Abstract
Objective
Prior research has suggested the possible role of oxidative stress in the pathogenesis of amyotrophic lateral sclerosis (ALS). Prospective data examining dietary antioxidants such carotenoids and vitamin C are limited.
Methods
Risk of ALS associated with carotenoid and vitamin C intake was investigated in 5 prospective cohorts: the National Institutes of Health – AARP Diet and Health Study, the Cancer Prevention Study II Nutrition Cohort, the Multiethnic Cohort, the Health Professionals Follow-up Study, and the Nurses Health Study. ALS deaths were documented using the National Death Index and confirmed nonfatal ALS cases were included from HPFS and NHS. A total of 1153 ALS deaths occurred among 1,100,910 participants (562,942 men; 537,968 women). Participants were categorized into cohort-specific quintiles of intake for dietary variables. We applied Cox proportional hazards regression to calculate cohort-specific risk ratios (RR), and pooled results using random-effects methods.
Results
A greater total major carotenoids intake was associated with a reduced risk of ALS (pooled, multivariable-adjusted RR for the highest to the lowest quintile: 0.75; 95% CI: 0.61 to 0.91; P trend = 0.004). Individually, higher dietary intakes of β-carotene and lutein were inversely associated with ALS risk. The pooled multivariable RRs comparing the highest to the lowest quintile for β- carotene and lutein were 0.85 (95% CI: 0.64–1.13; P trend=0.03) and 0.79 (95% CI: 0.64 to 0.96; P trend=0.01), respectively. Lycopene, β-cryptoxanthin, and vitamin C were not associated with reduced risk of ALS.
Interpretation
Consumption of foods high in carotenoids may help prevent or delay onset of ALS.
INTRODUCTION
Previous research has implicated a potential role of oxidative stress in the pathogenesis of amyotrophic lateral sclerosis (ALS). By-products of oxidative damage were reported in patients with sporadic ALS, and mutations in the gene coding copper/zinc superoxide dismutase (SOD1), which is important in the cellular antioxidant defense, are a cause of familial ALS.1–4
Reduced risk of ALS among long-term vitamin E supplement users as reported in prior prospective studies supports a role of oxidative stress and also suggests that other antioxidants derived from supplement or dietary sources like vitamin C or carotenoids could also favorably affect ALS risk.5,6 Animal studies of familial ALS indicated that although vitamin C supplementation prior to disease failed to delay age at onset, it did significantly slow progression of paralysis.7 However, several small case control studies did not observe an association between vitamin C intake and ALS progression. To our knowledge, the relationship between carotenoid intake and ALS risk has been examined in only 3 previous case-control studies.8–10 One case-control study8 with 153 ALS cases in Japan reported inverse trends across quartiles of β-carotene and another with 107 ALS cases found a lower risk of ALS among individuals with a high lycopene intake.10 However, these studies were also generally small in size and vulnerable to selection and recall biases. Therefore, we conducted a pooled analysis of 5 large prospective studies including the Nurses’ Health Study (NHS), the Health Professionals Follow-up Study (HPFS), the Cancer Prevention Study II-Nutrition Cohort (CPS-II Nutrition), the Multiethnic Cohort Study (MEC), and the National Institutes of Health – AARP Diet and Health Study (NIH-AARP) compromised over 1 million participants and over 1000 documented cases of ALS to examine whether higher intakes of vitamin C and carotenoids are associated with a reduced risk of ALS.
SUBJECTS, MATERIALS AND METHODS
The NHS began in 1976 when 121,700 women registered nurses aged 30 to 55 responded to a mailed questionnaire assessing disease history and lifestyle characteristics; 103,298 participants first completed a food frequency questionnaire (FFQ) in 1980.11 The HPFS, comprising 51,529 male health professionals aged 40 to 65 began in 1986 with participants answering a similar questionnaire.12 Subsequent follow-up of both studies continued through biennial questionnaires where participants reported major disease occurrence and information on various risk factors for chronic illnesses. The CPS-II Nutrition cohort (a subpopulation of the baseline CPS-II cohort) includes 86,404 men and 97,786 women aged 50 to 79 who were residents of 21 states with population based cancer surveillance and who completed a detailed mailed questionnaire assessing dietary and lifestyle factors in 1992.13,14 Updated exposure information was collected in 1997 and biennially thereafter. The MEC cohort study is comprised of 96,937 men and 118,843 women aged 45 to 75 with self-reported racial/ethnic backgrounds of African-American, Japanese-American, Latino, Native Hawaiian, and white.15 Participants lived primarily in Hawaii and California (Los Angeles) and completed a baseline mailed questionnaire in 1993–1996 as well as subsequent follow-up mailings every 5 years afterward. The NIH-AARP Diet and Health Study consists of 340,148 men and 227,021 women aged 50 to 71 who were residents of 6 states or 2 metropolitan areas that maintain high quality cancer registries.16 At the study baseline in 1995–1996, participants completed a mailed food frequency questionnaire. Roughly two-thirds of participants also completed a subsequent lifestyle questionnaire in 1996. All included studies were approved by the institutional review board at the institution where each study was conducted.
