GABAA channels described in the current study, liberated GABA could inhibit parasympathetic nerve release of acetylcholine in the airway but could counteract smooth muscle GABAA channel-mediated relaxation by stimulation of the GABAB receptor which in turn activates Gi, a signaling cascade classically linked to impairing airway smooth muscle relaxation.
Although we have previously described that GABA is immunohistochemically detectable in the guinea pig airway18, GABA staining predominated at the interface between the airway smooth muscle and surrounding epithelium in the absence of contractile stimuli and only sparse staining was noted within the airway smooth muscle layer itself18. This contrasted with the immunohistochemical localization observed for the GABAA channel which was localized throughout the airway smooth muscle layer.15 In the current study under unstimulated conditions GABA immunostaining was again diffusely visible near the smooth muscle layer (between smooth muscle and epithelium and between smooth muscle and cartilage/adventitia). However, GABA immunostaining increased dramatically over the smooth muscle layer following exposure to the pro-contractile selective neurokinin receptor 2 agonist (β-ala NKA fragment 4–10) supporting the possibility that endogenous GABA participates in modulation of airway smooth muscle tone.
Although HPLC detection and quantification of amino acid neurotransmitters has been performed extensively in neuronal tissues19 it has never been applied to airway tissues. Recent studies highlight the methodologic pitfalls involved in GABA analysis by HPLC20 which were taken into account in the current study. Using a validated approach we were able to identify discrete, symmetrical and reproducible GABA chromatograms derived from buffers into which intact airway tissues eluted GABA. In agreement with our immunohistochemical findings, we demonstrate that 10uM acetylcholine orβ-ala NKA fragment 4–10 increased eluted GABA levels. It is possible that physical manipulation or trauma to our dissected airway tissues induces release of GABA. To minimize this effect dissected tracheal rings were washed extensively until wash buffer concentrations of GABA were undetectable and then the tracheal rings were incubated undisturbed in a small volume of buffer in the absence or presence of a contractile agonist such that initial buffer concentrations were undetectable and GABA that subsequently eluted over 45 minutes was quantitated. Since we measured GABA that had permeated into the incubation buffer, our method does not directly assess the changes in GABA levels that occur within the interstitium of the airway smooth muscle layer. This is an important consideration as we likely are underestimating the magnitude of change in endogenous airway GABA levels that airway smooth muscle GABAA channels are actually exposed to. The current study does not allow us to determine the cellular source of GABA. Taken together, our immunohistochemical and HPLC findings demonstrate that airway GABA levels increase following procontractile stimuli and raise the intriguing possibility that liberated GABA acting at airway smooth muscle GABAA channels may contribute to relaxation thus functioning to counter-regulate the pro-contractile stimuli that induced its release.
The cellular source of GABA liberated into the airway was not determined in the present study but at least 3 structures in the airway appear to be candidate sources. The enzyme that synthesizes GABA (glutamic acid decarboxylase) was recently identified by us in airway smooth muscle (unpublished) and airway epithelial cells.16,17 Additionally, different types of nerve fibers (parasympathetic, nonadrenergic/noncholinergic) terminally branch in the airway and may contain GABA. As such, identifying the cellular source of GABA in the airway is an active topic of investigation in our laboratory. In the current study we provide evidence that the contractile agonists acetylcholine and β-ala NKA increase GABA release. There is evidence in the central nervous system22 and spinal cord23 that these agonists induce the release of GABA from neuronal cell types.
To determine if endogenous GABA enhances relaxation via activation of airway smooth muscle GABAA channels we conducted in vitro functional organ bath studies using intact guinea pig airway smooth muscle. In these studies, epithelial or neuronal contributions were eliminated by denuding the epithelium and by pretreatment with capsaicin and tetrodotoxin, respectively.
We initially examined the functional consequence of eliminating the ability of endogenous GABA to exert its prorelaxant effect via the airway smooth muscle GABAA channel. We used the GABAA channel antagonist gabazine, since it is water soluble (obviating the need for vehicle controls), is a competitive inhibitor of the GABAA channel (unlike picrotoxin, thereby allowing for a reversal of its effect), is not known to have nonspecific effects at targets other than the GABAA channel (like bicuculline methiodide)24, and has been successfully used by us in previous organ bath studies25. Gabazine increased smooth muscle force and had a greater effect under contracted conditions which correlates with conditions under which we demonstrated enhanced GABA release. The ability of the GABAA agonist muscimol to reverse gabazine’s effect further supported the specificity of gabazine’s effect at the GABAA channel. Another and perhaps more important consideration that may account for a larger effect of the GABAA antagonist under contracted conditions is the status of the membrane potential under baseline versus acetylcholine-contracted conditions. Opening of the GABAA channel in smooth muscle at resting membrane potential (approximately −45mV) may actually allow efflux of intracellular chloride favoring depolarization and contraction26. However, following an acetylcholine contraction in airway smooth muscle the chloride reversal potential is exceeded27 allowing for chloride entry and membrane hyperpolarization to occur (establishing a milieu that favors smooth muscle relaxation)28. However, in the current study we did not correlate resting membrane potential with resting muscle force.
