Abstract
Anti-RNA polymerase III (RNAP III) antibodies are highly specific for scleroderma (SSc) and associated with diffuse SSc and renal crisis. Coexistence of anti-RNAP III and other SSc autoantibodies is rarely documented. We report three cases with coexisting anti-RNAP III and anti-U1RNP. Autoantibodies in 3829 sera from rheumatology clinics were screened by immunoprecipitation. Anti-RNAP III-positive sera were also examined by immunofluorescence and anti-RNAP III ELISA. In total, 35 anti-RNAP III-positive sera were identified by immunoprecipitation, in which three had coexisting anti-U1RNP. All three were anti-RNAP III ELISA positive. Two had anti-RNAP I dominant (vs. RNAP III) reactivity and showed strong nucleolar staining. A case with anti-U1/U2RNP (U2RNP dominant) had systemic lupus erythematosus (SLE)–SSc overlap syndrome; however, the remaining two cases had SLE without signs of SSc. All three cases of anti-RNAP III + U1RNP fulfilled ACR SLE criteria but none in the group with anti-RNAP III alone (p = 0.0002). In contrast, only one case in the former group had sclerodermatous skin changes and Raynaud’s phenomenon, vs. 92% with scleroderma in the latter (p < 0.05). Although anti-RNAP III is highly specific for SSc, cases with coexisting anti-U1RNP are not so uncommon among anti-RNAP III positives (8%, 3/35) and may be SLE without features of SSc.
Keywords: anti-RNA polymerase III antibodies, anti-U1RNP antibodies, autoantibodies, scleroderma
Introduction
Several autoantibodies, such as anti-topoisomerase I (topo I), anti-RNA polymerase III (RNAP III), anti-centromere (ACA), anti-U3RNP/fibrillarin, anti-Th/To, anti-PM-Scl, and anti-U1RNP are associated with a diagnosis of scleroderma (systemic sclerosis, SSc). Among them, anti-topo I and anti-RNAP III antibodies are considered highly specific for SSc, and their detection in other diseases verified by reliable methods is rare.1–3 Each of these autoantibodies is associated with a unique clinical subset of SSc: anti-topo I, RNAP III, and U3RNP are associated with diffuse SSc; ACA and anti-Th/To with limited SSc; and anti-PM-Scl and anti-U1RNP with SSc overlap syndrome. In addition, SSc-related autoantibodies are linked with particular clinical manifestations, such as anti-topo I with severe interstitial lung disease (ILD), and anti-RNAP III with scleroderma renal crisis.1,2 An interesting yet unexplained characteristic that allows relatively clean clinical subsetting and analysis based on autoantibody specificity in SSc is the rare coexistence of more than one SSc-related autoantibody specificity.1
Anti-RNAP I/III was described as a disease marker of SSc over 20 years ago; however, immunoprecipitation (IP) has been the only method to test this specificity until several years ago when commercial enzyme-linked immunosorbent assay (ELISA) kits became widely available.2,3 Although coexistence of anti-RNAP III and other SSc-related autoantibodies is reported more frequently by ELISA,4–8 cases verified by IP are rarely described. We here report three cases of anti-RNAP III coexisting with anti-U1RNP verified by IP, two of them without any signs of SSc.
Patients and methods
Patients
All 1966 subjects enrolled in the University of Florida Center for Autoimmune Diseases (UFCAD) registry from 2000–2010 were studied. Diagnoses of the patients include 434 cases of systemic lupus erythematosus (SLE), 119 SSc, 85 cases of polymyositis/dermatomyositis (PM/DM), and various other diagnoses. In addition, 1466 sera (including 208 SLE) from University of North Carolina Hospitals, 149 (62 SLE, 67 PM/DM, 20 Sjögren’s syndrome) from University of Guadalajara, 248 sera (26 PM/DM, 57 SSc, 113 SLE, and 52 primary anti-phospholipid syndrome) from Spedali Civili di Brescia (Brescia, Italy) were also screened for autoantibodies. The total number of SLE sera tested was 817. Clinical information was from the database (patients from University of Florida and University of North Carolina) or charts (all institutes). The protocol was approved by the Institutional Review Board. This study meets and is in compliance with all ethical standards in medicine, and informed consent was obtained from all patients according to the Declaration of Helsinki.
