Abstract
Background
EGFR dysregulation occurs in both smoking-related and non-smoking-related NSCLC. In non-smoking-related NSCLC, dysregulation results primarily from mutation of EGFR while in smoking-related NSCLC the molecular mechanisms are incompletely understood. Activation of EGFR is associated with auto-phosphorylation of the receptor (p-EGFR) and has been shown in vitro to result in upregulation of cyclo-oxygenase 2 (COX-2). We sought to determine the relationship between activated EGFR (p-EGFR) and COX-2 in vivo and whether these are associated with clinical outcome in smoking-related NSCLC.
Methods
The expression of p-EGFR, EGFR, and COX-2 was studied by immunohistochemistry in 77 surgically-resected stage I/II non-small cell lung cancers (NSCLCs) from smokers. EGFR mutational status was determined by sequencing exons 18–21. Correlation of expression with clinical outcome and other biomarkers, including Ki-67 and microvessel density (MVD), was also examined.
Results
One tumor sample had EGFR mutation (L858R). EGFR overexpression, defined as membranous staining in more than 10% of cancer cells, was found in 37 patients (48.1%). Cytoplasmic staining of p-EGFR in at least 5% of cancer cells was found in 22 of 77 (28.6%) of tumor samples. Forty-five patients (58.4%) showed COX-2 overexpression (cytoplasmic granular staining in more than 10% of cancer cells). Expression of p-EGFR was significantly associated with COX-2 overexpression (p = 0.047), and showed a modest relationship with EGFR overexpression and high Ki-67 (p = 0.087, and 0.092, respectively). COX-2 overexpression also had a significant association with high Ki-67 expression (p = 0.011). No other significant associations were found with Ki-67 or MVD. Expression of p-EGFR was significantly related with a short disease free survival (p = 0.045) but not overall survival. However, neither EGFR nor COX-2 overexpression was associated with prognosis (p > 0.05).
Conclusions
In smoking-related NSCLC, activation of EGFR as reflected by receptor autophosphorylation is significantly associated with COX-2 overexpression and high proliferative activity in lung cancer cells. p-EGFR may better predict increased malignant potential and worse prognosis of early stage NSCLC than EGFR overexpression alone. Therapeutic strategies targeting both EGFR and COX-2 may be needed in smoking-related NSCLC.
Keywords: lung cancer, epidermal growth factor receptor, cyclo-oxygenase, survival
INTRODUCTION
Lung cancer remains the leading cause of cancer-related death in both sexes1 and non-small cell lung cancer (NSCLC) accounts for more than 85% of all lung cancers.2 Approximately 25% of patients with NSCLC present with early-stage disease (stage I and II)3 for whom surgical resection with appropriate lymph node staging has been the mainstay of treatment. The clinical outcome of patients with NSCLC treated with surgery is variable and the disease is also thought to have heterogeneous etiology. Although, smoking is strongly associated with lung cancer development, approximately 15% of patients who develop lung cancer have never smoked. Recently, the distinction between smoking-related NSCLC and non-smoking-related NSCLC became more distinct with the observation of frequent response to EGFR-TKI agents in non-smokers with advanced NSCLC4 and the subsequent identification of EGFR mutations5,6–primarily among never smokers.7 The importance of the differential mechanisms of EGFR dysregulation in the smoking and non-smoking-related NSCLC was highlighted by the marked difference in efficacy of erlotinib in these two subgroups.8
EGFR is a member of the ErbB receptor tyrosine kinase family and a 170-kDa single-pass transmembrane tyrosine kinase9. Specific ligands, such as epidermal growth factor (EGF) and transforming growth factor-α (TGF-α), bind to EGFR resulting in receptor dimerization and activation of tyrosine kinase, with receptor auto-phosphorylation and downstream signal transduction.10–12 Signaling through EGFR has been reported to induce proliferation, invasion, and angiogenesis of tumor cells.13,14 EGFR is frequently overexpressed in both cell lines and tissues of NSCLC15–17 and overexpression has been reported to be associated with higher incidence of lymph node metastasis, advanced stage, poor differentiation, and a worse prognosis.18–21 However, several reports, including a meta-analysis failed to confirm a correlation between overexpression of EGFR and patient outcome.17,22 In addition, no correlation was found between EGFR expression and tumor response to gefitinib, an EGFR tyrosine kinase inhibitor, in patients with NSCLC.23 Therefore, the clinical significance of EGFR expression in NSCLC is still controversial.
As EGFR activation after ligand binding is important for downstream signal transduction, the activated form of EGFR may have more significance in predicting tumor aggressiveness and a worse prognosis in NSCLC. Recently, in a series of 36 patients, expression of phosphorylated EGFR (p-EGFR) was found to be associated with poor prognosis.24 Although this was the first study to evaluate the clinical significance of an activated form of EGFR, the study population was relatively small and approximately half of patients had advanced stage disease.