End-point definition
ALS deaths were documented through linkage with the National Death Index in CPS-II Nutrition, NIH-AARP, and MEC. Participants with reported International Classification of Disease (9th version) codes of 335.2, which identifies motor neuron disease as the underlying cause of death or contributing cause of death were considered ALS cases. This method of ALS case ascertainment has been validated in a previous study where virtually all certificates indicating code 335.2 also list diagnosis of ALS upon medical record review.17
Incident ALS cases were included from the NHS and HPFS. To assess occurrence of ALS in each of these studies, participants completed health questionnaires biennially where he or she was asked to report new diagnosis of specific medical conditions (which initially did not include ALS) or “any other major illness.” Starting in 1992 for NHS and in 2000 for HPFS, a question asking about ALS specifically was added to questionnaires. Participants reporting ALS (including next of kin for decedents) authorized permission to contact the treating neurologist who was then asked to complete a questionnaire confirming the diagnosis of ALS as well as to rate certainty of diagnosis (definite, probable, or possible) and to release copies of participant medical records. The final confirmation for our study purposes was made by a neurologist with experience in ALS diagnosis (Dr. Guido J Falcone and Dr. Giancarlo Logroscino) We confirmed ‘definite’ or ‘probable’ ALS diagnosis by applying the El Escorial criteria using information abstracted from participant medical records or provided by the treating neurologist. We included only confirmed events in the analysis. Incident ALS cases for which we were unable to obtain medical records or contact the treating neurologist were classified as ‘possible’ and excluded from the primary analysis until death. Then he or she was included in all analyses as an ALS death if the disease was listed on the death certificate.
Assessment of diet and other covariates
Each cohort assessed dietary intake using semi-quantitative food frequency questionnaires (FFQs) designed for or adapted to each cohort.11,12,14–16,18 Participants reported routine intake of each food during the preceding year on a scale ranging from never or less than 1 time per month to 6 servings or more per day. Nutrient intakes were then calculated using the product of the frequency response and nutrient content by each specified portion size. Detailed information was collected on multivitamin use and supplemental vitamins C and E as well as β-carotene on each questionnaire. Supplemental dose and duration were also available in NHS, HPFS, and MEC.
For NIH-AARP and MEC dietary information was collected at baseline and for CPS-II Nutrition information on diet was collected at baseline and updated 7-years later. Diet was assessed at baseline and updated every 4 years in the NHS and HPFS. Validation and reproducibility studies for carotenoids and vitamin C intake were conducted in a subset of participants for all cohorts.12,18–24 For the HPFS, correlations between participant’s baseline FFQ and the average of two one-week diet records for total vitamin C and carotene intake were 0.92 and 0.64, respectively, and for dietary vitamin C, the correlation was 0.77.22 For NIH-AARP, correlations between an FFQ and two non-consecutive 24-hour diet recalls were 0.70 in men and 0.65 in women for vitamin C.24 In the NHS, the correlation between total and dietary vitamin C intake assessed by FFQ and by 3 one-week diet records were 0.76 and 0.64, respectively.25 Among non-smokers in the NHS, statistically significant adjusted correlations between plasma carotenoid markers and dietary measures from the FFQ were 0.48 for α-carotene, 0.27 for β-carotene, 0.32 for β-cryptoxanthin, 0.27 for lutein, and 0.21 for lycopene. For non-smoking men in the HPFS, significant adjusted correlations were generally slightly higher. Correlations between plasma carotenoid and dietary measures were 0.47 for α-carotene, 0.35 for β-carotene, 0.43 for β-cryptoxanthin, 0.40 for lutein, and 0.47 for lycopene. It should be noted, however, that these correlations between intake and plasma levels underestimate the validity of the long term dietary intakes (assessed on the FFQ), because are not corrected for 1) error in estimating plasma levels; and 2) short term variations (seasonal, etc.) in plasma levels.26 In CPS-II Nutrition, correlations between replicate administrations of the baseline FFQ were ≥ 0.65 for both dietary vitamin C and β-carotene while the correlation between baseline dietary assessment via FFQ and 4 24-hour diet recalls for vitamin C was 0.65 and for β-carotene was 0.38 in men and 0.30 in women.18 In MEC, weighted kappa coefficients comparing 324 diet-recalls with the baseline FFQ across 6 categories were 0.65 for use of vitamin C supplements and 0.49 for the less common β-carotene supplementation.23
Information on other covariates of interest including smoking status, height, weight, education level, and physical activity was collected at baseline for all cohorts.
Statistical analysis
We excluded 47,335 participants (4.3%) with extreme energy intake, which we defined as a loge transformed energy intake greater than 3 standard deviations above the study-specific mean loge transformed energy intake. In NIH-AARP we excluded 3.6% of participants who reported serious illnesses at baseline (leaving 328,108 men and 218,106 women). The duration of follow-up was calculated from baseline to the minimum time to ALS symptom onset (NHS, HPFS), ALS death or death from any other cause or the end of follow-up. The conclusion of follow-up was June 2008 for NHS, January 2008 for HPFS, December 2006 for CPS-II Nutrition, December 2007 for MEC, and December 2008 for NIH-AARP.
Because of the extended follow-up period in NHS and in order to reduce random measurement error associated with a single dietary exposure estimate, we divided follow-up time into two asymptotically uncorrelated segments 1980 to 1994 and 1994 to 2008 allowing us to update dietary information. Asymptotically, periods of follow-up time are uncorrelated even though they may be derived from the same persons.26 We used baseline dietary assessment for other cohorts. Within each cohort, we energy-adjusted all nutrients using the residual method.27 Participants from each of the 5 cohorts were then categorized into cohort-specific quintiles of intake for a given exposure, as differences in intake across studies may in part reflect differences in the instruments used to measure diet.
We also assessed the relationship between ALS and vitamin C supplemental intake (derived from supplement use and multivitamin use) categorized as never users, <400 mg/d, 400-<700mg/day, and ≥700mg/day for each cohort.
We applied Cox proportional hazards regression stratified by age in years to calculate cohort-specific hazard ratios and associated 95% confidence intervals. For CPS-II Nutrition, MEC, and NIH-AARP analyses were conducted in men and women separately. We pooled estimates using DerSimonian and Laird methods for random effects and assessed heterogeneity using Q statistics.28 Within study specific multivariate Cox models, we adjusted for potential confounders, such as BMI (continuous), physical activity (approximate tertiles), education level (<high school, high school, >high school), smoking status (non-smoker, past, current), total vitamin E intake (quartiles), as uniformly as possible across all studies. We tested for trend across all categorical analyses of vitamin C and carotenoids by creating a new variable where participants in a certain category were assigned the median value for that category and added this variable to the model as a continuous covariate.26
We assessed effect modification by age (≤60 years,>60 years at baseline), smoking status (ever smoker, never smoker at baseline), and vitamin E supplement use (yes, no at baseline). To address the possibility that participants could be experiencing symptoms of ALS at the time of questionnaire completion, we conducted a lagged analysis excluding the first 4 years of follow-up in each cohort.