Immunoprecipitation
Autoantibodies in sera from patients were screened by IP using 35S-methionine-labeled K562 cell extracts. Anti-RNAP III, anti-U1RNP and other autoantibodies were determined using reference sera.9,10 Analysis of RNA components of immunoprecipitates was by urea-PAGE and silver staining (Bio-Rad Laboratories, Inc., Hercules, CA, USA).11
Immunofluorescence antinuclear antibodies
Immunofluorescence antinuclear (ANA)/cytoplasmic antibodies (HEp-2 ANA slides; INOVA Diagnostics, San Diego, CA, USA) were tested using a 1:80-diluted human serum and DyLight488 donkey IgG F(ab)’2 anti-human IgG (gamma chain specific, 1:200 dilution; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA).12
ELISA
Sera were tested for IgG anti-RNAP III antibodies using a commercial ELISA kit (QUANTA Lite® RNA Pol III, INOVA Diagnostics, San Diego, CA, USA) following the manufacturer’s instruction.
Statistical analysis
Data between groups were compared by Fisher’s exact test (frequency) or Mann–Whitney test (levels) using Prism 5.0 for Macintosh (GraphPad Software, Inc., San Diego, CA, USA); p < 0.05 was considered significant.
Results
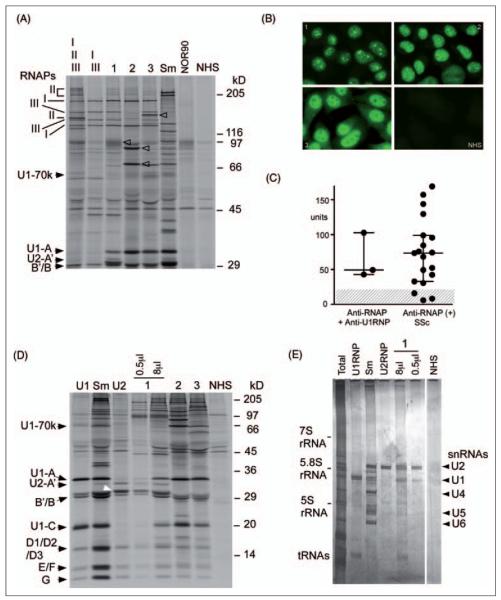
Autoantibodies to RNA polymerase I/III were detected by IP in 21 patients from UFCAD, 11 from North Carolina, one from Guadalajara, and two from Brescia. Virtually all sera with anti-RNAP III also had anti-RNAP I, and usually the intensity of RNAP III was dominant, or RNAP I and III were similar.13,14 Three of 35 anti-RNAP III sera (8%) had coexisting anti-U1RNP, including one that had predominant anti-U2RNP reactivity (Figure 1A). Intensity of RNAP I in IP appeared to be stronger than that of RNAP III in cases 1 and 2 (I > III, Table 1), different from the pattern of the majority of patients with SSc,14 but consistent with strong nucleolar staining in these sera (Figure 1B, panels 1 and 2). Intensity of RNAP I and III was comparable in case 3 (I = III, Table 1), and positive nucleolar staining of this serum was less clear when the nuclear staining was strong (Figure 1B, panel 3); this is consistent with similar observations described for many anti-RNAP I/III-positive SSc patients not reported to be nucleolar positive in routine clinical laboratories.15 In any case, these results are consistent with known distributions of RNAPs; RNAP III and II localize to the nuclei, whereas RNAP I localizes to nucleoli.16,17 Levels of anti-RNAP III were tested by ELISA, comparing cases with anti-RNAP III + U1RNP vs. anti-RNAP III-positive SSc (Figure 1C). All three in the former group were anti-RNAP III ELISA positive, and their levels were not lower than those in the latter. The presence of anti-U1RNP antibodies by IP is shown in Figure 1D. A serum sample from case 1 immunoprecipitated U1 and U2snRNP (Figure 1D and 1E) with unusual U2RNP dominant reactivity,18,19 as shown in protein IP (Figure 1D) and analysis of immunoprecipitated RNA components using a small amount (0.5 μl) of serum (Figure 1E, lane).