Cyclooxygenase-2 (COX-2) overexpression has also been described in human malignancies, including colon, head and neck, and NSCLC,25–28 in which COX-2 expression is correlated with poor prognostic outcome.27 Although the exact molecular mechanisms underlying the activity of COX-2 remain unclear, interaction between EGFR and COX-2 pathway has been suggested. For example, EGFR activation stimulates COX-2 production in colon cancer cells resulting in increased mitotic activity.29 Furthermore, EGFR ligand-induced biological effects can be blocked by a selective COX-2 inhibitor.30 Thus, there appears to be a relationship between activated EGFR (p-EGFR) and COX-2 activity in cultured tumor cells. In H&N cancer, smokers have higher levels of COX-2 in the oral mucosa, which is thought to result from signaling through EGFR.31 However, a study of 172 patients with NSCLC failed to find an association between EGFR expression and COX-2 expression.32
To better understand the role EGFR signaling plays in smoking-related NSCLC and it’s relationship to in vivo expression of COX-2, we evaluated the expression of EGFR, p-EGFR and COX-2 expression in early-stage resected NSCLC from a group of smokers, and investigated their correlation with other biomarkers, including Ki-67 proliferation index and microvessel density (MVD).
MATERIALS AND METHODS
Study Population
Seventy-seven patients who were current or former smokers (>100 cigarettes in their lifetime) with previously untreated NSCLCs from the Durham Veterans Affairs Hospital were included in this study. The characteristics of patients were as Table 1. Morphological classification of the carcinomas was assigned according to the WHO criteria; 39 patients had squamous cell carcinomas, and 38 had non-squamous cell carcinomas (Table 1). All patients were staged at the time of surgery following the guidelines of the American Joint Committee on Cancer Staging33 and all patients had mediastinal lymph node evaluation at the time of surgery by mediastinoscopy and/or mediastinal lymph node sampling as currently recommended for early stage NSCLC.34 Fifty-nine patients had stage I tumors and 18 patients had stage II tumors. All patients had surgical resection of the primary tumor. Six patients received adjuvant or neoadjuvant therapy; two patients received adjuvant chemotherapy (T2N0 and T2N1) on clinical trials, one patient received neoadjuvant combined chemotherapy/radiation (T2N1) on a clinical trial, and three patients received post-operative radiation therapy for positive margins (one T1N0 and two T1N1). The median follow-up of surviving patients at the time of analysis was 35 months (range, 4–84 months). Follow-up data were obtained from medical records. Survival times were measured from the date of surgery to the time of death or time of last follow-up observation.
Table 1.
Characteristics of Patients
| Characteristics | No. (%) | |
|---|---|---|
| Age | Median | 69 |
| Range | 51 – 86 | |
| Gender | Male | 77 (100.0) |
| Female | 0 (0.0) | |
| Histology | Squamous | 39 (50.6) |
| Adeno | 27 (35.1) | |
| Large cell | 6 (7.8) | |
| Untyped | 5 (6.5) | |
| Pathologic stage | I | 59 (76.6) |
| II | 18 (23.4) | |
| T stage | 1 | 34 (44.2) |
| 2 | 40 (51.9) | |
| 3 | 3 (3.9) | |
| N stage | 0 | 67 (87.0) |
| 1 | 10 (13.0) | |
| Surgery | Lobectomy | 56 (72.7) |
| Pneumonectomy | 6 (7.8) | |
| Wedge resection | 15 (19.5) | |
| Survival | Alive | 44 (57.1) |
| Dead | 33 (42.9) | |
| Recurrence | Non-recurrence | 55 (71.4) |
| Recurrence | 22 (28.6) |
Immunohistochemistry
From formalin-fixed, paraffin-embedded tissues, 5 μm sections were cut and placed onto positively charged glass slides. Tissue sections were deparaffinized and rehydrated. Antigen retrieval was performed by enzyme digestion for 5 min at 37 °C for EGFR. For p-EGFR and COX-2, sections were microwaved in 0.01 M citrate buffer (pH 6.0) twice for 5 minutes each at 600 W. After antigen retrieval, endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 10 min. After incubation with blocking solution for 10 min, sections were incubated with primary antibodies at the dilutions and conditions as in table 2. Sections were incubated with labeled HRP for 5 min and color was developed by 5 min incubation in diaminobezidine (DAB) solution. Slides were counterstained with hematoxylin. The detailed information of primary antibodies used in this study was as Table 2. Monoclonal antibody to phosphorylated EGFR recognizes residues of tyrosine 1068 of human EGFR, thus, only EGFRs phophorylated at tyrosine 1068 could be detected with this antibody. Blocking solution, labeled HRP and DAB chromogen were from Detection Kit, K4007 (Dako, Copenhagen, Denmark). Two investigators (S. J. K. and Z. N. R.) evaluated all slides independently and differences between the two observers were resolved by consensus. For EGFR staining, only membranous staining was considered as positive and the percentage of positive tumor cells were measured after three areas of maximum EGFR expression were selected for each case. A cut-off value of 10% positive cells was defined as overexpression of EGFR to avoid inclusion of scattered positivity of same intensity found in normal bronchial tissue as previously described.35 For COX-2, cytoplasmic granular staining was considered positive to avoid non-specific staining. Percentage of positive tumor cells was assessed as above mentioned method for EGFR and a same cut-off value was used for COX-2 overexpression as previously described.36 Positive expression of p-EGFR was defined as when more than 5% of cells had cytoplasmic staining as previously described.37
Table 2.