Under tonic exposure to high concentrations of GABA, classic neuronal GABAA channels desensitize (as is seen within the synaptic cleft following vesicular release of GABA)29. However, there is emerging evidence that subunit composition plays an important role in determining the threshold for GABAA channel desensitization30. Indeed, two electrophysiologically distinct classes of GABAA channels have been identified in neurons; the classic synaptic chloride channels with fast kinetics and rapid inactivation (collectively termed “phasic”), and the slower extra-synaptic channels which are responsive to lower concentrations of GABA and display slower desensitization properties (collectively termed “tonic”)30,31. Interestingly, tonic GABAA channels require the inclusion of either an α4,α5, or α6 subunit with a δ subunit and we have previously demonstrated that airway smooth muscle GABAA channels express the δ subunit in addition to having α subunit expression limited toα4 and α5 subunits. This finding indicates that the repertoire of subunits required in neurons to form tonic GABAA channels are also expressed in airway smooth muscle15.
To illustrate the functional role of endogenous GABA in promoting relaxation of contracted tissues, an anesthetic with positive allosteric effects at the GABAA channel (i.e., propofol) was utilized. Since positive allosterism enhances the native effect of a ligand at its target channel/receptor (and by itself cannot activate the target channel/receptor) it provides a unique opportunity to demonstrate a ligand’s endogenous effect. We demonstrate a dose dependent improvement in relaxation of a substance P contraction by propofol which was partially reversed by low dose GABAA channel antagonism. This partial reversal may indicate that propofol relaxation of airway smooth muscle may involve additional mechanisms or that insufficient concentrations of gabazine were employed to demonstrate complete reversal of propofol. However, we were limited in the concentrations of gabazine (5uM) that could be used in these propofol studies because as illustrated above, higher concentrations of gabazine (100uM) increase muscle force by blocking relaxation induced by endogenous GABA.
This latter set of experiments adds yet another possible explanation for propofol’s preferential protection from reflex-induced bronchoconstriction during intubation which has traditionally been attributed to its action on airway efferent parasympathetic cholinergic nerves and less convincingly to post-junctional modulation of L-type calcium channels 32 or inositol phosphate signaling 33 in airway smooth muscle itself. However, these previous studies have illustrated these smooth muscle effects only at concentrations of propofol (>100uM) above those typically achieved clinically 34–36. Several studies in airway tissues from humans and animal models have shown that propofol can attenuate contractile responses from acetylcholine 37–39, histamine 40 and endothelin 39, but only at concentrations of 100–300uM. These previous studies which only demonstrated a propofol effect at high concentrations have largely focused on cellular signaling pathways involved in the initiation and maintenance of contraction (L-type calcium channels 32, intracellular calcium changes 40,41 or inositol phosphate synthesis 33). Conversely, in the present study, lower concentrations of propofol (20uM) facilitated airway smooth muscle relaxation likely due to different signaling pathways regulating relaxation28 as opposed to those regulating contraction.
Although the measurement of plasma propofol concentrations during the administration of clinically relevant doses of propofol is complex, induction doses of propofol (2–3 mg/kg i.v.) typically result in peak plasma concentrations of 60–80uM 34–36 while maintenance infusions of propofol reportedly achieve approximately 30uM concentrations 42–45. Although the concentration in individual tissue compartments is unknown, high tissue uptake of propofol by the lung (30% of bolus dose) has been reported 46. Of note, the pro-relaxant effect we demonstrate with propofol occurs at a concentration (20uM) that is comparable to levels typically achieved during propofol used for either induction or maintenance of anesthesia.
In summary, the findings of 1) endogenous airway GABA levels existing at measurable levels in the airway, 2) GABA levels increasing following pro-contractile stimuli, 3) selective blockade of endogenous GABA activation of airway smooth muscle GABAA channels resulting in increased muscle force, 4) GABAA channel antagonism is greater under contracted versus resting muscle tone and 5) positive allosteric agonism with propofol enhances endogenous GABA mediated relaxation of precontracted tissue provides evidence of a prorelaxant GABAergic system existing in airway smooth muscle that contributes to the modulation of contractile force.