All statistical analyses were conducted using SAS, version 9.2 (SAS Institute, Cary, North Carolina).
RESULTS
Over a total of 13,370,698 person-years (median follow-up time: 11 years, 5th percentile: 5 years, 95th percentile: 28 years), 1153 cases of ALS occurred among 562,942 men and 537,968 women across the 5 cohorts. Following exclusion of participants with implausible energy intakes, there remained 1093 cases of ALS and 1,053,575 participants (543,433 men; 510142 women). Individuals with high dietary carotenoid intakes tended to exercise more, have more advanced education, have higher intakes of dietary vitamin C, and were generally more likely to take vitamin C and E supplements (Table 1). Smoking was increasingly less common, while age as well as body mass index were roughly constant across quintiles of dietary carotenoid intake. Participant characteristics were distributed similarly across quintiles of dietary vitamin C intake. The proportion of individuals taking vitamin C supplements at baseline ranged from 19.4% in NIH-AARP to 58% in MEC. Median intakes of dietary carotenoids and vitamin C were relatively consistent across studies and ranged from 8.8 mg/day in CPS-II Nutrition to 15.5 mg/day in the HPFS for total major carotenoid intake, and from 121 mg/day in NHS in 1980 to 160 mg/day in HPFS for dietary vitamin C intake.
Table 1.
Mean or frequency of selected age-adjusteda characteristics of the 5 included cohorts.
| STUDY | No. of ALS cases (men/women) |
Baseline Cohort Sizeb |
Quintiles of Dietary Carotenoid Intake |
||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Nurse Health Study (1980–2004) | 101 | 92 221 | |||||
| Age (years)c | 45.9 | 46.4 | 47.0 | 47.1 | 47.7 | ||
| Body Mass Index (kg/m2) | 24.6 | 24.4 | 24.5 | 24.4 | 24.1 | ||
| Current Smokers (%) | 33.2 | 30.0 | 28.5 | 27.3 | 25.3 | ||
| High Level of Physical Activity (%) | 19.9 | 23.8 | 25.2 | 28.1 | 32.8 | ||
| Vitamin C Supplement Use (%) | 15.6 | 17.3 | 19.4 | 20.5 | 24.0 | ||
| Vitamin E Supplement Use (%) | 10.7 | 11.5 | 13.1 | 14.1 | 16.7 | ||
| Dietary Vitamin C Intake (mg/day) | 90.4 | 115.9 | 130.9 | 144.0 | 172.3 | ||
|
Health Professional’s Follow-up Study (1986 – 2004) |
63 | 49 934 | |||||
| Age (years)c | 55.2 | 54.4 | 54.5 | 54.7 | 55.1 | ||
| Body Mass Index (kg/m2) | 25.5 | 25.5 | 25.5 | 25.6 | 25.5 | ||
| Current Smokers (%) | 13.6 | 10.4 | 9.0 | 8.3 | 7.0 | ||
| High Level of Physical Activity (%) | 26.5 | 30.4 | 33.7 | 36.4 | 39.8 | ||
| Vitamin C Supplement Use (%) | 30.5 | 33.9 | 37.5 | 39.5 | 41.5 | ||
| Vitamin E Supplement Use (%) | 16.0 | 18.3 | 19.7 | 22.6 | 24.6 | ||
| Dietary Vitamin C Intake (mg/day) | 125.4 | 152.3 | 170.5 | 189.6 | 228.5 | ||
| Multiethnic Cohort Study (1993–1997) | 140 (83/57) | 205 914 | |||||
| Age (years)c | 59.9 | 59.1 | 59.9 | 60.8 | 61.4 | ||
| Male, (%) | 43.3 | 45.7 | 46.4 | 45.8 | 44.0 | ||
| High School Education or less (%) | 50.6 | 45.7 | 42.8 | 41.2 | 38.8 | ||
| Body Mass Index (kg/m2) | 26.2 | 26.1 | 26.0 | 25.8 | 25.6 | ||
| Current Smokers (%) | 21.5 | 17.2 | 14.5 | 13.3 | 11.4 | ||
| High Level of Physical Activity (%) | 24.8 | 28.2 | 30.4 | 32.5 | 35.3 | ||
| Vitamin C Supplement Use (%) | 65.1 | 61.6 | 58.0 | 64.9 | 50.7 | ||
| Vitamin E Supplement Use (%) | 72.9 | 70.4 | 67.8 | 64.9 | 59.7 | ||
| Dietary Vitamin C Intake (mg/day) | 97.1 | 130.0 | 154.7 | 183.0 | 244.8 | ||
|
Cancer Prevention Study II – Nutrition Cohort (1992–2005) |
221 (126/95) | 165 683 | |||||
| Age (years)c | 63.1 | 63.1 | 63.1 | 63.3 | 63.1 | ||
| Male, (%) | 35.2 | 40.3 | 45.6 | 52.5 | 62.7 | ||
| High School Education or less (%) | 44.2 | 35.5 | 29.9 | 25.6 | 21.8 | ||
| Body Mass Index (kg/m2) | 26.0 | 26.0 | 26.0 | 26.0 | 25.9 | ||
| Current Smokers (%) | 12.2 | 9.4 | 7.9 | 7.2 | 6.5 | ||
| High Level of Physical Activity (%) | 28.8 | 34.5 | 38.1 | 42.3 | 46.5 | ||
| Vitamin C Supplement Use (%) | 42.1 | 44.8 | 46.3 | 47.9 | 51.0 | ||
| Vitamin E Supplement Use (%) | 16.0 | 18.0 | 19.6 | 21.6 | 26.1 | ||
| Dietary Vitamin C Intake (mg/day) | 97.5 | 119.7 | 134.2 | 151.2 | 177.2 | ||
| NIH – AARP Diet and Health Study (1995–2005) | 568 (384/184) | 539 823 | |||||
| Age (years)c | 61.7 | 61.7 | 61.6 | 61.6 | 61.6 | ||
| Male, (%) | 55.4 | 58.7 | 60.8 | 62.7 | 48.4 | ||
| High School Education or less (%) | 33.8 | 26.9 | 24.2 | 22.4 | 21.5 | ||
| Body Mass Index (kg/m2) | 27.0 | 27.1 | 27.1 | 27.1 | 27.0 | ||
| Current Smokers (%) | 18.0 | 12.5 | 10.6 | 9.5 | 9.3 | ||
| High Level of Physical Activity (%) | 14.7 | 16.8 | 18.7 | 21.0 | 25.6 | ||
| Vitamin C Supplement Use (%) | 34.8 | 35.9 | 41.7 | 44.9 | 49.5 | ||
| Vitamin E Supplement Use (%) | 32.0 | 35.9 | 38.7 | 42.0 | 46.9 | ||
| Dietary Vitamin C Intake (mg/day) | 113.3 | 143.2 | 163.0 | 182.8 | 229.4 | ||
Values are means (SD) or percentages and are standardized to the age distribution of the specific study population;
After exclusions for implausible energy intake
Value is not age-adjusted.