Immunohistochemistry Techniques
| Antibody | Clone | Company (location) | Dilution (duration) | Antigen retrieval method |
|---|---|---|---|---|
| EGFR | H11 (monoclonal/mouse) | Dako (Copenhagen, Denmark) | 1:200 (30 min) | Enzyme digestion (Proteinase K, Dako) |
| COX-2 | COX229 (monoclonal/mouse) | Zymed (San Francisco, CA) | 1:200 (60 min) | Heat, citrate buffer |
| p-EGFR | 1H12 (monoclonal/mouse) | Cell Signaling (Beverly, MA) | 1:500 (overnight) | Heat, citrate buffer |
| CD31 | JC70A (monoclonal/mouse) | Dako (Copenhagen, Denmark) | 1:20 (60 min) | Heat, citrate buffer |
| Ki-67 | NCL Ki67 (monoclonal/mouse) | Novocastra Lab (Newcastle, UK) | 1:500 (overnight) | Heat, citrate buffer |
Previous Immunohistochemistry
The expression of Ki-67, and microvessel density (MVD) have been analyzed in this series as previously described (Table 2).38 This immunohistochemistry results were compared with the staining of EGFR, p-EGFR, and COX-2.
EGFR Mutational Analysis
Two to three paraffin-embedded, formalin-fixed tissue sections of 8 μm thickness were scraped from slides, placed in 1.5 ml vials, dewax by washing twice in 1 mL xylene (30 min each), centrifuged for 10 min, followed by two ethanol washes (100% and 75%; 15 min each). After air drying, the tissue pellets were digested overnight at 50°C with 250 μl of proteinase K (200 μg/ml) in 50 mM Tris, 1 mM EDTD, and 0.5% Tween 20. Finally, the lysate was boiled for 10 min to inactivate the proteinase K and the 5 uL supernatant used as template for PCR.
Exons 18, 19, 20 and 21, which contain all EGFR mutations identified in lung cancer, were amplified by nested PCR. The primers used in this study were adopted from.6 PCR was performed in a total volume of 25 μL, containing 0.5 unit Platinum Taq (Invitrogen, catalog number 10966-026), 0.2mM dNTP, 1.5mM MgCl2, 20 mM Tris-HCl (pH 8.4), 50 mM KCl and 0.2 μM each primer. The reaction began at 94C (2 min), followed by 35 cycles of 94C (30 sec), 55C (30 sec) and 72C (1 min), and ended with a single final step at 72C (7 min). The first PCR product (1 μL) was further amplified with second primer pair in the same conditions. PCR products were then purified by QIAquick PCR purification kit (Qiagen, catalog number 28104), and subjected to dye-terminator sequencing (ABI 3730) using the second forward PCR primer. Samples harboring mutations were resequenced on the opposite strand and confirmed in an independent PCR reaction.
Statistical Analysis
The Fisher’s exact test was applied to assess the association between categorical variables. Disease free survival and overall survival were calculated using the Kaplan-Meier method and compared by the log-rank test. The Cox proportional hazards regression model was used for multivariate analyses. All the statistical analyses were performed using a statistical software package (SPSS, Version 10.0, Inc., Chicago, IL). Statistical significance was defined as p values less than 0.05. All p values were two-sided.
RESULTS
EGFR Mutational Analysis
We screened all 77 tumor samples for EGFR mutation using sequence analysis. One tumor sample (2281) had a heterozygous T to G change at nucleotide 2573 of the coding sequence corresponding to the commonly reported L858R mutation. No sequence variants were detected in the remaining 76 tumor samples, confirming that the predominant tumor type included in this study is smoking-related.