Figure 1. Anti-RNA polymerases and UsnRNPs autoantibodies.
A. 8% SDS-PAGE analysis of immunoprecipitation resolving the high molecular weight components of RNAPs. 35S-methionine-labeled K562 cell extract was immunoprecipitated by serum samples from patients with anti-RNA polymerases and anti-U1RNP and controls. Samples were analyzed by 8% SDS-PAGE. Lane I, II, III, anti-RNAP I/II/III; I ,III, anti-RNAP I/III; lanes 1–3, cases 1–3 with anti-RNAP III and -U1RNP as listed in Table 1; Sm, anti-Sm; NOR90, anti-NOR90, NHS, normal human serum. The positions of the two largest subunits of RNA polymerase I, II, and III, components of U1 and U2 snRNPs, and molecular weight markers are indicated. Arrowheads indicate NOR90, Ku (p70/p80), and RNA helicase A in lanes 1–3, respectively. B. Immunofluorescence. HEp-2 ANA slide was stained with sera from cases 1 – 3 and normal human serum (NHS) at 1:80 dilutions. C. Anti-RNAP III levels by ELISA. Sera from the three cases with anti-RNAP III and -U1RNP vs. anti-RNAP III alone (n=19) were tested by ELISA. Cut-off (<20 units) is shown by shaded area. D. 12.5% SDS-PAGE analysis of the U1RNP components. Immunoprecipitated samples by sera with anti-RNAP III and -U1RNP (1–3) and controls were analyzed by 12.5% SDS-PAGE. Positions of components of U1RNP (left) and U2-B” (white arrowhead) and molecular weight markers are indicated. U1, Sm, U2, prototype human serum for anti-U1RNP, Sm, and U2RNP, respectively; NHS, normal human serum; 0.5μl, 8μl, amount of serum used for immunoprecipitation. E. Analysis of immunoprecipitated RNA components. RNA components immunoprecipitated by sera were analyzed by urea-PAGE and silver staining. Total, total RNA; U1RNP, Sm, U2RNP, prototype human sera for each specificity. Positions of U1, 2, 4, 5, and 6 snRNA are shown.
Table 1.
Three cases with anti-RNA polymerase III and -U1RNP antibodies
| Case | 1 | 2 | 3 |
|---|---|---|---|
| Anti-RNAP | I, II, III | I, II, III | I, II, III |
| I>III=II | I>II>>>III | I=III>II | |
| Anti-RNAPIII ELISA (>20 U, positive) | 49 U | 43-63 U | 103 U |
| Coexisting antibodies | U1/U2RNP (U2>U1) | U1RNP | U1RNP |
| NOR90 | Ku | RHA | |
| Diagnosis | SLE–SSc | SLE | SLE |
| Race/gender | Caucasian/F | African Am/F | Mexican/F |
| Age (onset/last visit) | 42/66 | 38/42 | 22/50 |
| Observation (after anti-RNAP III) (years) | 2 | 4 | 5 |
| Proximal scleroderma | − | − | − |
| Sclerodactyly | + | − | - |
| Pitting scar | + | − | − |
| ILD | + | − | − |
| Raynaud’s phenomenon | + | − | − |
| SLE criteria | Arthritis | Discoid | Photosensitivity |
| Serositis | Arthritis | Arthritis, Nephritis | |
| Anti-dsDNA | Serositis | Thrombocytopenia | |
| ANA | Hematologic | AIHA, Leukopenia | |
| Anti-dsDNA, ANA | Anti-Sm, ANA | ||
| Other | Sjogren’s syndrome | ||
| Hypothyroidism |
RNAP, RNA polymerases; RHA, RNA helicase A; F, female; ANA, antinuclear antibodies; AIHA, autoimmune hemolytic anemia.