Acknowledgments
This work was supported by National Institutes of Health (Bethesda, Maryland) grant GM065281 (CWE) and was presented in part at the 2008 American Society of Anesthesiologists annual meeting in Orlando, Florida on October 20, 2008.
References
- 1.Eder W, Ege MJ, von ME. The asthma epidemic. N Engl J Med. 2006;355:2226–35. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 2.Sanderson MJ, Delmotte P, Bai Y, Perez-Zogbhi JF. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc Am Thorac Soc. 2008;5:23–31. doi: 10.1513/pats.200704-050VS. [DOI] [PubMed] [Google Scholar]
- 3.Rooke GA, Choi JH, Bishop MJ. The effect of isoflurane, halothane, sevoflurane, and thiopental/nitrous oxide on respiratory system resistance after tracheal intubation. Anesthesiology. 1997;86:1294–9. doi: 10.1097/00000542-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Eames WO, Rooke GA, Wu RS, Bishop MJ. Comparison of the effects of etomidate, propofol, and thiopental on respiratory resistance after tracheal intubation. Anesthesiology. 1996;84:1307–11. doi: 10.1097/00000542-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Pizov R, Brown RH, Weiss YS, Baranov D, Hennes H, Baker S, Hirshman CA. Wheezing during induction of general anesthesia in patients with and without asthma: a randomized blinded trial. Anesthesiology. 1995;82:1111–6. doi: 10.1097/00000542-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Brown RH, Wagner EM. Mechanisms of bronchoprotection by anesthetic induction agents: propofol versus ketamine. Anesthesiology. 1999;90:822–8. doi: 10.1097/00000542-199903000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Wiklund CU, Lindsten U, Lim S, Lindahl SG. Interactions of volatile anesthetics with cholinergic, tachykinin, and leukotriene mechanisms in isolated Guinea pig bronchial smooth muscle. Anesthesia and Analgesia. 2002;95:1650–5. doi: 10.1097/00000539-200212000-00032. [DOI] [PubMed] [Google Scholar]
- 8.Yamakage M, Namiki A. Cellular mechanisms of airway smooth muscle relaxant effects of anesthetic agents. J Anesth. 2003;17:251–8. doi: 10.1007/s00540-003-0194-4. [DOI] [PubMed] [Google Scholar]
- 9.Prakash YS, Iyanoye A, Ay B, Sieck GC, Pabelick CM. Store-operated Ca2+ influx in airway smooth muscle: Interactions between volatile anesthetic and cyclic nucleotide effects. Anesthesiology. 2006;105:976–83. doi: 10.1097/00000542-200611000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama T, Penheiter AR, Penheiter SG, Chini EN, Thompson M, Warner DO, Jones KA. Differential effects of volatile anesthetics on M3 muscarinic receptor coupling to the Galphaq heterotrimeric G protein. Anesthesiology. 2006;105:313–24. doi: 10.1097/00000542-200608000-00014. [DOI] [PubMed] [Google Scholar]
- 11.O’Shea SM, Wong LC, Harrison NL. Propofol increases agonist efficacy at the GABA(A) receptor. Brain Res. 2000;852:344–8. doi: 10.1016/s0006-8993(99)02151-4. [DOI] [PubMed] [Google Scholar]
- 12.Moore CT, Wilson CG, Mayer CA, Acquah SS, Massari VJ, Haxhiu MA. A GABAergic inhibitory microcircuit controlling cholinergic outflow to the airways. J Appl Physiol. 2004;96:260–70. doi: 10.1152/japplphysiol.00523.2003. [DOI] [PubMed] [Google Scholar]
- 13.Tohda Y, Ohkawa K, Kubo H, Muraki M, Fukuoka M, Nakajima S. Role of GABA receptors in the bronchial response: studies in sensitized guinea-pigs. Clinical and Experimental Allergy. 1998;28:772–7. doi: 10.1046/j.1365-2222.1998.00289.x. [DOI] [PubMed] [Google Scholar]
- 14.Chapman RW, Danko G, Rizzo C, Egan RW, Mauser PJ, Kreutner W. Prejunctional GABA-B inhibition of cholinergic, neurally-mediated airway contractions in guinea-pigs. Pulmonary Pharmacology. 1991;4:218–24. doi: 10.1016/0952-0600(91)90014-t. [DOI] [PubMed] [Google Scholar]