EGFR, COX-2, and p-EGFR immunohistochemistry
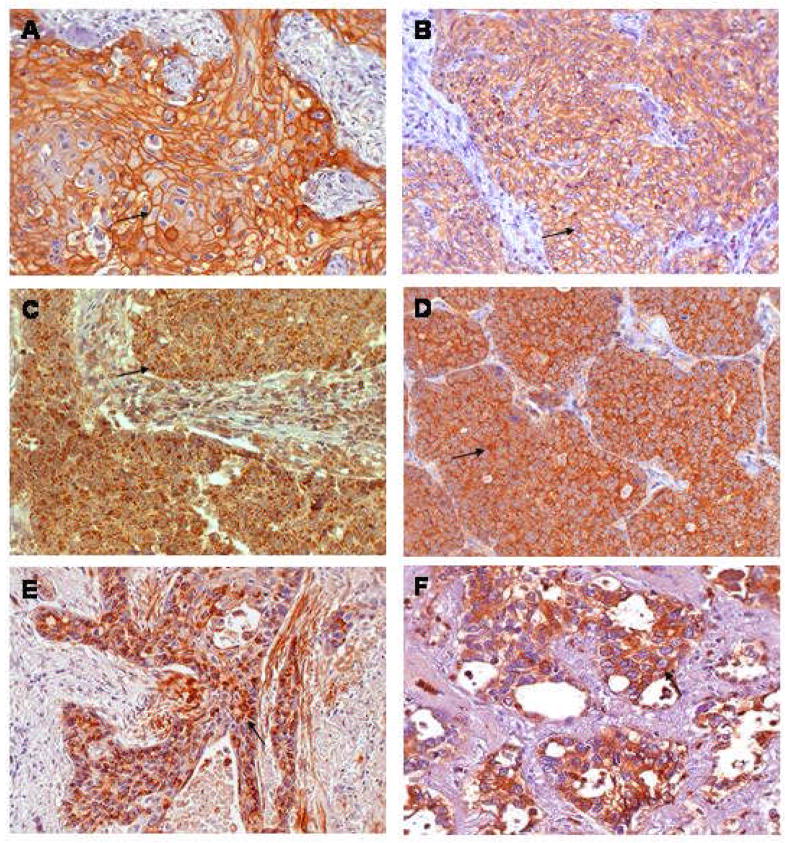
Among tumor samples from 77 patients, 37 (48.1%) showed EGFR expression in more than 10% of cancer cells, which was predominantly membraneous staining (Fig. 1). COX-2 overexpression, defined as granular cytoplasmic staining in > 5% of cells, occurred in 45 of 77 (58.4%) of tumors (Fig. 1). Presence of activated EGFR, defined as 5% of cells positive for p-EGFR, was observed in 22 (28.6 %) patients (Fig. 1). EGFR overexpression was significantly associated with squamous cell carcinoma (p < 0.05); 28 of 39 (71.8 %) squamous cell carcinomas had overexpression of EGFR compared to only 9 of 38 (23.6%) of non-squamous cell carcinomas. However, there was no significant correlation of COX-2 overexpression or p-EGFR expression with histology.
Figure 1.
Representative Photomicrographs of Immunohistochemistry.
Other pathological parameters, including tumor size, nodal involvement, and pathologic stage failed to show any significant association with overexpression of EGFR or COX-2 or positive p-EGFR expression (data not shown).
Associations of EGFR, COX-2, and p-EGFR with Ki-67 and MVD
Expression of p-EGFR was significantly associated with COX-2 overexpression (p = 0.047), and showed a modest relationship with EGFR overexpression (p = 0.087). However, no association was found between EGFR and COX-2 overexpression. COX-2 overexpression had a clear association with high Ki-67 expression (p = 0.011) (Table 3). Expression of p-EGFR had a marginal significance with high Ki-67 index (p = 0.092). MVD showed no association with p-EGFR, EGFR, and COX-2 overexpression (Table 3).
Table 3.
Association between expressions of EGFR, p-EGFR, COX-2, Ki-67, and MVD
| Variables | p-EGFR expression | EGFR overexpression | COX-2 overexpression | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Negative | Positive | P valuea | Negative | Positive | P valuea | Negative | Positive | P valuea | |
| EGFR overexpression | |||||||||
| Negative | 31 | 9 | 0.087 | ||||||
| Positive | 21 | 16 | |||||||
| COX-2 overexpression | |||||||||
| Negative | 26 | 6 | 0.047 | 19 | 13 | 0.356 | |||
| Positive | 26 | 19 | 21 | 24 | |||||
| Ki-67 | |||||||||
| < median value | 22 | 17 | 0.497 | 30 | 9 | 0.092 | 22 | 17 | 0.011 |
| ≥ median value | 18 | 20 | 22 | 16 | 10 | 28 | |||
| Microvessel density | |||||||||
| < median value | 20 | 17 | 0.821 | 24 | 13 | 0.808 | 13 | 24 | 0.356 |
| ≥ median value | 20 | 20 | 28 | 12 | 19 | 21 | |||
Survival analysis
Thirty-three of 77 patients died during the follow-up period. The cause of death was primary tumor recurrence (n = 19) and other causes (n = 14) including infection, thus, the cancer-related death rate was 24.7% (19/77). To examine the importance of each marker to survival, univariate analysis was performed. When patients were divided into two groups based on the status of EGFR, COX-2, and p-EGFR, positive expression of p-EGFR showed statistically significant differences in disease free survival as illustrated by Kaplan-Meier curves (Figure 2). However, overexpression of EGFR and COX-2 was not associated with worse disease free survival (Figure 2). In univariate analysis of overall survival, positive expression of p-EGFR failed to show a significant relationship with shorter overall survival. There was no significant association of EGFR and COX-2 overexpression with overall survival, although COX-2 overexpression showed a marginal significance (p = 0.081).