Clinical features of the three interesting cases of coexisting anti-RNAP III and anti-U1RNP are summarized in Table 1. Case 1 is a 66-year-old Caucasian female who developed Raynaud’s phenomenon and skin tightening in 1982 and was diagnosed with CREST syndrome. Later, during the 1990s she developed deforming arthritis and sicca symptoms, and also had a diagnosis of hypothyroidism. She was admitted to a hospital in June 2006 for chest pain thought to be due to pleuropericarditis with a pericardial effusion, and was referred to the UFCAD. Sclerodactyly, digital pitting scars, flexion contracture and subluxation of metacarpophalangeal and proximal interphalangeal joints, ulnar deviation, subcutaneous calcinosis, teleangiectasia, and ILD, were noted. ANA (speckled pattern, titer > 1: 1280), anti-dsDNA (Crithidia, 1: 20) and anti-RNAPI/III were positive. She was classified as having a SSc–SLE overlap syndrome.
Case 2 was included in the previous study on anti-RNAP II autoantibodies in SLE and SLE overlap syndrome.9 This patient already had SLE at the initial visit, and six sequential sera over a 4-year period were tested by IP and anti-RNAP III ELISA. IP patterns were nearly identical in all sera. The anti-RNAP III ELISA units did not change significantly, fluctuating between 43 and 63 units (data not shown). No Raynaud’s phenomenon or sclerodermatous features were noted during the 4-year observation.
Case 3 is a Mexican female with a long history of SLE. She developed thrombocytopenia in May 1982 at age 22 during her first pregnancy, and also had discoid rash and leukopenia. She developed autoimmune hemolytic anemia during her second pregnancy in 1983, and ANA and anti-Sm antibodies were detected. A diagnosis of SLE was made and she was treated with prednisone and hydroxychloroquine. During 1990s she had flares of SLE with thrombocytopenia and hemolytic anemia, discoid rash, polyarthritis, nephrotic syndrome, and chronic renal failure. She was treated with prednisone and azathioprine, followed by cyclophosphamide from 1994 to 2010. No Raynaud’s phenomenon or scleroderma features were noted during observation.
Clinical features of the three cases of anti-RNAP III + anti-U1RNP were compared with those in patients with anti-RNAP III alone (Table 2). All three cases in the former group fulfilled American College of Rheumatology criteria of SLE, but none in the latter group (p = 0.0002). In contrast, only one case in the former group had sclerodermatous skin changes or Raynaud’s phenomenon (p < 0.05).
Table 2.
Clinical features of patients with anti-RNAP III+anti-U1RNP vs. anti-RNAP III
| Anti-RNAP III+U1RNP | Anti-RNAP III | |
|---|---|---|
| n | 3 | 32 |
| SLE (ACR criteria) | 100%a | 0%a |
| Overlap syndrome | 33% | 0% |
| Scleroderma (ACR criteria) | 33%b,c | 91%b (−97%c) |
| Diffuse cutaneous scleroderma | 0%d | 77%d |
| Any sclerodermatous changes (sclerodactyly or proximal scleroderma) | 33%e | 91%e |
| Raynaud’s phenomenon | 33%f | 86%f |
| Anti-RNAP III ELISA positive | 100% | 84% |
p=0.0002.
p=0.0474.
p=0.0148; includes a case that can be considered systemic sclerosis sine scleroderma (one with ILD, one with Raynaud’s phenomenon, dilated cardiomyopathy and an episode consistent with scleroderma renal crisis).
p=0.0243; the number in anti-RNAP III groups is 17/22 due to availability of charts for review.
p=0.0474.
p=0.0913; the number in anti-RNAP III group is 19/22.
Discussion
To date, detection of anti-RNAP III in patients with a diagnosis other than SSc is uncommon.1 Most previous studies did not find any positives in non-SSc systemic autoimmune rheumatic diseases by IP.6,13,20 One study reported anti-RNAP III in one of 138 patients with SLE (case 2 in Table 1).9 Only one of 434 SLE patients from UFCAD (case 1, SLE–SSc overlap syndrome) and one of 62 Mexican patients with SLE (case 3) were positive in the present study. Although anti-RNAP III ELISA is a reliable test, there have been more positives in patients other than SSc reported, but prevalence is usually less than a few percent.4,6,21 Positives in non-SSc patients were usually only weakly positive;5,6,21 many of them were negative by IP and considered false-positive results caused by reactivity with contaminating bacterial components.6,21
Mutual exclusiveness of SSc-related autoantibodies in each patient is well described.1–3 Most studies using IP confirmed the absence of coexistence of anti-RNAP III with other SSc-related autoantibodies.5,13,22 A case of SLE with the coexistence of anti-RNAP I/II/III with anti-U1RNP and Ku antibodies by IP, reported previously,9 is included in the present study as case 2. In studies using anti-RNAP III ELISA, the coexistence of other SSc-related autoantibodies has been reported more frequently.4–8 However, many cases in this category had only low levels of reactivity,7 and at least some were confirmed to be IP negative,5 suggesting that many of these may not be true cases of coexistence. Alternatively, it is possible that patients with low levels of anti-RNAP III tend to have coexisting SSc-related autoantibodies. Thus, although anti-RNAP III ELISA is a clinically useful assay, false positives still occur as discussed above. In cases of anti-RNAP III ELISA positives with a diagnosis other than SSc or coexistence of other SSc-related autoantibodies, in particular at low levels of reactivity, cautious interpretation is advised and confirmation by IP would be ideal.