High intakes of total dietary carotenoid were associated with a lower risk of ALS: individuals in the highest quintile experienced a 25% lower risk of ALS when compared with those in lowest in the pooled multivariate analysis (Table 2, relative risk (RR)= 0.75; 95% CI:0.61–0.91; p for trend = 0.004). Smoking is a well-known modulator of carotenoid levels, and among never smokers the effect was stronger where the pooled multivariate RR comparing the highest to the lowest quintiles was 0.65 (362 cases; 95% CI: 0.44–0.97). Individually, β-carotene and lutein were associated with a reduced risk of ALS (Figure; Table 3). The pooled multivariate RR comparing individuals in the top quintile with those in the bottom was 0.85 (95% CI: 0.64 to 1.13; p for trend = 0.03) for β-carotene and 0.79 (95% CI 0.64 to 0.96; p for trend = 0.01): for lutein. Dietary α-carotene, β-cryptoxanthin and lycopene were not individually associated with risk of ALS (all p for trend >0.2). Post-hoc analyses using deciles indicated stronger associations for β- carotene and lutein where we observed 26% and 30% reductions in risk of ALS comparing the top to the bottom decile for β- carotene and lutein, respectively (β-carotene: RR = 0.74; 95% CI: 0.55 to 1.00; P for trend = 0.007; lutein: 0.70; 95% CI: 0.52 to 0.96; P for trend = 0.003). We observed no association between β-carotene supplement use and risk of ALS; the pooled multivariate RR was 0.88 (95% CI: 0.65–1.20).
Table 2.
Pooled estimates of risk of amyotrophic lateral sclerosis associated with cohort specific quintiles of total major dietary carotenoid intake
|
Quintiles of Dietary Carotenoid Intake |
|||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Pbtrend | Pcheterogeneity | |
| Total Dietary Carotenoid Intake | |||||||
| Cases | 234 | 229 | 222 | 229 | 179 | ||
| Age-adjusted HR (95% CI)d | 1.00 [ref] | 0.90 (0.70–1.17) | 0.93 (0.77–1.11) | 0.95 (0.79–1.14) | 0.74 (0.60–0.91) | 0.003 | 0.57 |
| Multivariate-adjusted HR (95% CI)e | 1.00 [ref] | 0.91 (0.71–1.18) | 0.94 (0.78–1.13) | 0.96 (0.79–1.15) | 0.75 (0.61–0.91) | 0.004 | 0.65 |
Includes intake from β-carotene, α-carotene, lutein, lycopene and β-cryptoxanthin.
Ptrend calculated using median value for each quintile
P- value for heterogeneity is calculated from the Q-statistic and is used to quantify differences between studies.
Adjused for age (years) and sex,
Adjused for age (years), sex, smoking status (never, past, current), total vitamin E intake (quartiles), body mass index (<23, 23-<25, 25-<30, ≥30), physical activity (low, average, high), educational level (<high school, high school, >high school), and total calories.
Figure 1. Study-specific and pooled multivariable relative risks (95% CI) of ALS for 2,500µg/day increase in intake of β-carotene1.