Figure 2.
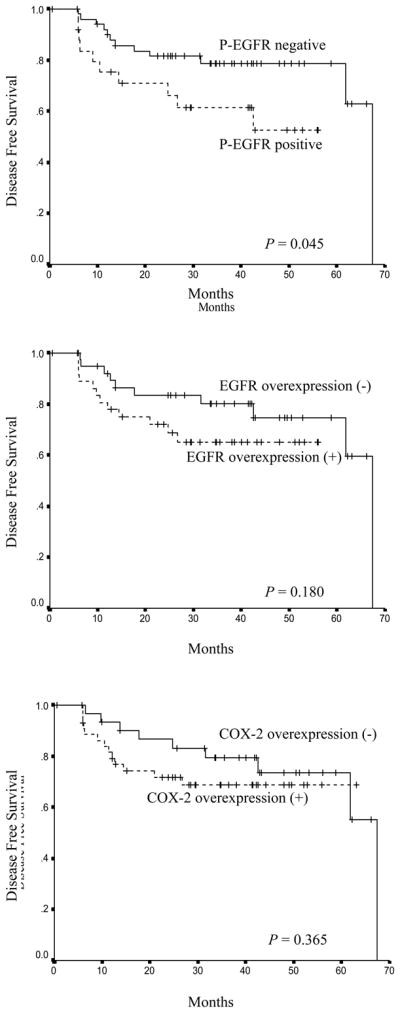
Effect of p-EGFR Expression and EGFR and COX-2 Over-expression on Disease-Free Survival in Early Stage Smoking-Related Non-Small Cell Lung Cancer. The method of Kaplan-Meier was used and groups were compared with the log-rank test. A. p-EGFR expression in > 5% of tumor cells (positive) versus < 5% (negative). B. EGFR overexpression in > 10% of tumor cells (positive) versus < 10% (negative). C. COX-2 overexpression in > 5% of tumor cells (positive) versus < 5% (negative).
In multivariate analysis including all three markers and other risk factors; age, pathologic tumor-node-metastasis stage, race and histology, only pathologic stage was found to be an independent prognostic indicator for poor overall survival. Positive expression of p-EGFR and overexpression of COX-2 and EGFR failed to show independent prognostic significance.
DISCUSSION
EGFR is composed of an extracellular ligand-binding domain, transmembrane segment, and an intracellular tyrosine kinase domain.39 Intracellular tyrosine kinase domain is activated by homo- or heterodimerization after ligand binding.40,41 Subsequently, intracellular proteins involved in signaling pathways are phosphorylated and activated, resulting in modulation of gene transcription.41 Dysregulated activation of EGFR has been reported in various malignant disorders including lung, breast, colorectal, and, head and neck leading to malignant transformation and tumor growth through the inhibition of apoptosis, cellular proliferation, promotion of angiogenesis, and metastasis.42,43 Dysregulated activation of EGFR results from overexpression of EGFR,44 ligand-independent activation by mutation of EGFR,44,45 and production of ligand leading to autocrine activation.46 However, lack of correlation between EGFR overexpression and patient outcome has been reported by several in prior studies.17,22 This might support EGFR overexpression itself does not reflect role of EGFR promoting tumor aggressiveness.
Three major signaling pathways mediating the downstream effects of EGFR activation at the cellular level include: (1) Ras-Raf-MAP kinase pathway, (2) Pphosphatidylinositol 3-kinase (PI-3 K)/Akt pathway, (3) Janus tyrosine kinase (Jak)/signal transducers and activators of transcription (STAT) pathway.47–49 Ligand-induced receptor activation results in the auto-phosphorylation of its own tyrosine residues creating binding sites for signal transduction molecules. Several auto-phosphorylation sites have been identified in the human EGFR and these sites are clustered in the C-terminal tail of the receptor.50 Tyrosine 1068 (Tyr 1068) is one of the major auto-phosphorylation sites and auto-phosphorylation of Tyr 1068 allows direct binding of Grb2, an adaptor protein.13,51 This binding results in activation of the Ras-Raf-MAP kinase through Grb2/Sos-1 signaling pathway.52
In this study, we have used monoclonal antibody to phosphorylated EGFR recognizing Tyr 1068 of human EGFR and demonstrated a significant relationship with disease free survival. Even though EGFR overexpression showed a marginal relation with p-EGFR expression (p = 0.087), EGFR overexpression failed to show a significant relation with disease free survival (p = 0.181). This suggests p-EGFR expression rather than EGFR overexpression may reflect functional significance of EGFR activation. Furthermore, p-EGFR expression was correlated with COX-2 overexpression (p = 0.047). Our results are consistent with the prior report of a lack of association of EGFR and COX-2 expression.32 EGFR activation have been reported to result in COX-2 expression in vitro53 and a transcription factor, NF-kB has been regarded as a mediator of this pathway.54 This is supported by the presence of NF-kB binding motif in the promoter region of COX-2 gene. Thus, auto-phosphorylation of EGFR activates the downstream signal transduction leading to increased COX-2 expression.