The two patients with anti-RNAP III without signs of SSc in this study were somewhat surprising, considering the high disease specificity of anti-RNAP III. When anti-RNAP III developed in these patients, or how many years anti-RNAP III can precede the clinical manifestation of SSc, is unknown. Nevertheless, these patients were observed for several years after confirmation of the presence of anti-RNAP III. It is interesting to speculate that presence of anti-U1RNP antibodies affects the disease phenotype or progression. For example, TLR stimulation of type I IFN production by the anti-U1RNP-UsnRNPs immune complex23,24 may create an environment that is not optimal for the expression of the anti-RNAP III-related disease phenotype. It is also possible that the proper environment for anti-U1RNP production may be incompatible with development of anti-RNAP III-associated SSc features. This situation could be somewhat similar to different autoantibody production in animal models of SLE, in which NZB/NZW F1 mice produce anti-RNA helicase A vs. anti-snRNPs,25 or SJL/J mice produce anti-fibrillarin (U3RNP) vs. anti-ribosomal P antibodies,26 depending on different environments, as we reported. Another possibility is the potential effects of treatment for SLE, such as high-dose steroid and immunosuppressive therapy, which might have prevented the development of SSc-related features.
The clinical significance of anti-RNAP III detected in SLE patients is, of course, not known. Patients with anti-RNAP III can present without sclerodermatous skin changes but with internal organ involvement consistent with SSc, known as systemic sclerosis sine sclerodema.27–29 A patient with SLE with anti-RNAP III antibodies who developed renal crisis and was considered a SLE–sine scleroderma overlap syndrome has been described,30 suggesting that patients with SLE who develop acute renal failure may benefit from testing for anti-RNAP III antibodies. In some cases, internal organ involvement such as renal or lung disease may precede skin manifestation of SSc, and the detection of anti-RNAP III will provide useful diagnostic and prognostic information.
Anti-RNAP III antibodies confirmed by IP analysis in SLE are rare and found only in 0.3% of cases, based on our experience. It is estimated that around 1% of cases of anti-U1RNP-positive SLE also have anti-RNAP III. Although coexistence of anti-RNAP III and -U1RNP has been rarely reported, our data of 3/35 (8%) of anti-RNAP III-positive patients with coexisting anti-U1RNP suggest that it is not so uncommon. When anti-U1RNP antibodies are detected in patients, testing for anti-RNAP III antibodies is not always considered, but the present data suggest that careful evaluation of anti-RNAP III is necessary in SLE patients with anti-U1RNP.
In summary, we report three cases of coexisting anti-RNAP III and anti-U1RNP antibodies, one with SLE–SSc overlap syndrome and two cases with SLE without signs of SSc. Since anti-RNAP III-positive patients can develop life-threatening SSc internal organ involvement, the significance of anti-RNAP III in SLE will need to be evaluated carefully.
Acknowledgements
We would like to thank Marlene Sarmiento, Annie Chan and Matthew Paulus for assistance with clinical data collection and Jason YF Chan and Steven J Ross for technical assistance.
Funding Supported by NIH grant R01-AR40391 and M01R00082 from the US Public Health Service and by generous gifts from Lupus Link, Inc. (Daytona Beach, FL) and Mr Lewis M Schott to the University of Florida Center for Autoimmune Disease.