- 15.Mizuta K, Xu D, Pan Y, Comas G, Sonett JR, Zhang Y, Panettieri RA, Jr, Yang J, Emala CW., Sr GABAA receptors are expressed and facilitate relaxation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1206–16. doi: 10.1152/ajplung.00287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang YY, Wang S, Liu M, Hirota JA, Li J, Ju W, Fan Y, Kelly MM, Ye B, Orser B, O’Byrne PM, Inman MD, Yang X, Lu WY. A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat Med. 2007;13:862–7. doi: 10.1038/nm1604. [DOI] [PubMed] [Google Scholar]
- 17.Mizuta K, Osawa Y, Mizuta F, Xu D, Emala CW. Functional expression of GABAB receptors in airway epithelium. American Journal of Respiratory Cell and Molecular Biology. 2008;39:296–304. doi: 10.1165/rcmb.2007-0414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osawa Y, Xu D, Sternberg D, Sonett JR, D’Armiento J, Panettieri RA, Emala CW. Functional expression of the GABAB receptor in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;291:L923–31. doi: 10.1152/ajplung.00185.2006. [DOI] [PubMed] [Google Scholar]
- 19.van der ZM, Oldenziel WH, Rea K, Cremers TI, Westerink BH. Microdialysis of GABA and glutamate: analysis, interpretation and comparison with microsensors. Pharmacol Biochem Behav. 2008;90:135–47. doi: 10.1016/j.pbb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Rea K, Cremers TI, Westerink BH. HPLC conditions are critical for the detection of GABA by microdialysis. Journal of Neurochemistry. 2005;94:672–9. doi: 10.1111/j.1471-4159.2005.03218.x. [DOI] [PubMed] [Google Scholar]
- 21.Jooste E, Zhang Y, Emala CW. Rapacuronium preferentially antagonizes the function of M2 versus M3 muscarinic receptors in guinea pig airway smooth muscle. Anesthesiology. 2005;102:117–24. doi: 10.1097/00000542-200501000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Bailey CP, Maubach KA, Jones RS. Neurokinin-1 receptors in the rat nucleus tractus solitarius: pre- and postsynaptic modulation of glutamate and GABA release. Neuroscience. 2004;127:467–79. doi: 10.1016/j.neuroscience.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Seddik R, Schlichter R, Trouslard J. Corelease of GABA/glycine in lamina-X of the spinal cord of neonatal rats. Neuroreport. 2007;18:1025–9. doi: 10.1097/WNR.0b013e3281667c0c. [DOI] [PubMed] [Google Scholar]
- 24.Druzin M, Haage D, Johansson S. Bicuculline free base blocks voltage-activated K+ currents in rat medial preoptic neurons. Neuropharmacology. 2004;46:285–95. doi: 10.1016/j.neuropharm.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Gallos G, Gleason NR, Zhang Y, Pak SW, Sonett JR, Yang J, Emala CW. Activation of endogenous GABAA channels on airway smooth muscle potentiates isoproterenol-mediated relaxation. Am J Physiol Lung Cell Mol Physiol. 2008;295:L1040–7. doi: 10.1152/ajplung.90330.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chipperfield AR, Harper AA. Chloride in smooth muscle. Prog Biophys Mol Biol. 2000;74:175–221. doi: 10.1016/s0079-6107(00)00024-9. [DOI] [PubMed] [Google Scholar]
- 27.Wang YX, Kotlikoff MI. Muscarinic signaling pathway for calcium release and calcium-activated chloride current in smooth muscle. Am J Physiol. 1997;273:C509–19. doi: 10.1152/ajpcell.1997.273.2.C509. [DOI] [PubMed] [Google Scholar]
- 28.Kotlikoff MI, Kamm KE. Molecular mechanisms of beta-adrenergic relaxation of airway smooth muscle. Annu Rev Physiol. 1996;58:115–41. doi: 10.1146/annurev.ph.58.030196.000555. [DOI] [PubMed] [Google Scholar]
- 29.Bianchi MT, Haas KF, Macdonald RL. Structural determinants of fast desensitization and desensitization-deactivation coupling in GABAa receptors. Journal of Neuroscience. 2001;21:1127–36. doi: 10.1523/JNEUROSCI.21-04-01127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56:763–70. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 32.Yamakage M, Hirshman CA, Croxton TL. Inhibitory effects of thiopental, ketamine and propofol on voltage-dependent Ca2+ channels in porcine tracheal smooth muscle cells. Anesthesiology. 1995;83:1274–82. doi: 10.1097/00000542-199512000-00018. [DOI] [PubMed] [Google Scholar]