Tumor cell proliferation, a characteristic of tumor aggressiveness, is also associated with EGFR activation.13 Thus, auto-phosphorylation of EGFR promotes tumor cell proliferation through Ras-Raf-MAP kinase pathway. In this study, high Ki-67 index was significantly related with COX-2 overexpression (p = 0.011). Expression of p-EGFR also showed a modest relation with high Ki-67 (p = 0.092). This supports that EGFR may play a role augmenting activity of proliferation in tumor cells via downstream signal transduction or induction of COX-2 expression. Microvessel density (MVD) was not significantly related with p-EGFR expression and COX-2 overexpression (p > 0.05) in this study. EGFR and COX-2 have been demonstrated to play a role in tumor-associated angiogenesis, inducing the synthesis of angiogenic factors such as VEGF.55,56 However, other studies of EGFR and COX-2 expression also failed to find a correlation with MVD in NSCLC.28,57,58 The wide range of techniques in use to detect EGFR and COX-2 as well as differences in interpreting immunohistochemistry results might explain heterogeneity of reports. It is also possible that MVD may not reflect true tumor-associated new vessel formation. Thus, further study should be warranted using a marker to detect newly developed blood vessels such as endoglin (CD105).59
Although p-EGFR expression was significantly associated with worse disease free survival in univariate analysis, it failed to show independent prognostic significance in multivariate analysis. Several pitfalls of immunohistochemistry might lead to the bias underestimating true prognostic significance of p-EGFR expression such as subjective judgment of positive expression and the lack of standardized cut-off points for positive expression. A true association between p-EGFR expression and overall survival might have been confounded by the relatively large fraction of the patients in this study who died of causes other than lung cancer. Thus, standardization of techniques to determine p-EGFR expression and further study with a larger study population is warranted to further assess the prognostic significance of p-EGFR in smoking-related NSCLC.
While lung cancer has long been recognized as a collection of heterozygous diseases with diverse clinical and molecular characteristics, recent advances have allowed us to study a more homogeneous population by selecting all smokers and confirming a low incidence of the EGFR mutation, which is strongly associated with non-smoking tumors. Thus our results may not be comparable to studies that have included more heterogeneous patients, especially in regions of the world with higher rates of EGFR mutation.
In conclusion, p-EGFR expression may better predict worse disease free survival than EGFR overexpression itself, and this might be associated with COX-2 overexpression and high tumor cell proliferation. This suggests the role of p-EGFR expression as a prognostic indicator in early stage smoking-related NSCLC. Thus, evaluation of p-EGFR expression could contribute to define a subset of patients with higher risk of relapse after surgery and help to establish a postoperative treatment strategy such as targeted treatment against EGFR.
Acknowledgments
This work was supported by NCI grant R21-CA91565, U01-CA96123, and a VA merit review award (to MJK). We thank Marguerite Adkins for secretarial support.
References
- 1.Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer. 1995;75:191–202. doi: 10.1002/1097-0142(19950101)75:1+<191::aid-cncr2820751307>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Fry WA, Phillips JL, Menck HR. Ten-year survey of lung cancer treatment and survival in hospitals in the United States: a national cancer data base report. Cancer. 1999;86:1867–1876. doi: 10.1002/(sici)1097-0142(19991101)86:9<1867::aid-cncr31>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Miller VA, Kris MG, Shah N, et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:1103–1109. doi: 10.1200/JCO.2004.08.158. [DOI] [PubMed] [Google Scholar]
- 5.Lynch TJ, Bell DW, Sordella R, et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non-Small-Cell Lung Cancer to Gefitinib. N Engl J Med. 2004 doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 6.Paez JG, Janne PA, Lee JC, et al. EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy. Science. 2004 doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 7.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shepherd F, Pereira J, Ciuleanu T, et al. A randomized placebo-controlled trial of erlotinib in patients with advanced non-small cell lung cancer (NSCLC) following failure of 1st line or 2nd line chemotherapy. A National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) trial. J Clin Oncol. 2004;22:7022. [Google Scholar]