Footnotes
Conflict of interests RWB and TTW are employees of INOVA Diagnostics Inc. All other authors have no competing interests.
References
- 1.Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35:35–42. doi: 10.1016/j.semarthrit.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Steen VD. The many faces of scleroderma. Rheum Dis Clin North Am. 2008;34:1–15. doi: 10.1016/j.rdc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Satoh M, Vazquez-Del Mercado M, Chan EK. Clinical interpretation of antinuclear antibody tests in systemic rheumatic diseases. Mod Rheumatol. 2009;19:219–228. doi: 10.1007/s10165-009-0155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santiago M, Baron M, Hudson M, Burlingame RW, Fritzler MJ. Antibodies to RNA polymerase III in systemic sclerosis detected by ELISA. J Rheumatol. 2007;34:1528–1534. [PubMed] [Google Scholar]
- 5.Parker JC, Burlingame RW, Webb TT, Bunn CC. Anti-RNA polymerase III antibodies in patients with systemic sclerosis detected by indirect immunofluorescence and ELISA. Rheumatology (Oxford) 2008;47:976–979. doi: 10.1093/rheumatology/ken201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satoh T, Ishikawa O, Ihn H, et al. Clinical usefulness of anti-RNA polymerase III antibody measurement by enzyme-linked immunosorbent assay. Rheumatology (Oxford) 2009;48:1570–1574. doi: 10.1093/rheumatology/kep290. [DOI] [PubMed] [Google Scholar]
- 7.Cavazzana I, Angela C, Paolo A, Stefania Z, Angela T, Franco F. Anti-RNA polymerase III antibodies: a marker of systemic sclerosis with rapid onset and skin thickening progression. Autoimmun Rev. 2009;8:580–584. doi: 10.1016/j.autrev.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Meyer O, De Chaisemartin L, Nicaise-Roland P, et al. Anti-RNA polymerase III antibody prevalence and associated clinical manifestations in a large series of French patients with systemic sclerosis: a cross-sectional study. J Rheumatol. 2010;37:125–130. doi: 10.3899/jrheum.090677. [DOI] [PubMed] [Google Scholar]
- 9.Satoh M, Ajmani AK, Ogasawara T, et al. Autoantibodies to RNA polymerase II are common in systemic lupus erythematosus and overlap syndrome. Specific recognition of the phosphorylated (IIO) form by a subset of human sera. J Clin Invest. 1994;94:1981–1989. doi: 10.1172/JCI117550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh M, Langdon JJ, Hamilton KJ, et al. Distinctive immune response patterns of human and murine autoimmune sera to U1 small nuclear ribonucleoprotein C protein. J Clin Invest. 1996;97:2619–2626. doi: 10.1172/JCI118711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamasaki Y, Yamada H, Nozaki T, et al. Unusually high frequency of autoantibodies to PL-7 associated with milder muscle disease in Japanese patients with polymyositis/dermatomyositis. Arthritis Rheum. 2006;54:2004–2009. doi: 10.1002/art.21883. [DOI] [PubMed] [Google Scholar]
- 12.Yamasaki Y, Narain S, Hernandez L, et al. Autoantibodies against the replication protein A complex in systemic lupus erythematosus and other autoimmune diseases. Arthritis Res Ther. 2006;8:R111–R120. doi: 10.1186/ar2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okano Y, Steen VD, Medsger TAJ. Autoantibody reactive with RNA polymerase III in systemic sclerosis. Ann Intern Med. 1993;119:1005–1013. doi: 10.7326/0003-4819-119-10-199311150-00007. [DOI] [PubMed] [Google Scholar]
- 14.Satoh M, Kuwana M, Ogasawara T, et al. Association of autoantibodies to topoisomerase I and the phosphorylated (IIO) form of RNA polymerase II in Japanese scleroderma patients. J Immunol. 1994;153:5838–5848. [PubMed] [Google Scholar]