- 33.Lin CC, Shyr MH, Tan PP, Chien CS, Pan SL, Wang CC, Chiu CT, Yang CM. Mechanisms underlying the inhibitory effect of propofol on the contraction of canine airway smooth muscle. Anesthesiology. 1999;91:750–9. doi: 10.1097/00000542-199909000-00028. [DOI] [PubMed] [Google Scholar]
- 34.Fan SZ, Yu HY, Chen YL, Liu CC. Propofol concentration monitoring in plasma or whole blood by gas chromatography and high-performance liquid chromatography. Anesthesia and Analgesia. 1995;81:175–8. doi: 10.1097/00000539-199507000-00036. [DOI] [PubMed] [Google Scholar]
- 35.Dowrie RH, Ebling WF, Mandema JW, Stanski DR. High-performance liquid chromatographic assay of propofol in human and rat plasma and fourteen rat tissues using electrochemical detection. J Chromatogr B Biomed Appl. 1996;678:279–88. doi: 10.1016/0378-4347(95)00475-0. [DOI] [PubMed] [Google Scholar]
- 36.Conti G, Utri DD, Vilardi V, De Blasi RA, Pelaia P, Bufi M, Rosa G, Gaspareto G. Propofol induces bronchodilation in mechanically ventilated chronic obstructive pulmonary disease (COPD) patients. Acta Anaesthesiologica Scandinavica. 1993;37:105–9. doi: 10.1111/j.1399-6576.1993.tb03609.x. [DOI] [PubMed] [Google Scholar]
- 37.Cheng EY, Mazzeo AJ, Bosnjak ZJ, Coon RL, Kampine JP. Direct relaxant effects of intravenous anesthetics on airway smooth muscle. Anesthesia and Analgesia. 1996;83:162–8. doi: 10.1097/00000539-199607000-00028. [DOI] [PubMed] [Google Scholar]
- 38.Ouedraogo N, Marthan R, Roux E. The effects of propofol and etomidate on airway contractility in chronically hypoxic rats. Anesthesia and Analgesia. 2003;96:1035–41. doi: 10.1213/01.ANE.0000053236.52491.69. [DOI] [PubMed] [Google Scholar]
- 39.Hashiba E, Sato T, Hirota K, Hashimoto Y, Matsuki A. The relaxant effect of propofol on guinea pig tracheal muscle is independent of airway epithelial function and beta-adrenoceptor activity. Anesthesia and Analgesia. 1999;89:191–6. doi: 10.1097/00000539-199907000-00034. [DOI] [PubMed] [Google Scholar]
- 40.Ouedraogo N, Roux E, Forestier F, Rossetti M, Savineau JP, Marthan R. Effects of intravenous anesthetics on normal and passively sensitized human isolated airway smooth muscle. Anesthesiology. 1998;88:317–26. doi: 10.1097/00000542-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Belouchi NE, Roux E, Savineau JP, Marthan R. Interaction of extracellular albumin and intravenous anaesthetics, etomidate and propofol, on calcium signalling in rat airway smooth muscle cells. Fundam Clin Pharmacol. 2000;14:395–400. doi: 10.1111/j.1472-8206.2000.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 42.Hirota K, Ebina T, Sato T, Ishihara H, Matsuki A. Is total body weight an appropriate predictor for propofol maintenance dose? Acta Anaesthesiologica Scandinavica. 1999;43:842–4. doi: 10.1034/j.1399-6576.1999.430810.x. [DOI] [PubMed] [Google Scholar]
- 43.Hoymork SC, Raeder J. Why do women wake up faster than men from propofol anaesthesia? British Journal of Anaesthesia. 2005;95:627–33. doi: 10.1093/bja/aei245. [DOI] [PubMed] [Google Scholar]
- 44.Handa-Tsutsui F, Kodaka M. Propofol concentration requirement for laryngeal mask airway insertion was highest with the ProSeal, next highest with the Fastrach, and lowest with the Classic type, with target-controlled infusion. Journal of Clinical Anesthesia. 2005;17:344–7. doi: 10.1016/j.jclinane.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Luo W, Li YH, Yang JJ, Tian J, Xu JG. Cerebrospinal fluid and plasma propofol concentration during total intravenous anaesthesia of patients undergoing elective intracranial tumor removal. J Zhejiang Univ Sci B. 2005;6:865–8. doi: 10.1631/jzus.2005.B0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuipers JA, Boer F, Olieman W, Burm AG, Bovill JG. First-pass lung uptake and pulmonary clearance of propofol: assessment with a recirculatory indocyanine green pharmacokinetic model. Anesthesiology. 1999;91:1780–7. doi: 10.1097/00000542-199912000-00032. [DOI] [PubMed] [Google Scholar]