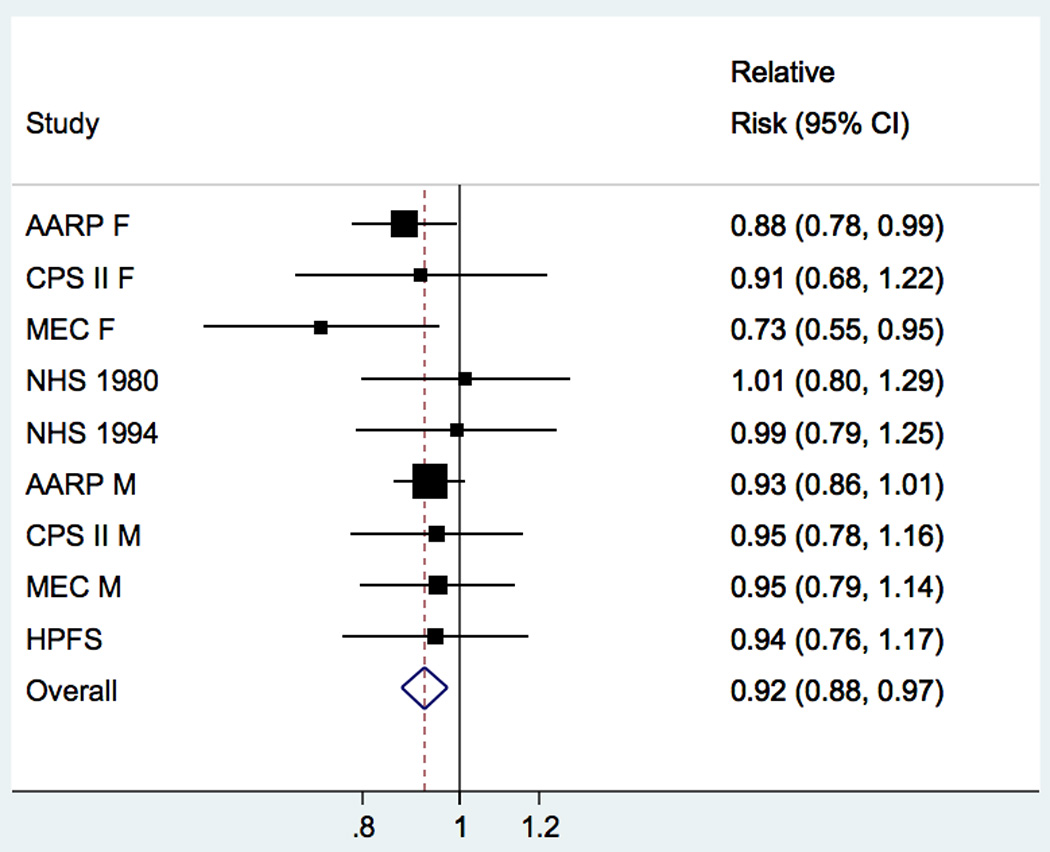
Study-specific and pooled multivariable relative risk (RR) and 95% confidence interval (CI) of amyotrophic lateral sclerosis for a 2,500µg change in intake of β-carotene Pooled RR = 0.92 (95% CI: 0.88–0.97; P = 0.003). In men, a 2500μg increase in β-carotene was associated with a 6% reduced risk of ALS whereas in women the effect appeared slightly stronger and a 2500μg increase in β-carotene was associated with a 10% reduced risk although these results did not significantly differ from one another. The squares and horizontal lines correspond to the study specific multivariable RR and 95% CI, respectively. The inverse of the variance is used to calculate study weight and is represented by the area of the square. The diamond displays the pooled multivariable RR and 95% CI.
1There is approximately 2,500µg β-carotene in 1/3 of a medium sized carrot. Increment is based on mean standard deviations of mean intake of nutrients across studies. Among women, β-carotene intake ranged from 2206μg per day in CPS-II Nutrition to 3892μg in MEC and among men, β-carotene intake ranged from 2750μg per day in CPS-II Nutrition to 4094μg in HPFS.
Table 3.
Pooled estimates of risk of amyotrophic lateral sclerosis associated with cohort specific quintiles of dietary carotenoid intake
|
Quintiles of Dietary Carotenoid Intake |
|||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Patrend | Pbheterogeneity | |
| α-carotene | |||||||
| Cases | 232 | 205 | 218 | 224 | 214 | ||
| Age-adjusted HR (95% CI)c | 1.00 [ref] | 0.84 (0.70–1.02) | 0.88 (0.73–1.06) | 0.86 (0.66–1.11) | 0.83 (0.69–1.00) | 0.15 | 0.66 |
| Multivariate-adjusted HR (95% CI)d | 1.00 [ref] | 0.85 (0.71–1.03) | 0.90 (0.74–1.08) | 0.88 (0.68–1.14) | 0.84 (0.70–1.02) | 0.21 | 0.69 |
| β-carotene | |||||||
| Cases | 215 | 249 | 207 | 217 | 205 | ||
| Age-adjusted HR (95% CI)c | 1.00 [ref] | 1.09 (0.85–1.41) | 0.90 (0.70–1.16) | 0.92 (0.76–1.11) | 0.85 (0.64–1.12) | 0.02 | 0.54 |
| Multivariate-adjusted HR (95% CI)d | 1.00 [ref] | 1.10 (0.86–1.41) | 0.91 (0.71–1.17) | 0.93 (0.76–1.13) | 0.85 (0.64–1.13) | 0.03 | 0.53 |
| β-cryptoxanthin | |||||||
| Cases | 214 | 213 | 233 | 230 | 203 | ||
| Age-adjusted HR (95% CI)c | 1.00 [ref] | 0.96 (0.79–1.16) | 1.03 (0.85–1.25) | 0.98 (0.79–1.22) | 0.86 (0.71–1.04) | 0.18 | 1.00 |
| Multivariate-adjusted HR (95% CI)d | 1.00 [ref] | 0.98 (0.81–1.19) | 1.05 (0.87–1.27) | 1.01 (0.82–1.24) | 0.88 (0.72–1.07) | 0.26 | 1.00 |
| Lutein | |||||||
| Cases | 220 | 257 | 222 | 210 | 184 | ||
| Age-adjusted HR (95% CI)c | 1.0 [ref] | 1.14 (0.95–1.37) | 0.94 (0.73–1.21) | 0.91 (0.75–1.10) | 0.80 (0.64–1.00) | 0.02 | 0.21 |
| Multivariate-adjusted HR (95% CI)d | 1.0 [ref] | 1.14 (0.95–1.37) | 0.94 (0.74–1.20) | 0.91 (0.75–1.10) | 0.79 (0.64–0.96) | 0.01 | 0.26 |
| Lycopene | |||||||
| Cases | 238 | 235 | 208 | 211 | 201 | ||
| Age-adjusted HR (95% CI)c | 1.00 [ref] | 0.97 (0.76–1.23) | 0.87 (0.66–1.15) | 0.92 (0.74–1.13) | 0.89 (0.74–1.08) | 0.28 | 0.53 |
| Multivariate-adjusted HR (95% CI)d | 1.00 [ref] | 0.98 (0.77–1.25) | 0.89 (0.68–1.16) | 0.94 (0.78–1.14) | 0.91 (0.75–1.10) | 0.36 | 0.62 |
Ptrend calculated using median value for each quintile
P- value for heterogeneity is calculated from the Q-statistic and is used to quantify differences in trend between studies.