- 9.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 10.Raben D, Helfrich BA, Chan D, et al. ZD1839, a selective epidermal growth factor receptor tyrosine kinase inhibitor, alone and in combination with radiation and chemotherapy as a new therapeutic strategy in non-small cell lung cancer. Semin Oncol. 2002;29:37–46. doi: 10.1053/sonc.2002.31521. [DOI] [PubMed] [Google Scholar]
- 11.Cohen S. The epidermal growth factor (EGF) Cancer. 1983;51:1787–1791. doi: 10.1002/1097-0142(19830515)51:10<1787::aid-cncr2820511004>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 12.Derynck R. Transforming growth factor alpha. Cell. 1988;54:593–595. doi: 10.1016/s0092-8674(88)80001-1. [DOI] [PubMed] [Google Scholar]
- 13.Jorissen RN, Walker F, Pouliot N, et al. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 14.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–2799. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 15.Brabender J, Danenberg KD, Metzger R, et al. Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer Is correlated with survival. Clin Cancer Res. 2001;7:1850–1855. [PubMed] [Google Scholar]
- 16.Pastorino U, Andreola S, Tagliabue E, et al. Immunocytochemical markers in stage I lung cancer: relevance to prognosis. J Clin Oncol. 1997;15:2858–2865. doi: 10.1200/JCO.1997.15.8.2858. [DOI] [PubMed] [Google Scholar]
- 17.Rusch V, Klimstra D, Venkatraman E, et al. Overexpression of the epidermal growth factor receptor and its ligand transforming growth factor alpha is frequent in resectable non-small cell lung cancer but does not predict tumor progression. Clin Cancer Res. 1997;3:515–522. [PubMed] [Google Scholar]
- 18.Fontanini G, Vignati S, Bigini D, et al. Epidermal growth factor receptor (EGFr) expression in non-small cell lung carcinomas correlates with metastatic involvement of hilar and mediastinal lymph nodes in the squamous subtype. Eur J Cancer. 1995;31A:178–183. doi: 10.1016/0959-8049(93)00421-m. [DOI] [PubMed] [Google Scholar]
- 19.Veale D, Ashcroft T, Marsh C, et al. Epidermal growth factor receptors in non-small cell lung cancer. Br J Cancer. 1987;55:513–516. doi: 10.1038/bjc.1987.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai WW, Chen FF, Wu MH, et al. Immunohistochemical analysis of epidermal growth factor receptor family members in stage I non-small cell lung cancer. Ann Thorac Surg. 2001;72:1868–1876. doi: 10.1016/s0003-4975(01)03207-6. [DOI] [PubMed] [Google Scholar]
- 21.Tateishi M, Ishida T, Mitsudomi T, et al. Immunohistochemical evidence of autocrine growth factors in adenocarcinoma of the human lung. Cancer Res. 1990;50:7077–7080. [PubMed] [Google Scholar]
- 22.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37 (Suppl 4):S9–15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 23.Cortes-Funes H, Soto Parra H. Extensive experience of disease control with gefitinib and the role of prognostic markers. Br J Cancer. 2003;89 (Suppl 2):S3–8. doi: 10.1038/sj.bjc.6601476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanematsu T, Yano S, Uehara H, et al. Phosphorylation, but not overexpression, of epidermal growth factor receptor is associated with poor prognosis of non-small cell lung cancer patients. Oncol Res. 2003;13:289–298. doi: 10.3727/096504003108748348. [DOI] [PubMed] [Google Scholar]
- 25.Masunaga R, Kohno H, Dhar DK, et al. Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. Clin Cancer Res. 2000;6:4064–4068. [PubMed] [Google Scholar]
- 26.Gallo O, Franchi A, Magnelli L, et al. Cyclooxygenase-2 pathway correlates with VEGF expression in head and neck cancer. Implications for tumor angiogenesis and metastasis. Neoplasia. 2001;3:53–61. doi: 10.1038/sj.neo.7900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HS, Youm HR, Lee JS, et al. Correlation between cyclooxygenase-2 and tumor angiogenesis in non-small cell lung cancer. Lung Cancer. 2003;42:163–170. doi: 10.1016/s0169-5002(03)00290-3. [DOI] [PubMed] [Google Scholar]
- 28.Khuri FR, Wu H, Lee JJ, et al. Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res. 2001;7:861–867. [PubMed] [Google Scholar]
- 29.Coffey RJ, Hawkey CJ, Damstrup L, et al. Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci U S A. 1997;94:657–662. doi: 10.1073/pnas.94.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vadlamudi R, Mandal M, Adam L, et al. Regulation of cyclooxygenase-2 pathway by HER2 receptor. Oncogene. 1999;18:305–314. doi: 10.1038/sj.onc.1202307. [DOI] [PubMed] [Google Scholar]
- 31.Moraitis D, Du B, De Lorenzo MS, et al. Levels of cyclooxygenase-2 are increased in the oral mucosa of smokers: evidence for the role of epidermal growth factor receptor and its ligands. Cancer Res. 2005;65:664–670. [PubMed] [Google Scholar]
- 32.Richardson CM, Richardson D, Swinson DE, et al. Cyclooxygenase-2 protein levels are independent of epidermal growth factor receptor expression or activation in operable non-small cell lung cancer. Lung Cancer. 2005;48:47–57. doi: 10.1016/j.lungcan.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 34.Smythe WR. Treatment of stage I non-small cell lung carcinoma. Chest. 2003;123:181S–187S. [PubMed] [Google Scholar]