- 15.Yamasaki Y, Honkanen-Scott M, Hernandez L, et al. Nucleolar staining cannot be used as a screening test for the scleroderma marker anti-RNA polymerase I/III antibodies. Arthritis Rheum. 2006;54:3051–3056. doi: 10.1002/art.22043. [DOI] [PubMed] [Google Scholar]
- 16.Hirakata M, Okano Y, Pati U, et al. Identification of autoantibodies to RNA polymerase II. Occurrence in systemic sclerosis and association with autoantibodies to RNA polymerases I and III. J Clin Invest. 1993;91:2665–2772. doi: 10.1172/JCI116505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones E, Kimura H, Vigneron M, Wang Z, Roeder RG, Cook PR. Isolation and characterization of monoclonal antibodies directed against subunits of human RNA polymerases I, II, and III. Exp Cell Res. 2000;254:163–172. doi: 10.1006/excr.1999.4739. [DOI] [PubMed] [Google Scholar]
- 18.Mimori T, Hinterberger M, Pettersson I, Steitz JA. Autoantibodies to the U2 small nuclear ribonucleoprotein in a patient with scleroderma-polymyositis overlap syndrome. J Biol Chem. 1984;259:560–565. [PubMed] [Google Scholar]
- 19.Craft J, Mimori T, Olsen TL, Hardin JA. The U2 small nuclear ribonucleoprotein particle as an autoantigen. J Clin Invest. 1988;81:1716–1724. doi: 10.1172/JCI113511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bardoni A, Rossi P, Salvini R, Bobbio-Pallavicini F, Caporali R, Montecucco C. Autoantibodies to RNA-polymerases in Italian patients with systemic sclerosis. Clin Exp Rheumatol. 2003;21:301–306. [PubMed] [Google Scholar]
- 21.Kuwana M, Okano Y, Pandey JP, Silver RM, Fertig N, Medsger TA., Jr Enzyme-linked immunosorbent assay for detection of anti-RNA polymerase III antibody: analytical accuracy and clinical associations in systemic sclerosis. Arthritis Rheum. 2005;52:2425–2432. doi: 10.1002/art.21232. [DOI] [PubMed] [Google Scholar]
- 22.Codullo V, Cavazzana I, Bonino C, et al. Serologic profile and mortality rates of scleroderma renal crisis in Italy. J Rheumatol. 2009;36:1464–1469. doi: 10.3899/jrheum.080806. [DOI] [PubMed] [Google Scholar]
- 23.Savarese E, Chae OW, Trowitzsch S, et al. U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood. 2006;107:3229–3234. doi: 10.1182/blood-2005-07-2650. [DOI] [PubMed] [Google Scholar]
- 24.Lee PY, Kumagai Y, Li Y, et al. TLR7-dependent and Fc{gamma}R-independent production of type I interferon in experimental mouse lupus. J Exp Med. 2008;205:2995–3006. doi: 10.1084/jem.20080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida H, Satoh M, Behney KM, et al. Effect of an exogenous trigger on the pathogenesis of lupus in NZB NZW (F1) mice. Arthritis Rheum. 2002;46:2235–2244. doi: 10.1002/art.10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satoh M, Hamilton KJ, Ajmani AK, et al. Induction of anti-ribosomal P antibodies and immune complex glomerulonephritis in SJL mice by pristane. J Immunol. 1996;157:3200–3206. [PubMed] [Google Scholar]
- 27.Molina JF, Anaya JM, Cabrera GE, Hoffman E, Espinoza LR. Systemic sclerosis sine scleroderma: an unusual presentation in scleroderma renal crisis. J Rheumatol. 1995;22:557–560. [PubMed] [Google Scholar]
- 28.Phan TG, Cass A, Gillin A, Trew P, Fertig N, Sturgess A. Anti-RNA polymerase III antibodies in the diagnosis of scleroderma renal crisis sine scleroderma. J Rheumatol. 1999;26:2489–2492. [PubMed] [Google Scholar]
- 29.Poormoghim H, Lucas M, Fertig N, Medsger TA., Jr Systemic sclerosis sine scleroderma: demographic, clinical, and serologic features and survival in forty-eight patients. Arthritis Rheum. 2000;43:444–451. doi: 10.1002/1529-0131(200002)43:2<444::AID-ANR27>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 30.Horn HC, Ottosen P, Junker P. Renal crisis in asclerodermic scleroderma–lupus overlap syndrome. Lupus. 2001;10:886–888. doi: 10.1191/096120301701548382. [DOI] [PubMed] [Google Scholar]