Adjusted for age (years) and sex.
Adjused for age (years), sex, smoking status (never, past, current), total vitamin E intake (quartiles), body mass index (<23, 23-<25, 25-<30, ≥30), physical activity (low, average, high), educational level (<high school, high school, >high school), and total calories.
There was no association between supplemental use of vitamin C and risk of ALS (Table 4). The pooled multivariable RR for those with the highest supplemental intake (≥700 mg/day) compared with no supplemental intake was 1.04 (95% CI: 0.81 to 1.35). We also observed no association for dietary vitamin C and risk of ALS (Table 5; quintile 5 vs. quintile 1: RR: 0.87; 95% CI: 0.72 to 1.06; P for trend=0.22). After excluding supplement users, we observed no association for dietary vitamin C intake and risk of ALS (RR: 0.90; 95% CI: 0.68–1.19; P for trend=0.42).
Table 4.
Pooled estimates of risk of amyotrophic lateral sclerosis associated by categories of vitamin C supplement intake
| Vitamin C Supplement | Non Users | <400 mg/day | 400–700 mg/day | ≥700 mg/day | Patrend | Pbheterogeneity |
|---|---|---|---|---|---|---|
| Men | ||||||
| Cases | 284 | 203 | 96 | 73 | ||
| Multivariate-adjusted HR (95% CI)c | 1.00 [ref] | 1.08 (0.62–1.87) | 1.13 (0.70–1.83) | 0.98 (0.60–1.62) | 0.90 | 0.57 |
| Women | ||||||
| Cases | 171 | 140 | 69 | 57 | ||
| Multivariate-adjusted HR (95% CI)c | 1.00 [ref] | 0.86 (0.64–1.17) | 1.01 (0.71–1.45) | 0.99 (0.67–1.46) | 0.45 | 0.75 |
| Pooled analysis | ||||||
| Multivariate-adjusted HR (95% CI)c | 1.00 [ref] | 0.99 (0.72–1.37) | 1.13 (0.88–1.45) | 1.04 (0.81–1.35) | 0.68 | 0.82 |
Ptrend calculated using median value for each quintile
P- value for heterogeneity is calculated from the Q-statistic and is used to quantify differences between studies.
Adjused for age (years), sex, smoking status (never, past, current), total vitamin E intake (quartiles), body mass index (<23, 23-<25, 25-<30, ≥30), physical activity (low, average, high), educational level (<high school, high school, >high school), and total calories.
Table 5.
Pooled estimates of risk of amyotrophic lateral sclerosis associated with cohort specific quintiles of dietary vitamin C intake
|
Quintiles of Dietary Vitamin C Intake |
|||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Patrend | Pbheterogeneity | |
| Dietary Vitamin C | |||||||
| Cases | 219 | 216 | 226 | 223 | 209 | ||
| Age-adjusted HR (95% CI)c | 1.00 [ref] | 0.92 (0.74–1.14) | 0.94 (0.76–1.17) | 0.91(0.76–1.10) | 0.85 (0.71–1.03) | 0.13 | 0.87 |
| Multivariate-adjusted HR (95% CI)d | 1.00 [ref] | 0.94 (0.76–1.17) | 0.97 (0.80–1.19) | 0.94 (0.77–1.13) | 0.87 (0.72–1.06) | 0.22 | 0.73 |
|
Dietary Vitamin C among non-users of vitamin C supplement |
|||||||
| Cases | 111 | 128 | 126 | 135 | 112 | ||
| Age-adjusted HR (95% CI)c | 1.00 [ref] | 1.06 (0.82–1.39) | 1.03 (0.80–1.34) | 1.08 (0.84–1.39) | 0.90 (0.69–1.17) | 0.38 | 0.68 |
| Multivariate-adjusted HR (95% CI)d | 1.00 [ref] | 1.08 (0.81–1.43) | 1.04 (0.80–1.35) | 1.08 (0.83–1.41) | 0.90 (0.68–1.19) | 0.42 | 0.66 |
Ptrend calculated using median value for each quintile
P- value for heterogeneity is calculated from the Q-statistic and is use to quantify differences between studies.
Adjusted for age (years) and sex.
Adjused for age (years), sex, smoking status (never, past, current), total vitamin E intake (quartiles), body mass index (<23, 23-<25, 25-<30, ≥30), physical activity (low, average, high), educational level (<high school, high school, >high school), and total calories.
For the 4-year lagged analysis, results were similar, albeit slightly attenuated as expected. The pooled multivariable RRs comparing individuals in the top quintile to the bottom were 0.78 (95% CI: 0.62 to 0.97) for total major carotenoid intake, 0.88 (95% CI: 0.67–1.16) for β-carotene, and 0.80 (95% CI: 0.62 to 1.04) for lutein. Similarly, neither dietary nor supplemental vitamin C intake was associated with risk of ALS in the lagged analysis.
We observed no significant evidence of effect modification by age or vitamin E supplement use in any of the 5 cohorts. Results were also unchanged when we adjusted for smoking using continuous pack-years in multivariable models. Results did not differ in models with ALS death as the outcome (in CPS-II Nutrition, HPFS, and NHS) rather than incidence.
DISCUSSION
Results from this pooled analysis of 5 prospective cohorts suggest a potential inverse relation between total intake of major carotenoids and ALS risk. Specifically, high intakes of β-carotene and lutein were associated with reduced ALS risk. Neither supplemental use nor high dietary intake of vitamin C appeared to affect risk of ALS. Long-term vitamin C supplementation was also not associated with ALS risk.