- 35.Selvaggi G, Novello S, Torri V, et al. Epidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non-small-cell lung cancer. Ann Oncol. 2004;15:28–32. doi: 10.1093/annonc/mdh011. [DOI] [PubMed] [Google Scholar]
- 36.Ali-Fehmi R, Che M, Khalifeh I, et al. The effect of cyclooxygenase-2 expression on tumor vascularity in advanced stage ovarian serous carcinoma. Cancer. 2003;98:1423–1429. doi: 10.1002/cncr.11650. [DOI] [PubMed] [Google Scholar]
- 37.Han SW, Hwang PG, Chung DH, et al. Epidermal growth factor receptor (EGFR) downstream molecules as response predictive markers for gefitinib (Iressa, ZD1839) in chemotherapy-resistant non-small cell lung cancer. Int J Cancer. 2005;113:109–115. doi: 10.1002/ijc.20550. [DOI] [PubMed] [Google Scholar]
- 38.Kim SJ, Rabbani ZN, Vollmer RT, et al. Carbonic Anhydrase IX in Early-Stage Non-Small Cell Lung Cancer. Clin Cancer Res. 2004;10:7925–7933. doi: 10.1158/1078-0432.CCR-04-0636. [DOI] [PubMed] [Google Scholar]
- 39.Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37 (Suppl 4):S3–8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 40.Olayioye MA, Neve RM, Lane HA, et al. The ErbB signaling network: receptor heterodimerization in development and cancer. Embo J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 42.Salomon DS, Brandt R, Ciardiello F, et al. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 43.Mendelsohn J. Targeting the epidermal growth factor receptor for cancer therapy. J Clin Oncol. 2002;20:1S–13S. [PubMed] [Google Scholar]
- 44.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 45.Moscatello DK, Holgado-Madruga M, Godwin AK, et al. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res. 1995;55:5536–5539. [PubMed] [Google Scholar]
- 46.Prenzel N, Zwick E, Daub H, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 47.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 48.Weinstein-Oppenheimer CR, Blalock WL, Steelman LS, et al. The Raf signal transduction cascade as a target for chemotherapeutic intervention in growth factor-responsive tumors. Pharmacol Ther. 2000;88:229–279. doi: 10.1016/s0163-7258(00)00085-1. [DOI] [PubMed] [Google Scholar]
- 49.Calo V, Migliavacca M, Bazan V, et al. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197:157–168. doi: 10.1002/jcp.10364. [DOI] [PubMed] [Google Scholar]
- 50.Bishayee A, Beguinot L, Bishayee S. Phosphorylation of tyrosine 992, 1068, and 1086 is required for conformational change of the human epidermal growth factor receptor c-terminal tail. Mol Biol Cell. 1999;10:525–536. doi: 10.1091/mbc.10.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rojas M, Yao S, Lin YZ. Controlling epidermal growth factor (EGF)-stimulated Ras activation in intact cells by a cell-permeable peptide mimicking phosphorylated EGF receptor. J Biol Chem. 1996;271:27456–27461. doi: 10.1074/jbc.271.44.27456. [DOI] [PubMed] [Google Scholar]
- 52.Zwick E, Hackel PO, Prenzel N, et al. The EGF receptor as central transducer of heterologous signalling systems. Trends Pharmacol Sci. 1999;20:408–412. doi: 10.1016/s0165-6147(99)01373-5. [DOI] [PubMed] [Google Scholar]
- 53.Mestre JR, Chan G, Zhang F, et al. Inhibition of cyclooxygenase-2 expression. An approach to preventing head and neck cancer. Ann N Y Acad Sci. 1999;889:62–71. doi: 10.1111/j.1749-6632.1999.tb08724.x. [DOI] [PubMed] [Google Scholar]
- 54.Beraud C, Henzel WJ, Baeuerle PA. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-kappaB activation. Proc Natl Acad Sci U S A. 1999;96:429–434. doi: 10.1073/pnas.96.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruns CJ, Solorzano CC, Harbison MT, et al. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926–2935. [PubMed] [Google Scholar]
- 56.O’Byrne KJ, Koukourakis MI, Giatromanolaki A, et al. Vascular endothelial growth factor, platelet-derived endothelial cell growth factor and angiogenesis in non-small-cell lung cancer. Br J Cancer. 2000;82:1427–1432. doi: 10.1054/bjoc.1999.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koukourakis MI, Giatromanolaki A, O’Byrne KJ, et al. bcl-2 and c-erbB-2 proteins are involved in the regulation of VEGF and of thymidine phosphorylase angiogenic activity in non-small-cell lung cancer. Clin Exp Metastasis. 1999;17:545–554. doi: 10.1023/a:1006780710148. [DOI] [PubMed] [Google Scholar]
- 58.Cox G, Jones JL, O’Byrne KJ. Matrix metalloproteinase 9 and the epidermal growth factor signal pathway in operable non-small cell lung cancer. Clin Cancer Res. 2000;6:2349–2355. [PubMed] [Google Scholar]
- 59.Saad RS, Liu YL, Nathan G, et al. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in colorectal cancer. Mod Pathol. 2004;17:197–203. doi: 10.1038/modpathol.3800034. [DOI] [PubMed] [Google Scholar]