Strengths of this study include the large number of participants and cases, extended follow-up period, and use of validated dietary assessment tools. Another primary strength of our analysis is the study’s prospective design, which minimizes recall bias that may be particularly problematic in case-control studies of dietary exposures. Furthermore, our study included ALS patients with various educational and socioeconomic backgrounds thus limiting selection bias which may arise when patients are recruited solely from tertiary care centers.30,31 Though these cohorts have been previously used to assess associations with vitamin E intake, we consider a formal correction for multiple comparisons unnecessary, because both analyses were based on specific and separate a priori hypotheses. Further, analyses of individual carotenoids were examined only following an overall significant association with total major carotenoid intake.
One limitation of our analysis is that ALS death rather than incidence is used in CPS-II Nutrition, MEC, and NIH-AARP. However, due to the rapidly progressive nature of ALS (median survival 1.5 to 3 years), we assume ALS death to be a reasonable proxy for incidence.31–33 Furthermore, previous research indicated that 70 to 90% of ALS cases of motor neuron diseases were identified through the use of death certificates.34,35 Symptoms of ALS could have influenced participants’ health behaviors or questionnaire responses at baseline, but the consistent results obtained following exclusion of the first 4 years of follow-up suggest this bias to be unlikely. Another limitation is that changes in diet could have occurred over follow-up. Nevertheless, when we re-analyzed the data taking into account updated dietary information results were consistent with those presented.
β-carotene and lutein are derived largely from dark green vegetables, which are also high in vitamin E and other phytochemicals. The persistence, in our study, of an inverse relation between carotenoid intake and ALS risk in analyses adjusted for vitamin E intake suggests that vitamin E alone cannot explain the inverse relations reported. However, a role of other compounds cannot be excluded.
The associations we observed for carotenoids are biologically plausible. The antioxidant activity of carotenoids occurs primarily through quenching of multiple types of reactive oxygen species. Individual carotenoids have different antioxidant properties. For example, the polarization of a carotenoid affects how it is orientated in the cell membrane. β-carotene congregates in the inner portion of the cell membrane, reacting with oxygen radicals and contributes to membrane stability. Lutein, a more polar xanthophyll, spans across the membrane and is more effective in protecting against by-products fromperoxyl radicals.38 The resulting reduction in oxidative reactions may help prevent mitochondrial distress, interruptions of glial glutamate transport, protein aggregation, or inflammation, thereby preventing motor neuron death.39–40
The failure to observe any association between vitamin C and reduced risk of ALS is consistent with several previous case-control studies as well as a prospective study that included the full Cancer Prevention Study of which the included CPS-II Nutrition cohort is a more detailed subset.5,8,9 As a water-soluble antioxidant, vitamin C may have differential biologic effects than other lipid-soluble antioxidants, such as carotenoids.7
To summarize, results from this pooled analysis of 5 large prospective studies of over 1 million people and almost 1100 cases of ALS indicate that high dietary intakes and supplemental use of vitamin C appear not to affect risk of ALS. Our results do, however, suggest that intake of foods high in carotenoids may help to prevent or delay the onset of ALS. Further research including food based analyses may suggest possible dietary characteristics associated with ALS prevention.
Acknowledgments
Acknowledgement statement (including conflict of interest and funding sources): This work was supported by grant R01 NS045893 from the NIH/National Institute of Neurological Diseases and Stroke. Nurses Health Study is funded by NIH program project P01 CA87969 and Health Professional Follow-up Study by NIH program project P01 CA055075.
REFERENCES
- 1.Bowling AC, Schulz JB, Brown RH, Jr, Beal MF. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J. Neurochem. 1993;61(6):2322–2325. doi: 10.1111/j.1471-4159.1993.tb07478.x. [DOI] [PubMed] [Google Scholar]
- 2.Ferrante RJ, Browne SE, Shinobu LA, et al. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J. Neurochem. 1997;69(5):2064–2074. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosen DR, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 4.Bonnefont-Rousselot D, Lacomblez L, Jaudon M, et al. Blood oxidative stress in amyotrophic lateral sclerosis. J. Neurol. Sci. 2000;178(1):57–62. doi: 10.1016/s0022-510x(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 5.Ascherio A, Weisskopf MG, O’reilly EJ, et al. Vitamin E intake and risk of amyotrophic lateral sclerosis. Ann. Neurol. 2005;57(1):104–110. doi: 10.1002/ana.20316. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, O’Reilly ÉJ, Weisskopf MG, et al. Vitamin E intake and risk of amyotrophic lateral sclerosis: a pooled analysis of data from 5 prospective cohort studies. Am. J. Epidemiol. 2011;173(6):595–602. doi: 10.1093/aje/kwq416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padayatty SJ, Katz A, Wang Y, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22(1):18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto K, Kihira T, Kobashi G, et al. Fruit and vegetable intake and risk of amyotrophic lateral sclerosis in Japan. Neuroepidemiology. 2009;32(4):251–256. doi: 10.1159/000201563. [DOI] [PubMed] [Google Scholar]
- 9.Veldink JH, Kalmijn S, Groeneveld G-J, et al. Intake of polyunsaturated fatty acids and vitamin E reduces the risk of developing amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatr. 2007;78(4):367–371. doi: 10.1136/jnnp.2005.083378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longnecker MP, Kamel F, Umbach DM, et al. Dietary intake of calcium, magnesium and antioxidants in relation to risk of amyotrophic lateral sclerosis. Neuroepidemiology. 2000;19(4):210–216. doi: 10.1159/000026258. [DOI] [PubMed] [Google Scholar]
- 11.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 12.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338(8765):464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 13.Garfinkel L. Selection, follow-up, and analysis in the American Cancer Society prospective studies. Natl Cancer Inst Monogr. 1985;67:49–52. [PubMed] [Google Scholar]
- 14.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94(2):500–511. doi: 10.1002/cncr.10197. [DOI] [PubMed] [Google Scholar]
- 15.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am. J. Epidemiol. 2000;151(4):346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am. J. Epidemiol. 2001;154(12):1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 17.Weisskopf MG, McCullough ML, Calle EE, et al. Prospective study of cigarette smoking and amyotrophic lateral sclerosis. Am. J. Epidemiol. 2004;160(1):26–33. doi: 10.1093/aje/kwh179. [DOI] [PubMed] [Google Scholar]
- 18.Flagg EW, Coates RJ, Calle EE, Potischman N, Thun MJ. Validation of the American Cancer Society Cancer Prevention Study II Nutrition Survey Cohort Food Frequency Questionnaire. Epidemiology. 2000;11(4):462–468. doi: 10.1097/00001648-200007000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Hunter DJ, Sampson L, Stampfer MJ, et al. Variability in portion sizes of commonly consumed foods among a population of women in the United States. Am. J. Epidemiol. 1988;127(6):1240–1249. doi: 10.1093/oxfordjournals.aje.a114916. [DOI] [PubMed] [Google Scholar]
- 20.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 21.Ascherio A, Stampfer MJ, Colditz GA, et al. Correlations of vitamin A and E intakes with the plasma concentrations of carotenoids and tocopherols among American men and women. J. Nutr. 1992;122(9):1792–1801. doi: 10.1093/jn/122.9.1792. [DOI] [PubMed] [Google Scholar]
- 22.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am. J. Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127-1136. [DOI] [PubMed] [Google Scholar]
- 23.Murphy SP, Wilkens LR, Hankin JH, et al. Comparison of two instruments for quantifying intake of vitamin and mineral supplements: a brief questionnaire versus three 24-hour recalls. Am. J. Epidemiol. 2002;156(7):669–675. doi: 10.1093/aje/kwf097. [DOI] [PubMed] [Google Scholar]
- 24.Thompson FE, Kipnis V, Midthune D, et al. Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health Study. Public Health Nutr. 2008;11(2):183–195. doi: 10.1017/S1368980007000419. [DOI] [PubMed] [Google Scholar]
- 25.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 26.Breslow N, Day N, et al. Statistical Methods in Cancer Research. Vol. 2 The Design and Analysis of Cohort Studies. Lyon, France: International; 1987. [PubMed] [Google Scholar]
- 27.Willett WC, et al. Nutritional Epidemiology. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.Logroscino G, Traynor BJ, Hardiman O, et al. Descriptive epidemiology of amyotrophic lateral sclerosis: new evidence and unsolved issues. J. Neurol. Neurosurg. Psychiatr. 2008;79(1):6–11. doi: 10.1136/jnnp.2006.104828. [DOI] [PubMed] [Google Scholar]
- 30.Armon C. An evidence-based medicine approach to the evaluation of the role of exogenous risk factors in sporadic amyotrophic lateral sclerosis. Neuroepidemiology. 2003;22(4):217–228. doi: 10.1159/000070562. [DOI] [PubMed] [Google Scholar]
- 31.del Aguila MA, Longstreth WT, Jr, McGuire V, Koepsell TD, van Belle G. Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology. 2003;60(5):813–819. doi: 10.1212/01.wnl.0000049472.47709.3b. [DOI] [PubMed] [Google Scholar]
- 32.Magnus T, Beck M, Giess R, et al. Disease progression in amyotrophic lateral sclerosis: predictors of survival. Muscle Nerve. 2002;25(5):709–714. doi: 10.1002/mus.10090. [DOI] [PubMed] [Google Scholar]
- 33.Drory VE, Birnbaum M, Korczyn AD, Chapman J. Association of APOE epsilon4 allele with survival in amyotrophic lateral sclerosis. J. Neurol. Sci. 2001;190(1–2):17–20. doi: 10.1016/s0022-510x(01)00569-x. [DOI] [PubMed] [Google Scholar]
- 34.O’Malley F, Dean G, Elian M. Multiple sclerosis and motor neurone disease: survival and how certified after death. J Epidemiol Community Health. 1987;41(1):14–17. doi: 10.1136/jech.41.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckley J, Warlow C, Smith P, et al. Motor neuron disease in England and Wales, 1959–1979. J. Neurol. Neurosurg. Psychiatr. 1983;46(3):197–205. doi: 10.1136/jnnp.46.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albarracin SL, Stab B, Casas Z, et al. Effects of natural antioxidants in neurodegenerative disease. Nutr Neurosci. 2012;15(1):1–9. doi: 10.1179/1476830511Y.0000000028. [DOI] [PubMed] [Google Scholar]
- 37.Han R-M, Zhang J-P, Skibsted LH. Reaction dynamics of flavonoids and carotenoids as antioxidants. Molecules. 2012;17(2):2140–2160. doi: 10.3390/molecules17022140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trotti, Rolfs, Danbolt, Brown, Hediger SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter. Nat. Neurosci. 1999;2(9):848. doi: 10.1038/12227. [DOI] [PubMed] [Google Scholar]
- 39.Trotti D, Aoki M, Pasinelli P, et al. Amyotrophic lateral sclerosis-linked glutamate transporter mutant has impaired glutamate clearance capacity. J. Biol. Chem. 2001;276(1):576–582. doi: 10.1074/jbc.M003779200. [DOI] [PubMed] [Google Scholar]
- 40.Kruman II, Pedersen WA, Springer JE, Mattson MP. ALS-linked Cu/Zn-SOD mutation increases vulnerability of motor neurons to excitotoxicity by a mechanism involving increased oxidative stress and perturbed calcium homeostasis. Exp. Neurol. 1999;160(1):28–39. doi: 10.1006/exnr.1999.7190. [DOI] [PubMed] [Google Scholar]


